Abstract
Deficiency in monoamine oxidase A (MAOA), an enzyme that degrades serotonin and norepinephrine, has recently been shown to be associated with aggressive behavior in men of a Dutch family. A line of transgenic mice was isolated in which transgene integration caused a deletion in the gene encoding MAOA, providing an animal model of MAOA deficiency. In pup brains, serotonin concentrations were increased up to ninefold, and serotonin-like immunoreactivity was present in catecholaminergic neurons. In pup and adult brains, norepinephrine concentrations were increased up to twofold, and cytoarchitectural changes were observed in the somatosensory cortex. Pup behavioral alterations, including trembling, difficulty in righting, and fearfulness were reversed by the serotonin synthesis inhibitor parachlorophenylalanine. Adults manifested a distinct behavioral syndrome, including enhanced aggression in males.
In the debate surrounding advances in genetic research on aggressive behavior (1), transgenic animal models carrying single gene defects are of critical importance. We describe transgenic mice lacking MAOA as a result of the integration of an interferon β (IFN-β) transgene into the gene encoding MAOA, and report that MAOA-deficient males show increased aggressiveness. MAOA and monoamine oxidase B (MAOB) are mitochondrially located enzymes with overlapping substrate specificities and tissue distributions. They inactivate neuroactive amines such as serotonin, dopamine, and norepinephrine. MAOA and MAOB are encoded by separate genes that are closely linked on the X chromosome, and they share 70% similarity in amino acid sequence (2). The loss of both MAO genes may be implicated in the severe mental retardation of some patients with Norrie disease (3), and recently a family has been described in which a point mutation in the gene encoding MAOA abolishes MAOA catalytic activity and is associated with impulsive aggression (4).
We generated mice transgenic for IFN-β by injecting an IFN-β minigene into one-cell embryos of C3H/HeJ (C3H) mice. The minigene was devised to evaluate the potential of the gene encoding IFN-β for antiviral gene therapy. We describe the transgenic line Tg(H2-IFN-β)8 (Tg8), which was initially characterized by the X-linked recessive abnormal behavior of mouse pups.
By Southern (DNA) blot hybridization and polymerase chain reaction (PCR), a single minigene copy was found in Tg8 DNA (5). This transgene was transmitted by X-linked inheritance. By reverse transcriptase-mediated PCR (RT-PCR), IFN-β RNA was detected in the testis and spleen but not in the brain. Because this suggested that the abnormal behavior of Tg8 mice was not due to IFN-β, we examined the behavior of the F2 progeny of Tg8 females mated to knockout males lacking the IFN-β receptor (6). F2 and F3 pups having the IFN-β transgene but no IFN-β receptor displayed the same abnormal behavior as Tg8 pups and were used to rule out the possibility of IFN-β affecting the phenotypic features of Tg8 mice.
By inverse PCR (7) done on Tg8 DNA with transgene primers, we obtained 2.0 kb of the two genomic regions flanking the transgene. We then isolated the junctions by inverse PCR done on C3H DNA with primers derived from the flanking regions, and it appeared that transgene integration had caused a genomic deletion of at least 1 kb. The 2-kb flanking sequences and 1-kb deleted sequences were not found in the GenBank and European Molecular Biology Laboratory databases but were mapped to a 3-million–base pair (bp) X-chromosome region containing several genes (8), including the genes encoding MAOA and MAOB. RT-PCR done on total RNA from Tg8 spleen or testes, with two primers derived from rat MAOA exons 1 and 8 (9), showed that the MAOA RNA of Tg8 mice, instead of occurring as a single species as did the MAOA RNA of C3H mice, consisted of at least four smaller species (Fig. 1A). PCR on Tg8 DNA indicated that exons 2 and 3 were missing. The deletion encompassing MAOA exons 2 and 3 was estimated by Southern blot analysis to span 17 kb.
Fig. 1.
MAOA RNA of Tg8 mice (A) Representation of the Tg8 gene encoding MAOA between exons 1 and 8 (the gene for MAOA probably has 15 exons) and structure of the four species of MAOA RNA detected by RT-PCR. The species containing IFN-β sequences (an exon of 199 bp) results from splicing events between legitimate MAOA splice sites and cryptic IFN-β splice sites (SA and SD, acceptor and donor sites present in antisense IFN-β RNA). (B) Sequence of SA and SD, and sequence of MAOA exon 2 acceptor splice site. The MAOA exon 2 acceptor has a strong putative branch site sequence (TGTTAAC), as opposed to the cryptic acceptor SA. (C) C3H MAOA sequences obtained by RT-PCR between exons and 8. The Tg8 RNA splice between exons 1 and 4 causes a reading frame shift, and the IFN-β exon of the hybrid RNA species does not restore the normal reading frame. Internal initiation of translation may occur at ACAATG within exon 4.
Any truncated polypeptide that could be synthesized from the various Tg8 MAOA RNA species (Fig. 1C) was expected to be devoid of catalytic activity, because MAO exon 2 encodes part of the βαβ unit that is required to position the cofactor FAD (10). Using serotonin as a substrate, we found that MAOA activity was abolished in the brain and liver (Table 1). In contrast, MAOB activity on phenylethylamine was not altered (11).
Table 1.
MAOA activity in the brain and liver of Tg8 and C3H mice. MAOA activity (in nanomoles per 20 min per milligram of protein) was determined by use of 14C-labeled serotonin (5-HT) as substrate (23) and, when necessary, by addition of 10−6 M l-deprenyl (dep) to inhibit interference by MAOB, which was abundant in liver tissue (as compared with MAOA, MAOB has less affinity for 5-HT and greater affinity for l-deprenyl). Each value corresponds to one mouse (average of a duplicated sample, with less than 10% variation). Similar results were obtained with other sets of mice of various ages (8 to 400 days old).
| Tissue | C3H at day |
Tg8 at day |
||||
|---|---|---|---|---|---|---|
| 14 | 30 | 90 | 14 | 30 | 90 | |
| Brain | 20.9 | 22.0 | 14.0 | 0.1* | 0.4 | 0.4 |
| Liver | 7.1 | 5.5 | 3.9 | 2.5 | 1.5 | 1.4 |
| Liver + dep | 3.9 | 2.9 | 1.8 | 0.1 | 0.1 | 0.1 |
Twenty-five percent above a blank that had no homogenate.
All Tg8 pups displayed the same pattern of altered behavior, which varied with age. Chronic administration of the serotonin synthesis inhibitor parachlorophenylalanine (PCPA) [300 mg per kilogram of body weight per day, subcutaneous (SC)] reversed to normal all the behavioral traits of Tg8 pups, whereas none of the traits were modified by the catecholamine synthesis inhibitor alpha-methylparatyrosine (300 mg/kg per day, SC). In newborns, the first sign of abnormal behavior was intense head nodding. Between days 5 and 10, the pattern included (i) trembling upon locomotion and suspension by the tail, (ii) prolonged righting (Fig. 2A), (iii) moving backward instead of pivoting when placed on a new surface, and (iv) prolonged and stronger reactions to pinching. These behaviors could be reproduced in C3H pups, but with less intensity, by daily injection of the MAOA inhibitor clorgyline (30 mg/kg per day, SC). Between days 11 and 16, Tg8 mice showed (i) frantic running and falling over, jumping, or prompt digging to hide under woodshavings in response to moderate sound and movement; (ii) sleep accompanied by violent shaking and jumps causing frequent dispersion of littermates; (iii) propensity to bite the experimenter; (iv) hunched posture; and (v) bat and ball postures with hindlimb crossing upon suspension by the tail (12). Postural traits were well reproduced in 12-day-old C3H pups by a single injection of clorgyline, whereas the other behaviors, particularly auditory startle, were not fully obtained even when clorgyline was administered from birth (30 mg/kg per day).
Fig. 2.
Behavioral alterations of Tg8 mice. (A) Righting responses. Pups were separated from their dam, isolated in a bare cage at 20°C for 3 min to wake them up, and placed on their backs. The graph gives the mean time for the pups to turn over with an upper limit of 1 min (means of the mean of five consecutive time points for each pup ± SEM). Tg8 pups turned over with forceful movements and excitation, whereas C3H pups acted calmly. (B) Aggression between Tg8 male cage mates. The plot represents a 1-day survey of skin wounds in 2- to 7-month-old males housed in groups form the time of weaning (12 cages; in the one cage of unwounded 4-month-old mice, two individuals were wounded 2 week later). In the control survey, no wounded individuals were found among 2- to 8-month-old C3H males housed in groups from the time of weaning (173 males in 22 cages). Skin wounds were not present in Tg8 and C3H female groups. (C) Latency to the first appearance of biting attack in resident-intruder tests after a long period of breeding. Each 6-month-old resident was given a single 10-min encounter in his home cage with a 2-month-old C3H intruder. The 30 Tg8 residents and 30 C3H residents were housed with a female from the age of 2 months and reared several litters. The 60 intruders were housed in groups of 10 from the time of weaning; attack latency (mean ± SEM) of Tg8 and C3H residents was 74 ± 10s and 237 ± 18 s, respectively (t test; t(58) = 7.90, P < 0.0001). (D) Latency to the first appearance of biting attack in resident-intruder tests after a period of isolation. Tg8 and C3H males were individually housed for 5 weeks beginning at weaning and were given single 3-min encounters (a short time to allow further testing) in their home cages with 2-month-old C3H intruders. Attack latency of Tg8 and C3H residents was 110 ± 12 s and 172 ± 4 s, respectively (P < 0.0001). One month later, the residents were given single 10-min encounters in their home cages with 2-month-old BALB/cJ intruders: Attack latency of Tg8 and C3H residents was 27 ± 6 s and 252 ± 35 s, respectively (P < 0.0001).
Adult mice had a different pattern of behavioral alterations. Males housed in groups from the time of weaning showed signs of offensive aggressive behavior (13) (Fig. 2B), notably bite wounds on genitals and rump, which were most apparent from the age of 3 months (areas scabbed over, with fur missing; in 6- to 12-week-old individuals, wounds were detected by palpation). We investigated aggression in Tg8 males in two different resident-intruder tests (Fig. 2, C and D). Tg8 resident males attacked the intruder faster than did C3H residents. In the interval preceding the onset of attack, C3H residents displayed intense social investigation and home cage checking, whereas Tg8 residents adopted a static, hunched, fluffed-fur posture after the first olfactory stimulus. Alterations were also seen in mating behavior. When 2-month-old virgin Tg8 males were tested individually for 30 min with a nonreceptive virgin C3H female, the courtship differed from that of C3H males in that it was disrupted by episodes of grasping [12 ± 2 griping events (mean ± SEM, n = 5)] that were reflected by increased frequency and intensity of female squeaking (113 ± 20 versus 28 ± 8 squeaks, P < 0.001). In the Porsolt’s swim test (14), Tg8 adults, instead of mostly floating, made persistent attempts to escape, which corresponds to what is observed when normal mice receive MAO inhibitors and other antidepressants. For 9-week-old mice, the time spent immobile in water, in a test of 4 min (after 2 min for habitation), was 200 ± 7 s for C3H females (mean ± SEM, n = 15), 84 ± 12 s for Tg8 females, 156 ± 12 s for C3H males, and 37 ± 8 s for Tg8 males (P < 0.001). In the open field test as described by Chen et al. (1), Tg8 adults stayed a longer time in the center, with much hesitation as to which direction to take, and it remains to be determined whether this behavior corresponds to sensory or cognitive deficits or to reduced fear. For 12-week-old mice, the time spent in the center of the field was 10 ± 2 s for C3H females (mean ± SEM, n = 13), 93 ± 34 s for Tg8 females, 20 ± 4 s for C3H males, and 118 ± 27 s for Tg8 males (P < 0.001). In the beam-walking test, Tg8 adults grasped the edge of the beam with hindlimbs while walking (15), whereas C3H adults were sure-footed (it should be noted that adult Tg8 and C3H mice are blind, as they carry a retinal degeneration gene).
A particular behavioral test to distinguish MAOA-deficient adults from normal adults consisted in giving an injection of l-deprenyl [15 mg/kg, intraperitoneal (IP)] or lazabemide (60 mg/kg, IP) (16), two MAOB inhibitors differing in several pharmacological properties. These treatments did not overtly affect C3H behavior but caused, in Tg8 adults, restlessness and attentional deficit, which disrupted social interaction, feeding, and self-grooming, beginning within 2 hours after injection and lasting for at least 3 hours (17).
In the rodent brain, MAOB activity is low at birth and increases during the first month (partly due to gliogenesis), whereas MAOA activity is close to adult amounts from the first week on (18). This developmental difference may explain why the phenotype of Tg8 mouse pups was more severe than that of adults, and why l-deprenyl did not aggravate the behavioral traits of 12-day-old Tg8 mice.
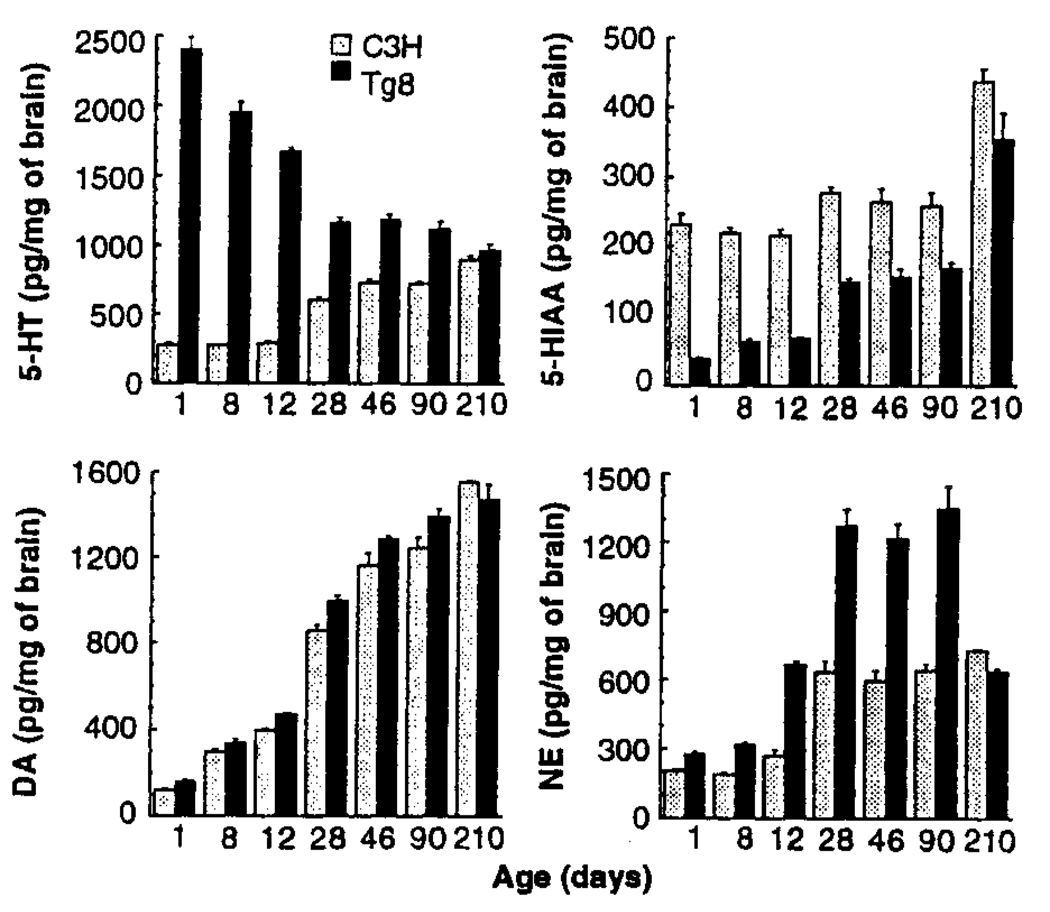
Comparison of the amount of serotonin (5-HT), dopamine (DA), and norepinephrine (NE) in Tg8 and C3H brains showed an increase in all three amines in Tg8 brains (Fig. 3). The elevation in DA was slight, although the DA metabolite dihydroxyphenylacetic acid was markedly decreased (3.5 times less at 3 months). The amount of 5-HT was considerably increased in Tg8 pups (ninefold at day 1 and sixfold at day 12) and returned to normal in older mice, whereas the amount of the 5-HT metabolite 5-HIAA (probably produced by MAOB) was considerably decreased in Tg8 pups and returned to normal in older mice. The MAOB inhibitor l-deprenyl (15 mg/kg, IP) elicited within 3 hours a greater increase in 5-HT in Tg8 than in C3H brains (2.5-fold versus 1.2-fold in 4-month-old males) and caused the near disappearance of 5-HIAA in Tg8 brains but no decrease in 5-HIAA in C3H brains. This suggests that the role of MAOB in 5-HT oxidation in the brain in vivo is more important than can be predicted from in vitro tests (Table 1).
Fig. 3.
Amounts of 5-HT, 5-HIAA, DA, and NE in whole brains from Tg8 and C3H mice. Values from HPLC assays (24) are expressed in picogram per milligram of wet brain and represent the mean ± SEM (n = 4, 5, 7, 8, 4, 4, and 2). Mice were of both sexes and various ages (except for the 3-month-old mice, which were males only) and were housed in groups according to sex beginning at weaning (except for the 7-month-old mice, which were breeding pairs).
In 7-day-old Tg8 pups, the density of fibers stained by 5-HT immunochemistry was enhanced in several brain regions (such as the striatum, cerebral cortex, and the hilus of the dentate gyrus), and 5-HT–like staining was abnormally present in catecholaminergic neurons of the locus coeruleus and nigral complex (A8, A9, and A10) (Fig. 4, D and E). These neurons showed no immunoreactivity to the 5-HT–synthesizing enzyme tryptophan hydroxylase (TPH), and during chronic administration of the TPH inhibitor PCPA (300 mg/kg per day, SC), they did not remain immunoreactive to antibody to 5-HT. Thus, the presence of 5-HT immunoreactivity probably corresponds to 5-HT uptake (19) and the likely absence of MAOB in this category of neurons (20). In contrast, in 10-week-old Tg8 mice, the distribution and density of 5-HT-immunoreactive cell bodies and fibers appeared normal. One of the possibilities to explain the difference between pups and adults is that 5-HT may be captured by MAOB-rich cells in adults (such as mature glial cells) (20).
Fig. 4.
Abnormal 5-HT immunostaining in 7-day-old Tg8 brains. (A through C) Coronal sections of C3H brain. (D through F) Corresponding sections of Tg8 brain. 5-HT polyclonal antibody was used at a dilution of 1:15,000 and was revealed by a streptavidin-biotin-peroxidase complex and by diaminobenzidine. In the locus coeruleus (Ic) [(A) and (D)], substantia nigra (sn), and ventral tegmental area (vta) [(B) and (E)], 5-HT-like immunoreactivity is present in terminal fibers and medial forebrain bundle (mfb) in C3H and Tg8 mice but is abnormally present in cell bodies in Tg8 mice [arrows in (D) and (E)]. Four different 5-HT antisera (three polyclonal and one monoclonal) revealed the same staining pattern. Double immunofluorescent staining for 5-HT and the catecholamine synthesizing enzyme tyrosine hydroxylase showed that these 5-HT-like immunoreactive cell bodies were catecholaminergic. In the somatosensory cortex [(C) and (F)], clusters of 5-HT-immunoreactive fibers delineate barrels in layer IV in C3H mice (C), whereas they form a continuous band in layer IV in Tg8 mice (F). Scale bar, 225 µm.
It is well documented that in the somatosensory cortex of normal pups, serotonergic afferents show a pattern that coincides with cylindrical aggregates (barrels) of granule cells in layer IV (21). Instead of this disjunctive pattern (Fig. 4C), the somatosensory cortex of Tg8 pups showed a continuous band of 5-HT immunostaining (Fig. 4F). In Tg8 pups and adults, Nissl and cytochrome oxidase chemistry revealed at complete absence of barrelfield in the somatosensory cortex, whereas barrelettes were present in the trigeminal and thalamic nuclei bridging the sensory periphery to the cortex (11). Neonatal administration of PCPA (300 mg/kg per day, SC) partly restored the capacity to form cortical barrels. A role for serotonin in barrelfield formation could be considered because, for example, thalamic afferents in the barrels of normal pups express large amounts of 5-HT1B receptors (22). Tg8 pup cortices that were stained for the 5-HT1B receptors did not show the barrel pattern that was found in C3H cortices (11). It will be interesting to check for the presence of the barrelfield in the progeny of Tg8 mice mated to diverse 5-HT receptor knockouts.
This study shows that MAOA-deficient mouse pups have a dramatically altered serotonin metabolism and severe behavioral alterations, both phenomena being linked. The behavioral traits of adults may be related to persisting defects in monoamine metabolism or to structural alterations such as the one we demonstrated in the cerebral cortex, an issue that pharmacological interventions may help to clarify. The finding that MAOA-deficient males with a C3H/HeJ genetic background display enhanced aggression under standard rearing conditions supports the idea that the particularly aggressive behavior of the few known human males lacking MAOA is not fostered by an unusual genetic background or complex psychosocial stressors but is a more direct consequence of MAOA deficiency.
Contributor Information
Olivier Cases, Centre National de la Recherche Scientifique (CNRS), Unité de Recherche Associée (URA) 1343, Institut Curie, 91405 Orsay, France.
Isabelle Seif, Centre National de la Recherche Scientifique (CNRS), Unité de Recherche Associée (URA) 1343, Institut Curie, 91405 Orsay, France.
Joseph Grimsby, Department of Molecular Pharmacology and Toxicology, University of Southern California, Los Angeles, CA 90033, USA.
Patricia Gaspar, Institut National de la Sant é et de la Recherche Médicale, Unité 106, Hôpital de la Salpêtriėre, 75651, Paris, France.
Kevin Chen, Department of Molecular Pharmacology and Toxicology, University of Southern California, Los Angeles, CA 90033, USA.
Sandrine Pournin, CNRS, URA 361, Institut Pasteur, 75724 Paris, France.
Ulrike Müller, Institute of Molecular Biology I, University of Zürich, 8093 Zürich, Switzerland.
Michel Aguet, Genentech, South San Francisco, CA 94080.
Charles Babinet, CNRS, URA 361, Institut Pasteur, 75724 Paris, France.
Jean Chen Shih, Department of Molecular Pharmacology and Toxicology, University of Southern California, Los Angeles, CA 90033, USA.
Edward De Maeyer, Centre National de la Recherche Scientifique (CNRS), Unité de Recherche Associée (URA) 1343, Institut Curie, 91405 Orsay, France.
REFERENCES AND NOTES
- 1.Mann CC. Science. 1994;264:1686. doi: 10.1126/science.8209246. [DOI] [PubMed] [Google Scholar]; Barinaga M. ibid. :1690. [Google Scholar]; Saudou F, et al. ibid. 1994;265:1875. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]; Chen C, Rainnie DG, Greene RW, Tonegawa S. ibid. 1994;266:291. doi: 10.1126/science.7939668. [DOI] [PubMed] [Google Scholar]
- 2.Bach AWJ, et al. Proc. Natl. Acad. Sci. U.S.A. 1988;85:4934. [Google Scholar]; Chen Z-Y, Powell JF, Hsu Y-PP, Breakefield XO, Craig IW. Genomics. 1992;14:75. doi: 10.1016/s0888-7543(05)80286-1. [DOI] [PubMed] [Google Scholar]
- 3.Collins FA, et al. Am. J. Med. Genet. 1992;42:127. doi: 10.1002/ajmg.1320420126. [DOI] [PubMed] [Google Scholar]
- 4.Brunner HG, Nelen M, Breakefield XO, Ropers HH, van Oost BA. Science. 1993;262:578. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- 5.The IFN-β minigene is a 1588-bp Acc I-Apa I fragment derived from plasmid pKb-IFN-β [ Seif I, De Maeyer E, Rivière I, De Maeyer-Guignard J. J. Virol. 1991;65:664. doi: 10.1128/jvi.65.2.664-671.1991. ]. The Tg8 transgene is identical to the minigene, except for a 64-bp terminal deletion in the herpes virus thymidine kinase (TK) region and a 148-bp deletion in the promoter region from the major histocompatibility gene H-2Kb (H2).
- 6.Müller U, et al. Science. 1994;264:1918. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 7. Ochman H, Gerber AS, Hartl DL. Genetics. 1988;120:621. doi: 10.1093/genetics/120.3.621. Inverse PCR was done without prior size fractionation and isolation of restriction fragments.
- 8.This is a region between the DXHS32 and A-raf loci [ Brown SDM, et al. Mamm. Genome. 1993;4:S269. doi: 10.1007/BF00360846. ].
- 9.Kuwahara T, Takamoto S. A. Ito. Agric. Biol. Chem. 1990;54:253. [PubMed] [Google Scholar]
- 10.Grimsby J, et al. Proc. Natl. Acad. Sci. U.S.A. 1991;88:3637. [Google Scholar]; Wierenga RK, et al. J. Mol. Biol. 1986;187:101. doi: 10.1016/0022-2836(86)90409-2. [DOI] [PubMed] [Google Scholar]
- 11.Cases O, et al. unpublished data. [Google Scholar]
- 12.Whishaw IQ, Schallert T, Kolb B. J. Comp. Physiol. Psychol. 1981;95:85. doi: 10.1037/h0077760. [DOI] [PubMed] [Google Scholar]
- 13.Maxson SC. In: Techniques for the Genetic Analysis of Brain and Behavior: Focus on the Mouse. Goidowitz D, Wahlstein D, Wimer RE, editors. Amsterdam: Elsevier; 1992. pp. 349–373. [Google Scholar]
- 14.Porsolt RD, Bertin A, Jalfre M. Arch. Int. Pharmacodyn. 1977;229:327. [PubMed] [Google Scholar]; Borsini F, Meli A. Psychopharmacology. 1988;94:147. doi: 10.1007/BF00176837. [DOI] [PubMed] [Google Scholar]
- 15.Kolb B, Whishaw IQ. Can. J. Psychol. 1983;37:211. doi: 10.1037/h0080724. [DOI] [PubMed] [Google Scholar]
- 16.Haefely WE, et al. Adv. Neurol. 1990;53:505. [PubMed] [Google Scholar]
- 17.Gerson SC, Baldessarini RJ. Life Sci. 1980;27:1435. doi: 10.1016/0024-3205(80)90368-9. [DOI] [PubMed] [Google Scholar]
- 18.Tsang D, Ho KP, Wen HL. Dev. Neurosci. 1986;8:243. doi: 10.1159/000112258. [DOI] [PubMed] [Google Scholar]; Strolin Benedetti M, Dostert P, Tipton KF. Dev. Pharmacol. Ther. 1992;18:191. [PubMed] [Google Scholar]; Koide Y, Kobayashi K. Neurochem. Res. 1984;9:595. doi: 10.1007/BF00964506. [DOI] [PubMed] [Google Scholar]
- 19.Lichtensteiger W, et al. J. Neurochem. 1967;14:489. doi: 10.1111/j.1471-4159.1967.tb09548.x. [DOI] [PubMed] [Google Scholar]; Shaskan E, Snyder SH. J. Pharmacol. Exp. Ther. 1970;175:404. [PubMed] [Google Scholar]; Steinbusch HWM, Verhofstad AAJ, Joosten HWJ, Goldstein M. In: Cytochemical Methods in Neuroanatomy. Chan-Palay V, Palay SL, editors. Liss: New York; 1982. [Google Scholar]; Wallace JA, et al. Brain Res. Bull. 1982;9:117. doi: 10.1016/0361-9230(82)90127-7. [DOI] [PubMed] [Google Scholar]
- 20. Levitt P, Pintar JE, Breakefield XO. Proc. Natl. Acad. Sci. U.S.A. 1982;79:6385. doi: 10.1073/pnas.79.20.6385. Westlund KN, Denney RM, Kochersperger LM, Rose RM, Abell CW. Science. 1985;230:181. doi: 10.1126/science.3875898. In the normal brain, most catecholaminergic neurons of the locus coeruleus and nigral complex are rich in MAOA.
- 21.Woolsey T, Van der Loos H. Brain Res. 1970;17:205. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]; D’Amato RJ, et al. Proc. Natl. Acad. Sci. U.S.A. 1987;84:4322. [Google Scholar]; Rhoades RW, et al. J. Comp. Neurol. 1990;293:190. doi: 10.1002/cne.902930204. [DOI] [PubMed] [Google Scholar]; Osterheld-Hass MC, et al. Dev. Brain Res. 1994;77:189. doi: 10.1016/0165-3806(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 22.Bennett-Clarke CA, et al. Proc. Natl. Acad. Sci. U.S.A. 1993;90:153. doi: 10.1073/pnas.90.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu H-F, Chen K, Shih JC. Mol. Pharmacol. 1983;43:888. [PubMed] [Google Scholar]
- 24.For high-performance liquid chromatography (HPLC) assays, whole brain was homogenized by sonication in 0.1 M HCI04 containing 0.2% Na2S205. In the first assay, the HPLC system was composed of a 5-µm Ultrosphere column (Beckman, Fullerton, CA) and an LC-2A electrochemical detector (Bioanalytical Systems, West Lafayette, IN), and the mobile phase was an 80:20 mixture (pH 2.9) of 100 mM Na2H2PO4 and methanol, with 2.75 mM octane sulphonate, 0.1 mM EDTA, and 0.25 mM triethylamine. In the second assay (to refine NE determination), the HPLC system was a Coulochem II ESA with an HR-80 catecholamine column (ESA, Bedford, MA), and the mobile phase was an 88:6:6 mixture (pH 5.2) of 75 mM Na2H2PO4, methanol, and acetonitrile, with 2.75 mM octane sulphonate, 0.02 mM EDTA, and 0.7 mM triethylamine.
- 25.We thank D. Hervé, J.-P. Tassin, J. Adam, B. Courtier, L. Eusébe, and D. Haranger for participation; P. Avner for the DNA backcross panel; M. Da Prada for Iazabemide; and R. Hen, M. Jouvet, J. P. Changeux, I. Gresser, L. Abbott, E. Lauret, V. Vieillard, R. Zawatzky, and J. De Maeyer-Guignard for discussion. Supported by CNRS, the Agence Nationale de Recherches sur le Sida, the Association pour la Recherche sur le Cancer, and National Institute of Mental Health grants RO1 MH 37020, R37 MH 39085, and K05 MH 00796