Abstract
Background
FOXP3+ regulatory T cells (Treg) suppress innate and adaptive immune responses and are critical for intestinal immune homeostasis. Our objective was to define the postnatal developmental regulation of Treg in relationship to other T cells in the human intestinal tract.
Methods
We analyzed 41 small and 18 large intestinal paraffin-embedded tissue samples from preterm and term infants with and without necrotizing enterocolitis (NEC) for presence of CD3+, CD4+, CD8+, and FOXP3+ cells by immunohistochemistry. We compared labeled cells against age, gestational age (GA), or (corrected) postmenstrual age (PMA).
Results
The GA ranged from 23 to 40 weeks, with a mean of 32 (SD 4.7) weeks. Independent of age, GA, or PMA, the numbers of CD4+ cells were higher in the small compared to the large intestine (p=0.046), except in patients with NEC. FOXP3+ cells could be detected as early as 23 weeks GA in both large and small bowel, and similar quantities were detected at the highest GA examined (40 weeks). We saw no statistically significant effect of GA, age, or PMA on total number of FOXP3+ cells, or by comparing FOXP3+ to CD4+ or FOXP3+to CD8+ ratios, suggesting intact ontogeny of Treg in intestinal tissue early in gestation.
Conclusion
Human infants exhibit presence of mucosal FOXP3+ cells in the small and large intestinal mucosa at birth and as early as 23 weeks GA. The frequency of FOXP3+ cells and the ratios of FOXP3+ to CD4+ or CD8+ cells do not change with increasing intrauterine development or postnatal age.
Keywords: Enterocolitis, necrotizing, FOXP3 protein, human, immunity, mucosal, immunohistochemistry, infant development, T-lymphocyte
Introduction
Forkhead box protein 3 (FOXP3) is a transcription factor predominantly expressed in IL-2Rα (CD25)high CD4+ T cells and its expression is required for regulatory T lymphocyte (Treg) generation and maintenance. Although the validity of FOXP3 to define human Treg is debated (1), currently it remains the most commonly used and unambiguous marker available to identify Treg in mice and humans (2-5). Treg suppress innate and adaptive immune responses, and abnormalities in numbers and/or function of these cells have been associated with autoimmune diseases, chronic infections and cancer (6). In patients with X-chromosome linked mutations of FOXP3 (IPEX syndrome), early development of multi-organ autoimmune diseases, including enteropathy similar to inflammatory bowel syndrome, ensues (7). Intestinal immune homeostasis despite exposure to billions of bacteria is considered a unique feature of the mature human intestinal tract. The intestinal tract of the premature infant is prone to inflammatory complications such as necrotizing enterocolits (NEC) (8). It is therefore important to define the postnatal ontogeny of intestinal Treg because relative lack of these cells can lead to inflammatory enteropathy (9) and their ontogeny may explain why premature infants are at higher risk for NEC.
While Treg were first described as differentiating in the newborn thymus (10), Treg can also be converted in the periphery from non-Treg cells (11). Treg can be detected in the human fetal thymus at 13 weeks of gestation, approximately 4 weeks after the first appearance of adaptive T and B cells (12), suggesting an important role in tolerance to maternal antigens (13). Treg enter the fetal lymph nodes, spleen, liver and bone marrow by 14 to 17 weeks, where they acquire a primed/memory phenotype and are fully functional (13,14). Treg are abundant in cord blood and represent 15% of CD4+ T cells at 23 weeks and 7% at term, indicating a negative correlation with gestational age (15). Treg appear to be especially important in development in the early postnatal phase when proliferation of self-reactive T cells may be increased during a period of relative lymphopenia (14,16). In contrast, no data is available on the presence or ontogeny of fetal intestinal Treg.
The gastrointestinal tract is the largest immunological organ of the human body, and immune-mediated gastrointestinal disorders such as inflammatory bowel disease (IBD) have been associated with a relative lack of Treg, compared with non-IBD inflammatory controls (17-20). In preterm infants NEC is a severe, multifactorial disease of mainly the small intestinal tract characterized by dysregulated immunological and inflammatory host responses (7,21). Lower numbers or poor function of Treg may play a role in the NEC disease process. During fetal development, there is a linear increase in the density of lamina propria CD3+ cells between 12 and 16 weeks gestational age (GA) (22). After 11 weeks GA, the majority of T cells in the lamina propria are CD4+, whereas epithelial T cells are CD8+ (23). Culturing of fetal human small intestine with pokeweed mitogen resulted in high numbers of activated T cells and macrophages causing mucosal destruction (24). However in contrast to effector cells, the ontogeny of Treg in the human gastrointestinal tract remains unknown to date. It is conceivable that with increasing exposure to microbiota after birth intestinal Treg numbers also rise. Thus, the objective of this study was to define the postnatal developmental regulation of small and large intestinal Treg in preterm and term infants in relationship to other T cells.
MATERIALS AND METHODS
Tissue samples
Remnant human tissue samples not needed for diagnosis are made available for research purposes through the Vanderbilt Human Tissue Acquisition and Pathology Shared Resource (VICC). Between the years 2003 and 2006, we identified 59 surgical cases by International Classification of Diseases, 9th Revision (ICD-9) codes for NEC, perinatal spontaneous intestinal perforation (SIP), congenital intestinal obstruction (atresia, volvulus, intussusception, Hirschsprung's disease) from the records of our institution's pathology service.
Immunohistochemistry
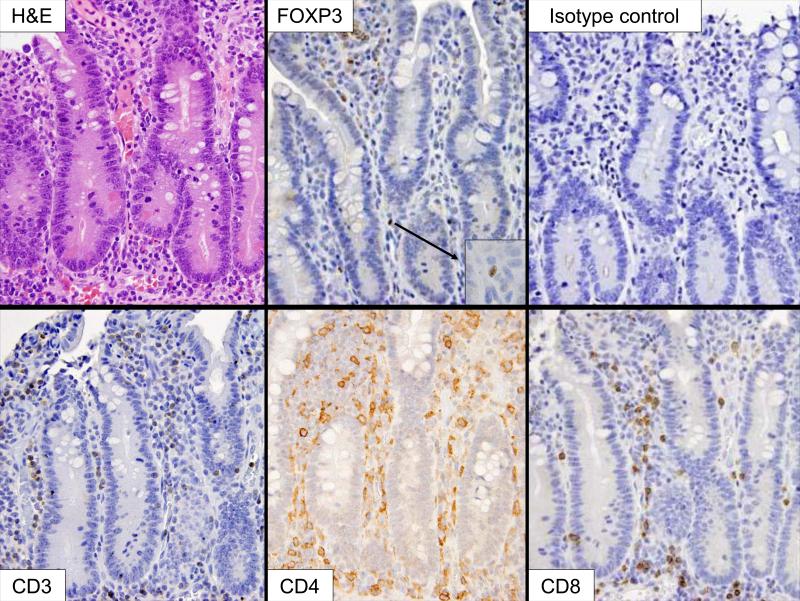
For immunohistochemistry 5 μm paraffin embedded sections were cut and placed on charged slides. Slides were deparaffinized and subjected to heat-induced epitope retrieval. Sections were immersed in either citrate (CD3 and FOXP3) or trilogy (CD4 and CD8) buffer retrieval solution (Cell Marque, Hot Springs, AR) and heated for 20 minutes in a high-pressure cooker. Endogenous peroxidase was neutralized with 0.3% hydrogen peroxide followed by a casein-based protein block (DakoCytomation, Carpinteria, CA) for nonspecific staining inhibition. Slides were incubated for either 20 minutes with anti-human CD3 (1:125) (DakoCytomation), 60 minutes with anti-human CD4 (1:40) (DakoCytomation), 30 minutes with anti-human CD8 (1:200) (DakoCytomation), or 60 minutes with mouse anti-human FOXP3 with the hFOXY clone (1:700) (eBioscience, San Diego, CA). The CD3, CD4 and CD8 slides were then rinsed with Tris Buffer Solution and the streptavidin-biotin detection system was used followed by application of DAB. Polymer based detection was used for the FOXP3 slides, and the murine Envision+ System, DAB/Peroxidase (DakoCytomation) was employed to produce localized, visible staining. The slides were counterstained with hematoxylin, dehydrated, and cover-slipped. Tonsillar follicular tissue was the positive control, showing nuclear expression of FOXP3 in scattered T cells within the interfollicular region. Negative controls were performed omitting the primary antibody. In addition, we used an isotype control antibody (1:500) (ChromPure mouse immunoglobulin G [IgG], Jackson ImmunoResearch Laboratories, West Grove, PA) on four tissue samples with the highest apparent number of FOXP3+ cells and counterstained with DAPI. Representative examples of staining patterns are illustrated in Figure 1. All slides were examined by two independent pathologists, who were blinded to the patient diagnosis during review. The number and distribution of CD3+, CD4+, CD8+, and FOXP3+ cells was determined per 10 high-power fields. Cells present in the lamina propria, not including large lymphoid aggregates, were included in the final count. Results were then averaged, ratios of CD3+, CD4+, and CD8+ cells to FOXP3+ cells calculated, and reported as a number of positive cells per single high-power field.
Figure 1.
Small bowel mucosa from a 34 weeks gestational age preterm infant with gastroschisis showing mild epithelial erosions. The intact lamina propria contains a mixed population of lymphocytes, as demonstrated by the CD3, CD4 and CD8 immunohistochemical stains. Immunohistochemistry for FOXP3 illustrates nuclear staining in lamina propria lymphocytes (insert). (H&E, 400X; insert 1000X).
Ethical considerations
The study was approved by the Vanderbilt University Institutional Review Board. Stored intestinal tissue samples were de-identified and only demographic data pertinent to the study design (diagnosis and indication for tissue resection, age at time of tissue resection, gestational age, and sex) were collected from patient records.
Statistical analysis
Baseline demographics and variables were assessed using the Wilcoxon rank sum test for continuous variables and Fisher's exact tests for proportions. Counts of positively stained cells underwent logarithmic transformation to stabilize variance. Ordinary linear regression models were used to relate the cell counts and age (including gestational and postmenstrual age) and sites (small intestine versus large intestine). All analyses were carried out with R version 2.7.0 (25).
RESULTS
We studied 59 surgical cases, evenly distributed between males and females (47% females). Forty-one samples (69%) were from the small and 18 (31%) from the large intestine. Four colon samples were full-thickness colon biopsies for Hirschsprung's disease. All other cases were small or large bowel resections. The GA ranged from 23 to 40 weeks, with a mean GA of 32 (SD 4.7) weeks. A list of the analyzed tissue types is shown in Table 1. The medians with lower and upper quartiles for demographic values and cell counts separated by small or large intestinal tissue are illustrated in Table 2.
Table 1.
Tissue types included in the study
| Small intestine | Large intestine | Combined | |
|---|---|---|---|
| N = 41 | N = 18 | N = 59 | |
| Congenital atresia | 19 | 0 | 19 |
| Necrotizing enterocolitis | 12 | 6 | 18 |
| Spontaneous intestinal perforation | 5 | 0 | 5 |
| Hirschsprung's disease | 0 | 8 | 8 |
| Stricture | 0 | 3 | 3 |
| Enterostomy closure | 2 | 0 | 2 |
| Mesenteric infarct | 2 | 0 | 2 |
| Meconium cyst | 1 | 0 | 1 |
| Functional obstruction | 0 | 1 | 1 |
Table 2.
Descriptive statistics by intestinal site
| Small intestine |
Large intestine |
Combined |
P values |
|
|---|---|---|---|---|
| N = 41 | N = 18 | N = 59 | ||
| Gestational age (weeks) | 32.0 (28.0-35.0) | 32.5 (29.0-36.0) | 32.0 (28.5-36.0) | P = 0.401 |
| Age (weeks) | 2.1 (0.7-3.4) | 2.3 (1.5-4.2) | 2.1 (0.70-4.0) | P = 0.301 |
| Postmenstrual age (weeks) | 34.6 (31.3-37.1) | 36.2 (34.3-37.5) | 35.1 (32.1-37.4) | P = 0.131 |
| Sex: Female | 46% (19) | 50% (9) | 47% (28) | P = 1.02 |
| Tissue type: NEC | 29% (12) | 33% (6) | 31% (18) | P = 0.772 |
| FOXP3+ | 0.3 (0.1-1.4) | 0.7 (0.2-1.5) | 0.4 (0.1-1.5) | P = 0.491 |
| CD3+ | 17.9 (10.1-27.6) | 10.8 (8.4-19.0) | 15.0 (9.0-26.0) | P = 0.121 |
| CD4+ | 19.6 (9.1-28.8) | 9.2 (7.1-17.9) | 16.5 (7.7-24.0) | P = 0.0461 |
| CD8+ | 12.1 (3.1-18.5) | 5.7 (3.3-9.5) | 7.7 (3.0-16.1) | P = 0.0871 |
| FOXP3+ / CD4+ (%) | 2.4 (0.6-7.5) | 5.1 (2.4-9.9) | 3.9 (0.8-9.1) | P = 0.131 |
| FOXP3+ / CD8+ (%) | 6.4 (1.0-16.4) | 10.9 (5.8-7.0) | 7.0 (1.8-16.8) | P = 0.101 |
For continuous variables the median is followed by lower and upper quartiles in parenthesis. For categorical variables, numbers after percents are frequencies. Tests used:
Wilcoxon test
Fisher's exact test
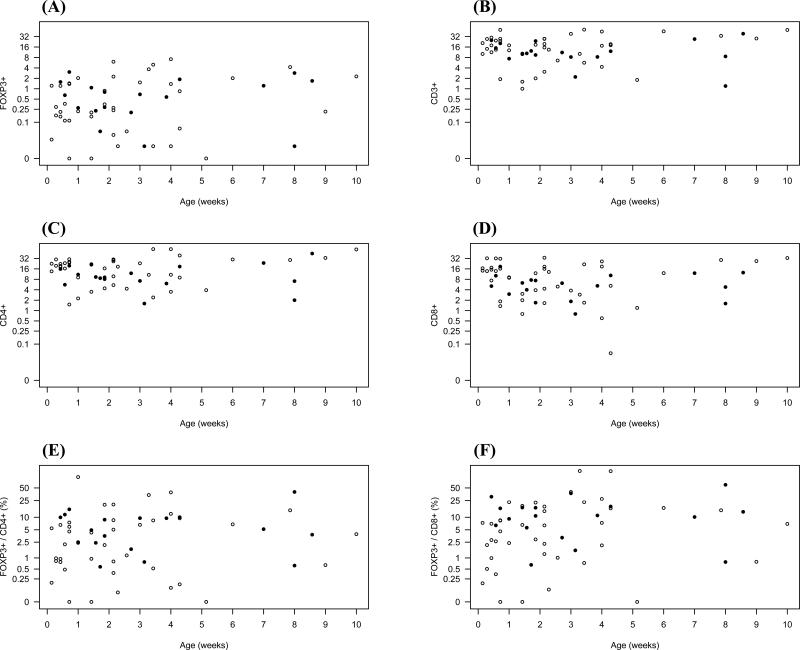
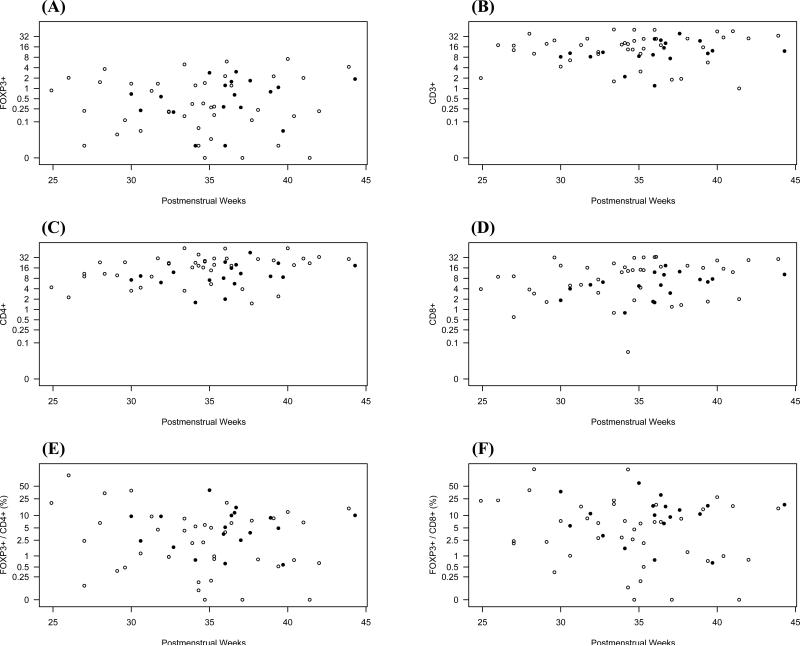
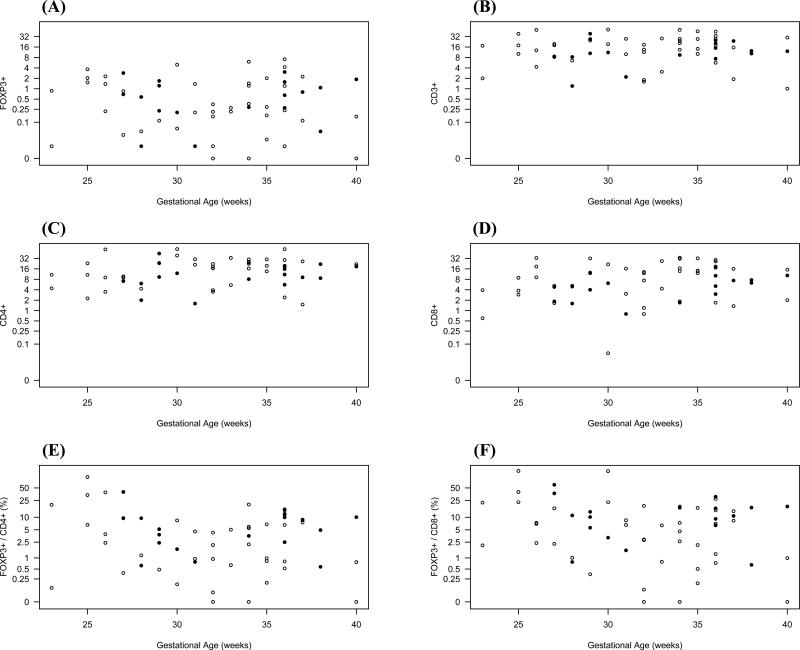
For the analysis of developmental regulation, we plotted the numbers of CD3+, CD4+, CD8+ and FOXP3+ cells per high-power field of all analyzed tissue specimens against increasing GA, chronological age, and PMA for the small or large intestines separately (Figures 2-4). FOXP3+ cells could be detected as early as 23 weeks GA in both large and small bowel, and similar quantities were detected at the highest GA examined (40 weeks). FOXP3+ cells were confined to the lamina propria, without infiltration of crypts or surface epithelium. Moreover, they were predominately localized to the deep lamina propria, although some were found in the superficial lamina propria. Independent of age, GA, or PMA, the numbers of CD4+ cells were higher in the small compared to the large intestine (p=0.046), and were evenly distributed throughout the lamina propria. We saw a small but statistically significant increase in CD4+ cells in the lamina propria with increasing PMA (p=0.008). In contrast to CD4+ cells, the number of CD3+, CD8+, and FOXP3+ cells did not increase with PMA. Numbers of all studied cell types were independent of age or GA in either the small or large intestine.
Figure 2.
Numbers of FOXP3+ (A), CD3+ (B), CD4+ (C), or CD8+ (D) cells per high-power field were plotted against increasing chronological age in weeks. Ratios of FOXP3+ cells to CD4+ cells and FOXP3+ to CD8+ cells are shown in (E) and (F), respectively. Samples from small intestine are shown as closed (•) and from large intestine as open (○) circles.
Figure 4.
Numbers of FOXP3+ (A), CD3+ (B), CD4+ (C), or CD8+ (D) cells per high-power field were plotted against increasing postmenstrual age. Postmenstrual age was defined as gestational age plus chronological age in weeks. Ratios of FOXP3+ cells to CD4+ cells and FOXP3+ to CD8+ cells are shown in (E) and (F), respectively. Samples from small intestine and large intestine are shown as in Figure 2.
Because the ratio of FOXP3+ to CD4+ or CD8+ cells may be a more important indicator of Treg presence than absolute counts of FOXP3+ cells, we plotted ratios of FOXP3+ to CD4+ or FOXP3+ to CD8+ cells for age, GA, and PMA independently (Figures 2-4). We found that the ratios of FOXP3+ to CD4+ or CD8+ cells were not developmentally regulated and did not change with postnatal exposure time to intestinal antigens.
To study the possible role of FOXP3+ cells in the risk to develop NEC, we compared the 18 tissue samples originating from NEC patients with the other intestinal disorders (controls) (Table 3). The main finding was a statistically significant decrease in CD3+, CD4+, and CD8+ cells in the NEC samples. There was a trend for lower numbers of FOXP3+ cells, but FOXP3+ to CD4+ or FOXP3+ to CD8+ ratios were not different. Higher numbers of CD4+ cells in the small versus large intestine continued to be present in the control group (p = 0.006) but not in the NEC group (p=0.6) because NEC patients had relatively lower numbers of CD4+ cells in the small intestine. Even though NEC tissue samples originated from younger and more premature infants, excluding all NEC samples in the analysis did not change the finding that the number of FOXP3+ cells or FOXP3+ to CD4+ or CD8+ ratios in the lamina propria of the small or large intestine were independent from age, GA, or PMA.
Table 3.
Descriptive statistics by NEC versus control
| Control |
NEC |
Combined |
P Values |
|
|---|---|---|---|---|
| N = 41 | N = 18 | N = 59 | ||
| Gestational Age (weeks) | 35.0 (32.0-36.0) | 28.0 (26.0-30.0) | 32.0 (28.5-36.0) | P < 0.0011 |
| Age (weeks) | 1.7 (0.6-3.4) | 2.9 (2.0-4.0) | 2.1 (0.7-4.0) | P = 0.0281 |
| Postmenstrual Age (weeks) | 36.1 (34.3-39.1) | 31.2 (29.3-34.2) | 35.1 (32.1-37.4) | P < 0.0011 |
| Sex: Female | 46% (19) | 50% (9) | 47% (28) | P = 1.02 |
| Site: Large intestine | 29% (12) | 33% (6) | 31% (18) | P = 0.772 |
| FOXP3+ | 0.7 (0.2-1.7) | 0.2 (0.1-0.8) | 0.4 (0.1-1.5) | P = 0.121 |
| CD3+ | 18.0 (10.4-27.6) | 9.4 (3.4-17.8) | 15.0 (9.0-26.0) | P = 0.0111 |
| CD4+ | 19.7 (11.0-26.4) | 6.6 (4.0-10.6) | 16.5 (7.7-24.0) | P = 0.0011 |
| CD8+ | 12.1 (4.8-17.4) | 4.2 (1.3-8.2) | 7.7 (3.0-16.1) | P = 0.0041 |
| FOXP3+ / CD4+ (%) | 4.6 (0.8-8.8) | 3.2 (0.9-8.4) | 3.9 (0.8-9.1) | P = 0.781 |
| FOXP3+ / CD8+ (%) | 7.4 (1.0-16.8) | 6.7 (2.3-16.8) | 7.0 (1.8-16.8) | P = 0.811 |
For continuous variables the median is followed by lower and upper quartiles in parenthesis. For categorical variables, numbers after percents are frequencies. Tests used:
Wilcoxon test
Fisher's exact test
Discussion
Treg are critical for intestinal immune homeostasis, and the increased risk of premature infants for inflammatory intestinal complications, such as NEC, may be due to a relative lack of Treg in the intestinal lamina propria. Past studies examining the ontogeny of lymphocytes in fetal gastrointestinal tissue show that T cells are present as early as 14 weeks gestation, and well developed Peyer's patches with germinal centers and T cell rich zones are present by week 19 (26). Intraepithelial and lamina propria lymphocytes are also present in these fetal intestinal tissues. Therefore while it has been established that there is a competent mucosal immune system present in the terminal ileum at early gestational age (27), little is known about the ontogeny of Treg in the human intestinal tract.
To foster our knowledge in human intestinal immune development, we examined the postnatal ontogeny of CD3+, CD4+, CD8+, and FOXP3+ cells in the gastrointestinal tract of human neonates. We found an abundance of all studied cell types in the lamina propria of infants as premature as 23 weeks GA. This is consistent with previous studies showing high levels of Treg in fetal lymph nodes, spleen, liver and bone marrow tissues. Some have hypothesized that Treg are not only suppressing self-reactive lymphocytes in fetal tissues, but maternal lymphocytes as well (13, 28).
The frequency of FOXP3+ cells and the ratios of FOXP3+ to CD4+ or CD8+ cells were independent of age, or PMA, indicating that in humans intestinal Treg are present immediately after birth. The only statistically significant difference detected with rising PMA was for CD4+ cells, perhaps representing part of the mounting immune response on exposure to an increased number of foreign intestinal antigens.
Our study faces the technical limitation of traditional immunohistochemistry methods that are constrained by the use of single cell surface antigens. For example, although 75% of T cells are CD4+, this antigen is also expressed by macrophages. While high FOXP3 expression is still considered a specific marker for Treg in humans (29,30), transient expression of FOXP3 has been observed in effector T cells and even high levels of FOXP3 expression may be insufficient to define a cell as a Treg (1). Polychromatic flow cytometry is currently the only technological platform that can suitably analyze the complex components of the immune system including the proper identification of human Treg through a panel of specific extracellular and intracellular fluorescent antibodies. Definitive characterization as Treg is performed by suppression assays, however flow cytometry or functional assays require freshly isolated cells. Gastrointestinal endoscopy to obtain biopsies is currently not feasible in premature infants and obtaining viable resected intestinal tissue from the human intestinal tract is difficult, as it often undergoes rapid autolysis. For these reasons immunohistochemistry of stored paraffin-embedded tissue samples was the only practical method for this initial study.
A second limitation is that 18 tissue samples (31%) originated from NEC patients. It is possible that the relatively high percentage of NEC cases may have skewed the data. As shown in Table 3, NEC samples had overall lower cell counts than controls. NEC patients were also of younger age, GA, and PMA than controls. Therefore, if data skewing had occurred, we would expect to find lower FOXP3+ cells in younger and/or more premature infants. In contrast, the frequency of FOXP3+ cells and the ratios of FOXP3+ to CD4+ or CD8+ cells were independent of age, GA, or PMA in our study.
In summary we demonstrate that FOXP3+ cell are present in the postnatal small and large intestinal lamina propria as early as 23 weeks GA. While the frequency of CD4+ cells increased with PMA, the total numbers of FOXP3+ cells and the ratios of FOXP3+ to CD4+ or CD8+ cells were independent of age, GA, or PMA, indicating a potential for intestinal immune regulation immediately after birth in human infants.
Future work will need to confirm this postnatal ontogeny data by multicolor flow cytometry and functional assays to investigate the role of Treg in neonatal diseases and adult disorders that originate in infancy. This will include isolation, phenotypic characterization and functional analysis of viable Treg isolated from small or large intestinal tissue from preterm and term infants.
Figure 3.
Numbers of FOXP3+ (A), CD3+ (B), CD4+ (C), or CD8+ (D) cells per high-power field were plotted against increasing gestational age. Gestational age was calculated from the first day of the last menstrual period in weeks. Ratios of FOXP3+ cells to CD4+ cells and FOXP3+ to CD8+ cells are shown in (E) and (F), respectively. Samples from small intestine and large intestine are shown as in Figure 2.
Acknowledgements
This work was supported in part by the Marshall Klaus Perinatal Research Award given to J.-H. W. by the American Academy of Pediatrics, Section on Perinatal Pediatrics in 2005, the Vanderbilt Physician Scientist Development Program Award (J.-H. W.), and the Vanderbilt Digestive Disease Research Center (DK058404). We thank Drs. Michael Rosen, Kay Washington, Jim Crowe, Jr., and Mark Frey for critical review of the manuscript and helpful discussions.
Footnotes
New address: Department of Laboratories, A6901, Children's Hospital and Regional Medical Center, 4800 Sand Point Way NE, Seattle, WA 98105
References
- 1.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roncarolo MG, Gregori S. Is FOXP3 a bona fide marker for human regulatory T cells? Eur J Immunol. 2008;38:925–927. doi: 10.1002/eji.200838168. [DOI] [PubMed] [Google Scholar]
- 3.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 4.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 5.Bennet CL, Christie J, Ramsdell F, et al. The immune dysregulation polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 6.O'Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004;10:801–805. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]
- 7.Wildin RS, Ramsdell F, Peake J, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nature Genetics. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 8.Guthrie SO, Gordon PV, Thomas V, Thorp JA, Peabody J, Clark RH. Necrotizing enterocolitis among neonates in the United States. J Perinatol. 2003;23:278–285. doi: 10.1038/sj.jp.7210892. [DOI] [PubMed] [Google Scholar]
- 9.Izcue A, Powrie F. Special regulatory T-cell review: Regulatory T cells and the intestinal tract--patrolling the frontier. Immunology. 2008;123:6–10. doi: 10.1111/j.1365-2567.2007.02778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and Autoimmunity: Production of CD25+CD4+ Naturally Anergic and Suppressive T Cells as a Key Function of the Thymus in Maintaining Immunologic Self-Tolerance. J Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- 11.Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darrasse-Jeze G, Marodon G, Salomon BL, Catala M, Klatzmann D. Ontogeny of CD4+CD25+ regulatory/suppressor T cells in human fetuses. Blood. 2005;105:4715–4721. doi: 10.1182/blood-2004-10-4051. [DOI] [PubMed] [Google Scholar]
- 13.Izcue A, Powrie F. Prentatal tolerance-a role for regulatory T cells? Eur J Immunol. 2005;35:379–382. doi: 10.1002/eji.200525996. [DOI] [PubMed] [Google Scholar]
- 14.Cupedo T, Nagasawa M, Weijer K, Blom B, Spits H. Development and activation of regulatory T cells in the human fetus. Eur J Immunol. 2005;35:383–390. doi: 10.1002/eji.200425763. [DOI] [PubMed] [Google Scholar]
- 15.Takahata Y, Nomura A, Takada H, et al. CD25+CD4+ T cells in human cord blood: an immunoregulatory subset with naïve phenotype and specific expression of forkhead box p3 (Foxp3) gene. Exp Hematol. 2004;32:622–629. doi: 10.1016/j.exphem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Min B, McHugh R, Sempowski GD, Mackall C, Foucras G, Paul WE. Neonates support lymphopenia-induced proliferation. Immunity. 2003;18:131–140. doi: 10.1016/s1074-7613(02)00508-3. [DOI] [PubMed] [Google Scholar]
- 17.Maul J, Loddenkemper C, Mundt P, et al. Peripheral and intestinal regulatory CD4+CD25high T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–1878. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 18.Yu QT, Saruta M, Avanesyan A, Fleshner PR, Banham A, Papadakis KA. Expression and functional characterization of FOXP3+CD4+ regulatory T cells in ulcerative colitis. Inflamm Bowel Dis. 2007;13:191–199. doi: 10.1002/ibd.20053. [DOI] [PubMed] [Google Scholar]
- 19.Veltkamp C, Ruhwald R, Geisem T, et al. CD4+CD25+ cell depletion from the normal CD4+ T cell pool prevents tolerance toward the intestinal flora and leads to chronic colitis in immunodeficient mice. Inflamm Bowel Dis. 2006;12:437–446. doi: 10.1097/00054725-200606000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Mottet C, Uhlig HH, Powrie F. Cutting Edge: Cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 21.Nanthakumar NN, Fusunyan RD, Sanderson I, Walker WA. Inflammation in the developing human intestine: A possible pathophysiologic contribution to necrotizing enterocolitis. PNAS. 2000;97:6043–6048. doi: 10.1073/pnas.97.11.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacDonald TT, Spencer J. Evidence that activated mucosal T cells play a role in the pathogenesis of enteropathy in human small intestine. J Exp Med. 1988;167:1341–1349. doi: 10.1084/jem.167.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacDonald TT, Spencer J. Ontogeny of the gut-associated lymphoid system in man. Acta Paediatr Suppl. 1994;83:3–5. doi: 10.1111/j.1651-2227.1994.tb13219.x. [DOI] [PubMed] [Google Scholar]
- 24.Lionetti P, Breese E, Braegger CP, Murch SH, Taylor J, MacDonald TT. T-cell activation can induce either mucosal destruction or adaptation in cultured human fetal small intestine. Gastroenterology. 1993;105:373–81. doi: 10.1016/0016-5085(93)90710-t. [DOI] [PubMed] [Google Scholar]
- 25.R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2008. ISBN 3-900051-07-0. URL http://www.R-project.org. [Google Scholar]
- 26.Spencer J, Isaacson PG, Walker-Smith JA, MacDonald TT. Heterogeneity in intraepithelial lymphocyte subpopulations in fetal and postnatal human small intestinte. J Pediatr Gastroenterol Nutr. 1989;9:173–177. doi: 10.1097/00005176-198908000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Spencer J, MacDonald TT, Finn T, Isaacson PG. The development of gut associated lymphoid tissue in the terminal ileum of fetal human intestine. Clin Exp Immunol. 1986;64:536–543. [PMC free article] [PubMed] [Google Scholar]
- 28.Hall JM, Lingenfelter P, Adams SL, Lasser D, Hansen JA, Bean MA. Detection of maternal cells in human umbilical cord blood using fluorescence in situ hybridization. Blood. 1995;86:2829–2832. [PubMed] [Google Scholar]
- 29.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 30.Sakaguchi S. Regulatory T cells in the past and for the future. Eur J Immunol. 2008;38:901–937. doi: 10.1002/eji.200890012. [DOI] [PubMed] [Google Scholar]