Abstract
Atrial fibrillation (AF) is a growing clinical problem associated with increased morbidity and mortality. Currently available antiarrhythmic drugs (AADs), although highly effective in acute cardioversion of paroxysmal AF, are generally only moderately successful in long-term maintenance of sinus rhythm. The use of AADs is often associated with an increased risk of ventricular proarrhythmia, extracardiac toxicity, and exacerbation of concomitant diseases such as heart failure. AF is commonly associated with intracardiac and extracardiac disease, which can modulate the efficacy and safety of AAD therapy. In light of the multifactorial intracardiac and extracardiac causes of AF generation, current development of anti-AF agents is focused on modulation of ion channel activity as well as on upstream therapies that reduce structural substrates. The available data indicate that multiple ion channel blockers exhibiting potent inhibition of peak INa with relatively rapid unbinding kinetics, as well as inhibition of late INa and IKr, may be preferable for the management of AF when considering both safety and efficacy.
Introduction
Atrial fibrillation (AF) is the most commonly encountered sustained arrhythmia, affecting an estimated 2.3 million people in the US alone.1 Advancing age is a major risk factor for AF and the prevalence of this condition is rising at an alarming rate with ageing of the population.1 Of the two principal options for the management of AF, rhythm control—which aims to restore and maintain sinus rhythm—is believed to be preferable to rate control, in which ventricular rate is regulated while the atria continue to fibrillate.1-3 However, currently available approaches for rhythm control have important limitations, which are discussed below, making rate control preferable in some cases, particularly in older patients with relatively few symptoms of AF.
Rhythm control of AF can be achieved with antiarrhythmic drugs (AADs), catheter ablation techniques, or electrical cardioversion. AADs are often successfully used to maintain sinus rhythm following catheter ablation or cardioversion.4 Most AADs currently available for rhythm control of AF have poor safety profiles and inadequate long-term efficacy, making long-term pharmacologic rhythm control in patients with AF a challenge.5,6 The results of a number of multicenter, randomized, and prospective clinical trials, including AFFIRM,7 and the AF-CHF,8 RACE,9 and PIAF10 studies, suggest that rhythm control strategy with AADs is not superior to rate control in terms of survival, and may be associated with an increase in rate of hospitalization. Adverse effects of AADs, such as ventricular proarrhythmia and extracardiac toxicity, are likely to negate the limited ability of these agents to maintain sinus rhythm. The post-hoc analysis of the AFFIRM data showed that patients maintained in sinus rhythm had a better survival rate than those in whom AF persisted.11
Mounting evidence indicates that for some AF cases—particularly relatively young (<65 years of age) symptomatic patients—catheter ablation is superior to currently available AADs for long-term maintenance of sinus rhythm.12,13 However, despite progress in catheter ablation technologies and improvements in the success of these techniques, AADs remain first-line therapy for rhythm control of AF1 and are expected to do so for the foreseeable future.
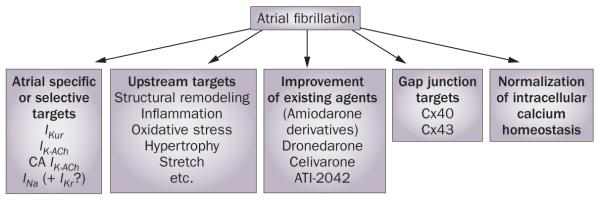
AF is commonly associated with atrial electrical and structural abnormalities as well as with a constellation of intracardiac and extracardiac diseases, including heart failure, hypertension, coronary artery disease, myocardial infarction, and valvular heart defects, which can develop independently of AF but may promote and be aggravated by the arrhythmia. Each of these AF-associated abnormalities and diseases, as well as numerous mediating factors of these disorders, can also be pharmacological targets for AF treatment and may alter the safety and anti-AF efficacy of AAD therapy. Development of anti-AF medications, therefore, is currently focused on modulation of ion channel activity as well as on upstream therapies that target these intracardiac and extracardiac factors that induce or promote structural remodeling (Figure 1). Other preclinical investigations are directed at pharmacological modulation of gap junctions and intracellular calcium activity. In this Review, we discuss current and novel pharmacological approaches to rhythm control in patients with AF.
Figure 1.
Current prominent investigational strategies for rhythm control of atrial fibrillation. Abbreviations: CA, constitutively active; Cx, connexin; IK-ACh, acetylcholine-regulated inward rectifying potassium current; IKr, rapidly activating delayed rectified potassium current; IKur, ultra-rapid delayed rectifier potassium current; INa, early sodium current. Modified from Burashnikov, A. & Antzelevitch, C. Ann. Noninvasive Electrocardiol. 14, 290–300 (2009).
Modulation of ion channel activity
Most AADs in current clinical use exert their anti-AF actions via inhibition of cardiac ion channels, particularly during acute administration (Table 1). Chronic treatment can additionally remodel ion channel expression,14 cause degradation of specific ion channels (for example, the ultra-rapid delayed rectifier potassium current [IKur] as reported with quinidine15), or influence atrial electrical and structural remodeling, as observed with amiodarone.16,17 Anti-AF drugs can be very effective in the short term, with up to 80–90% efficacy for the termination of paroxysmal AF and some forms of persistent AF.18-20 However, these agents are generally only moderately effective for long-term rhythm control of paroxysmal AF, and even less so in the case of persistent AF.1 The long-term (1–1.5 years) efficacy of amiodarone, which is arguably the best available AAD for the maintenance of sinus rhythm, ranges from 34% to 65%.12,21
Table 1.
Drugs used for rhythm control in atrial fibrillation
| Agent | Primary mechanisms of action |
Primary indication | Current status |
|---|---|---|---|
| Amiodarone | Multiple ion channel block. Atrial-selective peak INa block |
Maintenance of sinus rhythm | Off-label use |
| Dronedarone | Multiple ion channel block |
Maintenance of sinus rhythm, reduction of cardiovascular hospitalization |
FDA approved |
| Dofetilide | IKr block | Acute cardioversion and maintenance of sinus rhythm |
FDA approved |
| Sotalol | IKr block | Maintenance of sinus rhythm | FDA approved |
| Ibutilide | IKr block | Acute cardioversion | FDA approved |
| Propafenone | Peak INa block | Acute cardioversion and maintenance of sinus rhythm |
FDA approved |
| Flecainide | Peak INa block | Acute cardioversion and maintenance of sinus rhythm |
FDA approved |
| Vernakalant | Peak INa and IKur block | Acute cardioversion | Investigational |
| Ranolazine | Atrial-selective peak INa and IKr block |
Acute cardioversion and maintenance of sinus rhythm |
Off-label use |
| AZD7009 | Atrial-selective peak INa and IKr block |
Acute cardioversion | Investigational, abandoned |
| AVE0118 | Atrial selective IKur, Ito, IK-ACh block |
Maintenance of sinus rhythm | Investigational, abandoned |
The principal complication of all anti-AF drugs is that they are associated with an increased risk of life-threatening ventricular arrhythmias, induction of multi-organ toxicity, or worsening of coexisting disease.1,22 Severe ventricular proarrhythmia has been observed with administration of sodium-channel blockers (class IC agents, such as flecainide and propafenone) in patients with structural heart disease.23 Agents that potently inhibit the rapidly activating delayed rectifier potassium current (IKr) can produce long QT syndrome and related life-threatening polymorphic ventricular tachycardia (torsade de pointes [TdP]).1 These drugs include class III agents such as dofetilide, ibutilide, and sotalol or class IA agents such as quinidine. Amiodarone is a class III drug that induces TdP only rarely, but is often associated with the development of extracardiac multi-organ toxicity with long-term use.24,25 Amiodarone-associated death is caused largely by pulmonary complications.26 The worsening of coexisting disease, such as heart failure, has also been associated with the use of AADs. In the ANDROMEDA study,22 the use of dronedarone in patients with severe left ventricular (LV) systolic dysfunction increased mortality, apparently unrelated to AF or ventricular proarrhythmia. Although amiodarone is generally not associated with excess all-cause mortality, the death rate for amiodarone use in patients with NYHA class IV heart failure was reported to be greater than for placebo in SCD-HeFT.27 Notably, however, the prognosis of patients after a CHF-related hospitalization is very bad regardless of the therapy administered.28 These adverse effects and limitations of currently available AADs restrict the scope of these agents in the management of patients with AF.
Atrial-selective ion channel block
The atrial-selective approach to the treatment of AF was conceived with the aim of reducing the risk of ventricular arrhythmias. The channels responsible for the IKur, the acetylcholine-regulated inward rectifying potassium current (IK-ACh), and the constitutively active (CA)-IK-Ach (which does not require acetylcholine or muscarinic receptors for activation) are present in atria, but largely absent from the ventricles. These channels are, therefore, commonly referred to as being atrial-specific.29,30 While this strategy is attractive in theory, the available data indicate that blockade of IKur alone might not effectively suppress AF.31-33 Indeed, when administered at concentrations that effectively suppress AF, currently available IKur blockers potently inhibit other currents as well. For example, vernakalant and AZD7009 also block the early sodium current (INa) and AVE0118 also blocks the transient outward potassium current (Ito), IK-ACh, Ca-IK-ACh, and INa.34-38 The anti-AF actions of AZD7009 and vernakalant appear to be largely the result of inhibition of INa rather than IKur.32 AZD7009 reduces excitability and conduction velocity preferentially in the atria of dogs in vivo,39 indicating atrial-selective sodium-channel block. While a number of other purported IKur blockers have been shown to produce atrial-selective effective refractory period (ERP) prolongation and to suppress experimental AF,40 whether pure IKur block can effectively suppress AF has yet to be demonstrated.30,33 Selective inhibition of IKur neither prevents nor terminates acetylcholine-mediated AF in canine atria.41 Notably, IKur density is progressively reduced with acceleration of activation rate;42 therefore, the contribution of this current in AF may be relatively small. However, under conditions associated with triangulation of atrial action potential morphology (electrical remodeling or rapid activation rates), IKur blockers promote prolongation of action potential duration at 90% repolarization (APD90).43 IKur density has also been reported to be reduced in cells isolated from the atria of patients with chronic AF.37,44 By contrast to remodeled atria, where IKur block slightly prolongs APD90, selective IKur inhibition abbreviates APD90 in healthy atria (Figure 2).41,45 This finding could explain the occurrence of AF with IKur inhibition in ‘healthy’ canine atria41 and the association between AF and a mutation in KCNA5, the gene that encodes the α subunit of the IKur channel.46
Figure 2.
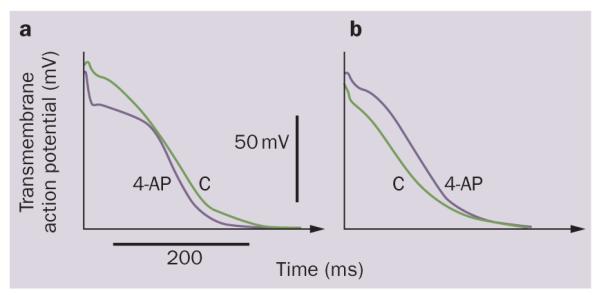
Opposite effect of IKur inhibition on the action potential in healthy and remodeled atria. Block of IKur with 4-aminopyridine (50 μM). APD90 is abbreviated in a | ‘healthy’ (plateau-shaped action potential), but prolonged in b | ‘remodeled’ (triangular-shaped action potential) canine coronary-perfused atrial preparations. Abbreviations: 4-AP, 4-aminopyridine; APD90, action potential duration at 90% repolarization; C, control. Modified from Burashnikov, A. & Antzelevitch, C. Heart Rhythm 5, 1304–1309 (2008) and Burashnikov, A. et al. Am. J. Physiol. Heart Circ. Physiol. 286, H2393–H2400 (2004).
Vagal activity can contribute to the initiation of paroxysmal AF,47,48 and so blocking parasympathetic activity could help maintain sinus rhythm in these patients. IK-ACh block with tertiapin-Q prolongs atrial APD and suppresses AF in experimental models.49,50 Interestingly, CA-IKACh is only marginally present in healthy nonfibrillating human or canine atria and is significantly increased in the atria of patients with chronic AF and in canine tachycardia-remodeled atria,49,51-53 indicating that this current is not only atrial-specific, but is also a pathology-specific target.54 At present, there are no available drugs that selectively block CA-IKACh.
Atrial-selective multiple ion channel block
Several cardiac ion channels, including fast INa sodium channels30,55 and IKr potassium channels, 56-64 respond in an atrial-selective or predominant manner when blocked with specific drugs, despite being present in both atria and ventricles. In canine coronary-perfused cardiac preparations, atrial-selective or predominant INa blockers, such as amiodarone (long-term treatment) and ranolazine, effectively suppress AF with little or no effects in the ventricles.31,55,63,65 Ranolazine and amiodarone exert these actions by atrial-selective or atrial-predominant depression of INa–dependent parameters leading to increased diastolic threshold of excitation and induction of postrepolarization refractoriness (Figures 3 and 4).55,63,66,67 In superfused pulmonary vein preparations, both ranolazine and long-term amiodarone also effectively suppress intracellular calcium-dependent delayed (DAD) and late phase 3 early after depolarization (EAD)-induced triggered activity.66,67 AZD7009 is also an atrial-selective INa blocker.39 Although the clinical anti-AF efficacy of amiodarone, ranolazine, and AZD7009 has been documented,19,25,68-70 the degree to which the efficacy of these multiple ion channel blockers depends on their potency to depress sodium-channel-dependent parameters remains to be elucidated.
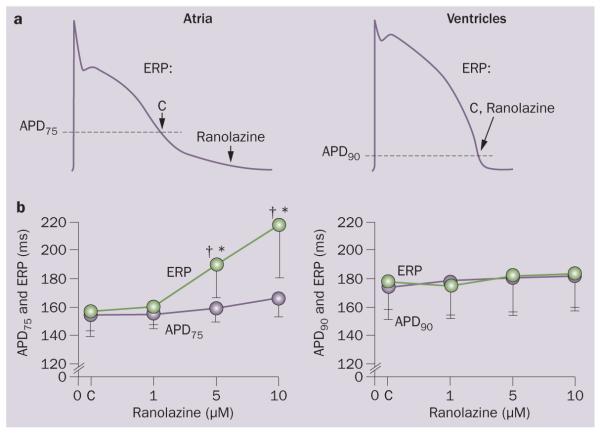
Figure 3.
Ranolazine induces atrial-selective prolongation of the ERP and development of PRR. The PRR is the difference between ERP and APD75 in atria and between ERP and APD90 in ventricles. ERP corresponds to APD75 in atria and APD90 in ventricles. Cycle length, 500 ms. Schematic illustration of induction of postrepolarization refractoriness with ranolazine in the atrium but not in the ventricle. a | The arrows illustrate the position on the action potential corresponding to the end of the ERP in atria and ventricles and the effect of ranolazine to shift the end of the ERP in atria but not ventricles. Summary data of the effect of ranolazine to induce PRR in atria but not in ventricles. b | *P <0.05 versus control. ‡P <0.05 versus APD75 values in atria and APD90 in ventricles; (n = 5–18). Abbreviations: APD90, action potential duration at 90% repolarization; C, control; CL, cycle length; ERP, effective refractory period. Modified from Burashnikov, A. et al. Circulation 116, 1449–1457 (2007).
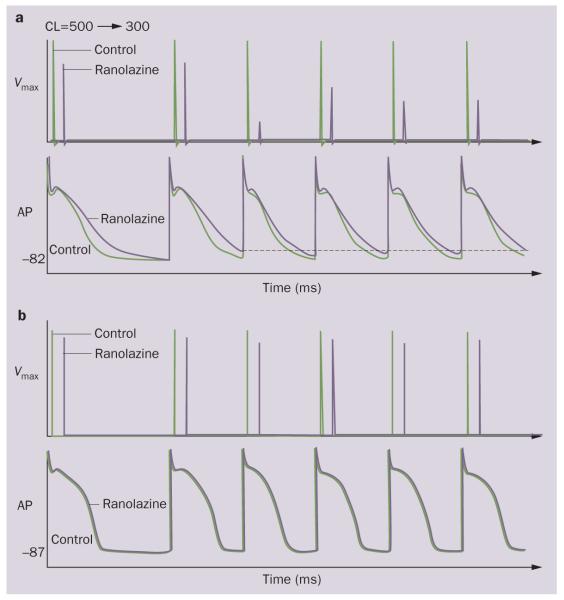
Figure 4.
Ranolazine produces a much greater rate-dependent inhibition of the maximal action potential upstroke velocity (Vmax) in atria than in ventricles. Shown are Vmax and action potential recordings obtained from coronary-perfused canine right atrium a | and left ventricle b | before (C) and after ranolazine (10 μM). Ranolazine prolongs late repolarization in the atria, but not in the ventricles (due to IKr inhibition) and acceleration of rate leads to elimination of the diastolic interval, during which much of the recovery from sodium-channel block occurs, contributing to the atrial selectivity of the drug. Abbreviations: AP, action potential; CL, cycle length. Modified from Antzelevitch, C. & Burashnikov, A. J. Electrocardiol. 42, 543–548 (2009).
It should be emphasized that the nature and degree of atrial selectivity of AADs observed in normal hearts may be different in remodeled hearts or those associated with various pathologies, including myocardial infarction, long QT syndrome, heart failure, and hypertrophy. The pharmacological responses to sodium-channel and potassium-channel blockers can be very different, and even opposite (Figure 2), in healthy versus diseased atria and ventricles; both augmentation and reduction in blocking efficacy have been reported.32,45,71 These altered substrates and pharmacological sensitivities could explain why atrial-selective agents (ranolazine, AVE0118, chronic amiodarone) can successfully suppress ventricular arrhythmias encountered under pathophysiological conditions.24,68,72
A number of factors are likely to contribute to the atrial selectivity of INa blockers, including a more depolarized resting membrane potential (RMP), more negative half-inactivation voltage (V0.5), and more gradual phase 3 of the action potential in atrial cells as compared with ventricular cells (Figure 3).32,55,73 As a consequence, a large proportion of sodium channels are inactivated at the normal RMP in atrial cells. The fraction of resting channels is, therefore, smaller in atrial cells than in ventricular cells at RMP. Atrial cells show a greater accumulation of use-dependent sodium-channel block, because much of the recovery from sodium-channel block commonly occurs during the resting state of the channel.74,75 Rapid kinetics of recovery from inactivation is thought to contribute to the atrial selectivity of sodium-channel blockers. Drugs, such as propafenone, that exhibit slow dissociation from the sodium channel show little or no atrial selectivity, whereas agents that dissociate rapidly, such as ranolazine and amiodarone, tend to be highly atrial-selective in their inhibition of INa-dependent parameters.55,76 Our current understanding of the mechanisms of atrial selectivity of INa blockers, as well as potential applications of atrial-selective INa block for rhythm control of AF, have been discussed in more detail eslewhere.31,32,77 Mechanisms by which depression of INa by drugs with rapid unbinding kinetics lead to AF termination are discussed in an elegant study by Comtois and colleagues.78
Selective inhibition of IKr prolongs APD and ERP to a greater extent in the atria than in the ventricles at both normal and rapid heart rates (Figure 4).56-62 Atrial-predominant prolongation of APD and ERP by ranolazine and chronic amiodarone (both IKr blockers) is thought to contribute to their atrial-selective, use-dependent inhibition of sodium-channel-dependent parameters in the atria by causing greater abbreviation of the diastolic interval (Figure 4). Much of the recovery of sodium channels from inactivation occurs during the diastolic interval and curtailment of this interval promotes accumulation of INa block. Under bradycardic conditions or following long pauses, however, it is the ventricles rather than atria that develop substantial APD prolongation, EADs, and TdP with selective IKr inhibition.79,80 The proarrhythmic effects of IKr block can be counteracted with a concomitant block of late INa. In remodeled atria, in which APD is abbreviated, prolongation of APD90 secondary to IKur block can promote accumulation of INa block preferentially by reducing diastolic interval in the atria but not in ventricles.32 The reduced ability of IKr block to prolong APD in remodeled atria can be overcome with additional inhibition of IKur/Ito.81
Practical clinical experience indicates that multiple ion channel blockers that can block INa with rapid dissociation kinetics are generally better suited for rhythm control of AF than are selective ion channel blockers, because the former have a more favorable risk:benefit ratio.32 With the exception of IKr blockers, such as dofetilide, currently used AADs (amiodarone, dronedarone, flecainide, propafenone) and promising investigational AADs (vernakalant and AZD7009) inhibit multiple ion channels. Notably, selective inhibition of INa, which at present can be achieved only with INa blockers with rapid binding and unbinding kinetics (for example, lidocaine and mexiletine), is not very effective in the clinical management of AF.1
Several anti-AF agents, including amiodarone, dronedarone, vernakalant, and ranolazine, are effective in the clinic and do not, or only rarely, produce ventricular proarrhythmia. These relatively safe AADs have two features in common; firstly they all inhibit INa with relatively rapid kinetics and, secondly, they all also block IKr. As discussed, rapidly dissociating INa blockers tend to be atrial selective, whereas slowly dissociating blockers are not.32 These characteristics coupled with the ability of these drugs to inhibit late INa, which keeps the effects of their IKr block in check, adds to their electrical safety profile. Late INa inhibition plays a key role in the suppression of ventricular arrhythmias in a variety of pathological conditions, such as long QT syndrome, acute ischemia, and heart failure.82-84 Slowly dissociating INa blockers, which also block late INa,85 promote arrhythmogenesis in structurally compromised ventricles because they slow conduction and induce unidirectional block, thus providing the substrate for the development of re-entry.23
In summary, the available data indicate that multiple ion channel blockers exhibiting potent inhibition of fast INa with relatively rapid unbinding kinetics, as well as inhibition of late INa and IKr seem to be best suited for the management of AF. The addition of IKur block to this cocktail is theorized to increase atrial selectivity and anti-AF ability in remodeled atria.32 The long-term adverse effects of AADs are difficult to predict, as shown by the results of CAST,23 and the SWORD86 and ANDROMEDA trials.22 However, agents, such as dronedarone and ranolazine, that have the above ion-channel profile seem to be relatively safe in the long-term for some,87,88 but perhaps not all,22 AF pathologies.
Improved derivatives of existing drugs
Another approach for the development of new drugs involves modification of molecules that have demonstrated anti-AF efficacy. Many AADs in current use are derivatives of existing drugs; for example, flecainide and propafenone stem from procainamide and propranolol, respectively. One notable example of this approach is dronedarone, which was approved by the FDA in July 2009 and is an amiodarone derivative lacking the iodine moiety believed to be responsible for the multi-organ toxicity associated with amiodarone use. Dronedarone has been found to be significantly more effective than placebo in maintaining sinus rhythm in patients with AF, and is largely free of extra-cardiac toxicity.89 Dronedarone also has rate-control properties,89,90 and AADs that have combined rhythm-control and rate-control effects may be of particular interest in future AAD development. The anti-AF efficacy of dronedarone, however, appears to be inferior to that of amiodarone.88 In the landmark, randomized placebo-controlled study, ATHENA,91 dronedarone substantially reduced incidence of cardiac hospitalization and cardiovascular-related death in patients with AF.91 Of the enrolled patients, 21% had NYHA class II or III chronic heart failure, 12% had an LV ejection fraction of less than 45%, and 60% had coronary artery disease.91 The amelioration of several comorbidities, including reduced incidence of AF, stroke, and acute coronary syndrome and a decrease in blood pressure, was observed with the use of dronedarone in ATHENA.90,92 These dronedarone-related positive outcomes are likely to be interrelated and contribute to the decrease in cardiovascular-related hospitalization and death.91,92 However, all-cause mortality was not statistically significantly reduced by dronedarone use in ATHENA (5% for dronedarone versus 6% for placebo, P = 0.18).91 In another large trial (ANDROMEDA),22 dronedarone was associated with increased mortality in patients with severe heart failure and LV systolic dysfunction (NYHA class III and IV), which was likely to be the result of worsening chronic heart failure. Whether the increase in mortality in ANDROMEDA was related to dronedarone itself, or to unrelated confounding factors, remains unknown. The initially postulated hypothesis accounting for the increase in mortality in ANDREOMEDA (that is, discontinuation of treatment with angiotensin-converting-enzyme [ACE] inhibitors because of elevation in serum creatinine level) remains an issue of debate.88 A negative inotropic effect of dronedarone resulting from inhibition of ICa-L93 could have contributed to worsening of severe heart failure, leading to increased mortality.88
Other examples of efforts to improve existing AADs include AZD1305 and AVE1231, which are derivatives of AZD7009 and AVE0118, respectively.94,95 AZD7009 has been found to be effective in acute cardioversion of both paroxysmal and persistent AF, with a small risk for induction of ventricular arrhythmias.19,70 Although AVE0118 effectively suppresses AF in several experimental AF models,36,38 its clinical efficacy turned out to be disappointing and its clinical use for long-term management of AF is limited by first-pass hepatic metabolism. These negative outcomes apparently explain the termination of the further development of AVE0118.
Gap-junction therapy
Conduction disturbances are associated with many forms of cardiac arrhythmia, including AF. Conduction abnormalities in the heart can occur as a consequence of disturbances in sodium-channel or calcium-channel activity, gap-junction abnormalities (impairing cell–cell communications), and structural changes in the myocardium.96 Gap junctions—comprised of proteins called connexins (Cx)—are complexes that connect myocardial cells through low-resistance pathways. Cx40, Cx43, and Cx45 are found in the human heart. Cx40 is commonly recognized as a potential target for atrial-specific treatment of AF because it is found in atrial, but not ventricular, myocardium.97 Cx40 is, however, present in the ventricular conduction system.97
To date, experimental studies have shown that improved conduction with the gap-junction modulator rotigaptide may exert antiarrhythmic action in some AF pathologies (models of chronic mitral regurgitation AF and acute ischemia AF), but not others (models of heart failure or atrial tachypacing).98,99 GAP-134—a dipeptide that behaves similarly to rotigaptide by specifically enhancing gap-junction conductance—has been shown to improve conduction and to reduce inducibility of AF in canine experimental models.100,101 The clinical applicability of this approach is yet to be determined.
Intracellular calcium homeostasis
A growing body of evidence implicates abnormal intracellular calcium homeostasis in the development of AF, and normalization of sarcoplasmic reticulum (SR) calcium release as a potential therapeutic approach.102-105 Abnormal intracellular calcium handling can promote AF by directly inducing intracellular calcium-mediated DAD, EAD, and late phase 3 EAD-induced triggered activity, modulating electrical remodeling, and affecting a number of signaling cascades involved in structural remodeling.105 An increase in spontaneous SR Ca2+ release, as well as a significant SR calcium leak, have been observed in atrial myocytes isolated from AF patients and dogs with tachypacing-induced atrial remodeling.103,104 This SR calcium leak may be mediated by protein kinase a hyper-phosphorylation and calstabin2 (a ryanodine receptor inhibitory subunit, FKBP12.6).104
Although pharmacological modulation of these arrhythmogenic mechanisms might be of benefit, the challenge is to regulate SR calcium release and intracellular calcium loading, without compromising myocardial contractility. Ranolazine limits sodium loading, primarily via inhibition of early INa, and so can also reduce calcium loading associated with conditions that predispose to the development of AF, and suppress triggered AF activity induced by DAD, EAD, and late phase 3 EAD.66 Amiodarone has been reported to exert similar actions to ranolazine in both atria and pulmonary veins.63,67 Other sodium-channel-blocking agents used in the management of AF, including vernakalant, flecainide, propafenone, AZD7009, as well as β-blockers, are thought to act in part by modulating calcium homeostasis and suppressing triggered activity.
‘Pill-in-the-pocket’ approach to therapy
The idea of limiting AAD administration to critical times was introduced because the chance of an adverse reaction is more likely with long-term administration of these agents. Acute termination of paroxysmal AF with class IC agents has proved to be a reasonable approach in some patients with AF.18 This has been labeled a ‘pill-in-the-pocket’ approach. Preliminary results indicate that a single dose of ranolazine (2 g) is effective for this strategy.106 In addition, ranolazine is safe in patients with structural heart diseases,87 pointing to a much wider potential applicability of the pill-in-the-pocket approach in patients with AF. Short-term administration of AADs is also used for maintenance of sinus rhythm following cardioversion (electrically or by catheter radiofrequency oblation) or postoperatively.4,107 Short-term application of an AAD can be of a greater benefit than risk in some, but not all patients with AF. All-cause mortality and the rate of AF recurrence have been shown to be greater in patients treated with amiodarone episodically when compared with those who received amiodarone continuously (patients with NYHA class III and IV chronic heart failure were excluded from the study).108
Upstream therapy
The recognition that atrial structural remodeling can lead to the induction of AF109 has led to the development of ‘upstream therapies’, which target arrhythmogenic structural remodeling in the atria, factors that promote such remodeling, or both.110,111 Structural remodeling encompasses a number of pathological changes, including increased interstitial fibrosis, fibroblast proliferation, dilatation, hypertrophy, pathological collagen accumulation and its abnormal distribution or redistribution, and is often associated with stretch, oxidative stress, inflammation, or ischemia.112,113 These factors are in turn induced by, or associated with, hypertension, heart failure, or coronary artery disease.110-113 Studies of drugs designed to directly or indirectly mitigate these precipitating factors, such as ACE inhibitors, angiotensin II receptor blockers (ARBs), omega-3 polyunsaturated fatty acids, and statins, have yielded variable results.110,111,114-117 The precise value of upstream therapy in the treatment of AF varies substantially by AF pathology.111,116-118 Preoperative statin and omega-3 therapy has been consistently associated with a reduction in postoperative AF.119-121 A number of clinical studies have indicated that ARBs and statins might be of particular benefit for patients with AF and severe ventricular dysfunction and heart failure,122-124 but not in those without heart disease.114,125,126
Most of the current clinical data on upstream AF therapy are, however, derived from observational studies that were not sufficiently powered, and these findings do not warrant rejection or widespread use of any of the approaches.111,115-117 The results of several large randomized placebo-controlled trials are now available. In the GISSI-AF trial,114 treatment with the ARB valsartan did not reduce the recurrence of AF (only 8% of the enrolled patients had chronic heart failure or LV systolic dysfunction). In another large clinical trial (ACTIVE-I), the ARB irbesartan also did not substantially reduce the composite of stroke, myocardial infarction, and vascular death in patients with AF and hypertension, but this agent was associated with a reduced rate of hospitalization from heart failure.127 Several large, randomized placebo-controlled trials testing the anti-AF efficacy of various types of upstream therapy are underway, including studies assessing antiarrhythmic ability of omega-3-acid ethyl esters115 and olmesartan (the ANTIPAF trial)128 in the prevention of recurrence of paroxysmal AF. Interestingly, because atria commonly develop structural remodeling to a greater extent than do the ventricles,129-133 structural remodeling is a potential atrial-selective target for upstream therapy of AF.134
Future directions
The ultimate goal of pharmacological therapy for AF is to improve patient morbidity (and, thus, quality of life) and reduce mortality. To date, all large, placebo-controlled, randomized clinical trials evaluating the ability of anti-AF AADs to improve all-cause mortality have yielded neutral outcomes at best.1,89,90,135,136 However, morbidity (and quality of life) of patients with AF can be significantly improved with the use of AADs.90,137 The results of ATHENA, which demonstrated significant reductions in cardiovascular-related morbidity and mortality in dronedarone-treated patients with AF,91,92 may encourage a change of focus in the management of AF, from electrocardiographically-derived measures (that is, rhythm control and rate control) toward more general end points (such as morbidity and mortality).138 This novel paradigm is related to the fact that the positive outcomes with dronedarone can be achieved in spite of the rather limited ability of this agent to maintain sinus rhythm. In the combined analysis of EURIDIS and ADONIS, AF recurrence (after 1 year of follow-up) occurred in 64% patients randomly assigned to dronedarone, as compared with 75% patients taking placebo.89
Several major questions about the pharmacological management of patients with AF need to be addressed in future studies. Because the primary end point of ATHENA was unique (time to first cardiovascular hospitalization or death), future studies need to determine if dronedarone is superior to the other AADs in prolonging survival free of cardiovascular hospitalization. Of note, secondary analysis of data from DIAMOND137 suggests that dofetilide reduces hospitalization in patients with AF and chronic heart failure without improving all-cause mortality. In addition, amiodarone significantly reduced arrhythmic death compared with placebo in patients with myocardial infarction (LV ejection fraction ≤40%), but did not improve all-cause mortality in EMIAT139 and CAMIAT.140
The introduction of atrial-selective sodium-channel blockade for the management of AF needs to be further explored and developed as an approach to achieving safe and effective rhythm control.32,141 Available studies suggest that although novel agents demonstrating anti-AF efficacy are multiple ion channel blockers, they potently inhibit early INa (for example, vernakalant, dronedarone, AZD7009, ranolazine)34,35,55,142 and most, if not all, are atrial-selective INa blockers. We have reviewed this subject elsewhere.32 Studies have revealed a unique synergism when predominantly inactivated-state and activated-state INa blockers are combined. The combination of long-term therapy with amiodarone63 and acute administration of ranolazine,55 both of which are atrial-selective ion channel blockers, resulted in a profound atrial-selective use-dependent inhibition of INa and depression of INa-dependent parameters in isolated canine atria, leading to a potent effect of the drug combination for the prevention of AF.143 Studies of the molecular differences between atrial and ventricular sodium-channels are needed to enable us to better understand the basis for atrial selectivity of pharmacological agents and design ever more selective drugs. Experimental observations suggest that studies specifically designed to evaluate the potential clinical role of atrial-selective sodium-channel blockers, such as ranolazine and amiodarone, as antiarrhythmics are warranted.
Conclusions
The ultimate goal of AF therapy is to improve patient quality-of-life and reduce mortality. Ongoing research aimed at development of new pharmacological strategies for the management of AF includes a wide range of approaches, targeting both AF-related electrical and nonelectrical (intracardiac and extracardiac) abnormalities. While success to date has been modest, the identification of atrial-selective agents and targets, as well as the development of upstream therapies, hold promise for the development of effective and safe new treatments.
Key points.
Atrial fibrillation (AF) is a growing clinical problem associated with increased morbidity and mortality
Currently available antiarrhythmic drugs (AADs) can be highly effective in acute cardioversion of AF, but are only moderately successful in long-term maintenance of sinus rhythm and may induce adverse effects
AF is commonly associated with atrial electrical and structural abnormalities as well as extracardiac disease, which may induce or promote AF and determine the efficacy and safety of AAD therapy
Current development of anti-AF agents is focused on alteration of ion channel activity as well as upstream therapies that reduce structural substrates
Multiple ion channel blockers exhibiting inhibition of fast INa and late INa, IKr, and IKur are likely to be atrial-selective and may be best suited for the management of AF
Preventing or reversing atrial structural remodeling (‘upstream therapy’) seems to be beneficial for some AF pathologies, such as postoperative AF or AF associated with severe heart failure
Acknowledgments
Acknowledgments Supported by grant HL47678 from NHLBI (C. Antzelevitch) and NYS and Florida Grand Lodges F. & A. M.
Footnotes
Competing interests C. Antzelevitch has declared associations with the following companies: AstraZeneca, Cardiome, Gilead Sciences, Inc., Lundbeck, and Solvay. See the article online for full details of the relationships. A. Burashnikov declares no competing interests.
Review criteria We have attempted to cite the most relevant papers concerning the respective statements, arguments, and hypotheses discussed in this Review. These references were taken from searches of the PubMed database using appropriate terms, including “atrial fibrillation”, “anti-arrhythmic agents”, and “upstream therapy”. The literature search was limited to full-text articles in the English language. Because of space limitations, we have restricted our citations to the most pertinent publications.
References
- 1.Fuster V, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation) J. Am. Coll. Cardiol. 2006;48:854–906. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Reiffel JA. Rate versus rhythm control pharmacotherapy for atrial fibrillation: where are we in 2008? JAFIB. 2008;1:31–47. doi: 10.4022/jafib.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naccarelli GV, Gonzalez MD. Atrial fibrillation and the expanding role of catheter ablation: do antiarrhythmic drugs have a future? J. Cardiovasc. Pharmacol. 2008;52:203–209. doi: 10.1097/FJC.0b013e318175dd73. [DOI] [PubMed] [Google Scholar]
- 4.Roux JF, et al. Antiarrhythmics after ablation of atrial aibrillation (5A Study) Circulation. 2009;120:1036–1040. doi: 10.1161/CIRCULATIONAHA.108.839639. [DOI] [PubMed] [Google Scholar]
- 5.Cain ME, Curtis AB. Rhythm control in atrial fibrillation—one setback after another. N. Engl. J. Med. 2008;358:2725–2727. doi: 10.1056/NEJMe0803289. [DOI] [PubMed] [Google Scholar]
- 6.Savelieva I, Camm J. Anti-arrhythmic drug therapy for atrial fibrillation: current anti-arrhythmic drugs, investigational agents, and innovative approaches. Europace. 2008;10:647–665. doi: 10.1093/europace/eun130. [DOI] [PubMed] [Google Scholar]
- 7.Wyse DG, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N. Engl. J. Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 8.Roy D, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N. Engl. J. Med. 2008;358:2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 9.Van GI, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N. Engl. J. Med. 2002;347:1834–1840. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 10.Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation—Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomised trial. Lancet. 2000;356:1789–1794. doi: 10.1016/s0140-6736(00)03230-x. [DOI] [PubMed] [Google Scholar]
- 11.Corley SD, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109:1509–1513. doi: 10.1161/01.CIR.0000121736.16643.11. [DOI] [PubMed] [Google Scholar]
- 12.Jais P, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation. 2008;118:2498–2505. doi: 10.1161/CIRCULATIONAHA.108.772582. [DOI] [PubMed] [Google Scholar]
- 13.Calkins H, et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: Two systematic literature reviews and meta analysis. Circ. Arrhythm. Electrophysiol. 2009;2:349–361. doi: 10.1161/CIRCEP.108.824789. [DOI] [PubMed] [Google Scholar]
- 14.Le Bouter S, et al. Long-term amiodarone administration remodels expression of ion channel transcripts in the mouse heart. Circulation. 2004;110:3028–3035. doi: 10.1161/01.CIR.0000147187.78162.AC. [DOI] [PubMed] [Google Scholar]
- 15.Schumacher SM, et al. Antiarrhythmic drug-induced internalization of the atrial-specific K+ channel Kv1.5. Circ. Res. 2009;104:1390–1398. doi: 10.1161/CIRCRESAHA.108.192773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shinagawa K, Shiroshita-Takeshita A, Schram G, Nattel S. Effects of antiarrhythmic drugs on fibrillation in the remodeled atrium: insights into the mechanism of the superior efficacy of amiodarone. Circulation. 2003;107:1440–1446. doi: 10.1161/01.cir.0000055316.35552.74. [DOI] [PubMed] [Google Scholar]
- 17.Ashikaga H, et al. Transmural dispersion of myofiber mechanics: implications for electrical heterogeneity in vivo. J. Am. Coll. Cardiol. 2007;49:909–916. doi: 10.1016/j.jacc.2006.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alboni P, et al. Outpatient treatment of recent-onset atrial fibrillation with the “pill-in-the-pocket” approach. N. Engl. J. Med. 2004;351:2384–2391. doi: 10.1056/NEJMoa041233. [DOI] [PubMed] [Google Scholar]
- 19.Geller JC, et al. Rapid conversion of persistent atrial fibrillation to sinus rhythm by intravenous AZD7009. J. Clin. Pharmacol. 2009;49:312–322. doi: 10.1177/0091270008329549. [DOI] [PubMed] [Google Scholar]
- 20.Banchs JE, et al. Efficacy and safety of dofetilide in patients with atrial fibrillation and atrial flutter. J. Interv. Card. Electrophysiol. 2008;23:111–115. doi: 10.1007/s10840-008-9290-6. [DOI] [PubMed] [Google Scholar]
- 21.Roy D, et al. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N. Engl. J. Med. 2000;342:913–920. doi: 10.1056/NEJM200003303421302. [DOI] [PubMed] [Google Scholar]
- 22.Kober L, et al. Increased mortality after dronedarone therapy for severe heart failure. N. Engl. J. Med. 2008;358:2678–2687. doi: 10.1056/NEJMoa0800456. [DOI] [PubMed] [Google Scholar]
- 23.CAST Investigators Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N. Engl. J. Med. 1989;321:406–412. doi: 10.1056/NEJM198908103210629. [DOI] [PubMed] [Google Scholar]
- 24.Singh BN. Amiodarone as paradigm for developing new drugs for atrial fibrillation. J. Cardiovasc. Pharmacol. 2008;52:300–305. doi: 10.1097/FJC.0b013e31818914b6. [DOI] [PubMed] [Google Scholar]
- 25.Zimetbaum P. Amiodarone for atrial fibrillation. N. Engl. J. Med. 2007;356:935–941. doi: 10.1056/NEJMct065916. [DOI] [PubMed] [Google Scholar]
- 26.Steinberg JS, et al. Analysis of cause-specific mortality in the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Circulation. 2004;109:1973–1980. doi: 10.1161/01.CIR.0000118472.77237.FA. [DOI] [PubMed] [Google Scholar]
- 27.Bardy GH, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N. Engl. J. Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 28.Solomon SD, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116:1482–1487. doi: 10.1161/CIRCULATIONAHA.107.696906. [DOI] [PubMed] [Google Scholar]
- 29.Nattel S, Carlsson L. Innovative approaches to anti-arrhythmic drug therapy. Nat. Rev. Drug Discov. 2006;5:1034–1049. doi: 10.1038/nrd2112. [DOI] [PubMed] [Google Scholar]
- 30.Burashnikov A, Antzelevitch C. How do atrial-selective drugs differ from antiarrhythmic drugs currently used in the treatment of atrial fibrillation? JAFIB. 2008;1:98–107. doi: 10.4022/jafib.v1i1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burashnikov A, Antzelevitch C. Atrial-selective sodium channel blockers: do they exist? J. Cardiovasc. Pharmacol. 2008;52:121–128. doi: 10.1097/FJC.0b013e31817618eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burashnikov A, Antzelevitch C. Atrial-selective sodium channel block for the treatment of atrial fibrillation. Expert Opin. Emerg. Drugs. 2009;14:233–249. doi: 10.1517/14728210902997939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ehrlich JR, Nattel S. Atrial-selective pharmacological therapy for atrial fibrillation: hype or hope? Curr. Opin. Cardiol. 2009;24:50–55. doi: 10.1097/HCO.0b013e32831bc336. [DOI] [PubMed] [Google Scholar]
- 34.Fedida D. Vernakalant (RSD1235): a novel, atrial-selective antifibrillatory agent. Expert Opin. Investig. Drugs. 2007;16:519–532. doi: 10.1517/13543784.16.4.519. [DOI] [PubMed] [Google Scholar]
- 35.Carlsson L, Chartier D, Nattel S. Characterization of the in vivo and in vitro electrophysiological effects of the novel antiarrhythmic agent AZD7009 in atrial and ventricular tissue of the dog. J. Cardiovasc. Pharmacol. 2006;47:123–132. doi: 10.1097/01.fjc.0000196242.04384.c3. [DOI] [PubMed] [Google Scholar]
- 36.Blaauw Y, et al. “Early” class III drugs for the treatment of atrial fibrillation: efficacy and atrial selectivity of AVE0118 in remodeled atria of the goat. Circulation. 2004;110:1717–1724. doi: 10.1161/01.CIR.0000143050.22291.2E. [DOI] [PubMed] [Google Scholar]
- 37.Christ T, et al. Pathology-specific effects of the IKur/Ito/IK, ACh blocker AVE0118 on ion channels in human chronic atrial fibrillation. Br. J. Pharmacol. 2008;154:1619–1630. doi: 10.1038/bjp.2008.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burashnikov A, et al. The atrial-selective potassium channel blocker AVE0118 prolongs effective refractory period in canine atria by inhibiting sodium channels. Heart Rhythm. 2009;6:S98. [Google Scholar]
- 39.Goldstein RN, Khrestian C, Carlsson L, Waldo AL. AZD7009: a new antiarrhythmic drug with predominant effects on the atria effectively terminates and prevents reinduction of atrial fibrillation and flutter in the sterile pericarditis model. J. Cardiovasc. Electrophysiol. 2004;15:1444–1450. doi: 10.1046/j.1540-8167.2004.04354.x. [DOI] [PubMed] [Google Scholar]
- 40.Ford JW, Milnes JT. New drugs targeting the cardiac ultra-rapid delayed-rectifier current (IKur): rationale, pharmacology and evidence for potential therapeutic value. J. Cardiovasc. Pharmacol. 2008;52:105–120. doi: 10.1097/FJC.0b013e3181719b0c. [DOI] [PubMed] [Google Scholar]
- 41.Burashnikov A, Antzelevitch C. Can inhibition of IKur promote atrial fibrillation? Heart Rhythm. 2008;5:1304–1309. doi: 10.1016/j.hrthm.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng J, Xu D, Wang Z, Nattel S. Ultrarapid delayed rectifier current inactivation in human atrial myocytes: properties and consequences. Am. J. Physiol. 1998;275:H1717–H1725. doi: 10.1152/ajpheart.1998.275.5.H1717. [DOI] [PubMed] [Google Scholar]
- 43.Courtemanche M, Ramirez RJ, Nattel S. Ionic targets for drug therapy and atrial fibrillation-induced electrical remodeling: insights from a mathematical model. Cardiovasc. Res. 1999;42:477–489. doi: 10.1016/s0008-6363(99)00034-6. [DOI] [PubMed] [Google Scholar]
- 44.Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circ. Res. 1997;80:772–781. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]
- 45.Wettwer E, et al. Role of IKur in controlling action potential shape and contractility in the human atrium: influence of chronic atrial fibrillation. Circulation. 2004;110:2299–2306. doi: 10.1161/01.CIR.0000145155.60288.71. [DOI] [PubMed] [Google Scholar]
- 46.Olson TM, et al. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum. Mol. Genet. 2006;15:2185–2191. doi: 10.1093/hmg/ddl143. [DOI] [PubMed] [Google Scholar]
- 47.Bettoni M, Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation. 2002;105:2753–2759. doi: 10.1161/01.cir.0000018443.44005.d8. [DOI] [PubMed] [Google Scholar]
- 48.Pappone C, et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004;109:327–334. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]
- 49.Cha TJ, et al. Kir3-based inward rectifier potassium current: potential role in atrial tachycardia remodeling effects on atrial repolarization and arrhythmias. Circulation. 2006;113:1730–1737. doi: 10.1161/CIRCULATIONAHA.105.561738. [DOI] [PubMed] [Google Scholar]
- 50.Hashimoto N, Yamashita T, Tsuruzoe N. Tertiapin, a selective, IKACh blocker, terminates atrial fibrillation with selective atrial effective refractory period prolongation. Pharmacol. Res. 2006;54:136–141. doi: 10.1016/j.phrs.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 51.Dobrev D, et al. The G protein-gated potassium current, IKACh is constitutively active in patients with chronic atrial fibrillation. Circulation. 2005;112:3697–3706. doi: 10.1161/CIRCULATIONAHA.105.575332. [DOI] [PubMed] [Google Scholar]
- 52.Voigt N, et al. Differential phosphorylation-dependent regulation of constitutively active and muscarinic receptor-activated, IKACh channels in patients with chronic atrial fibrillation. Cardiovasc. Res. 2007;74:426–437. doi: 10.1016/j.cardiores.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 53.Ehrlich JR, et al. Characterization of a hyperpolarization-activated time-dependent potassium current in canine cardiomyocytes from pulmonary vein myocardial sleeves and left atrium. J. Physiol. 2004;557:583–597. doi: 10.1113/jphysiol.2004.061119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ravens U. Potassium channels in atrial fibrillation: targets for atrial and pathology-specific therapy? Heart Rhythm. 2008;5:758–759. doi: 10.1016/j.hrthm.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 55.Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116:1449–1457. doi: 10.1161/CIRCULATIONAHA.107.704890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spinelli W, Parsons RW, Colatsky TJ. Effects of WAY-123,398, a new class-III antiarrhythmic agent, on cardiac refractoriness and ventricular fibrillation threshold in anesthetized dogs—a comparison with UK-68798, E-4031, and dl-sotalol. J. Cardiovasc. Pharmacol. 1992;20:913–922. doi: 10.1097/00005344-199212000-00011. [DOI] [PubMed] [Google Scholar]
- 57.Wiesfeld AC, et al. Rate-dependent effects of the class III antiarrhythmic drug almokalant on refractoriness in the pig. J. Cardiovasc. Pharmacol. 1996;27:594–600. doi: 10.1097/00005344-199604000-00021. [DOI] [PubMed] [Google Scholar]
- 58.Baskin EP, Lynch JJ., Jr. Differential atrial versus ventricular activities of class III potassium channel blockers. J. Pharmacol. Exp. Ther. 1998;285:135–142. [PubMed] [Google Scholar]
- 59.Stump GL, Wallace AA, Regan CP, Lynch JJ., Jr. In vivo antiarrhythmic and cardiac electrophysiologic effects of a novel diphenylphosphine oxide IKur blocker (2-isopropyl-5-methylcyclohexyl) diphenylphosphine oxide. J. Pharmacol. Exp. Ther. 2005;315:1362–1367. doi: 10.1124/jpet.105.092197. [DOI] [PubMed] [Google Scholar]
- 60.Wang J, Feng J, Nattel S. Class III antiarrhythmic drug action in experimental atrial fibrillation. Differences in reverse use dependence and effectiveness between d-sotalol and the new antiarrhythmic drug ambasilide. Circulation. 1994;90:2032–2040. doi: 10.1161/01.cir.90.4.2032. [DOI] [PubMed] [Google Scholar]
- 61.Echt DS, et al. Prolongation of the human monophasic action potential by sotalol. Am. J. Cardiol. 1982;50:1082–1086. doi: 10.1016/0002-9149(82)90421-0. [DOI] [PubMed] [Google Scholar]
- 62.Buchanan LV, et al. Antiarrhythmic and electrophysiologic effects of intravenous ibutilide and sotalol in the canine sterile pericarditis model. J. Cardiovasc. Electrophysiol. 1996;7:113–119. doi: 10.1111/j.1540-8167.1996.tb00506.x. [DOI] [PubMed] [Google Scholar]
- 63.Burashnikov A, et al. Atrial-selective effects of chronic amiodarone in the management of atrial fibrillation. Heart Rhythm. 2008;5:1735–1742. doi: 10.1016/j.hrthm.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burashnikov A, Antzelevitch C. New pharmacological strategies for the treatment of atrial fibrillation. Ann. Noninvasive Electrocardiol. 2009;14:290–300. doi: 10.1111/j.1542-474X.2009.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burashnikov A, Di Diego JM, Zygmunt AC, Belardinelli L, Antzelevitch C. Atrial-selective sodium channel block as a strategy for suppression of atrial fibrillation. Ann. N. Y. Acad. Sci. 2008;1123:105–112. doi: 10.1196/annals.1420.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sicouri S, Glass A, Belardinelli L, Antzelevitch C. Antiarrhythmic effects of ranolazine in canine pulmonary vein sleeve preparations. Heart Rhythm. 2008;5:1019–1026. doi: 10.1016/j.hrthm.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sicouri S, Belardinelli L, Carlsson L, Antzelevitch C. Potent antiarrhythmic effects of chronic amiodarone in canine pulmonary vein sleeve preparations. J. Cardiovasc. Electrophysiol. 2009;20:803–810. doi: 10.1111/j.1540-8167.2009.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scirica BM, et al. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non ST-segment elevation acute coronary syndrome: results from the Metabolic Efficiency with Ranolazine for Less Ischemia in Non ST-Elevation Acute Coronary Syndrome Thrombolysis in Myocardial Infarction 36 (MERLIN-TIMI 36) randomized controlled trial. Circulation. 2007;116:1647–1652. doi: 10.1161/CIRCULATIONAHA.107.724880. [DOI] [PubMed] [Google Scholar]
- 69.Murdock DK, Overton N, Kersten M, Kaliebe J, Devecchi F. The effect of ranolazine on maintaining sinus rhythm in patients with resistant atrial fibrillation. Indian Pacing Electrophysiol. J. 2008;8:175–181. [PMC free article] [PubMed] [Google Scholar]
- 70.Crijns HJ, et al. Safe and effective conversion of persistent atrial fibrillation to sinus rhythm by intravenous AZD7009. Heart Rhythm. 2006;3:1321–1331. doi: 10.1016/j.hrthm.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 71.Duytschaever M, Blaauw Y, Allessie M. Consequences of atrial electrical remodeling for the anti-arrhythmic action of class IC and class III drugs. Cardiovasc. Res. 2005;67:69–76. doi: 10.1016/j.cardiores.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 72.Billman GE, Kukielka M. Novel transient outward and ultra-rapid delayed rectifier current antagonist, AVE0118, protects against ventricular fibrillation induced by myocardial ischemia. J. Cardiovasc. Pharmacol. 2008;51:352–358. doi: 10.1097/FJC.0b013e31816586bd. [DOI] [PubMed] [Google Scholar]
- 73.Li GR, Lau CP, Shrier A. Heterogeneity of sodium current in atrial vs epicardial ventricular myocytes of adult guinea pig hearts. J. Mol. Cell Cardiol. 2002;34:1185–1194. doi: 10.1006/jmcc.2002.2053. [DOI] [PubMed] [Google Scholar]
- 74.Carmeliet E, Mubagwa K. Antiarrhythmic drugs and cardiac ion channels: mechanisms of action. Prog. Biophys. Mol. Biol. 1998;70:1–72. doi: 10.1016/s0079-6107(98)00002-9. [DOI] [PubMed] [Google Scholar]
- 75.Hondeghem LM, Katzung BG. In: Mechanism of Action of Antiarrhythmic Drugs in Physiology and Pathophysiology of the Heart. Sperelakis N, editor. Kluwer Academic Publishers; 1995. pp. 589–603. [Google Scholar]
- 76.Burashnikov A, Belardinelli L, Antzelevitch C. Ranolazine and propafenone both suppress atrial fibrillation but ranolazine unlike propafenone does it without prominent effects on ventricular myocardium. Heart Rhythm. 2007;4:S163. [Google Scholar]
- 77.Antzelevitch C, Burashnikov A. Atrial selective sodium channel block as a novel strategy for the management of atrial fibrillation. J. Electrocardiol. 2009;42:543–548. doi: 10.1016/j.jelectrocard.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Comtois P, et al. Mechanisms of atrial fibrillation termination by rapidly unbinding Na+ channel blockers. Insights from mathematical models and experimental correlates. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H1489–H1504. doi: 10.1152/ajpheart.01054.2007. [DOI] [PubMed] [Google Scholar]
- 79.Antzelevitch C, et al. The M cell: its contribution to the ECG and to normal and abnormal electrical function of the heart. J. Cardiovasc. Electrophysiol. 1999;10:1124–1152. doi: 10.1111/j.1540-8167.1999.tb00287.x. [DOI] [PubMed] [Google Scholar]
- 80.Burashnikov A, Antzelevitch C. Late-phase 3 EAD. A unique mechanism contributing to initiation of atrial fibrillation. PACE. 2006;29:290–295. doi: 10.1111/j.1540-8159.2006.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blaauw Y, Schotten U, van HA, Neuberger HR, Allessie MA. Cardioversion of persistent atrial fibrillation by a combination of atrial specific and non-specific class III drugs in the goat. Cardiovasc. Res. 2007;75:89–98. doi: 10.1016/j.cardiores.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 82.Antzelevitch C. Electrical heterogeneity, cardiac arrhythmias, and the sodium channel. Circ. Res. 2000;87:964–965. doi: 10.1161/01.res.87.11.964. [DOI] [PubMed] [Google Scholar]
- 83.Antzelevitch C, et al. Electrophysiologic properties and antiarrhythmic actions of a novel anti-anginal agent. J. Cardiovasc. Pharmacol. Therapeut. 2004;9(Suppl 1):S65–S83. doi: 10.1177/107424840400900106. [DOI] [PubMed] [Google Scholar]
- 84.Shryock JC, Belardinelli L. Inhibition of late sodium current to reduce electrical and mechanical dysfunction of ischaemic myocardium. Br. J. Pharmacol. 2008;153:1128–1132. doi: 10.1038/sj.bjp.0707522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Persson F, Andersson B, Duker G, Jacobson I, Carlsson L. Functional effects of the late sodium current inhibition by AZD7009 and lidocaine in rabbit isolated atrial and ventricular tissue and Purkinje fibre. Eur. J. Pharmacol. 2007;558:133–143. doi: 10.1016/j.ejphar.2006.11.040. [DOI] [PubMed] [Google Scholar]
- 86.Waldo AL, et al. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival with oral d-sotalol. Lancet. 1996;348:7–12. doi: 10.1016/s0140-6736(96)02149-6. [DOI] [PubMed] [Google Scholar]
- 87.Koren MJ, Crager MR, Sweeney M. Long-term safety of a novel antianginal agent in patients with severe chronic stable angina: the Ranolazine Open Label Experience (ROLE) J. Am. Coll. Cardiol. 2007;49:1027–1034. doi: 10.1016/j.jacc.2006.10.067. [DOI] [PubMed] [Google Scholar]
- 88.Zimetbaum PJ. Dronedarone for atrial fibrillation--an odyssey. N. Engl. J. Med. 2009;360:1811–1813. doi: 10.1056/NEJMp0902248. [DOI] [PubMed] [Google Scholar]
- 89.Singh BN, et al. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N. Engl. J. Med. 2007;357:987–999. doi: 10.1056/NEJMoa054686. [DOI] [PubMed] [Google Scholar]
- 90.Davy JM, et al. Dronedarone for the control of ventricular rate in permanent atrial fibrillation: the Efficacy and safety of dRonedArone for the cOntrol of ventricular rate during atrial fibrillation (ERATO) study. Am. Heart J. 2008;156:527.e1–527e9. doi: 10.1016/j.ahj.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 91.Hohnloser SH, et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N. Engl. J. Med. 2009;360:668–678. doi: 10.1056/NEJMoa0803778. [DOI] [PubMed] [Google Scholar]
- 92.Connolly SJ, et al. Analysis of stroke in ATHENA: a placebo-controlled, double-blind, parallel-arm trial to assess the efficacy of dronedarone 400 mg BID for the prevention of cardiovascular hospitalization or death from any cause in patients with atrial fibrillation/atrial flutter. Circulation. 2009;120:1174–1180. doi: 10.1161/CIRCULATIONAHA.109.875252. [DOI] [PubMed] [Google Scholar]
- 93.Gautier P, et al. Electrophysiologic characterization of dronedarone in guinea pig ventricular cells. J. Cardiovasc. Pharmacol. 2003;41:191–202. doi: 10.1097/00005344-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 94.Carlsson L, Andersson B, Linhardt G, Lofberg L. Assessment of the ion channel-blocking profile of the novel combined ion channel blocker AZD1305 and its proarrhythmic potential versus dofetilide in the methoxamine-sensitized rabbit in vivo. J. Cardiovasc. Pharmacol. 2009;54:82–89. doi: 10.1097/FJC.0b013e3181ac62c9. [DOI] [PubMed] [Google Scholar]
- 95.Wirth KJ, et al. In vitro and in vivo effects of the atrial selective antiarrhythmic compound AVE1231. J. Cardiovasc. Pharmacol. 2007;49:197–206. doi: 10.1097/FJC.0b013e318032002f. [DOI] [PubMed] [Google Scholar]
- 96.Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol. Rev. 2004;84:431–488. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- 97.Ehrlich JR, Biliczki P, Hohnloser SH, Nattel S. Atrial-selective approaches for the treatment of atrial fibrillation. J. Am. Coll. Cardiol. 2008;51:787–792. doi: 10.1016/j.jacc.2007.08.067. [DOI] [PubMed] [Google Scholar]
- 98.Guerra JM, Everett TH, Lee KW, Wilson E, Olgin JE. Effects of the gap junction modifier rotigaptide (ZP123) on atrial conduction and vulnerability to atrial fibrillation. Circulation. 2006;114:110–118. doi: 10.1161/CIRCULATIONAHA.105.606251. [DOI] [PubMed] [Google Scholar]
- 99.Shiroshita-Takeshita A, Sakabe M, Haugan K, Hennan JK, Nattel S. Model-dependent effects of the gap junction conduction-enhancing antiarrhythmic peptide rotigaptide (ZP123) on experimental atrial fibrillation in dogs. Circulation. 2007;115:310–318. doi: 10.1161/CIRCULATIONAHA.106.665547. [DOI] [PubMed] [Google Scholar]
- 100.Laurent G, et al. Effects of chronic gap junction conduction–enhancing antiarrhythmic peptide GAP-134 administration on experimental atrial fibrillation in dogs. Circ. Arrhythm. Electrophysiol. 2009;2:171–178. doi: 10.1161/CIRCEP.108.790212. [DOI] [PubMed] [Google Scholar]
- 101.Rossman EI, et al. The gap junction modifier, GAP-134 [(2S, 4R)-1-(2-aminoacetyl)-4-benzamido-pyrrolidine-2-carboxylic acid], improves conduction and reduces atrial fibrillation/flutter in the canine sterile pericarditis model. J. Pharmacol. Exp. Ther. 2009;329:1127–1133. doi: 10.1124/jpet.108.150102. [DOI] [PubMed] [Google Scholar]
- 102.Burashnikov A, Antzelevitch C. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation. 2003;107:2355–2360. doi: 10.1161/01.CIR.0000065578.00869.7C. [DOI] [PubMed] [Google Scholar]
- 103.Hove-Madsen L, et al. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation. 2004;110:1358–1363. doi: 10.1161/01.CIR.0000141296.59876.87. [DOI] [PubMed] [Google Scholar]
- 104.Vest JA, et al. Defective cardiac ryanodine receptor regulation during atrial fibrillation. Circulation. 2005;111:2025–2032. doi: 10.1161/01.CIR.0000162461.67140.4C. [DOI] [PubMed] [Google Scholar]
- 105.Dobrev D, Nattel S. Calcium handling abnormalities in atrial fibrillation as a target for innovative therapeutics. J. Cardiovasc. Pharmacol. 2008;52:293–299. doi: 10.1097/FJC.0b013e318171924d. [DOI] [PubMed] [Google Scholar]
- 106.Murdock DK, Kersten M, Kaliebe J, Larrian G. The use of oral ranolazine to convert new or paroxysmal atrial fibrillation: a reveiw of experience with implications for possible “pill in the pocket” approach to atrial fibrillation. Indian Pacing Electrophysiol. J. 2009;9:260–267. [PMC free article] [PubMed] [Google Scholar]
- 107.Kirchhof P, et al. Early and comprehensive management of atrial fibrillation: proceedings from the 2nd AFNET/EHRA consensus conference on atrial fibrillation entitled ‘research perspectives in atrial fibrillation’. Europace. 2009;11:860–885. doi: 10.1093/europace/eup124. [DOI] [PubMed] [Google Scholar]
- 108.Ahmed S, et al. Continuous vs episodic prophylactic treatment with amiodarone for the prevention of atrial fibrillation: a randomized trial. JAMA. 2008;300:1784–1792. doi: 10.1001/jama.300.15.1784. [DOI] [PubMed] [Google Scholar]
- 109.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 110.Goette A, Bukowska A, Lendeckel U. Non-ion channel blockers as anti-arrhythmic drugs (reversal of structural remodeling) Curr. Opin. Pharmacol. 2007;7:219–224. doi: 10.1016/j.coph.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 111.Savelieva I, Camm J. Statins and polyunsaturated fatty acids for treatment of atrial fibrillation. Nat. Clin. Pract. Cardiovasc. Med. 2008;5:30–41. doi: 10.1038/ncpcardio1038. [DOI] [PubMed] [Google Scholar]
- 112.Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ. Arrhythm. Electrophysiol. 2008;1:62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- 113.Van Wagoner DR. Oxidative stress and inflammation in atrial fibrillation: role in pathogenesis and potential as a therapeutic target. J. Cardiovasc. Pharmacol. 2008;52:306–313. doi: 10.1097/FJC.0b013e31817f9398. [DOI] [PubMed] [Google Scholar]
- 114.Disertori M, et al. Valsartan for prevention of recurrent atrial fibrillation. N. Engl. J. Med. 2009;360:1606–1617. doi: 10.1056/NEJMoa0805710. [DOI] [PubMed] [Google Scholar]
- 115.Pratt CM, Reiffel JA, Ellenbogen KA, Naccarelli GV, Kowey PR. Efficacy and safety of prescription omega-3-acid ethyl esters for the prevention of recurrent symptomatic atrial fibrillation: a prospective study. Am. Heart J. 2009;158:163–169. doi: 10.1016/j.ahj.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 116.Dawe DE, Ariyarajah V, Khadem A. Is there a role for statins in atrial fibrillation? Pacing Clin. Electrophysiol. 2009;32:1063–1072. doi: 10.1111/j.1540-8159.2009.02440.x. [DOI] [PubMed] [Google Scholar]
- 117.Barra S, Silvestri N, Vitagliano G, Madrid A, Gaeta G. Angiotensin II receptor blockers in the prevention of atrial fibrillation. Expert Opin. Pharmacother. 2009;10:1395–1411. doi: 10.1517/14656560902973736. [DOI] [PubMed] [Google Scholar]
- 118.Ehrlich JR, Nattel S. Novel approaches for pharmacological management of atrial fibrillation. Drugs. 2009;69:757–774. doi: 10.2165/00003495-200969070-00001. [DOI] [PubMed] [Google Scholar]
- 119.Liakopoulos OJ, et al. Statins for prevention of atrial fibrillation after cardiac surgery: a systematic literature review. J. Thorac. Cardiovasc. Surg. 2009;138:678–686. doi: 10.1016/j.jtcvs.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 120.Calo L, et al. N-3 fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a randomized, controlled trial. J. Am. Coll. Cardiol. 2005;45:1723–1728. doi: 10.1016/j.jacc.2005.02.079. [DOI] [PubMed] [Google Scholar]
- 121.Heidt MC, et al. Beneficial effects of intravenously administered N-3 fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a prospective randomized study. Thorac. Cardiovasc. Surg. 2009;57:276–280. doi: 10.1055/s-0029-1185301. [DOI] [PubMed] [Google Scholar]
- 122.Murray KT, et al. Inhibition of angiotensin II signaling and recurrence of atrial fibrillation in AFFIRM. Heart Rhythm. 2004;1:669–675. doi: 10.1016/j.hrthm.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 123.Healey JS, et al. Prevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: a meta-analysis. J. Am. Coll. Cardiol. 2005;45:1832–1839. doi: 10.1016/j.jacc.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 124.Maggioni AP, et al. Effects of rosuvastatin on atrial fibrillation occurrence: ancillary results of the GISSI-HF trial. Eur. Heart J. 2009;30:2327–2336. doi: 10.1093/eurheartj/ehp357. [DOI] [PubMed] [Google Scholar]
- 125.Salehian O, et al. Impact of ramipril on the incidence of atrial fibrillation: results of the Heart Outcomes Prevention Evaluation study. Am. Heart J. 2007;154:448–453. doi: 10.1016/j.ahj.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 126.Almroth H, et al. Atorvastatin and persistent atrial fibrillation following cardioversion: a randomized placebo-controlled multicentre study. Eur. Heart J. 2009;30:827–833. doi: 10.1093/eurheartj/ehp006. [DOI] [PubMed] [Google Scholar]
- 127.Cleland JG, et al. Clinical trials update from the European Society of Cardiology Meeting 2009: AAA, RELY, PROTECT, ACTIVE-I, European CRT survey, German pre-SCD II registry, and MADIT-CRT. Eur. J. Heart Fail. 2009;11:1214–1219. doi: 10.1093/eurjhf/hfp162. [DOI] [PubMed] [Google Scholar]
- 128.Goette A, et al. Angiotensin II antagonist in paroxysmal atrial fibrillation (ANTIPAF) trial: rationale and study design. Clin. Drug Investig. 2007;27:697–705. doi: 10.2165/00044011-200727100-00005. [DOI] [PubMed] [Google Scholar]
- 129.Nakajima H, et al. Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-b1 transgene in the heart. Circ. Res. 2000;86:571–579. doi: 10.1161/01.res.86.5.571. [DOI] [PubMed] [Google Scholar]
- 130.Hanna N, Cardin S, Leung TK, Nattel S. Differences in atrial versus ventricular remodeling in dogs with ventricular tachypacing-induced congestive heart failure. Cardiovasc. Res. 2004;63:236–244. doi: 10.1016/j.cardiores.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 131.Verheule S, et al. Increased vulnerability to atrial fibrillation in transgenic mice with selective atrial fibrosis caused by overexpression of TGF-beta1. Circ. Res. 2004;94:1458–1465. doi: 10.1161/01.RES.0000129579.59664.9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Adam O, et al. Role of Rac1 GTPase activation in atrial fibrillation. J. Am. Coll. Cardiol. 2007;50:359–367. doi: 10.1016/j.jacc.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 133.Burstein B, Libby E, Calderone A, Nattel S. Differential behaviors of atrial versus ventricular fibroblasts: a potential role for platelet-derived growth factor in atrial-ventricular remodeling differences. Circulation. 2008;117:1630–1641. doi: 10.1161/CIRCULATIONAHA.107.748053. [DOI] [PubMed] [Google Scholar]
- 134.Burashnikov A. Are there atrial selective/predominant targets for “upstream” atrial fibrillation therapy? Heart Rhythm. 2008;5:1294–1295. doi: 10.1016/j.hrthm.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 135.Lafuente-Lafuente C, Mouly S, Longas-Tejero MA, Bergmann JF. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst. Rev. 2007;17 doi: 10.1002/14651858.CD005049.pub2. CD005049. [DOI] [PubMed] [Google Scholar]
- 136.Torp-Pedersen C, et al. Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish Investigations of Arrhythmia and Mortality on Dofetilide Study Group. N. Engl. J. Med. 1999;341:857–865. doi: 10.1056/NEJM199909163411201. [DOI] [PubMed] [Google Scholar]
- 137.Pedersen OD, et al. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (DIAMOND) substudy. Circulation. 2001;104:292–296. doi: 10.1161/01.cir.104.3.292. [DOI] [PubMed] [Google Scholar]
- 138.Hohnloser SH. Dronedarone in clinical context. The 2009 Atrial Fibrillation Forum; Orlando, USA. 14 November 2009. [Google Scholar]
- 139.Julian DG, et al. Randomised trial of effect of amiodarone on mortality in patients with left-ventricular dysfunction after recent myocardial infarction: EMIAT. European Myocardial Infarct Amiodarone Trial Investigators. Lancet. 1997;349:667–674. doi: 10.1016/s0140-6736(96)09145-3. [DOI] [PubMed] [Google Scholar]
- 140.Cairns JA, Connolly SJ, Roberts R, Gent M. Randomised trial of outcome after myocardial infarction in patients with frequent or repetitive ventricular premature depolarisations: CAMIAT. Canadian Amiodarone Myocardial Infarction Arrhythmia Trial Investigators. Lancet. 1997;349:675–682. doi: 10.1016/s0140-6736(96)08171-8. [DOI] [PubMed] [Google Scholar]
- 141.Antzelevitch C, Burashnikov A. Atrial-selective sodium channel block as a novel strategy for the management of atrial fibrillation. J. Electrocardiol. 2009;42:543–548. doi: 10.1016/j.jelectrocard.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lalevee N, Nargeot J, Barrere-Lemaire S, Gautier P, Richard S. Effects of amiodarone and dronedarone on voltage-dependent sodium current in human cardiomyocytes. J. Cardiovasc. Electrophysiol. 2003;14:885–890. doi: 10.1046/j.1540-8167.2003.03064.x. [DOI] [PubMed] [Google Scholar]
- 143.Sicouri S, Burashnikov A, Belardinelli L, Antzelevitch C. Synergistic electrophysiologic and antiarrhythmic effects of the combination of ranolazine and chronic amiodarone in canine atria. Circ. Arrhythm. Electrophysiol. doi: 10.1161/CIRCEP.109.886275. doi:10.1161/CIRCEP.109.886275. [DOI] [PMC free article] [PubMed] [Google Scholar]