Abstract
Spinal cord injury (SCI) is a devastating condition characterized by disruption of axonal connections, failure of axonal regeneration, and loss of motor and sensory function. The therapeutic promise of neural stem cells has been focused on cell replacement but many obstacles remain in getting neuronal integration following transplantation into the injured CNS. In this study we investigated the neurotransmitter identity and axonal growth potential of neural progenitors following grafting into adult rats with a dorsal column lesion. We found that using a combination of neuronal and glial restricted progenitors (NRP and GRP) produced graft-derived glutamatergic and GABAergic neurons within the injury site with minimal axonal extension. Administration of brain derived neurotrophic factor (BDNF) with the graft promoted modest axonal growth from grafted cells. In contrast, injecting a lentiviral vector expressing BDNF rostral to the injury generated a neurotrophin gradient and promoted directional growth of axons for up to 9 mm. Analysis of animals injected with BDNF lentivirus (at 2.5 and 5.0 mm) showed significantly more axons and significantly longer axons than in control animals injected with GFP lentivirus. However, only the 5.0 mm BDNF group showed preference for extension in the rostral direction. We concluded that NRP/GRP grafts can be used to produce excitatory and inhibitory neurons while neurotrophin gradients can guide axonal growth from graft-derived neurons toward putative targets. Together, they can serve as a building block for neuronal cell replacement of local circuits and formation of neuronal relays.
Keywords: neural stem cells, neurotrophin gradients, dorsal columns, brain-derived neurotrophic factor, cell transplantation
Introduction
Neural stem cells (NSCs) have the potential to repair damaged neuronal circuits after spinal cord injury (SCI). Several studies have demonstrated functional recovery following the transplantation of NSCs; however, this recovery is most likely due to glial-mediated processes such as neuroprotection, trophic support of host circuitry, and remyelination (reviewed, Belegu et al. 2007). For NSCs to contribute to the repair of neuronal circuitry after SCI, they must produce neurons that show appropriate phenotypes after transplantation. The phenotypes include projection neurons, local interneurons, and mature neurotransmitter identities. The goal of this study is to determine the capacity of neuronal restricted precursors (NRP) and glial restricted precursors (GRP) to generate these phenotypes in the injured spinal cord.
The injured spinal cord presents many obstacles to cell replacement—and neuronal replacement in particular—a fact that is underscored by the failure of endogenous repair mechanisms after SCI (Darian-Smith 2009). We showed previously that NRP can survive and differentiate in the injured spinal cord when grafted with GRP that provide a favorable niche (Lepore and Fischer 2005). NRP only produce neurons (Han et al. 2002), whereas GRP only produce astrocytes and oligodendrocytes (Han et al. 2004). Long-term experiments with NRP/GRP transplants showed that cells survive and form synaptic structures up to 15 months after transplantation (Lepore et al. 2006). The focus of the current study was to determine if NRP/GRP grafts can generate multiple neurotransmitter identities in the injured spinal cord and extend long axons out of the injury site toward a putative target. Differentiation into glutamatergic, GABAergic, or cholinergic neurons would indicate that NRP could contribute to the repair of long axonal tracts, intraspinal circuits, or damaged motor units, respectively. Serotonergic differentiation would make NRP good candidates for replacing lost neuromodulatory input below the injury level (Eaton et al. 2008). We found that NRP differentiate into glutamatergic and GABAergic neurons but show no evidence of becoming cholinergic or serotonergic neurons.
The role of neurotrophin factors as chemoattractants has been well established (Paves and Saarma 1997) and led to diverse applications in the injured central nervous system (CNS), including the use of neurotrophin-rich matrices (Piantino et al. 2006), intrathecal catheters (Ramer et al. 2000), and transgenic cells that express neurotrophins (Himes et al. 2001). Injections and catheter methods are limited by their ability to deliver long-term administration of neurotrophins without inducing inflammation and tissue damage (Jones and Tuszynski 2001). Transgenic cells produce a favorable environment for injured axons but generally fail to produce axonal growth beyond the injury site (Himes et al. 2001; Tuszynski et al. 2003). The development of lentiviral vectors provides a method for long-term delivery of neurotrophins with limited damage and/or inflammation and the potential to create directional gradients (Blesch 2004). Taylor et al. (2006) demonstrated that an in vivo neurotrophin gradient can be established in the injured spinal cord using NT-3 lentivirus and that this gradient can lead to host axon regeneration across a cellular transplant but only a short distance into distal host tissue. Viral vectors have also proved useful in directing axonal growth from dorsal root ganglion (DRG) grafts in the brain (Jin et al. 2008; Ziemba et al. 2008). Because NRP/GPR grafts produce neurons in the injured spinal cord, we reasoned that neurotrophin gradients applied after SCI and NRP/GRP transplantation would be likely to promote long, directional axon growth from NRP. We found that one treatment with brain-derived neurotrophic factor (BDNF) was sufficient to induce axon extension from NRP/GRP grafts and that BDNF gradients induced long, directional axon growth from NRP grafts. These findings demonstrate that NRP/GPR grafts could have the potential to reconnect disrupted tracts and circumvent the poor regenerative capacity of damaged CNS neurons.
Methods and Materials
Animal Subjects and Experimental Design
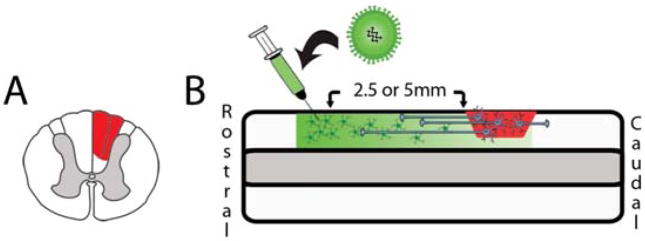
Adult (250–300 g) female Sprague-Dawley rats were divided into 6 groups (groups 1, 2, 6 n=3; groups 3 to 5, n=6). Group 1 received a unilateral dorsal column lesion at C4 (Fig. 1A) and an acute transplant of NRP/GRP suspended in the collagen-based matrix Vitrogen (Cohesion, Palo Alto, CA). Group 2 received the same injury and cell transplant, but cells were suspended in Vitrogen + 50 μg/ml BDNF to examine the effects of local administration of BDNF on grafted cells. Groups 3 to 5 received a dorsal column injury and acute transplant of NRP/GRP followed by vector injection 7 days later. Group 3 received 2 injections of GFP vector 2.5 mm rostral to the center of the transplant at a depth of 0.5 mm and 1.0 mm (Fig 1B). Groups 4 and 5 followed the same timeline with regard to injury, acute cell transplant, and vector injections but received injections of BDNF lentivirus at 2.5 mm or 5.0 mm rostral to the center of the transplant, respectively. All animals in groups 1–5 were sacrificed 5 weeks after injury and perfused with 0.9% saline followed by 4% paraformaldehyde. Group 6 received an injection of BDNF lentivirus but no injury or graft to confirm production of a BDNF gradient.
Fig. 1.
Experimental design. Animals were given a unilateral dorsal column partial hemisection at C4 (A). NRP/GRP suspended in Vitrogen at 200,000 cells/μl were transplanted acutely into the cavity. 1 week after injury and cell transplantation lentivirus was injected either 2.5 mm or 5.0 mm rostral to the injury site to induce axon extension from grafted cells (B).
Harvest and Culture of NRP/GRP
The NRP and GRP were harvested from E13.5 spinal cord of Fischer 344 rats expressing the AP transgene (Lepore et al. 2004). Time-pregnant females were sacrificed at E13.5 and the embryos were placed in DMEM/F12 (Invitrogen, Carlsbad, CA). Meninges were peeled from the cords after incubation in collagenase I (10 mg/ml)/dispase II (20 ng/ml) in Hank’s balanced salt solution for 8 min at room temperature. Cords were then treated with trypsin (0.5%)/EDTA for 20 min at 37°C. Cells were plated on poly-l-lysine and laminin in NRP complete medium [DMEM/F12, BSA (1 mg/ml), B27, basic fibroblast growth factor (10 μg/ml), Pen-Strep (100 IU/ml), N2 (10 μl/ml). and NT-3 (10 μg/ml)]. The medium was changed every 2 days.
Preparation of NRP/GRP for Transplantation
The NRP/GRP were cultured for 5 to 6 days prior to grafting. On the morning of transplantation, cells were dissociated from the culture dish with 0.05% trypsin-EDTA and viability was assessed using Trypan blue. Cells were suspended at 200,000 cells/μl in a bovine collagen-based grafting medium, Vitrogen. The grafting medium was 50% Vitrogen, 50% NRP basal medium pH 7.5. For experiments using exogenous BDNF in the grafting medium, 50 μg/ml of BDNF (R&D Systems, Minneapolis, MN) was added to the matrix prior to transplantation. Under these conditions the ratio of NRP:GRP is between 50:50 and 30:70 as reported previously (Lepore et al. 2006). Cells were ≥ 90% viable and used within 5 hrs of suspension in grafting medium.
Dorsal Column Injury and Cell Transplantation
Sprague Dawley rats were anesthetized by intraperitoneal injection of an XAK cocktail of xylazine (10 mg/kg; Webster Veterinary, Sterling, MA), acepromazine maleate (0.7 mg/kg; Webster Veterinary) and ketamine (95 mg/kg; Webster Veterinary). The skin was cleaned and treated with Betadine. The animal was elevated on a small pillow of gauze under the torso to separate the shoulders and vertebrae. Incisions were made through the muscle and skin, which were retracted. A laminectomy was performed at C4 to expose the spinal cord. The dura was cut with a 30-gauge needle along the rostral–caudal axis above the dorsal columns. A 30-gauge needle was used to make a unilateral incision in the right dorsal column to sever the tract, and a small piece of the right dorsal column was excised using light suction. Sutures (9-0) were placed in the dura on both sides of the lesion but not tightened. After achieving hemostasis in the spinal cord lesion, approximately 2 to 3 μl of cell suspension was injected directly into the cavity. The dura sutures were quickly tightened to keep the cells in the lesion site. A small piece of the nonadhesive sterile bandage Biobrane (Smith & Nephew, London) was placed over the spinal cord with the nonadhesive side down to prevent adhesions between the muscle and dura and the muscle was sutured. The skin was stapled. Animals were given cyclosporine A (10 mg/kg; Novartis, New York, NY) once daily via subcutaneous injection beginning 3 days prior to cell transplant and continuing for the remainder of the experiment. Bupranorphin (Webster Veterinary) was used for pain relief every 12 hrs for 3 days and then as needed for the remainder of the experiment. All animals were cared for in accordance with the guidelines established by the National Institutes of Health and the Drexel University College of Medicine Institutional Animal Care and Use Committee.
Vector Injection
One week after injury and cell grafting, the animals were injected with BDNF-GFP or GFP vector. The delay allowed NRP/GRP to differentiate in the injured adult without the addition of exogenous BDNF. Animals were anesthetized again with XAK, and the spinal cord was reexposed. The Biobrane bandage was removed and a 30-gauge needle was used to make a small puncture in the dura to allow for vector injection. A 10 μl-Hamilton syringe was fitted with a glass pipette with an external diameter between 70 and 100 μl and used to inject 1.25 μl of vector at 2 depths (1.0 mm and 0.5 mm) in the intact parenchyma of the spinal cord to ensure infection along the whole dorsal–ventral axis of the dorsal columns. The incision was closed, and the animals were cared for as stated previously. The pLV vector was modified to express GFP or huBDNF-IRES-GFP as described previously (Blesch 2004; Kwon et al. 2007). Vector preparations contained 114 μg/ml p24, 8 × 10 e8 TU/ml for BDNF and 150 μg/ml p24, 9 × 10 e8 TU/ml for GFP.
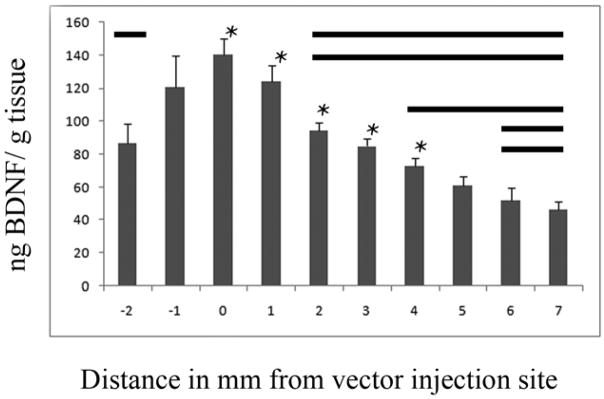
Group 6 was used to confirm that the BDNF vector could generate a gradient similar to the NT-3 gradient generated previously (Taylor et al. 2006). Animals (n=3) received a single injection of 2.5 μl of BDNF lentivirus into the dorsal column nucleus and sacrificed after two weeks to allow for expression of the transgene in host cells. Animals were perfused with 50ml of ice cold saline and spinal cords were removed, frozen, sectioned at 100 μm and pooled into 1mm groups for analysis. Each sample was sonicated in 40 μl/mg lysis buffer [Tris-HCl (50mM), NaCl (0.6M), Triton (0.2%), BSA (0.5%) and protease inhibitor tablets (Roche)] and centrifuged for 30 min at 4°C at 17,000 rpm. ELISA was conducted with a BDNF DuoSet ELISA kit (R&D) systems per the vendor’s instructions. Differences within groups were assessed using Tukey’s post-hoc analysis. We showed that BDNF lentivirus generated a peak expression of 140 ng BDNF/g wet tissue at the vector injection site and that the level of BDNF gradually decreased over 5mm (Fig. 2).
Fig. 2.
Injection of BDNF lentivirus in vivo creates a gradient of BDNF. Two weeks after 2.5 μl of BDNF lentivirus was injected into the dorsal column nucleus, levels of BDNF were measured with ELISA in 1mm bins relative to the injection site. We found a peak expression of 140 ng BDNF/g wet tissue at the injection site and a decreasing gradient of BDNF as distance from the injection site increased. Bold lines parallel to each * indicate groups are significantly different (p<0.05).
Tissue Collection for Histologic Analysis
Animals used for histologic analysis were sacrificed 5 weeks after injury/cell transplant with an overdose of Euthasol (Virbac Animal Health, Fort Worth, TX) and then perfused transcardially with 50 to 100 ml of ice-cold 0.9% saline and 500 ml of ice-cold 4% paraformaldehyde in phosphate buffer. The spinal cord and brain stem were removed and placed in 4% paraformaldehyde for 24 hrs followed by cryoprotection in 30% sucrose/0.1 M phosphate buffer at 4°C for at least 3 days. Spinal cords were embedded in Tissue-Tek O.C.T. mounting media (Fisher Scientific, Pittsburgh, PA) and cut sagittally in 20-μm sections. Tissue was collected on glass slides coated with poly-l-lysine and gelatin. Tissue was stored at 4°C until staining.
Alkaline Phosphatase Histochemical Analysis
This analysis was used to visualize transplants and examine process extension. Serial sections (each approximately 120 μm apart) were washed 3 times in phosphate buffered saline (PBS), then placed in 60°C-PBS for 1 hr to inactivate endogenous phosphatase activity. Sections were then washed in AP buffer [(Tris 100 mM pH 9.5),(MgCL2 50 mM), (NaCl 100 mM)] followed by staining in the dark for 2 hrs with AP staining solution [(1.0 mg/ml NBT, Sigma-Aldrich, St. Louis, MO), (5mM levamisole, Sigma-Aldrich), and (0.1 mg/ml BCIP, Sigma-Aldrich) in AP buffer)]. All steps were done at room temperature unless otherwise noted. The slides were coated with Vectashield (Vector Laboratories, Burlingame, CA) to form a coverslip. Slides were viewed using a Leica DMBRE microscope (Leica Microsystems, Bannockburn, IL).
Quantification of Neuronal Processes
Sagittal sections treated with AP histochemistry were imaged with a 5X lens and montaged into a single image using IPLab advanced image analysis software (Biovision, Exton, PA). Each montage image was overlayed with an image of a 0.01 mm/0.1 mm/1 mm micrometer taken with the same microscope and objective. Each image was divided into 1-mm bins, and processes were counted in each bin in both the rostral and caudal directions. The counting for each bin was capped at 20 processes. The bins were averaged across animals within each condition according to the distance from the center of the transplant. These averages were then compared using analysis of variance. Processes were counted only if they were not associated with a cell body and if they were at least 100 μm in length to ensure that only axonal, not glial, processes were counted. In addition, we examined the tissue with double immunofluorescence to verify the presence of NRP-derived axons.
Immunohistochemical Analysis
Slide-mounted tissue sections were treated for 5 min in 0.2% triton/PBS, washed 3 times in PBS for 5 min, and then blocked in 10% goat serum/PBS for 1 hr at room temperature. Sections were incubated with primary antibodies [βIII tubulin, 1:500, Covance, Princeton, NJ; AP, 1:200, AbD Serotec, Raleigh, NC; Vglut1, 1:10000, Vglut2, 1:2500, Millipore, Billerica, MA; glutamate, 1:500, Sigma-Aldrich; GAD 65/67, 1:2000, Chemicon (Millipore); ChAT, 1:100, Chemicon (Millipore); 5-HT, 1:20000, Immunostar, Hudson, WI) overnight at room temperature in 2% goat serum/PBS. Sections were then washed 3 times in PBS to remove unbound primary antibody. Sections were incubated with secondary antibodies (Gt × Rb FITC, Gt × Ms rhodamine, Gt × GP rhodamine, 1:200, Jackson ImmunoResearch, West Grove, PA) for 2 hrs, washed 3 more times in PBS, and coated with Vectashield (Vector Laboratories). Slides were viewed using a Leica DM5500B microscope (Leica) with Slidebook software (Intelligent Imaging Innovations, Denver CO).
Results
NRP/GRP Produce Glutamatergic and GABAergic Neurons in the Injured Spinal Cord
NRP/GRP harvested from E13.5 AP rats were cultured for 5 to 6 days prior to transplantation in a unilateral dorsal column injury. To determine the viability of using NRP for neuronal replacement in the injured spinal cord, we tested the ability of NRP to produce glutamatergic, GABAergic, cholinergic, and serotinergic neurons. NRP readily produced both glutamatergic (Fig. 3A–C, G–L) and GABAergic (Fig. 3D–F, M–O) neurons in the injured spinal cord. Glutamatergic neurons were present throughout the graft as shown with glutamate (Fig. 3A–C, G–I) and glutamate vesicular transporter 1&2 (Fig. 3J–L). GABAergic neurons were found in local clusters within the graft and shown by glutamate decarboxylase 65/67 (GAD 65/67) staining (Fig 3. D–F, M–O). Both glutamate and GABA are made through the same biochemical pathway, and GABAergic neurons express glutamatergic markers. However, because GABAergic markers were always present in distinct areas of the graft and glutamatergic markers were expressed throughout the graft, we concluded that both types of neurons are present.
Fig. 3.
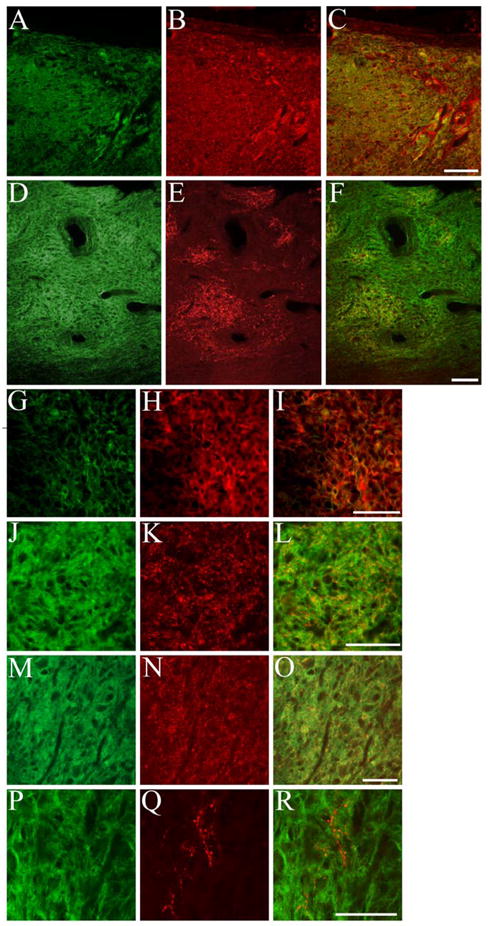
NRP differentiate into glutamatergic and GABAergic neurons after transplantation in the injured spinal cord. NRP/GRP suspended in Vitrogen at 200,000 cells/μl were transplanted into a dorsal column injury. AP+ NRP/GRP (A) survived and also expressed glutamate (B, C) throughout the injury site. Small clusters of AP+ cells (D) expressed the GABAergic marker GAD 65/67 (E, F). High magnification of AP+/glutamate+ cells (G–I) shows that the markers are present throughout the cytoplasm. High magnification of a cluster of AP+/GAD 65/67+ cells shows that GAD expression is localized to small puncta within AP+ cells (J–L). AP+ cells (M) also expressed Vglut 1+2 (N,O) throughout the graft. Although no AP+ cells (P) were double labeled with 5-HT, host (AP−) 5-HT+ axons (Q, R) were found in the perimeter of the graft. Scale bar: A–F 100 μm, G–R 50 μm.
NRP failed to produce definitive examples of cholinergic or serotinergic neurons after being grafted in the injured spinal cord. Choline acetyltransferase (ChAT) staining was negative throughout the graft site, indicating a failure of NRP to differentiate into motor neurons after transplant in the injured spinal cord (data not shown). Likewise, we noted a lack of AP/5-HT double labeled neurons, indicating a failure of NRP to differentiate into serotinergic neurons. Interestingly, we found many serotinergic fibers in the borders of the graft site (Fig. 3P–R). These fibers most likely originated in the host because they lacked the AP label and no 5-HT+ cell bodies were found within the lesion.
NRP/GRP Generate Neurons and Extend Processes When Grafted with BDNF
NRP/GRP were obtained from E13.5 AP rats and cultured for 5 to 6 days. Approximately 500,000 mixed NRP/GRP were grafted into the injured dorsal columns of adult rats at the C4 level. Cells were suspended in Vitrogen alone or in Vitrogen + 50 μg/ml BDNF. Animals were sacrificed 5 weeks after transplant and transplants were assessed histologically using serial sections. Control transplants without exogenous BDNF survived and filled the injury site (Fig. 4A); however, they showed a limited ability to extend axons into the surrounding host tissue. We found that the presence of BDNF in the grafting medium was sufficient to produce robust processes from grafted cells (Fig. 4B). Processes were several millimeters in length and grew in both rostral and caudal directions (Fig. 4C,D). Processes showed a clear preference to extend through white matter compared to gray matter (Fig. 4B), suggesting that BDNF is sufficient to stimulate axon extension through white matter but not through gray matter. The lack of process extension in the gray matter could be caused by synapse formation with host neurons (Lepore et al. 2006). Examination of processes at high magnification showed few AP+ cell bodies outside of the lesion site, suggesting that BDNF increased axon growth without promoting extensive cell migration.
Fig. 4.
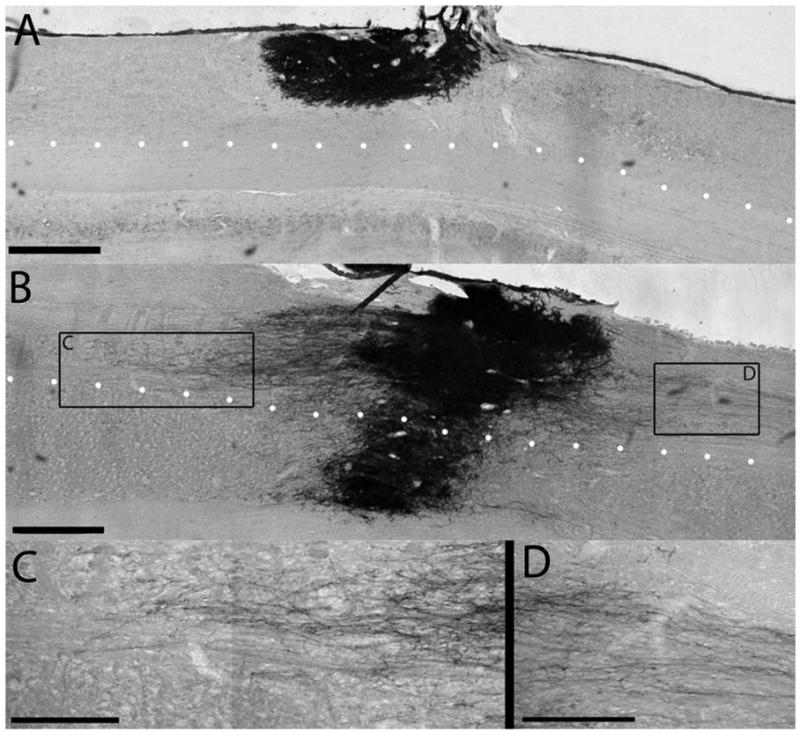
A single delivery of BDNF leads to axonal growth from NRP/GRP grafts in the injured spinal cord. Animals received a dorsal column injury at C4, followed by acute grafting of NRP/GRP in Vitrogen (A) or NRP/GRP in Vitrogen + 50 μg/ml BDNF (B). In the absence of BDNF, NRP/GRP survived at 5 weeks and filled the injury site as demonstrated by the histological stain for the AP transgene. When grafted with BDNF, NRP extended axons out of the graft site through the intact white matter. The dotted line outlines the boundary between dorsal white matter and central gray matter. NRP can extend axons through the white matter, but they are inhibited in the gray matter. NRP extend axons in both the rostral (C) and caudal (D) directions. Scale bar: A,B, 500 μm; C, D, 250 μm.
BDNF-GFP Lentivirus Causes Long Distance Axon Extension from Grafted Cells
To determine the effects of control GFP lentivirus injection on NRP/GRP transplants, we injected the virus 2.5 mm rostral to the graft/injury site 1 week post cell transplant. Animals were sacrificed 4 weeks after virus injection (5 weeks after grafting/injury). The spinal cord was cut in serial sagittal sections to determine transplant viability and process extension. Sagittal sections included the transplant and tissue 10 mm rostral to the transplant and 5 mm caudal to the transplant. NRP/GRP survived transplantation and the subsequent viral infection and filled the injury site at 5 weeks. Process extension after treatment with GFP vector was similar to that with Vitrogen-only controls (Fig. 5A, compare with 4A). Quantification of axon extension from the transplant showed that processes only extended 2 to 3 mm from the center of the transplant. We also saw a preference for grafted cells to extend axons caudally. These data show that lentivirus infection and GFP expression are both compatible with NRP/GRP transplants.
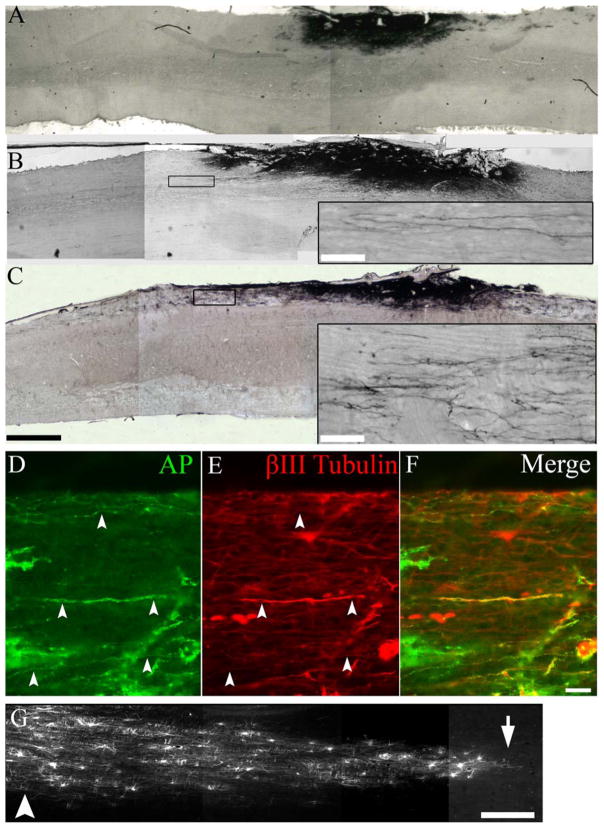
Fig. 5.
BDNF, but not GFP, lentiviral gradients led to robust axon extension from NRP/GRP grafts. After injection of the GFP lentivirus 2.5 mm rostral to the graft, NRP/GRP survived but did not extend many axons toward the injection site (A). After injection of BDNF lentivirus 2.5 mm rostral to the graft, NRP extended axons in both the rostral and caudal directions (B). After injection of BDNF lentivirus 5 mm rostral to the graft site, we saw pronounced axon extension in the rostral direction (C). Double immunostaining showed that AP+ axons (D, F) also expressed βIII tubulin (E, F). GFP labeling of infected cells (G) showed the rostral-caudal diffusion pattern of the lentiviral particles from the injection site (G, arrowhead) to the host-graft interface (G, arrow). Scale bars: A–C, 1 mm; insets, 50 μm; D–F, 100 μm; G, 500 μm.
To analyze the effects of a BDNF gradient on NRP/GRP transplants, BDNF-GFP lentivirus was injected 2.5 mm rostral (Fig. 5B) or 5.0 mm rostral (Fig. 5C) to the graft/lesion site 1 week post cell transplant. Animals were sacrificed 4 weeks after virus injection (5 weeks after grafting/injury). Spinal cords were cut in serial sagittal sections to examine transplant viability, process extension, and viral diffusion. In animals treated with BDNF lentivirus, the NRP/GRP transplants survived and filled the lesion cavity. We saw a dramatic increase in AP+ process extension following both 2.5-mm and 5.0-mm BDNF lentivirus injections. The processes typically projected from the transplant in a bundle but became less dense as distance from the graft increased (insets, Fig. 5B, C). Double immunofluorescence showed that the processes expressed the AP marker (Fig. 5D,F) and the axonal marker βIII tubulin (Fig. 5E,F). GFP labeling of host cells showed that the diffusion of lentiviral particles caused many cells to be infected near the vector injection site (Fig. 5G, arrowhead) and fewer cells to be infected near the graft-host interface (Fig. 5G, arrow). The constitutive presence of high levels of BDNF did not lead to unwanted expansion of the cell transplant.
To quantify the effect of BDNF gradients on NRP/GRP transplants, we counted the number of AP+ processes present in 1-mm bins of tissue both rostral and caudal to the transplant. Due to the high number of processes close to the transplant and the relatively few processes distal to the transplant, we capped the counting at 20 processes per 1mm bin. Processes were counted only if their length within the focal plane reached at least 100 μm and they did not originate from an AP+ cell body outside of the injury site. Because the processes weaved in and out of the focal plane and often of the section itself, following single processes for several millimeters through injured tissue was not feasible. The quantification is summarized in Figure 6. NRP/GRP transplants treated with a 2.5-mm BDNF lentivirus injection showed robust process extension, and some axons were found as far as 9 mm rostral to the graft site (Fig. 6A). A closer examination of the processes between +5 mm and -5 mm showed that the 2.5-mm, BDNF-treated animals exhibited no significant preference between rostral and caudal extensions, unlike the GFP-treated animals, which showed a preference for caudal extension (Fig. 6B).
Fig. 6.
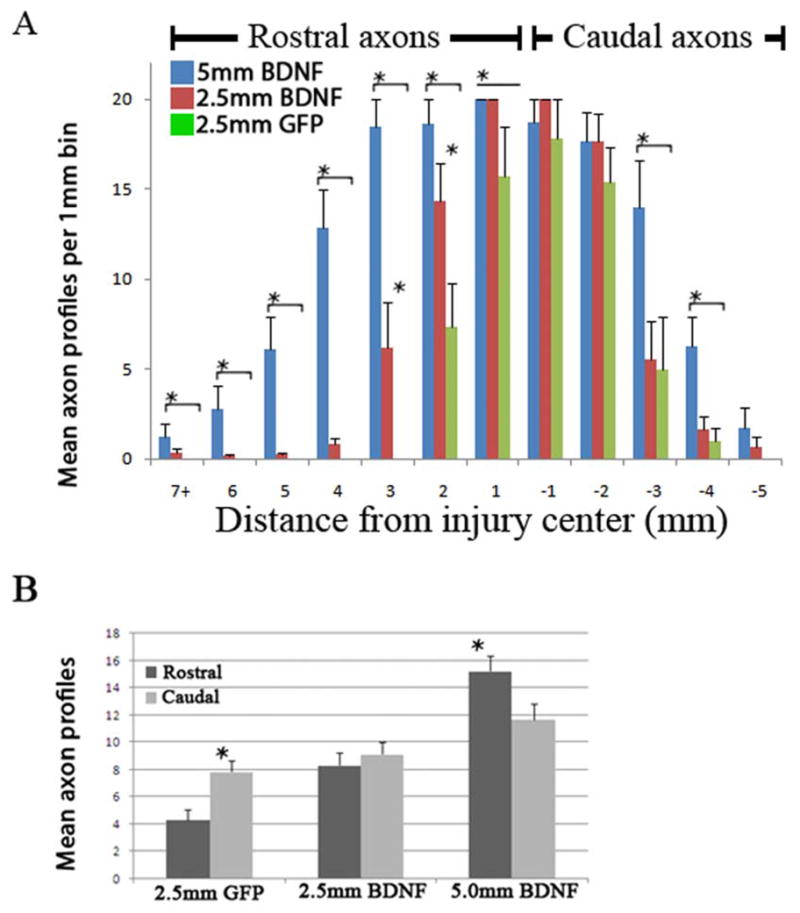
Axon extension from NRP/GRP grafts depends on the structure of the BDNF gradient. We counted the number of AP+ axons in animals treated with GFP and BDNF-GFP vectors. The spinal cord was divided into 1-mm bins and axon profiles were counted in those bins. Due to the high number of axon profiles close to the graft and lower numbers further from the graft, the counting was capped at 20 axons per 1 mm per 20-μm section. The number of axons is represented as mean axon profiles per 1 mm of spinal cord tissue. We saw more axons in both BDNF groups compared with the controls and more axons in animals receiving vector injections 5 mm rostral to the graft compared with those receiving injections 2.5 mm rostral to the graft (A). When we compared growth within 5 mm of the graft in the rostral and caudal directions, we found that the axons grew preferentially caudally in the GFP group, exhibited no preferential growth in the 2.5-mm BDNF group, and grew preferentially in the rostral direction in the 5-mm BDNF group (B) * p<0.05.
Animals in the 5.0-mm BDNF-treated group also showed robust axon extension. Quantification of these processes showed on average more processes than either the GFP- or 2.5-mm, BDNF-treated animals (Fig. 5A, p<0.01). However, in the 5-mm BDNF group, we found more axons in the rostral cord between +5 mm and -5 mm from the transplant center (Fig. 6B). This result indicates that NRP reacted differently to 5-mm BDNF treatments than to 2.5-mm BDNF and GFP treatments. Examination of tissue both rostral and caudal to the initial 15-mm sections did not reveal any AP+ processes further rostral or caudal to the injury site.
Discussion
We show that grafts containing NRP can generate glutamatergic and GABAergic neurons in injured spinal cord. These phenotypes are essential to the effective repair of local circuits of interneurons that are lost or damaged after SCI. We also examined the ability of NRP to extend axons beyond the injury site and through the surrounding white matter. We found that a onetime treatment of the graft with BDNF was sufficient to induce axon extension, but processes were relatively short. In contrast, administration of BDNF by lentiviral vectors generated a neurotrophin gradient that increased axon number and length and allowed directional growth toward putative targets. The ability of NRP to extend long axons makes them candidates for the reconnecting disrupted spinal tracts.
Analysis of Neurotransmitter Identities
Replacing neurons in the injured spinal cord requires that neurons differentiate into appropriate phenotypes. We tested NRP for their ability to generate neuronal phenotypes found in the spinal cord. The phenotypic potential of neuronal progenitors is dependent on the gradual restriction of the intrinsic program of cells (Mayer-Proschel et al. 1997) and instructive cues from the environment (Yang et al. 2000). The NRP were shown to generate glutamatergic and GABAergic neurons with small levels of other phenotypes such as cholinergic neurons (Kalyani et al. 1998) in vitro and after transplantation in the developing animal (Yang et al. 2000). It was not clear, however, whether the environment of the injured spinal cord would allow a similar pattern of neuronal differentiation. After transplantation, NRP differentiation could be directed by a variety of molecules produced by the host including growth factors (Tripathi and McTigue 2008), cytokines (Pineau and Lacroix 2007), and neurotransmitters (Panter et al. 1990) as well as factors from the grafted cells (Kamei et al. 2007). We found that NRP grafts readily became glutamatergic and GABAergic neurons but did not produce distinct populations of serotonergic or cholinergic neurons. However, these findings do not preclude NRP from being used for serotonergic or cholinergic replacement. NRP can be differentiated into cholinergic neurons in vitro (Kalyani et al. 1998) and embryonic stem cells can be predifferentiated in vitro and used for transplantation in animal models (Harper et al. 2004). The presence of both excitatory and inhibitory neurons indicates that NRP could be used to repair local spinal cord circuits composed of interneurons such as the central pattern generator, but excitatory neurons could also be used for reconnecting long tracts such as the dorsal columns following injury. We designed our experiments to minimize the effect of BDNF expression on the early stages of NRP differentiation by including a one week delay between cell transplantation and lentivirus injection. Indeed, we saw no apparent differences in neurotransmitter identities among experimental groups.
We considered two major explanations for the neurotransmitter heterogeneity found in the transplanted cells. NRP could be committed to a specific neuronal phenotype prior to transplantation or NRP fate could be directed by cues following grafting. Clonal analysis of NRP in vitro demonstrates that a single NRP can generate glutamatergic and GABAergic progeny (Kalyani et al. 1998), and transplantation of NRP into the developing CNS demonstrates that host cues can determine neurotransmitter identity (Yang et al. 2000). In addition, the distribution of the GABAergic neurons in local clusters indicates that distinct areas of the graft site are more conducive to GABAergic differentiation. If NRP were committed to a specific neurotransmitter identity prior to grafting we would expect an even distribution of GABAergic and glutamatergic neurons. We should also consider that during normal development young neurons change neurotransmitter identities, a common occurrence prior to synapse formation (Ruediger and Bolz 2007). We therefore concluded that, although NRP have the capacity to generate both excitatory and inhibitory neurons, the specific neurotransmitter identity depends on local cues and may vary until NRP integrate with host circuitry.
BDNF Initiates Axon Extension
NRP are grafted together with GRP into the site of the SCI to create a microenvironment conducive to survival and differentiation of neurons (Lepore and Fischer 2005), but this environment allows for only minimal axon extension. Studies of the developing CNS (Charron and Tessier-Lavigne 2005) and cultured neurons (Gallo and Letourneau 2004) have shown that axon extension and guidance reflect the interplay between attractive and repulsive signals. It appears that, despite the potential growth capacity of the embryonic neurons generated by NRP (Yang et al. 2000) and the presence of the permissive environment provided by the GRP at the graft site (Lepore and Fischer 2005), the lack of axon guidance factors (Bolsover et al. 2008) and the presence of inhibitory molecules including chondroitin sulfate proteoglycans present in the glial scar (Davies et al. 1999) prevent long-distance axon extension. To promote axonal growth and regeneration after SCI, BDNF and other growth factors have been used extensively via various delivery methods (Lu and Tuszynski 2008). To test whether NRP would be able to overcome the effects of inhibitory molecules in the presence of a neurotrophin, we grafted NRP with a single dose of BDNF included with the grafting media. NRP that were grafted in the presence of BDNF extended axons of several millimeters through the white matter. The biological activity of BDNF is short (Kishino et al. 2001); therefore, much of the axon extension occurred as a result of an initiation process that continued for a limited time. Axons most likely stop growing in the white matter due to the limited effects of a single application of BDNF. The abrupt change in axon profiles at the white matter–gray matter boundary indicates that the high density of neurons in the gray matter provides potential targets for the axons and that extension may end when NRP make synaptic connections with host cells.
Guiding NRP Axons along BDNF Gradients
Injections of viruses expressing growth factors can generate protein gradients through a process of virus diffusion and infection of host cells and promote growth of host axons following SCI (Taylor et al. 2006) or transplants of DRG neurons along the intact (Ziemba et al. 2008) and injured (Jin et al. 2008) corpus callosum. Our aim was to examine whether neurotrophin gradients generated by lentivirus injections could promote long distance axon growth as well as guide axon growth from NSCs grafted into the injured spinal cord. We found that animals with BDNF-lentivirus injected 2.5 mm or 5.0 mm rostral to the lesion had significantly more and significantly longer axons than GFP control animals. However, the 5.0-mm BDNF group showed significantly more axons in both the rostral and caudal directions as well as a preference for extension in the rostral direction relative to the 2.5-mm BDNF group (which showed no directional preference) and the control GFP group (which showed a caudal preference). These differences show that the distribution of BDNF plays a key role in initiation, growth, and axonal guidance of graft-derived neurons.
The pattern of axon extension, caudal preference without BDNF, no preference with a 2.5-mm gradient of BDNF, and rostral preference with a 5.0-mm gradient of BDNF suggests the action of a complex array of guidance molecules. The preference for NRP to extend axons caudally in the absence of BDNF (control GFP group) suggests that the rostral white matter is more inhibitory than the caudal white matter. This finding could be caused by the inflammation and glial responses that occur during the wallerian degeneration of the distal axon (Wang et al. 2008).
Differences between the 2.5-mm BDNF group and the 5.0-mm BDNF group indicate that the concentration of BDNF in and around the graft site is critical to axonal guidance. In vitro studies have shown that axons growing along BDNF gradients use an asymmetrical distribution of β-actin within extending growth cones (Yao et al. 2006) but that saturated neurons fail to demonstrate guidance (Ming et al. 1999) and can be repulsed by very high levels of BDNF near the cell body (Mai et al. 2009). We reason that in the 2.5-mm group, the high level of BDNF near the graft saturates the graft with BDNF and prevents the axons from following the gradient. Although the NRP are not effectively guided along the 2.5-mm gradient of BDNF, they are stimulated to grow axons out of the injury site and the BDNF appears to change the microenvironment enough for axons to overcome inhibition in the rostral white matter.
In the 5-mm group, the relatively lower levels of BDNF in and around the graft allow NRP to sense a gradient and grow toward the higher concentration of BDNF, resulting in effective axon guidance. However, the higher number of caudal axons compared with the other groups illustrates a need for more efficient control over the BDNF and possibly a diametric repulsive signal such as semaphorin 3A (Wright et al. 1995; Ziemba et al. 2008) to improve directional growth. These findings emphasize that when studying axon extension in the injured spinal cord, the presence, source, and patterning of all the molecules contributing to the microenvironment must be carefully considered.
Conclusions
We used an established model of SCI, a lesion of the dorsal columns, to demonstrate the ability of NRP/GRP grafts to generate glutamatergic and GABAergic neurons in the injured spinal cord and to extend long axons guided along a neurotrophin gradient. These findings, combined with evidence that graft-derived neurons can integrate with host circuits, suggest that NRP/GRP could be used to replace neurons in local circuits after SCI. The generation of excitatory neurons and their ability to extend long axons in a directional manner suggest that they can also be used to form neuronal relays to reconnect interrupted tracts. Future studies will examine the ability of these neurons to form functional synaptic connections with the host.
Acknowledgments
We are grateful for excellent technical assistance from Carla Tyler-Polsz, Maryla Obrocka, and Theresa Connors. We acknowledge Andrew Torre-Healy for his help with initial experiments and Mahendra S. Rao and Angelo Lepore for helpful discussions.
This work was supported by NIH grant NS055976, the Neilsen Foundation, and funds provided by Drexel University College of Medicine for the Spinal Cord Research Center.
References
- Belegu V, Oudega M, Gary DS, McDonald JW. Restoring function after spinal cord injury: promoting spontaneous regeneration with stem cells and activity-based therapies. Neurosurg Clin N Am. 2007;18(1):143–168. xi. doi: 10.1016/j.nec.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Blesch A. Lentiviral and MLV based retroviral vectors for ex vivo and in vivo gene transfer. Methods. 2004;33(2):164–172. doi: 10.1016/j.ymeth.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Bolsover S, Fabes J, Anderson PN. Axonal guidance molecules and the failure of axonal regeneration in the adult mammalian spinal cord. Restor Neurol Neurosci. 2008;26(2–3):117–130. [PubMed] [Google Scholar]
- Charron F, Tessier-Lavigne M. Novel brain wiring functions for classical morphogens: a role as graded positional cues in axon guidance. Development. 2005;132(10):2251–2262. doi: 10.1242/dev.01830. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C. Synaptic plasticity, neurogenesis, and functional recovery after spinal cord injury. Neuroscientist. 2009;15(2):149–165. doi: 10.1177/1073858408331372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SJ, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci. 1999;19(14):5810–5822. doi: 10.1523/JNEUROSCI.19-14-05810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton MJ, Pearse DD, McBroom JS, Berrocal YA. The combination of human neuronal serotonergic cell implants and environmental enrichment after contusive SCI improves motor recovery over each individual strategy. Behav Brain Res. 2008;194(2):236–241. doi: 10.1016/j.bbr.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Gallo G, Letourneau PC. Regulation of growth cone actin filaments by guidance cues. J Neurobiol. 2004;58(1):92–102. doi: 10.1002/neu.10282. [DOI] [PubMed] [Google Scholar]
- Han SS, Kang DY, Mujtaba T, Rao MS, Fischer I. Grafted lineage-restricted precursors differentiate exclusively into neurons in the adult spinal cord. Exp Neurol. 2002;177(2):360–375. doi: 10.1006/exnr.2002.7995. [DOI] [PubMed] [Google Scholar]
- Han SS, Liu Y, Tyler-Polsz C, Rao MS, Fischer I. Transplantation of glial-restricted precursor cells into the adult spinal cord: survival, glial-specific differentiation, and preferential migration in white matter. Glia. 2004;45(1):1–16. doi: 10.1002/glia.10282. [DOI] [PubMed] [Google Scholar]
- Harper JM, Krishnan C, Darman JS, Deshpande DM, Peck S, Shats I, Backovic S, Rothstein JD, Kerr DA. Axonal growth of embryonic stem cell-derived motoneurons in vitro and in motoneuron-injured adult rats. Proc Natl Acad Sci U S A. 2004;101(18):7123–7128. doi: 10.1073/pnas.0401103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himes BT, Liu Y, Solowska JM, Snyder EY, Fischer I, Tessler A. Transplants of cells genetically modified to express neurotrophin-3 rescue axotomized Clarke’s nucleus neurons after spinal cord hemisection in adult rats. J Neurosci Res. 2001;65(6):549–564. doi: 10.1002/jnr.1185. [DOI] [PubMed] [Google Scholar]
- Jin Y, Ziemba KS, Smith GM. Axon growth across a lesion site along a preformed guidance pathway in the brain. Exp Neurol. 2008;210(2):521–530. doi: 10.1016/j.expneurol.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LL, Tuszynski MH. Chronic intrathecal infusions after spinal cord injury cause scarring and compression. Microsc Res Tech. 2001;54(5):317–324. doi: 10.1002/jemt.1144. [DOI] [PubMed] [Google Scholar]
- Kalyani AJ, Piper D, Mujtaba T, Lucero MT, Rao MS. Spinal cord neuronal precursors generate multiple neuronal phenotypes in culture. J Neurosci. 1998;18(19):7856–7868. doi: 10.1523/JNEUROSCI.18-19-07856.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei N, Tanaka N, Oishi Y, Hamasaki T, Nakanishi K, Sakai N, Ochi M. BDNF, NT-3, and NGF released from transplanted neural progenitor cells promote corticospinal axon growth in organotypic cocultures. Spine. 2007;32(12):1272–1278. doi: 10.1097/BRS.0b013e318059afab. [DOI] [PubMed] [Google Scholar]
- Kishino A, Katayama N, Ishige Y, Yamamoto Y, Ogo H, Tatsuno T, Mine T, Noguchi H, Nakayama C. Analysis of effects and pharmacokinetics of subcutaneously administered BDNF. Neuroreport. 2001;12(5):1067–1072. doi: 10.1097/00001756-200104170-00040. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Liu J, Lam C, Plunet W, Oschipok LW, Hauswirth W, Di Polo A, Blesch A, Tetzlaff W. Brain-derived neurotrophic factor gene transfer with adeno-associated viral and lentiviral vectors prevents rubrospinal neuronal atrophy and stimulates regeneration-associated gene expression after acute cervical spinal cord injury. Spine. 2007;32(11):1164–1173. doi: 10.1097/BRS.0b013e318053ec35. [DOI] [PubMed] [Google Scholar]
- Lepore AC, Fischer I. Lineage-restricted neural precursors survive, migrate, and differentiate following transplantation into the injured adult spinal cord. Exp Neurol. 2005;194(1):230–242. doi: 10.1016/j.expneurol.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Lepore AC, Han SS, Tyler-Polsz CJ, Cai J, Rao MS, Fischer I. Differential fate of multipotent and lineage-restricted neural precursors following transplantation into the adult CNS. Neuron Glia Biol. 2004;1(2):113–126. doi: 10.1017/s1740925x04000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore AC, Neuhuber B, Connors TM, Han SS, Liu Y, Daniels MP, Rao MS, Fischer I. Long-term fate of neural precursor cells following transplantation into developing and adult CNS. Neuroscience. 2006;142(1):287–304. doi: 10.1016/j.neuroscience.2005.12.067. [DOI] [PubMed] [Google Scholar]
- Lu P, Tuszynski MH. Growth factors and combinatorial therapies for CNS regeneration. Exp Neurol. 2008;209(2):313–320. doi: 10.1016/j.expneurol.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai J, Fok L, Gao H, Zhang X, Poo MM. Axon initiation and growth cone turning on bound protein gradients. J Neurosci. 2009;29(23):7450–7458. doi: 10.1523/JNEUROSCI.1121-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Proschel M, Kalyani AJ, Mujtaba T, Rao MS. Isolation of lineage-restricted neuronal precursors from multipotent neuroepithelial stem cells. Neuron. 1997;19(4):773–785. doi: 10.1016/s0896-6273(00)80960-5. [DOI] [PubMed] [Google Scholar]
- Ming G, Song H, Berninger B, Inagaki N, Tessier-Lavigne M, Poo M. Phospholipase C-gamma and phosphoinositide 3-kinase mediate cytoplasmic signaling in nerve growth cone guidance. Neuron. 1999;23(1):139–148. doi: 10.1016/s0896-6273(00)80760-6. [DOI] [PubMed] [Google Scholar]
- Panter SS, Yum SW, Faden AI. Alteration in extracellular amino acids after traumatic spinal cord injury. Ann Neurol. 1990;27(1):96–99. doi: 10.1002/ana.410270115. [DOI] [PubMed] [Google Scholar]
- Paves H, Saarma M. Neurotrophins as in vitro growth cone guidance molecules for embryonic sensory neurons. Cell Tissue Res. 1997;290(2):285–297. doi: 10.1007/s004410050933. [DOI] [PubMed] [Google Scholar]
- Piantino J, Burdick JA, Goldberg D, Langer R, Benowitz LI. An injectable, biodegradable hydrogel for trophic factor delivery enhances axonal rewiring and improves performance after spinal cord injury. Exp Neurol. 2006;201(2):359–367. doi: 10.1016/j.expneurol.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Pineau I, Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol. 2007;500(2):267–285. doi: 10.1002/cne.21149. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Priestley JV, McMahon SB. Functional regeneration of sensory axons into the adult spinal cord. Nature. 2000;403(6767):312–316. doi: 10.1038/35002084. [DOI] [PubMed] [Google Scholar]
- Ruediger T, Bolz J. Neurotransmitters and the development of neuronal circuits. Adv Exp Med Biol. 2007;621:104–115. doi: 10.1007/978-0-387-76715-4_8. [DOI] [PubMed] [Google Scholar]
- Taylor L, Jones L, Tuszynski MH, Blesch A. Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord. J Neurosci. 2006;26(38):9713–9721. doi: 10.1523/JNEUROSCI.0734-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi RB, McTigue DM. Chronically increased ciliary neurotrophic factor and fibroblast growth factor-2 expression after spinal contusion in rats. J Comp Neurol. 2008;510(2):129–144. doi: 10.1002/cne.21787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski MH, Grill R, Jones LL, Brant A, Blesch A, Low K, Lacroix S, Lu P. NT-3 gene delivery elicits growth of chronically injured corticospinal axons and modestly improves functional deficits after chronic scar resection. Exp Neurol. 2003;181(1):47–56. doi: 10.1016/s0014-4886(02)00055-9. [DOI] [PubMed] [Google Scholar]
- Wang L, Hu B, Wong WM, Lu P, Wu W, Xu XM. Glial and axonal responses in areas of Wallerian degeneration of the corticospinal and dorsal ascending tracts after spinal cord dorsal funiculotomy. Neuropathology. 2008 doi: 10.1111/j.1440-1789.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- Wright DE, White FA, Gerfen RW, Silos-Santiago I, Snider WD. The guidance molecule semaphorin III is expressed in regions of spinal cord and periphery avoided by growing sensory axons. J Comp Neurol. 1995;361(2):321–333. doi: 10.1002/cne.903610209. [DOI] [PubMed] [Google Scholar]
- Yang H, Mujtaba T, Venkatraman G, Wu YY, Rao MS, Luskin MB. Region-specific differentiation of neural tube-derived neuronal restricted progenitor cells after heterotopic transplantation. Proc Natl Acad Sci U S A. 2000;97(24):13366–13371. doi: 10.1073/pnas.97.24.13366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Sasaki Y, Wen Z, Bassell GJ, Zheng JQ. An essential role for beta-actin mRNA localization and translation in Ca2+-dependent growth cone guidance. Nat Neurosci. 2006;9(10):1265–1273. doi: 10.1038/nn1773. [DOI] [PubMed] [Google Scholar]
- Ziemba KS, Chaudhry N, Rabchevsky AG, Jin Y, Smith GM. Targeting axon growth from neuronal transplants along preformed guidance pathways in the adult CNS. J Neurosci. 2008;28(2):340–348. doi: 10.1523/JNEUROSCI.3819-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]