Abstract
OBJECTIVE
A major determinant of the progression from insulin resistance to the development of overt type 2 diabetes is a failure to mount an appropriate compensatory β-cell hyperplastic response to maintain normoglycemia. We undertook the present study to directly explore the significance of the cell cycle protein cyclin D2 in the expansion of β-cell mass in two different models of insulin resistance.
RESEARCH DESIGN AND METHODS
We created compound knockouts by crossing mice deficient in cyclin D2 (D2KO) with either the insulin receptor substrate 1 knockout (IRS1KO) mice or the insulin receptor liver-specific knockout mice (LIRKO), neither of which develops overt diabetes on its own because of robust compensatory β-cell hyperplasia. We phenotyped the double knockouts and used RT-qPCR and immunohistochemistry to examine β-cell mass.
RESULTS
Both compound knockouts, D2KO/LIRKO and D2KO/IRS1KO, exhibited insulin resistance and hyperinsulinemia and an absence of compensatory β-cell hyperplasia. However, the diabetic D2KO/LIRKO group rapidly succumbed early compared with a relatively normal lifespan in the glucose-intolerant D2KO/IRS1KO mice.
CONCLUSIONS
This study provides direct genetic evidence that cyclin D2 is essential for the expansion of β-cell mass in response to a spectrum of insulin resistance and points to the cell-cycle protein as a potential therapeutic target that can be harnessed for preventing and curing type 2 diabetes.
The maintenance of an adequate and functional pancreatic β-cell mass dictates the body's ability to compensate for insulin resistance. Recent studies in autopsy samples from humans reported expansion of β-cell mass from infants through adolescence that was largely due to increased islet size (1). Further, humans with established type 2 diabetes exhibit a deficit in β-cell mass in comparison with their nondiabetic cohorts (2,3). Interestingly, obese, nondiabetic patients express a wide range of β-cell mass that is sufficient to maintain euglycemia up to a specific threshold, and crossing the threshold correlates with impaired fasting glucose and clinical diabetes (4).
While direct data in humans is lacking, studies in rodents clearly indicate that β-cell mass adaptively expands to compensate for both physiological and pathophysiological states of insulin resistance including pregnancy, onset of obesity, and high-fat feeding and after partial pancreatectomy (5–9). Although the adaptation is dependent on alterations in both the number and size of β-cells and generation of new β-cells from endogenous progenitors (10,11), recent studies point to replication as a primary mechanism for the physiological maintenance of adult β-cell mass (12) and in response to insulin resistance in both rodents and humans (9,12–15).
Replication is achieved by reentry of the β-cell into the cell cycle and relies on proteins regulating the G1 phase (13,16–19). Our previous work has established that cyclin D2, a G1/S cell-cycle regulator, is necessary for the postnatal expansion of β-cell mass (13). In these studies, we observed that in the absence of cyclin D2, the diminished β-cell mass established during the neonatal remodeling period was inadequate to sufficiently respond to metabolic demand for insulin in adult mice, leading to glucose intolerance but not frank diabetes (13). While these experiments indicate that cyclin D2 is important during early postnatal expansion of β-cells for the adult mouse to achieve its optimal β-cell mass, its importance in cell expansion in response to a pathophysiological demand for insulin is not known. Considering these observations, we explored whether a limited but adequate β-cell mass that is challenged by physiological stress would fall below a functional threshold required to maintain euglycemia, and eventually to promote the development of diabetes, using cyclin D2 knockout mice. To this end, we created compound double knockouts by breeding cyclin D2 (D2KO) mice with either mice that are deficient in insulin receptor substrate 1 (IRS1KO) (20,21) or mice with a knockout of the insulin receptor specifically in liver (LIRKO) (22). These models reflect the spectrum of insulin resistance and glucose intolerance observed in humans but do not develop frank diabetes in part because of compensatory β-cell expansion that can increase from 3-fold (IRS1KO) to 30-fold (LIRKO) largely by replication of β-cells (21,23).
Our results indicate that both D2KO/LIRKO and D2KO/IRS1KO double-knockout mice fail to show a β-cell compensatory response to insulin resistance, leading to overt diabetes that is secondary, in part, to a dramatic decrease in β-cell mass due to reduced β-cell replication. These data provide genetic evidence that cyclin D2 is essential for the compensatory increase in β-cell hyperplasia in response to insulin-resistant states.
RESEARCH DESIGN AND METHODS
Animal breeding and genotyping.
All studies and procedures were performed after approval according to the institutional animal committee regulations in both institutions. The creation and characterization of the LIRKO, IRS1KO, and D2KO mice has previously been described (20–22,24). All mice were maintained on a 12-h light/12-h dark cycle with ad libitum access to water and food, and a mixed mating scheme was adopted for breeding. In the first group, males and females homozygous for loxP sites (IRLox) and expressing Cre recombinase on an albumin promoter were bred with mice lacking cyclin D2. In the second group, males and females carrying either the insulin receptor substrate 1 or cyclin D2 alleles were used. In the D2KO/LIRKO study, the breeding generated mice homozygous for loxP (controls), LIRKO, D2KO/LIRKO, and D2KO, and in the D2KO/IRS1KO study the genotypes included IRS1KO (cycD2+/−;irs1−/−), D2KO/IRS1KO (cycD2−/−;irs1−/−), D2KO (cycD2−/−;irs1+/−), and their controls (cycD2+/−;irs1+/−). DNA extracted from tails was used for PCR-based genotyping (13,22). All studies included animals from a mixed genetic background. Pancreata were collected for immunohistochemical analyses (25), and islets were isolated using previously described methods (26).
Glucose and insulin tolerance tests and plasma insulin levels.
Following a 16-h fast, baseline blood glucose levels were measured from tail-vein samples using a glucometer (LifeScan, Milpitas, CA). Glucose tolerance tests were performed (25) in 7-week-old (D2KO/LIRKO) or 25-week-old (D2KO/IRS1KO) mice. Plasma insulin levels were measured by ELISA (Linco Research). Insulin tolerance tests were performed on 25-week-old mice (25).
Real-time RT–quantitative PCR.
Quantitative real-time RT-PCR was performed on total RNA samples extracted from islets (RNeasy Mini Kit; Qiagen, Valencia, CA). cDNA samples were amplified by using the SYBR Green PCR Master Mix (Applied Biosystems) and analyzed on an ABI PRISM 7900 sequence detection system (Applied Biosystems). All primer sequences are available in the online appendix, available at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0838/DC1.
Immunohistochemistry and β-cell mass analyses.
The pancreata were processed for immunohistochemical analyses as previously described (25). Primary antibodies included the following: mouse anti-glucagon (1:1,000; Sigma), guinea pig anti-insulin (1:500; Dako), rabbit anti-somatostatin (1:50; Abcam), rabbit anti–Ki-67 (1:50; BD Biosciences), cyclin D1 (1:800; Santa Cruz Biotechnology), rabbit anti–cyclin D2 (1:4,000; Santa Cruz Biotechnology), cyclin D3 (1:200; Santa Cruz Biotechnology), goat anti–PDX-1 (1:300; gift from C.V.E. Wright, PhD, Department of Cell and Developmental Biology, Vanderbilt University, Nashville, TN), rabbit anti-FoxO1 (1:50; Cell Signaling Technology), and rabbit anti-GLUT2 (1:100; Alpha Diagnostic). DAPI (Sigma-Aldrich) was used for nuclear staining. β-Cell size was assessed by quantifying the area of cells that costained with β-catenin (mouse monoclonal antibody; BD Biosciences) and insulin using ImageJ software (http://rsb.info.nih.gov/ij/). β-Cell mass was analyzed as previously described (27).
Statistical analyses.
All data are expressed as means ± SEM. Statistical significance was determined by an unpaired Student's t test or ANOVA as appropriate, and a P value <0.05 was used to reject the null hypothesis.
RESULTS
D2KO/LIRKO mice exhibit severe hyperglycemia and early diabetes.
We crossed the LIRKO mice with D2KO mice to generate compound double knockouts to investigate the requirement of cyclin D2 in the β-cell compensatory response to insulin resistance. Qualitatively similar data were obtained in females and males. Therefore, only data from males are reported. All mice were born in a normal Mendelian ratio, and we did not observe embryonic lethality. However, we observed an early lethality in the double knockouts, with several mice dying by age ∼3 months. Therefore, we phenotyped 7-week-old animals and used 10- to 12-week-old mice for islet isolation for gene expression or dissected the pancreas for immunohistochemistry.
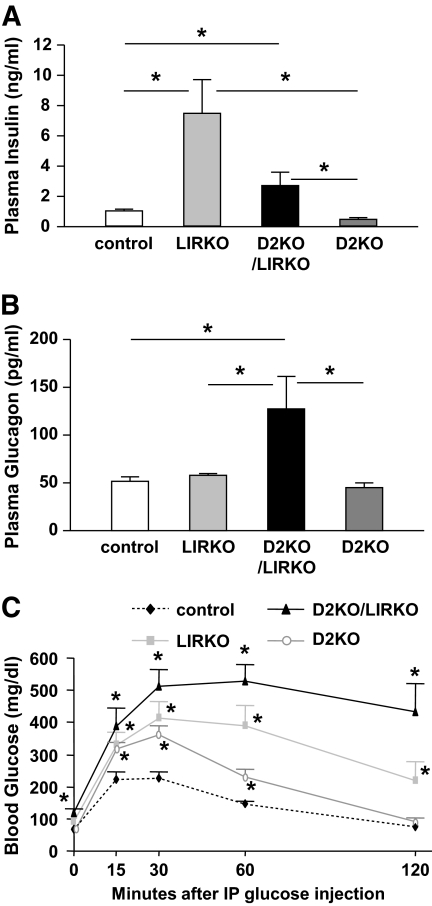
No significant differences were observed in body weights between LIRKO and control mice (Table 1), as reported previously (22). Consistent with previous data (23), LIRKOs exhibited hyperinsulinemia compared with controls (Fig. 1A), and no significant differences were observed in blood glucose between groups in 10- to 12-week-old mice. The D2KOs were smaller than the controls and exhibited mild hyperglycemia that was secondary to hypoinsulinemia (Table 1; Fig. 1A). On the other hand, the compound double knockouts showed a significant decrease in body weight, severe hyperglycemia (>600 mg/dl) (Table 1), and hyperinsulinemia, although their absolute insulin levels were still significantly lower than those in individual LIRKO mice (Fig. 1A). Further, the circulating glucagon levels were significantly increased in the double knockouts compared with those of all other groups (Fig. 1B).
TABLE 1.
Body weight and blood glucose of mice
| Body Weight (g) | P vs. control | Nonfasted blood glucose (mg/dl) | P vs. control | |
|---|---|---|---|---|
| 10–12 weeks of age | ||||
| Control | 24.8 ± 1.5 | 149 ± 9 | ||
| LIRKO | 24.2 ± 1.7 | NS | 198 ± 40 | NS |
| D2KO/LIRKO | 18.5 ± 1.4 | <0.01 | >600 | <0.000,001 |
| D2KO | 19.7 ± 1.4 | 0.024 | 188 ± 22 | 0.042 |
| 25 weeks of age | ||||
| Control | 23.1 ± 0.9 | 172 ± 5 | ||
| IRS1KO | 18.4 ± 1.1 | 0.012 | 176 ± 9 | NS |
| D2KO/IRS1KO | 13.5 ± 0.4 | <0.005 | 390 ± 41 | <0.005 |
| D2KO | 21.2 ± 0.8 | NS | 253 ± 18 | <0.005 |
Data are means ± SE (n = 5–9 male mice). NS, nonsignificant.
FIG. 1.
D2KO/LIRKO mice exhibit severe hyperglycemia and early diabetes. A and B: Plasma insulin (A) and glucagon (B) levels were measured at random fed states in control (white), LIRKO (light gray), D2KO/LIRKO (black), and D2KO (dark gray) mice (n = 4–10). *P < 0.05 as indicated by bars. C: Glucose tolerance tests were performed in 7-week-old mice, and blood glucose was measured at 0, 15, 30, 60, and 120 min after intraperitoneal (IP) injection of glucose (2 g/kg body wt). *P < 0.05 vs. controls; n = 4–8.
Our previous studies have shown that D2KO animals were glucose intolerant but were not severely diabetic despite a diminished β-cell mass (13). In this study, we observed severe intolerance as early as 7 weeks of age in both the LIRKO and D2KO/LIRKO mice compared with both the controls and the mildly glucose-intolerant D2KOs (Fig. 1C). These data indicate that absence of cyclin D2 in LIRKO mice leads to overt diabetes and early death in the double mutants.
Absence of cyclin D2 limits islet hyperplasia in LIRKO mice.
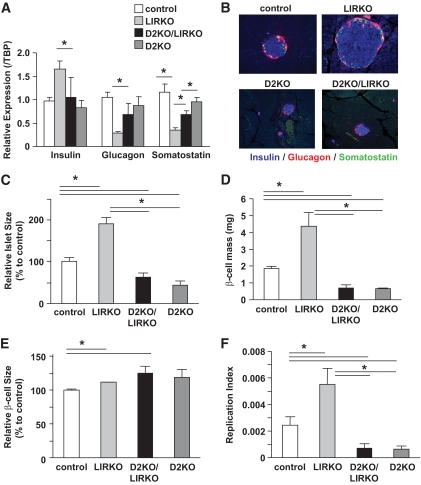
To evaluate the alterations in islet morphology, we analyzed pancreas sections by immunohistochemistry using a cocktail of antibodies to non–β-cell hormones (Fig. 2B). In parallel, we measured hormone gene expression using quantitative real-time RT-qPCR, and these results are presented in Fig. 2A. The significant increase in insulin gene expression observed in LIRKO islets was lost in the double knockout islets (Fig. 2A) and was consistent with the relatively lower circulating insulin levels in the double knockouts (Fig. 1A). On the contrary, the reduced glucagon gene expression observed in LIRKO islets was not detected in D2KO/LIRKO islets and in fact the circulating glucagon levels were increased in the double knockouts (Fig. 1B). Further, the decrease in somatostatin gene expression in the LIRKO group was attenuated in the D2KO/LIRKO islets.
FIG. 2.
Absence of cyclin D2 limits islet hyperplasia in LIRKO mice. A: Real-time RT-qPCR was performed on RNA extracted from islets of control, LIRKO, D2KO/LIRKO, and D2KO mice (n = 4–9). Results are normalized to TATA-binding protein (TBP) and expressed relative to controls. *P < 0.05 vs. groups as indicated. B: Triple immunostaining for insulin (blue), somatostatin (green), and glucagon (red) on pancreas sections from control, LIRKO, D2KO/LIRKO, and D2KO mice. A representative islet for each group at magnification 40× is presented (n = 4). C: Relative islet size was assessed from triple immunostaining (presented in B). Quantification with Image J software of islet area is presented (means ± SEM from n ≥10 islets counted per mouse; n = 4–6 in each group). D: β-Cell mass was assessed as described in research design and methods (n = 4–6). E: Relative β-cell size was assessed by coimmunostaining for β-catenin, insulin, and DAPI in pancreas sections from control, LIRKO, D2KO/LIRKO, and D2KO mice (n = 4–6). Quantification with Image J software of relative β-cell area is presented (means ± SEM from n ≥100 cells counted per mouse; n = 4–6 in each group). F: β-Cell proliferation was assessed by coimmunostaining for Ki-67, insulin, and DAPI in pancreas sections from control, LIRKO, D2KO/LIRKO, and D2KO mice. Respective replication index is presented. *P < 0.05 vs. groups as indicated; n = 4–6. In all cases, at least two to three pancreas sections were used for each animal. (A high-quality digital representation of this figure is available in the online issue.)
Examination of pancreas sections revealed a normal islet architecture and cell distribution in all groups (Fig. 2B). As expected, we observed robust islet hyperplasia in the LIRKO mice compared with islet hypoplasia in the D2KO pancreas. Despite the hyperinsulinemia and insulin resistance, the D2KO/LIRKO mice failed to show islet hyperplasia and presented a morphology that was largely similar to that observed in D2KO islets.
Whereas the size of islets is increased in the LIRKO group compared with that in controls, it was reduced in D2KO/LIRKO and D2KO mice (Fig. 2B and C). This absence of hyperplasia in D2KO/LIRKO islets was associated with a dramatic decrease in β-cell mass (Fig. 2D). Consistent with our previous report (23), LIRKO islets showed a 2.4-fold increase in β-cell mass compared with controls as a result of hyperplasia (Fig. 2F) rather than hypertrophy (Fig. 2E). Interestingly, quantification of β-cell size revealed a slight but significant increase in the double mutants, suggesting an attempt to compensate for the reduced β-cell mass (Fig. 2E). Therefore, to assess whether the absence of β-cell hyperplasia in D2KO/LIRKO pancreas was due to the inability of these cells to replicate in the absence of cyclin D2, we analyzed the expression of Ki-67, a marker of replication, by immunohistochemistry and measured the replication index by computing the ratio of Ki-67+/insulin+ double-positive cells to the total number of insulin+ cells (Fig. 2F). A decrease in replicating β-cells in the double knockouts compared with LIRKO islets suggested that the loss of hyperplasia in the D2KO/LIRKO mice is, in part, secondary to reduced β-cell proliferation. These data indicate that absence of cyclin D2 leads to a significantly reduced β-cell replication and a poor islet hyperplastic response to insulin resistance. Evaluation of cell death by transferase-mediated dUTP nick-end labeling immunostaining did not reveal significant differences between groups (data not shown).
Further evidence for a role for cyclin D2 in β-cell proliferation (13,28) emerges from our studies in the hyperplastic islets isolated from 4- to 6-month-old insulin-resistant LIRKO mice (22). We observed a significant increase in transcripts for all three cyclins that was consistent with enhanced β-cell replication in the LIRKOs (Fig. 3A). Further, immunostaining confirmed the expression of cyclins at the protein level, with cyclin D2 showing a more intense nuclear staining than that in controls (Fig. 3B).
FIG. 3.
Increased expression of cyclin D2 in LIRKO islets. A: Real-time RT-qPCR was performed on RNA extracted from islets of LIRKO (light gray) or control (white) mice (n = 4). Results are normalized to TATA-binding protein (TBP) and expressed relative to controls. *P < 0.05 for LIRKO vs. controls. B: Coimmunostaining of cyclin D2 (green) with insulin (red) and DAPI (blue) in pancreas sections from control and LIRKO mice. One to two representative islets for each group at magnification 40x are presented (n = 3). In all cases at least two to three pancreas sections were used for each animal. (A high-quality digital representation of this figure is available in the online issue.)
Regulation of pancreatic duodenal homeobox-1 and GLUT2 in D2KO/LIRKO islets.
We subsequently focused on examining the expression of forkhead box 1 (FoxO1) and pancreatic duodenal homeobox-1 (PDX-1)—two proteins known to be linked to β-cell proliferation (rev. in 14,29–32). Consistent with a previous report that PDX-1 is required for the compensatory β-cell growth response to insulin resistance (14), PDX-1 gene expression (Pdx-1) was significantly lower in the islets isolated from D2KO/LIRKO or D2KO mice than in those in controls (Fig. 4A). Further, PDX-1 protein was either virtually undetectable by immunostaining or severely reduced in D2KO/LIRKO β-cells in most of the islets from these two groups (Fig. 4B). On the other hand, islet gene expression for FoxO1 was not significantly different between groups (data not shown). However, evaluation of FoxO1 protein levels by immunostaining showed little nuclear but largely cytoplasmic staining in β-cells in the D2KO/LIRKO and D2KO islets compared with LIRKO or control islets (Fig. 4B).
FIG. 4.
Regulation of PDX-1 and GLUT2 expression in D2KO/LIRKO islets. A and C: Real time RT-qPCR was performed on RNA extracted from control, LIRKO, D2KO/LIRKO, and D2KO islets. Results are normalized to TATA-binding protein (TBP) and expressed relative to controls. *P < 0.05 in comparison with controls as indicated; n = 4. B: Coimmunostaining of PDX-1 (red) and FoxO1 (green) with DAPI (blue) in pancreas sections from control, LIRKO, D2KO/LIRKO, and D2KO mice. A representative islet for each group at magnification 40× is presented (n = 4). D: Coimmunostaining of GLUT2 (green) and insulin (red) with DAPI (blue) in pancreas sections from control, LIRKO, D2KO/LIRKO, and D2KO mice. A representative islet for each group at magnification 40× is presented (n = 4). In all cases, at least two to three pancreas sections were used for each animal. (A high-quality digital representation of this figure is available in the online issue.)
Considering that PDX-1 is known to regulate several β-cell–specific genes (33,34), we analyzed the expression of GLUT2, a key marker of β-cell function by real-time RT-qPCR and immunostaining. GLUT2 gene expression (SLC2A2) was decreased in islets from each knockout group but more severely reduced in D2KO/LIRKO islets (Fig. 4C), which correlated with the severe hyperglycemia in these mice compared with single-knockout counterparts (Table 1). At the protein level, GLUT2, like PDX-1, was either virtually absent or severely reduced in D2KO/LIRKO β-cells (Fig. 4D), thereby implicating a role for the transporter in the worsening of diabetes in the D2KO/LIRKO mice. These data indicate that a deficiency of cyclin D2 in the context of insulin resistance is associated with altered expression of PDX-1 and its downstream targets, with potential implications for poor β-cell proliferation and function.
D2KO/IRS1KO mice manifest hypoinsulinemia, glucose intolerance, and hyperglycemia.
In a parallel study, we crossed D2KO mice with another model of insulin resistance, the IRS1KOs, which exhibit mild insulin resistance and a compensatory increase in β-cell mass to maintain euglycemia (20,21). For clarity, we will refer to cycD2−/−;irs1+/− animals as D2KO, cycD2+/−;irs1−/− as IRS1KO, cycD2+/−;irs1+/− double heterozygotes as control, and cycD2−/−;irs1−/− as double knockouts (D2KO/IRS1KO). The IRS1KO animals exhibited reduced body mass at weaning and were easily identifiable (20). The D2KO/IRS1KO animals were generally smaller than IRS1KO or D2KO animals (Table 1). By age 25 weeks, D2KO/IRS1KO animals remained smaller than their IRS1KO counterparts, while there were no significant differences in the body weights of the controls or D2KO animals (Table 1). Thus, the body mass phenotype of the conventional IRS1KO (20,21) was also maintained in the compound D2KO/IRS1KO mice.
Insulin resistance in the individual IRS1KO and D2KO/IRS1KO mice was confirmed by insulin tolerance tests in 25-week-old animals (Fig. 5A). We then evaluated the ability of the D2KO/IRS1KO animals to secrete insulin in response to a glucose challenge. Plasma insulin levels measured before and 30 min after intraperitoneal glucose injection revealed that unlike the hyperinsulinemic response in the IRS1KO animals, a failure to show substantive insulin secretory response in injected glucose was observed in the D2KO/IRS1KO animals; the latter was similar to the hypoinsulinemic response to glucose challenge observed in the D2KO group (Fig. 5B). Taken together, these results indicate that D2KO/IRS1KO animals are as insulin resistant as their IRS1KO counterparts but fail to show robust compensatory hyperinsulinemia.
FIG. 5.
D2KO/IRS1KO animals are insulin resistant, hypoinsulinemic, and glucose intolerant. A: Insulin resistance was quantified by insulin tolerance tests. Insulin (0.75 mU/g body wt) was injected intraperitoneally, and blood was collected from tail veins of control (white), D2KO (gray), IRS1KO (striped), and D2KO/IRS1KO (hatched) mice. Data are expressed as area under the curve of glucose excursion. B: plasma insulin levels were measured before and 30 min after intraperitoneal injection of glucose in control, D2KO, IRS1KO, or D2KO/IRS1KO mice. C: Glucose tolerance test. Blood glucose was measured at 0, 15, 30, 60, and 120 min after intraperitoneal injection of glucose. *P < 0.05 and ***P < 0.005 in comparison with controls (A–C) or as indicated by bars. n = 7–8 for each group.
Consistent with previous reports (20), glucose disposal measured by glucose tolerance tests in IRS1KO animals was largely similar to that in control animals (Fig. 5C). In contrast, D2KO/IRS1KO animals were more severely glucose intolerant than the D2KO group, with blood glucose levels >400 mg/dl 2 h after glucose challenge (Fig. 5C). To confirm that these animals have overt diabetes (defined as nonfasting blood glucose levels >250 mg/dl [27]), we measured the nonfasting blood glucose levels over a period of 3 days. At age 25 weeks, D2KO animals exhibited mild hyperglycemia, whereas IRS1KO and control animals showed euglycemia and D2KO/IRS1KO animals were severely diabetic with blood glucose levels approaching 400 mg/dl (Table 1).
Diabetic D2KO/IRS1KO animals have disrupted islet architecture and severely diminished β-cell mass due to poor proliferation.
We next examined islet architecture, β-cell mass, and islet density in all groups. Consistent with previous reports, IRS1KO animals exhibited hyperplastic islets (20,35), while D2KO mice showed smaller islets than controls, although the islet architecture was normal (13,28). The islets of D2KO/IRS1KO mice were smaller than D2KO islets, exhibited abnormal architecture, and had visibly degranulated β-cells (Fig. 6A). Quantification confirmed a significant reduction in β-cell mass in the D2KO/IRS1KO animals (Fig. 6B) that was more severe than that in their D2KO counterparts. There were no significant changes in α-cell mass (data not shown). Measurement of the diameter of individual β-cells revealed slightly larger β-cells in the D2KO/IRS1KO group, suggesting an attempt at compensation to counter the decreased β-cell mass (Fig. 6C). Taken together, these data suggest that impaired β-cell replication likely accounted for the decrease in β-cell mass in the D2KO/IRS1KO mice.
FIG. 6.
D2KO/IRS1KO mice fail to exhibit compensatory β-cell replication and islet hyperplasia in response to insulin resistance. A: Coimmunostaining for insulin (green) and glucagon (red) in pancreas sections from control, D2KO, IRS1KO, and D2KO/IRS1KO mice as described in research design and methods. A representative islet for each group at magnification 20× is presented; n = 3. B: Quantification of β-cell mass in control (white), D2KO (gray), IRS1KO (striped), and D2KO/IRS1KO (hatched) mice. *P = 0.03 compared with controls (n = 3–5). C: Quantification of relative β-cell diameter. A minimum of 100 cells were measured per genotype. *P < 0.05, **P < 0.01, and ***P < 0.005 in comparison with control. D: Quantification of the proliferation index of replicating β-cells for each group in control (white), D2KO (gray), IRS1KO (striped), and D2KO/IRS1KO (hatched) mice. *P < 0.05 compared with controls; n = 3–5. (A high-quality digital representation of this figure is available in the online issue.)
To assess whether the diminished β-cell mass of D2KO/IRS1KO pancreas was due to an inability of β-cells to replicate as a result of the absence of cyclin D2, we measured the β-cell proliferation index as was done for the D2KO/LIRKO study. Since β-cell replication declines with age, we chose to examine younger, 6- to 8-week-old mice (36,37). IRS1KO β-cells showed a higher proliferation index in contrast to a significantly lower index in D2KO and D2KO/IRS1KO β-cells, underscoring the importance of cyclin D2 for β-cell replication in this model (Fig. 6D). The absence of transferase-mediated dUTP nick-end labeling–positive β-cells in 6- to 8-week-old mice in the D2KO/IRS1KO group suggested that apoptosis is unlikely to contribute to diminished β-cell mass at this age in the double mutants. However, in 25-week-old mice a higher rate of apoptosis was detectable in the D2KO and D2KO/IRS1KO islets, which is consistent with a recent report (38), although these differences did not reach statistical significance (data not shown). Taken together, our data indicate that cyclin D2 is required for replication-dependent expansion of β-cell mass in response to insulin resistance.
Upregulation of expression of alternative D-type cyclins fails to initiate proliferation in replication-competent β-cells.
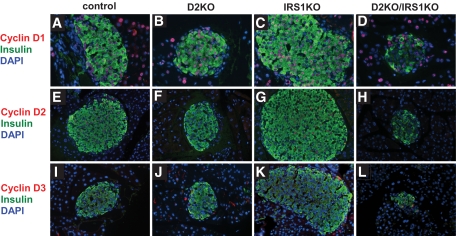
We next explored whether the absence of cyclin D2 in D2KO/IRS1KO animals resulted in an upregulation of other members of the D-type cyclin family using immunohistochemistry (Fig. 7). In control and IRS1KO pancreas sections, cyclin D1 was weakly expressed in both endocrine and exocrine cells (Fig. 7A and C) and cyclin D3 (Fig. 7I and K) was absent. In the D2KO pancreas, cyclin D1 was detected in both endocrine and exocrine cells (Fig. 7B) and cyclin D3 was weakly expressed in the nuclei of some β-cells (Fig. 7J). In contrast, in the D2KO/IRS1KO pancreas, cyclin D1 expression was present (Fig. 7D) and cyclin D3 was expressed strongly in virtually all nuclei compared with a low-intensity expression in D2KO pancreas (Fig. 7L). The enhanced expression of cyclin D3 and the presence of cyclin D1 in D2KO/IRS1KO animals indicated that despite their structural redundancy, these two D-type cyclins were unable to compensate for the absence of cyclin D2, further reinforcing the conclusion that cyclin D2 is essential for expansion of β-cell mass in response to insulin resistance.
FIG. 7.
Alterations in expression of D-type cyclins in D2KO/IRS1KO islets. Coimmunostaining for insulin (green) and cyclin (red) D1, D2, or D3 in pancreas sections from control, D2KO, IRS1KO, and D2KO mice. A representative islet for each group at magnification 20× is presented; n = 3. (A high-quality digital representation of this figure is available in the online issue.)
DISCUSSION
The presence of an adequate functional β-cell mass is critical for maintaining euglycemia in mammals. In states of altered metabolic demand, e.g., pregnancy or high-fat feeding, healthy β-cells maintain euglycemia, by increasing insulin secretion, through an elevated β-cell mass, or both (39). Indeed, <20% of obese insulin-resistant individuals develop type 2 diabetes, and a majority of insulin-resistant humans are capable of maintaining euglycemia by β-cell compensation (9,40). Recent studies focused on genomic analyses of type 2 diabetic patients have reported polymorphisms in genes that are close to those coding for key cell-cycle regulators (41,42); these experiments suggest that expansion of β-cell mass are linked to proteins in cell-cycle progression. In this study, we provide direct genetic evidence that the cell cycle protein cyclin D2 is essential for compensatory β-cell expansion in response to insulin resistance.
We have reported earlier that cyclin D2 is essential for the physiological remodeling of β-cell mass in the postnatal period (13,28). To address whether cyclin D2 is also required for β-cell expansion in states of insulin resistance, we crossed the cyclin D2 knockouts with two mouse models that exhibited varying degrees of insulin resistance. We chose the LIRKO model because it is tissue specific and is characterized by severe resistance and a dramatic β-cell hyperplastic response (22). For the second model, we used mice lacking IRS1, which exhibited mild insulin resistance but still manifested islet hyperplasia (20,21). The presence of a significantly increased number of proliferating β-cells in the islets from both models indicates the presence of hyperplasia—in part due to replication. Whereas imposition of cyclin D2 insufficiency led to diabetes in both models, severe hyperglycemia was evident in the D2KO/LIRKO compound knockouts at a much earlier age, suggesting that the severity of insulin resistance and the availability of cyclin D2 are important determinants of an appropriate islet hyperplastic response. On the other hand, in the IRS1KO mice, which develop mild-to-moderate postreceptor insulin resistance (20), the superimposition of cyclin D2 insufficiency also led to the development of diabetes. However, the compound D2KO/IRS1KO mice continued to live until age 25 weeks and more closely mimicked the human progression of insulin resistance–mediated type 2 diabetes compared with the D2KO/LIRKO model. Further, the reduced expression of two key β-cell–specific markers, PDX1 and GLUT2, suggests that absence of cyclin D2, in the context of insulin resistance, can directly influence β-cell function and proliferation.
Upstream signaling pathways (e.g., insulin/IGF-I) are crucial in the regulation of β-cell replication (rev. in 9) and may be linked with cyclin D2 in modulating β-cell mass. Indeed, we previously reported that increased β-cell replication in LIRKO mice correlates with increased insulin levels but not with glucose levels (23). Furthermore, in this same study, we crossed LIRKO mice with β-cell–specific insulin receptor knockout (βIRKO) mice, a model that exhibits β-cell hypoplasia and manifests a phenotype resembling human type 2 diabetes (25). Consistent with a role for the insulin receptor in modulating β-cell proliferation, βIRKO/LIRKO mice failed to develop islet hyperplasia and died as early as 8 weeks of age (23). Interestingly, islets and β-cells derived from βIRKO mice show reduced cyclin D2 protein expression (C.H. and R.N.K., unpublished data), and it is possible that this absence of the cell-cycle protein contributes to poor islet growth and development of age-dependent diabetes in these mutants (43,44). Recent experiments have also linked the cyclin/CDK4 complex with Akt in β-cell proliferation, indicating that Akt1 upregulates cyclin D1 and cyclin D2 levels and CDK4 activity (45).
Cyclin/cyclin-dependent kinase complexes are key nodes in the regulation of the G1-to-S transition during cell-cycle progression. For example, global knockouts of CDK4 in mice develop β-cell hypoplasia and diabetes (16,17). Previous work by our own group has shown that mice with a global knockout of p27, a cell-cycle inhibitor, regenerate β-cells more efficiently following streptozocin-induced diabetes (13), while other investigators have reported that β-cell–specific overexpression of p27 leads to islet hypoplasia and diabetes (46). Conversely, the cell-cycle inhibitor p21, which is the main target of the tumor suppressor p53, has recently been reported to be unessential for maintaining β-cell mass or function in vivo (47). In a similar manner, β-cell–specific deletion of pRb, a protein reported to be a central regulator of the cell cycle, leads to minimal defects in β-cell replication, mass, and function (48). Therefore, specific cell-cycle regulators play a critical role in β-cell proliferation regardless of the redundant expression of complementary family members. Intriguingly, Lavine et al. (49) have reported an inability to detect cyclin D2 protein in proliferating human islets that overexpressed prepro-cholecystokinin. Whether human β-cells utilize cyclin D2 for proliferation in a context-dependent manner or are dependent on another key cyclin protein requires further investigation.
While prior studies support a role for PDX-1 in β-cell proliferation, it is unclear whether proteins in the cyclin/CDK pathway are direct targets of this transcription factor. On the other hand, in our study the decreased β-cell mass in the compound knockouts and reduced expression of PDX-1 suggest that cyclin D2 is upstream of the pancreas-duodenum homeodomain transcription factor. It is likely there are intermediates that link these two important proteins, and additional studies are warranted to define their roles in the regulation of β-cell proliferation.
It is worth noting that the cyclin D2 mutants were global knockouts; therefore, it is possible that lack of cyclin D2 in putative precursors contributing to neogenesis also depends on cyclin D2–mediated cell-cycle reentry to facilitate enhanced β-cell mass. Nevertheless, our conclusion that cyclin D2 is necessary for replication-based β-cell expansion in insulin-resistant states is supported by lineage trace studies, which provide firm evidence that self-duplication is the primary source of new β-cells in adult rodents (12). Taken together, our studies support the hypothesis that cyclin D2, or its analog in humans, is a potential therapeutic target that can be harnessed to promote β-cell expansion in the treatment of type 1 diabetes and to delay the progression or prevent the development of type 2 diabetes.
Supplementary Material
ACKNOWLEDGMENTS
S.G. was supported by Ruth L. Kirschstein National Research Service Award GM07185. D.K. is the recipient of a research fellowship (Manpei Suzuki Diabetes Foundation, Japan) and a Juvenile Diabetes Research Foundation (JDRF) Postdoctoral Fellowship. This work was supported by grants from the National Institutes of Health (RO1 DK67536 and DK68721 [to R.N.K.] and DK068763 [to A.B.]), by Joslin DERC (Specialized Assay and Microscopy Cores) Grant DK36836, by the JDRF (to A.B.), and by the Larry Hillblom Research Foundation (to A.B.).
No potential conflicts of interest relevant to this article were reported.
We thank Lindsay Huse and Elizabeth Morgan for excellent assistance with preparation of the manuscript and P.C. Butler, MD, for discussions. We acknowledge the assistance of François Prodon of the C3M Cell Imaging Facility.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC: β-Cell replication is the primary mechanism subserving the postnatal expansion of β-cell mass in humans. Diabetes 2008; 57: 1584– 1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC: β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 2003; 52: 102– 110 [DOI] [PubMed] [Google Scholar]
- 3.Kloppel G, Lohr M, Habich K, Oberholzer M, Heitz PU: Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res 1985; 4: 110– 125 [DOI] [PubMed] [Google Scholar]
- 4.Ritzel RA, Butler AE, Rizza RA, Veldhuis JD, Butler PC: Relationship between β-cell mass and fasting blood glucose concentration in humans. Diabetes Care 2006; 29: 717– 718 [DOI] [PubMed] [Google Scholar]
- 5.Accili D: A kinase in the life of the beta cell. J Clin Invest 2001; 108: 1575– 1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonner-Weir S: Islet growth and development in the adult. J Mol Endocrinol 2000; 24: 297– 302 [DOI] [PubMed] [Google Scholar]
- 7.Bruning JC, Winnay J, Bonner-Weir S, Taylor SI, Accili D, Kahn CR: Development of a novel polygenic model of NIDDM in mice heterozygous for IR and IRS-1 null alleles. Cell 1997; 88: 561– 572 [DOI] [PubMed] [Google Scholar]
- 8.Lingohr MK, Dickson LM, McCuaig JF, Hugl SR, Twardzik DR, Rhodes CJ: Activation of IRS-2–mediated signal transduction by IGF-1, but not TGF-α or EGF, augments pancreatic β-cell proliferation. Diabetes 2002; 51: 966– 976 [DOI] [PubMed] [Google Scholar]
- 9.Assmann A, Hinault C, Kulkarni RN: Growth factor control of pancreatic islet regeneration and function. Pediatr Diabetes 2009; 10: 14– 32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu X, D'Hoker J, Stange G, Bonne S, De Leu N, Xiao X, Van De CM, Mellitzer G, Ling Z, Pipeleers D, Bouwens L, Scharfmann R, Gradwohl G, Heimberg H: Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 2008; 132: 197– 207 [DOI] [PubMed] [Google Scholar]
- 11.Manesso E, Toffolo GM, Saisho Y, Butler AE, Matveyenko AV, Cobelli C, Butler PC: Dynamics of beta-cell turnover: evidence for beta-cell turnover and regeneration from sources of beta-cells other than beta-cell replication in the HIP rat. Am J Physiol Endocrinol Metab 2009; 297: E323– E330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dor Y, Brown J, Martinez OI, Melton DA: Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004; 429: 41– 46 [DOI] [PubMed] [Google Scholar]
- 13.Georgia S, Bhushan A: Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J Clin Invest 2004; 114: 963– 968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulkarni RN, Jhala US, Winnay JN, Krajewski S, Montminy M, Kahn CR: PDX-1 haploinsufficiency limits the compensatory islet hyperplasia that occurs in response to insulin resistance. J Clin Invest 2004; 114: 828– 836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cozar-Castellano I, Fiaschi-Taesch N, Bigatel TA, Takane KK, Garcia-Ocana A, Vasavada R, Stewart AF: Molecular control of cell cycle progression in the pancreatic beta-cell. Endocr Rev 2006; 27: 356– 370 [DOI] [PubMed] [Google Scholar]
- 16.Rane SG, Dubus P, Mettus RV, Galbreath EJ, Boden G, Reddy EP, Barbacid M: Loss of cyclin-dependent kinase (Cdk4) expression causes insulin-deficient diabetes and cdk4 activation results in b-islet cell hyperplasia. Nat Genet 1999; 22: 44– 52 [DOI] [PubMed] [Google Scholar]
- 17.Tsutsui T, Hesabi B, Moons DS, Pandolfi PP, Hansel KS, Koff A, Kiyokawa H: Targeted disruption of CDK4 delays cell cycle entry with enhanced p27(Kip1) activity. Mol Cell Biol 1999; 19: 7011– 7019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Georgia S, Bhushan A: p27 regulates the transition of β-cells from quiescence to proliferation. Diabetes 2006; 55: 2950– 2956 [DOI] [PubMed] [Google Scholar]
- 19.Georgia S, Soliz R, Li M, Zhang P, Bhushan A: p57 and Hes1 coordinate cell cycle exit with self-renewal of pancreatic progenitors. Dev Biol 2006; 298: 22– 31 [DOI] [PubMed] [Google Scholar]
- 20.Araki E, Lipes MA, Patti ME, Bruning JC, Haag B, III, Johnson RS, Kahn CR: Alternative pathway of insulin signalling in mice with targeted disruption of the IRS-1 gene. Nature 1994; 372: 186– 190 [DOI] [PubMed] [Google Scholar]
- 21.Hennige AM, Ozcan U, Okada T, Jhala US, Schubert M, White MF, Kulkarni RN: Alterations in growth and apoptosis of insulin receptor substrate-1-deficient beta-cells. Am J Physiol Endocrinol Metab 2005; 289: E337– E346 [DOI] [PubMed] [Google Scholar]
- 22.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR: Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell 2000; 6: 87– 97 [PubMed] [Google Scholar]
- 23.Okada T, Liew CW, Hu J, Hinault C, Michael MD, Krtzfeldt J, Yin C, Holzenberger M, Stoffel M, Kulkarni RN: Insulin receptors in beta-cells are critical for islet compensatory growth response to insulin resistance. Proc Natl Acad Sci U S A 2007; 104: 8977– 8982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sicinski P, Donaher JL, Geng Y, Parker SB, Gardner H, Park MY, Robker RL, Richards JS, McGinnis LK, Biggers JD, Eppig JJ, Bronson RT, Elledge SJ, Weinberg RA: Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature 1996; 384: 470– 474 [DOI] [PubMed] [Google Scholar]
- 25.Kulkarni RN, Bruning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR: Tissue-specific knockout of the insulin receptor in pancreatic b cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell 1999; 96: 329– 339 [DOI] [PubMed] [Google Scholar]
- 26.Kulkarni RN, Wang ZL, Wang RM, Hurley JD, Smith DM, Ghatei MA, Withers DJ, Gardiner JV, Bailey CJ, Bloom SR: Leptin rapidly suppresses insulin release from insulinoma cells, rat and human islets and, in vivo, in mice. J Clin Invest 1997; 100: 2729– 2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kulkarni RN, Almind K, Goren HJ, Winnay JN, Ueki K, Okada T, Kahn CR: Impact of genetic background on development of hyperinsulinemia and diabetes in insulin receptor/insulin receptor substrate-1 double heterozygous mice. Diabetes 2003; 52: 1528– 1534 [DOI] [PubMed] [Google Scholar]
- 28.Kushner JA, Ciemerych MA, Sicinska E, Wartschow LM, Teta M, Long SY, Sicinski P, White MF: Cyclins D2 and D1 are essential for postnatal pancreatic beta-cell growth. Mol Cell Biol 2005; 25: 3752– 3762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitamura T, Ido KY: Role of FoxO proteins in pancreatic beta cells. Endocr J 2007; 54: 507– 515 [DOI] [PubMed] [Google Scholar]
- 30.Kitamura T, Nakae J, Kitamura Y, Kido Y, Biggs WH, Wright CV, White MF, Arden KC, Accili D: The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic beta cell growth. J Clin Invest 2002; 110: 1839– 1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gannon M, Ables ET, Crawford L, Lowe D, Offield MF, Magnuson MA, Wright CV: Pdx-1 function is specifically required in embryonic beta cells to generate appropriate numbers of endocrine cell types and maintain glucose homeostasis. Dev Biol 2008; 314: 406– 417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kushner JA, Ye J, Schubert M, Burks DJ, Dow MA, Flint CL, Dutta S, Wright CV, Montminy MR, White MF: Pdx1 restores beta cell function in Irs2 knockout mice. J Clin Invest 2002; 109: 1193– 1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H, Maechler P, Ritz-Laser B, Hagenfeldt KA, Ishihara H, Philippe J, Wollheim CB: Pdx1 level defines pancreatic gene expression pattern and cell lineage differentiation. J Biol Chem 2001; 276: 25279– 25286 [DOI] [PubMed] [Google Scholar]
- 34.Chakrabarti SK, James JC, Mirmira RG: Quantitative assessment of gene targeting in vitro and in vivo by the pancreatic transcription factor, Pdx1: importance of chromatin structure in directing promoter binding. J Biol Chem 2002; 277: 13286– 13293 [DOI] [PubMed] [Google Scholar]
- 35.Kulkarni RN, Winnay JN, Daniels M, Bruning JC, Flier SN, Hanahan D, Kahn CR: Altered function of insulin receptor substrate-1-deficient mouse islets and cultured beta-cell lines. J Clin Invest 1999; 104: R69– R75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teta M, Long SY, Wartschow LM, Rankin MM, Kushner JA: Very slow turnover of β-cells in aged adult mice. Diabetes 2005; 54: 2557– 2567 [DOI] [PubMed] [Google Scholar]
- 37.Tschen SI, Dhawan S, Gurlo T, Bhushan A: Age-dependent decline in β-cell proliferation restricts the capacity of β-cell regeneration in mice. Diabetes 2009; 58: 1312– 1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He LM, Sartori DJ, Teta M, Opare-Addo LM, Rankin MM, Long SY, Diehl JA, Kushner JA: Cyclin D2 protein stability is regulated in pancreatic beta-cells. Mol Endocrinol 2009; 23: 1865– 1875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Accili D: Lilly lecture 2003: the struggle for mastery in insulin action: from triumvirate to republic. Diabetes 2004; 53: 1633– 1642 [DOI] [PubMed] [Google Scholar]
- 40.Bonner-Weir S: Perspective: postnatal pancreatic beta cell growth. Endocrinology 2000; 141: 1926– 1929 [DOI] [PubMed] [Google Scholar]
- 41.Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, Bark CW, Goldstein JL, Watkins L, Xiang F, Saramies J, Buchanan TA, Watanabe RM, Valle TT, Kinnunen L, Abecasis GR, Pugh EW, Doheny KF, Bergman RN, Tuomilehto J, Collins FS, Boehnke M: A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 2007; 316: 1341– 1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, Barrett JC, Shields B, Morris AP, Ellard S, Groves CJ, Harries LW, Marchini JL, Owen KR, Knight B, Cardon LR, Walker M, Hitman GA, Morris AD, Doney AS, McCarthy MI, Hattersley AT: Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 2007; 316: 1336– 1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kulkarni RN, Kahn CR: Genetic models of insulin resistance: alterations in β-cell biology. In Molecular Basis of Pancreas Development and Function Habener JF, Hussain M: Eds. New York City, Kluwer Academic Publishers, 2001, p. 299– 323 [Google Scholar]
- 44.Mauvais-Jarvis F, Virkamaki A, Michael MD, Winnay JN, Zisman A, Kulkarni RN, Kahn CR: A model to explore the interaction between muscle insulin resistance and β-cell dysfunction in the development of type 2 diabetes. Diabetes 2000; 49: 2126– 2134 [DOI] [PubMed] [Google Scholar]
- 45.Fatrai S, Elghazi L, Balcazar N, Cras-Meneur C, Krits I, Kiyokawa H, Bernal-Mizrachi E: Akt induces β-cell proliferation by regulating cyclin D1, cyclin D2, and p21 levels and cyclin-dependent kinase-4 activity. Diabetes 2006; 55: 318– 325 [DOI] [PubMed] [Google Scholar]
- 46.Uchida T, Nakamura T, Hashimoto N, Matsuda T, Kotani K, Sakaue H, Kido Y, Hayashi Y, Nakayama KI, White MF, Kasuga M: Deletion of Cdkn1b ameliorates hyperglycemia by maintaining compensatory hyperinsulinemia in diabetic mice. Nat Med 2005; 11: 175– 182 [DOI] [PubMed] [Google Scholar]
- 47.Cozar-Castellano I, Haught M, Stewart AF: The cell cycle inhibitory protein p21cip is not essential for maintaining β-cell cycle arrest or β-cell function in vivo. Diabetes 2006; 55: 3271– 3278 [DOI] [PubMed] [Google Scholar]
- 48.Vasavada RC, Cozar-Castellano I, Sipula D, Stewart AF: Tissue-specific deletion of the retinoblastoma protein in the pancreatic β-cell has limited effects on β-cell replication, mass, and function. Diabetes 2007; 56: 57– 64 [DOI] [PubMed] [Google Scholar]
- 49.Lavine JA, Raess PW, Davis DB, Rabaglia ME, Presley BK, Keller MP, Beinfeld MC, Kopin AS, Newgard CB, Attie AD: Overexpression of pre-pro-cholecystokinin stimulates beta-cell proliferation in mouse and human islets with retention of islet function. Mol Endocrinol 2008; 22: 2716– 2728 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.