Abstract
One major advance in T cell based immunotherapy in the last twenty years has been the molecular definition of numerous viral and tumor antigens. Adoptive T-cell transfer has shown definite clinical benefit in the prophylaxis and treatment of viral infections that develop in pediatric patients after allogeneic transplant and in Epstein–Barr virus-associated post-transplant lymphoproliferative disease. Developing adoptive T cell therapies for other malignancies presents additional challenges. This article describes the recent advances in T cell based therapies for malignancy and infection in childhood and strategies to enhance the effector functions of T cells and optimize the cellular product, including gene modification and modulation of the host environment.
Keywords: Hematopoietic stem cell transplantation, pediatrics, adoptive immunotherapy, tumor vaccines, tumor antigens, lymphopenia, immune reconstitution, chimeric antigen receptor
TUMOR VACCINES
One major advance in T cell based immunotherapy in the last 20 years has been the molecular definition of numerous viral and tumor antigens. Immunodominant epitopes have been defined for major viral pathogens including Epstein Barr virus (EBV), Cytomegalovirus (CMV), adenovirus, human papilloma virus (HPV) and hepatitis B virus (HBV), that can be used to target infections in immunocompromised hosts or tumors that express viral antigens1. Many tumor antigens have also been identified in adult cancers, and some of these are expressed in pediatric tumors (Table 1). Current concepts in tumor immunology hold that tumor antigens comprise unique tumor-specific molecules or tumor-associated molecules that are rare on normal tissues but highly expressed on tumors. Unlike viral antigens, which generally induce vigorous immune responses in healthy hosts, “tumor antigens” are not naturally immunogenic, due to a combination of many factors including the immuno-evasive nature of cancer, which diminishes the presentation of antigens to the immune system, as well as co-expression of tumor associated antigens on normal tissues which begets immune tolerance. Tumor vaccine therapies posit that administration of tumor-specific or tumor-associated antigens in the context of immune co-stimulation will induce tumor specific immunity and result in antitumor effects.
Table 1.
Antigens Expressed on Pediatric Tumors
| ANTIGEN | PEDIATRIC TUMORS | REFERENCE |
|---|---|---|
| MAGE-1, -2, -3 | gliomas, medulloblastoma, neuroblastoma, osteosarcoma | 2–4 |
| GAGE | gliomas, medulloblastoma, neuroblastoma, ESFT | 2–5 |
| BAGE | AML | 2, 6 |
| XAGE | ESFT, alveolar rhabdomyosarcoma | 7 |
| NY-ESO-1 | Synovial sarcoma, osteosarcoma, neuroblastoma | 2, 8, 9 |
| PRAME | AML, Wilms tumor, neuroblastoma | 6, 10–12 |
| N-Myc | neuroblastoma | 13, 14 |
| Proteinase-3 | CML, AML, MDS | 15, 16 |
| WT1 | AML, ALL, rhabdomyosarcoma | 6, 17, 18 |
| Survivin | “universal” | 19–21 |
| Telomerase (hTERT) | “universal” | 20, 22, 23 |
| translocation breakpoints | Synovial sarcoma t(X;18); CML t(9;22), ALL t(12;21), DSRCT t(11;22), alveolar rhabdomyosarcoma t(2;13) | 24–27 |
| Mutant p53 | Variable across histologies | 28, 29 |
| HBV and HCV | Hepatocellular carcinoma | 30 |
| EBV EBNA2, 3 | EBV lymphoproliferative disorder | |
| EBV EBNA1, LMP-1, -2 | Hodgkin’s, nasopharyngeal carcinoma, EBV lymphoproliferative disorder, Burkitt lymphoma | 31, 32 |
Thus far, ample data is available from studies in adult cancer to conclude that tumor vaccines administered as single agents do not reliably induce regression of established tumors33. However, tumor burden is one critical factor that impacts the effectiveness of immunotherapy for cancer. Essentially all animal models of cancer demonstrate that minimal residual tumor burdens are more readily treated by the immune system than bulk tumors. In human studies, this is clearly demonstrated in the context of donor leukocyte infusions for chronic myelogenous leukemia (CML), which show an 85% response rate when tumor burdens are low and a <20% response rate in accelerated phase34. Therefore, with regard to tumor vaccines, randomized studies are needed to determine whether vaccines administered in the adjuvant setting can prevent tumor recurrence. Indeed, recent results using antigen loaded dendritic cell vaccine approach in men with advanced prostate cancer has shown benefit over placebo in a large phase 3 trial (31.7% vs. 23% 3 year survival and 25.8 months vs. 21.7 months median survival), raising the prospect that this may be the first cancer vaccine approved for general use by the FDA35. Studies of tumor vaccines in pediatric oncology have largely mirrored these principles. Several types of vaccines have been administered, all primarily aimed at delivering a tumor antigen (or antigens) in a manner that induces robust immune responses. Many different approaches to tumor-based vaccination are currently utilized and even within each approach, the choice of appropriate adjuvant, antigen, timing of vaccine, route, etc. remains under study (Table 2). While essentially all studies of tumor vaccines in pediatric oncology have demonstrated safety, only few instances of shrinkage of established tumors were observed 36–39. Thus, as with adult tumors, current efforts in pediatrics are focused on administering tumor vaccines in the setting of minimal residual disease (MRD) and/or combining vaccines with other cell based therapies for patients with established disease. This will be discussed further below.
Table 2.
Current approaches to tumor vaccination
| APPROACH | ANTIGEN | RESTRICTIONS | PROS | CONS |
|---|---|---|---|---|
| Peptide vaccines | 9–20 amino acids | HLA allele specific (e.g. HLA-A2) | Non-toxic, cheap to produce | Restricted to patients with one specific HLA alleles, targets only one epitope, requires adjuvant |
| Protein | Whole antigens | None | No restrictions based upon HLA type | Expensive to produce, unclear how best to administer |
| Pox Viruses | Whole antigens | Some concern in immuno-suppressed hosts | Can also administer costimulator y molecules | Anti-pox immune response limits repetitive administration |
| DNA | Whole antigens | None | Relatively simple to produce | May be better at boosting existing immune responses than inducing primary responses |
| Dendritic cells | Peptides or protein antigens or whole tumor cells | Requires harvest, not off-the-shelf | Individualize herapy, can iver multiple igens | Labor intensive, unclear how best to prepare DCs |
| Genetically engineered tumor cell banks | All antigens expressed by the tumor will be presented | None | Presentation of multiple antigens, can specifically modulate co-stimulation | Individual cell banks difficult to produce, allogeneic cells banks may or may not be as effective |
ADOPTIVE CELL THERAPIES
Adoptive T Cell Therapy for Infections
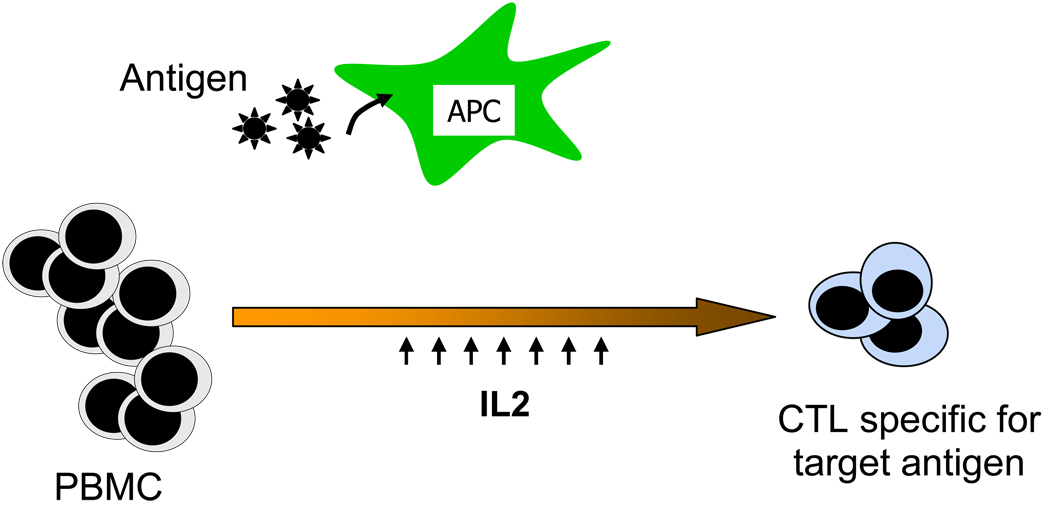
Infections are a major cause of morbidity and mortality in pediatric patients who are immunosuppressed following hematopoietic stem cell transplantation (HSCT).36 As risk clearly correlates with impaired virus specific immunity in the early post-transplant period, there is considerable interest in developing means to adoptively transfer a protective T cell response to more rapidly reconstitute immunity without transferring alloreactive T cells. Initial studies to evaluate such strategies targeted viruses such as CMV and EBV where the immune response is well defined and the approach has now been extended to other pathogens such as adenovirus, BK virus and aspergillus37–43. The methodology used in these studies has been to generate cytotoxic T cells ex vivo from the transplant donor by repeated stimulation of donor-derived peripheral blood mononuclear cells with antigen presenting cells expressing viral antigens (Figure 1). These cells are subsequently administered to the recipient either preemptively to prevent viral infection or to treat documented infections. To identify suitable viral antigens for such immunotherapeutic strategies, it is necessary to know which antigens are required for viral persistence. There must also be a source of the identified viral antigen suitable for clinical use and an appropriate antigen presenting cell that will effectively present viral antigen and will also produce the co-stimulation required to activate an effective T cell response.
Figure 1. Generating CTLs by Repeated Ex-vivo stimulation.
Low frequency virus specific CTLs in a peripheral blood mononuclear cell population are expanded by primary and secondary stimulation with antigen expressed on antigen presenting cells followed by expansion with IL2. The resulting population is enriched for T cells specific for the viral antigen.
Although the overall incidence of EBV-associated post-transplant lymphoproliferative disease (PTLD) following HSCT is less than 1%, the risk may be much higher in recipients with congenital immune deficiencies or in those who receive highly immunosuppressive conditioning regimens and T cell depleted grafts (which are becoming more commonly used as discussed elsewhere in this edition). EBV-PTLD is almost always derived from donor B cells, which express all EBV latency proteins and would normally be eliminated by an EBV specific immune response. The proliferating cells have the same phenotype and pattern of EBV gene expression as EBV transformed B lymphoblastoid cell lines (LCLs), and these can be readily prepared from any donor by infecting peripheral blood mononuclear cells with a laboratory strain of EBV. LCLs are excellent antigen presenting cells (APC) since they present EBV antigens efficiently on the cell surface with robust expression of co-stimulatory molecules. LCLs have been used after irradiation as effective stimulator cells to generate EBV-specific T cell lines from transplant donors. When EBV-specific cytotoxic T lymphocytes (CTLs) have been administered as prophylaxis or therapy for EBV lymphoma in high-risk HSCT recipients, they have been able to expand and reconstitute immunity to EBV. In addition they have been effective both in preventing EBV-LPD in high risk recipients and in treating patients who received CTL as for established EBV- LPD with sustained response rates of over 85% 1, 47,44.
The immune response to CMV is also well defined and several studies have transferred donor derived T cells specific for the immunodominant CMV pp65 protein to HSCT patients and shown that the transferred cells can prevent reactivation and treat CMV reactivation and disease in humans43, 45, 46. In these studies a number of sources of antigen were used including purified CMV antigen, CMV-infected cell lysates and peptides. Antigen presenting cells have included dendritic cells, fibroblasts and peripheral blood mononuclear cells. The first studies performed by the Seattle group infused CD8+ CMV-specific T cell clones, reactive against CMV virion proteins, and showed protection against CMV viremia and disease but long term persistence only in patients who recovered CD4+ CMV specific responses43. Peggs and colleagues produced CMV-specific CTLs using dendritic cells pulsed with CMV antigens as stimulator cells and after infusion saw rapid expansion and long term persistence of CMV immunity46. Several other groups have confirmed that adoptive transfer of donor-derived CMV-specific T cells reconstitutes immunity to CMV and can not only prevent transplant patients from developing CMV infection but treat active disease45.
The approaches described above target only one virus in a patient population that is at risk of infection with many viruses following transplant. Leen and colleagues therefore developed an approach to generate CTLs specific for three of the viruses that cause morbidity and mortality post transplant simultaneously; CMV, EBV and adenovirus40, 41. To achieve this they used mononuclear cells transduced with a recombinant adenoviral vector encoding the CMV antigen pp65 for the initial stimulation followed by stimulation with EBV-lymphoblastoid cell lines transduced with the same vector. Responding T cells were therefore exposed to all three antigens. In two sequential studies with CMV seropositive and seronegative donors they showed that both trivirus (CMV-, EBV- and Adenorvirus-specific) CTLs could expand in response to viral challenge and clear all three viruses in over 90% of patients with active viral disease. In preclinical studies this approach is being extended to also target BK virus38.
The methodologies described above for generating virus specific CTLs are complex and require considerable time. More rapid selection techniques are therefore being evaluated to provide virus specific T cells for transplant recipients in a timely manner when they have active infections. Two methodologies to select virus reactive T cells from donor blood have been evaluated in clinical trials. In the first, T cells specific for the CMV-derived antigens were selected from apheresis products obtained from donors by incubating cells with HLA-peptide tetramers (four joined MHC class I complexes that bind directly to T cell receptors of a particular specificity) specific for the viral peptides followed by selection with magnetic beads. After infusion, the cells were able to expand by several logs and reconstitute immunity to CMV47. A limitation of this approach is that the product has limited specificity for one epitope and is only available for some HLA types. A second rapid selection technique is gamma interferon capture assay where donor blood cells are briefly stimulated with antigen and cells are selected that respond to antigenic stimulus based upon gamma interferon secretion. Adenovirus specific donor T cells isolated by this technique were infused into nine children with systemic adenovirus infection post transplant and responses were seen in five of six evaluable patients40. An alternative to rapid selection is to develop banks of virus specific cells lines so that the most closely matched product can be accessed rapidly if a patient develops an infection. A recent Phase II study using banked EBV-specific CTLs to treat PTLD showed a response rate of 64% with no adverse events related to alloreactivity reported48. The above studies have all targeted viral antigens. However the T cell immune response may also be important for the clearance of other infections. The Perugia group has generated donor T-cell clones specific for Aspergillus and shown it is possible to transfer high-frequency T-cell responses associated with control of Aspergillus infections45.
Adoptive T Cell Therapy for Pediatric Malignancies
Developing adoptive T cell therapies for malignancy presents additional challenges. Although adoptively transferred T cells can in theory be redirected towards antigens that are relatively or absolutely restricted to the cancer cells, as discussed above, tumor specific antigens are not as well defined nor as immunogenic as viral antigens. In addition to tumor antigens defined in autologous hosts (Table 1), alloantigens selectively expressed on hematopoietic cells in the context of allogeneic HSCT are also a potential target and several groups are developing methodology for selection of such T cells based on the gamma interferon capture assay49. As described above EBV-PTLD has served as a prototype disease for successful targeting of viral antigens in cancer50. Other EBV-associated malignancies, such as Hodgkin lymphoma, some types of non-Hodgkin lymphoma (NHL) and nasopharyngeal carcinoma, have also been targeted using this strategy, but show lower response rates compared to EBV-CTL immunotherapy51, 52. These tumors, which develop in previously immunocompetent individuals, express a more restricted array of EBV-encoded antigens than EBV-PTLD with only the weakly immunogenic EBV antigens (EBNA1, LMP1 and LMP2) being expressed. They also possess a myriad of immune evasion mechanism that are active in the tumor microenvironment. To overcome these obstacles, investigators have developed ways to tailor CTL specificity to the subdominant tumor antigens expressed in EBV associated lymphomas by stimulating T cells with LMP (latent membrane protein) antigens transferred to antigen presenting cells (dendritic cells or LCLs) using adenoviral vectors. The resulting CTLs are enriched for T cells specific for LMP antigens and showed increased activity compared with EBV CTLs when administered to patients with EBV+ Hodgkin disease or NHL, either post-transplant or in the setting of relapsed disease53.
Although T cells specific for tumor antigens can be identified, most are present at a low frequency, may have receptors with low avidity for the tumor antigens and are commonly anergic. One strategy to overcome these limitations is to activate T cells ex vivo to circumvent these limitations and to overcome suppressive factors present in vivo thus augmenting the antitumor activity. In one study, allogeneic donor–derived leukemia reactive T cells were selected based on their ability to inhibit in vitro growth of CML progenitor cells, and subsequently expanded to generate CTL lines. When transferred to the recipient they were able to induce remission in a patient with recurrent CML54. However, this labor intensive process is not widely applicable. In a simpler approach, ex vivo-activated donor lymphocytes were expanded non-specifically by incubation with CD3- and CD28-coated beads and administered to 18 patients with relapsed lymphoreticular malignancies after HSCT. Objective responses were seen in eight patients, four of whom had a sustained response at a median 23 months of follow up55. Importantly however, such products may also contain alloreactive cells that can induce graft versus host disease (GVHD) and thus may be problematic when administered in the context of allogeneic HSCT.
An alternative approach to target tumor antigens is to genetically modify T cells with artificial antigen receptors to redirect their potent effector functions towards tumor cells. This has been achieved by expression of either αβ T cell receptor (TCR) heterodimer pairs or tumor antigen specific chimeric antigen receptors (CAR). High-avidity αβ-TCR heterodimer pairs are either generated by immunizing HLA-A2 transgenic mice with tumor antigen or cloned from human autologous CTL cultures56. This approach, albeit attractive, is limited to individuals with a particular HLA-type, mostly HLA-A2. Moreover, although αβ TCR T cells mediate antitumor activities in vitro, their in vivo effector functions may be limited by the inadvertent pairing between the native TCR and the transduced αβ chains. Such limitations may be overcome by using CARs which are artificial molecules custom made by fusing an extracellular variable domain derived from a high-affinity monoclonal antibody specific for a tumor-restricted antigen of interest to an intracellular signaling domain-usually derived from the ζ-signaling chain of the TCR57. Upon encounter of the specific antigen by the extracellular antibody-derived domain, the T cell-derived signaling domain initiates an intracellular signal that results in T cell activation. To promote cell activation and survival, investigators have incorporated additional signaling domains from co-stimulatory molecules to the intracellular portion of the CAR. Chimeric antigen receptors recognize antigens in an HLA-independent manner (like an antibody), and have thus overcome a major limitation of the αβ TCR. In addition, the CAR approach circumvents HLA molecule downregulation, an important mechanism of tumor evasion, and allows for recognition of unprocessed tumor antigens on the surface of the cell.58 Such artificial molecules can theoretically be designed to target any tumor-restricted or tumor-associated cell surface antigen of interest including those carbohydrate and glycolipid moieties such as the disialoganglioside GD2 in neuroblastoma. Genetically modified T cells have shown promising preclinical effector functions and CARs targeting CD20 and GD2 have already been evaluated in clinical trials in patients with lymphoma and neuroblastoma, respectively59, 60. Clinical responses were seen in some patients in both studies although the persistence of the transferred T cells was suboptimal. A number of trials are currently underway evaluating whether T cells genetically modified with a CAR targeting CD19 have activity in patients with relapsed CD19+ malignancies post transplant.
Among the multiple hurdles that must be crossed for adoptive T cell immunotherapy to be successful is the necessity for infused T cells to access the long term memory pool. There are concerns that excessive ex vivo stimulation can render T cells senescent, and unable to sustain long term proliferation required of memory T cells61. A recent study showed that it may be possible to take advantage of the longevity of virus specific CTLs and genetically incorporate antitumor specificities onto these cells. Two distinguishable GD2-specific CARs were transferred to EBV-CTL or primary T cells activated with OKT3 and IL2 administered to neuroblastoma patients in a phase I/II clinical trial and the EBV-specific CTLs did survive longer than T cells perhaps due to the costimulation received through their native receptor59.
HOST FACTORS PLAY AN ESSENTIAL ROLE IN DETERMINING THE EFFECTIVENESS OF T CELL BASED IMMUNOTHERAPY
Children with severe viral infection due to primary or secondary immunodeficiency and children with cancer are the primary pediatric populations for which T cell based immunotherapies are being developed. HSCT is a common cause of secondary immunodeficiency since it induces severe lymphocyte depletion, which typically lasts at least one year and may persist for several years following the procedure. Furthermore, common therapies for childhood cancer induce profound lymphocyte depletion and significant immunosuppressive effects result from cancer itself. Thus, patients receiving T cell based immunotherapies have alterations in host immunity that can impact the effectiveness of T cell based immunotherapy both positively and negatively. This section will describe the changes in immune physiology induced by T cell depletion and discuss the effects that these and other host factors play in enhancing or diminishing the effectiveness of T cell based immunotherapies for cancer or viral infection.
Unlike other marrow-derived populations, B cells and T cells require specialized microenvironments within the bone marrow and thymus respectively, to recapitulate primary development. The bone marrow microenvironment needed to support B cell lymphopoiesis remains functional throughout life, however, age-related changes occur within the thymus that limit the capacity for postnatal humans to regenerate T cells62. Many investigators have emphasized the importance of puberty and sex steroids in age associate thymic involution, but in fact, from birth onward there is a relatively linear decline in the relative mass and function of the thymus. As a result, adolescents have substantially diminished thymic function compared to younger children63, and the majority of patients in the fifth decade of life essentially show a complete inability to recover T cells via thymic-dependent pathways after T cell depletion64. Furthermore, even in very young children, the thymic microenvironment is exquisitely susceptible to damage by a variety of insults, including cytotoxic agents, viral infections, GVHD, and irradiation65, thus limiting that capacity for even young children with cancer or immunodeficiency to support thymic-dependent T cell regeneration.
When thymic-dependent T cell regeneration is limiting, T lymphocytes can be partially regenerated by thymic-independent homeostatic peripheral expansion. This process substantially increases T cell numbers and immune function, but it does not fully restore immune competence. Briefly, mature T cells, (either remaining within the host following the lymphopenia inducing insult, emerging from a diminished thymus, derived from maternal T cells or adoptively transferred through a stem cell graft or immunotherapy product) undergo vigorous mitotic expansion, which is dramatically enhanced compared to low level cycling that T cells normally undergo throughout life in the absence of lymphopenia. This cycling represents a combination of enhanced T cell proliferation toward cognate antigens (e.g. viral antigens present during lymphopenia)66, T cell proliferation in response to cross reactive antigens expressed by commensal flora in the gut, and T cell proliferation toward self-antigens, which do not induce substantial T cell cycling under lymphoreplete conditions, but can induce marked T cell proliferation in the setting of lymphopenia67. Thus, lymphopenia results in profound increases in global T cell cycling and increased responsiveness to antigens. These alterations in immune reactivity are primarily driven by interleukin-7 (IL-7), a stromal cell derived product that is a primary regulator of T cell homeostasis.
IL-7 is produced by non-lymphocytes including stroma within lymphoid tissues, and parenchymal cells in the skin, gut, kidney, etc. Nearly all T cells express the IL7 receptor and continually utilize this cytokine for survival68. When T cells are depleted, less IL-7 is utilized and IL-7 levels increase through accumulatation69. Normally, young children maintain serum IL-7 levels of 10–20 pg/ml, whereas healthy adults maintain IL-7 levels of 2–8 pg/ml. However, during lymphopenia, IL-7 levels increase to as high as 60 pg/ml. Rises in serum IL-7 levels in clinical settings associated with lymphopenia have been described following bone marrow transplantation, in human immunodeficiency virus (HIV) infection, following chemotherapy for cancer and in idiopathic CD4 lymphopenia. The increased availability of IL-7 drives the dramatic T cells cycling that occurs during lymphopenia (termed homeostatic peripheral expansion or HPE). Furthermore, treatment of non-lymphopenic mice, monkeys and humans70 with recombinant human IL-7 (rhIL-7) induces increases in T cell cycling (and, subsequently, T cell number) that closely resemble that seen during lymphopenia.
HPE efficiently increases T cell numbers, but does not generate new T cell specificities from HSCs, and therefore the T cell receptor repertoire of populations generated via this pathway remains limited, especially when depletion is severe. Furthermore, patients reliant on HPE for T cell regeneration have chronically diminished CD4+ counts, diminished CD4/CD8 ratios, and diminished numbers (but higher proportions) of suppressive CD4+ T cells. Therefore, the changes in immune physiology induced by T cell depletion enhance T cell reactivity but also results in chronic immune deficiencies. From an immunotherapist’s perspective, these changes are potentially exploitable, especially in the context of adoptive immunotherapy, which requires efficient expansion of adoptively transferred T cells. Indeed, recent non-randomized studies have suggested that induced lymphocyte depletion may actually enhance the efficacy of adoptive immunotherapy for cancer. Dudley et al, administered autologous tumor infiltrating lymphocytes harvested from patients with melanoma, expanded ex vivo and reinfused with rhIL-2 to patients with or without regimens to induce lymphopenia. In sequential non-randomized trials, they observed progressive increases in tumor response rates associated with increasing degrees of lymphocyte depletion. Similar results were seen in animal studies and in clinical trials wherein monoclonal antibodies targeting CD45 to induce lymphopenia appeared to augment the effectiveness of adoptive immunotherapy for nasopharyngeal carcinoma52. Thus, children who experience lymphocyte depletion due to congenital or acquired immunodeficiency, HSCT, or as a result of dose intensive chemotherapy for cancer, may be good candidates for T cell based therapies because the lymphopenia associated with their underlying disease can serve to increase the effectiveness of adoptive cell therapy.
Importantly, however, there are significant short and long-term toxicities associated with lymphopenia. Moreover, when the immunotherapy administered incorporates vaccines, which rely of endogenous T cells present within the host to mediate immune responses, chronic lymphopenia and limited repertoire diversity induced by T cell depletion may actually diminish the effectiveness of immune based therapies. This impact of reduced T cells number and restircted repertoire has been demonstrated in animal studies wherein lymphopenia diminishes the ability to control micrometastatic disease in cancer. Thus, future work seeks to replicate the beneficial aspects of lymphopenia in supporting T cell based immunoptherapy while avoiding the detrimental effects. This approach has been effective in animal studies, where targeted therapies that specifically deplete suppressive T cells and utilize rhIL7 to replicate the lymphopenic milieu in lymphoreplete hosts resulted in better outcomes following adoptive immunotherapy than when the same therapy was administered to lymphopenic hosts71.
Putting it all together, there is great interest in incorporating immune based therapies into existing standard therapies for childhood cancer. Since it is not uncommon for children with high-risk tumors to be rendered free of visible disease using standard multimodality therapy and since such patient populations are also profoundly lymphopenic upon completion of dose intensive therapy, this provides a certain “window of opportunity” for treating minimal residual disease in patients with high risk cancers. Indeed, “consolidative immunotherapy”, which combines tumor vaccines with therapies to enhance immune reconstitution has been piloted in patients with high-risk pediatric sarcomas. 72 Briefly, patients with metastatic and recurrent Ewing sarcoma and alveolar rhabdomyosarcoma undergo apheresis for collection of T cells prior to initiation of therapy. Following treatment with standard dose intensive chemotherapy and local therapy to attempt to induce a state of minimal residual disease, they receive infusion of autologous T cells as a source for homeostatic peripheral expansion and sequential tumor vaccines using dendritic cells. This approach demonstrated favorable survival using an intent-to-treat analysis, however conclusions regarding efficacy are hampered by issues of selection bias, and the lack of a randomized control arm. Despite these caveats, the study clearly demonstrated that all immunized patients, regardless of profound lymphopenia present at the time of vaccination, demonstrated the capacity to generate T cell responses to vaccination within 3 months following chemotherapy, indicating that vaccine induced T cell responses can be induced early after cytotoxic chemotherapy when combined with autologous T cell infusions. A subsequent study targeting patients with metastatic and recurrent pediatric sarcomas is underway with a modified DC vaccine, which incorporates approaches to deplete regulatory or suppressive CD4+ T cells and also incorporates rhIL-7 to enhance immune reconstitution.
In summary, an increased understanding of the biology of T cell mediated antiviral responses and tumor/ immune interactions have opened up real opportunities to harness T cells for clinical benefit in children with immunodeficiency associated infections and in children with cancer. Conceptually, the critical elements have been defined and clear proof-of-principle has been demonstrated. However, substantial work is needed to optimize these therapies, to broaden their applications beyond infection and enhance the effectiveness of tumor directed therapies and to simplify their administration so that they can be tested in large, controlled randomized studies. It is clear that if T cells are to be effective therapy for malignancies, CTLs must proliferate in vivo following infusion, whilst retaining their anti-tumor activity. Optimal proliferation depends on infusing T cells to an environment that promotes homeostatic expansion. The lymphopenia associated with post HSCT environment is similar to that in which autologous immunotherapy has been utilized. In addition with the emerging methodologies available to detect relapse following HSCT, there will be increasing numbers of patients who may benefit from these immunotherapeutic approaches instead of or as an adjunct to the non-specific graft versus tumor effect discussed elsewhere in this edition. Furthermore, infectious complications of HSCT are more frequent following T cell depleted allografts (also discussed elsewhere in this edition) for which infectious pathogen-specific adoptive therapies will play an important role. With increased knowledge of the optimum methodology for generation of T-cell products, and optimization of approaches to enhance the function of adoptively transferred, adoptive immunotherapy strategies may find increasing use to reduce the risk of relapse and prevent and treat infections post transplant.
Acknowledgments
N.A. is supported by grants from the Dana Foundation, the V-Foundation for Cancer Research and the American Brain Tumor Association. H.E.H. is the recipient of a Doris Duke Distinguished Clinical Scientist Award and the NIH/NCI P01 CA94237 and SPORE-in Lymphoma. This work was supported, in part, by the Intramural Research Program of the National Cancer Institute (C.L.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O'Reilly RJ, Doubrovina E, Trivedi D, Hasan A, Kollen W, Koehne G. Adoptive transfer of antigen-specific T-cells of donor type for immunotherapy of viral infections following allogeneic hematopoietic cell transplants. Immunol Res. 2007;38(1–3):237–250. doi: 10.1007/s12026-007-0059-2. [DOI] [PubMed] [Google Scholar]
- 2.Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev. 2002 Oct;188:22–32. doi: 10.1034/j.1600-065x.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- 3.Scarcella DL, Chow CW, Gonzales MF, Economou C, Brasseur F, Ashley DM. Expression of MAGE and GAGE in high-grade brain tumors: a potential target for specific immunotherapy and diagnostic markers. 1999 Feb;5(2):335–341. [PubMed] [Google Scholar]
- 4.Sahin U, Koslowski M, Tureci O, et al. Expression of cancer testis genes in human brain tumors. Clin Cancer Res. 2000 Oct;6(10):3916–3922. [PubMed] [Google Scholar]
- 5.Cheung IY, Cheung NK. Molecular detection of GAGE expression in peripheral blood and bone marrow: utility as a tumor marker for neuroblastoma. Clin Cancer Res. 1997 May;3(5):821–826. [PubMed] [Google Scholar]
- 6.Greiner J, Ringhoffer M, Taniguchi M, et al. mRNA expression of leukemia-associated antigens in patients with acute myeloid leukemia for the development of specific immunotherapies. Int J Cancer. 2004 Feb 20;108(5):704–711. doi: 10.1002/ijc.11623. [DOI] [PubMed] [Google Scholar]
- 7.Liu XF, Helman LJ, Yeung C, Bera TK, Lee B, Pastan I. XAGE-1, a new gene that is frequently expressed in Ewing's sarcoma. Cancer Res. 2000 Sep 1;60(17):4752–4755. [PubMed] [Google Scholar]
- 8.Rodolfo M, Luksch R, Stockert E, et al. Antigen-specific immunity in neuroblastoma patients: antibody and T-cell recognition of NY-ESO-1 tumor antigen. Cancer Res. 2003 Oct 15;63(20):6948–6955. [PubMed] [Google Scholar]
- 9.Soling A, Schurr P, Berthold F. Expression and clinical relevance of NY-ESO-1, MAGE-1 and MAGE-3 in neuroblastoma. Anticancer Res. 1999 May–Jun;19(3B):2205–2209. [PubMed] [Google Scholar]
- 10.Li CM, Guo M, Borczuk A, et al. Gene expression in Wilms' tumor mimics the earliest committed stage in the metanephric mesenchymal-epithelial transition. Am J Pathol. 2002 Jun;160(6):2181–2190. doi: 10.1016/S0002-9440(10)61166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oberthuer A, Hero B, Spitz R, Berthold F, Fischer M. The tumor-associated antigen PRAME is universally expressed in high-stage neuroblastoma and associated with poor outcome. Clin Cancer Res. 2004 Jul 1;10(13):4307–4313. doi: 10.1158/1078-0432.CCR-03-0813. [DOI] [PubMed] [Google Scholar]
- 12.Steinbach D, Hermann J, Viehmann S, Zintl F, Gruhn B. Clinical implications of PRAME gene expression in childhood acute myeloid leukemia. Cancer Genet Cytogenet. 2002 Mar;133(2):118–123. doi: 10.1016/s0165-4608(01)00570-2. [DOI] [PubMed] [Google Scholar]
- 13.Sarkar AK, Nuchtern JG. Lysis of MYCN-amplified neuroblastoma cells by MYCN peptide-specific cytotoxic T lymphocytes. Cancer Res. 2000;60(7):1908–1913. [PubMed] [Google Scholar]
- 14.Sarkar AK, Burlingame SM, Zang YQ, et al. Major histocompatibility complex-restricted lysis of neuroblastoma cells by autologous cytotoxic T lymphocytes. J Immunother. 2001 Jul–Aug;24(4):305–311. doi: 10.1097/00002371-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Molldrem JJ, Lee PP, Wang C, et al. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat Med. 2000 Sep;6(9):1018–1023. doi: 10.1038/79526. [DOI] [PubMed] [Google Scholar]
- 16.Molldrem J. Immune therapy of AML. Cytotherapy. 2002;4(5):437–438. doi: 10.1080/146532402320776099. [DOI] [PubMed] [Google Scholar]
- 17.Sugiyama H. Cancer immunotherapy targeting WT1 protein. Int J Hematol. 2002 Aug;76(2):127–132. doi: 10.1007/BF02982574. [DOI] [PubMed] [Google Scholar]
- 18.Oka Y, Tsuboi A, Elisseeva OA, Udaka K, Sugiyama H. WT1 as a novel target antigen for cancer immunotherapy. Curr Cancer Drug Targets. 2002 Mar;2(1):45–54. doi: 10.2174/1568009023334088. [DOI] [PubMed] [Google Scholar]
- 19.Andersen MH, thor SP. Survivin--a universal tumor antigen. Histol Histopathol. 2002 Apr;17(2):669–675. doi: 10.14670/HH-17.669. [DOI] [PubMed] [Google Scholar]
- 20.Gordan JD, Vonderheide RH. Universal tumor antigens as targets for immunotherapy. Cytotherapy. 2002;4(4):317–327. doi: 10.1080/146532402760271091. [DOI] [PubMed] [Google Scholar]
- 21.Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003 Jan;3(1):46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- 22.Vonderheide RH, Hahn WC, Schultze JL, Nadler LM. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999 Jun;10(6):673–679. doi: 10.1016/s1074-7613(00)80066-7. [DOI] [PubMed] [Google Scholar]
- 23.Vonderheide RH, Domchek SM, Schultze JL, et al. Vaccination of cancer patients against telomerase induces functional antitumor CD8+ T lymphocytes. Clin Cancer Res. 2004 Feb 1;10(3):828–839. doi: 10.1158/1078-0432.ccr-0620-3. [DOI] [PubMed] [Google Scholar]
- 24.Worley BS, van den Broeke LT, Goletz TJ, et al. Antigenicity of fusion proteins from sarcoma-associated chromosomal translocations. Cancer Res. 2001 Sep 15;61(18):6868–6875. [PubMed] [Google Scholar]
- 25.Yotnda P, Firat H, Garcia-Pons F, et al. Cytotoxic T cell response against the chimeric p210 BCR-ABL protein in patients with chronic myelogenous leukemia. J Clin Invest. 1998 May 15;101(10):2290–2296. doi: 10.1172/JCI488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yotnda P, Garcia F, Peuchmaur M, et al. Cytotoxic T cell response against the chimeric ETV6-AML1 protein in childhood acute lymphoblastic leukemia. J Clin Invest. 1998 Jul 15;102(2):455–462. doi: 10.1172/JCI3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van den Broeke LT, Pendleton CD, Mackall C, Helman LJ, Berzofsky JA. Identification and epitope enhancement of a PAX-FKHR fusion protein breakpoint epitope in alveolar rhabdomyosarcoma cells created by a tumorigenic chromosomal translocation inducing CTL capable of lysing human tumors. Cancer Res. 2006 Feb 1;66(3):1818–1823. doi: 10.1158/0008-5472.CAN-05-2549. [DOI] [PubMed] [Google Scholar]
- 28.Yanuck M, Carbone DP, Pendleton CD, et al. A mutant p53 tumor suppressor protein is a target for peptide-induced CD8+ cytotoxic T cells. Cancer Res. 1993;53:3257–3261. [PubMed] [Google Scholar]
- 29.Maher VE, Worley BS, Contois D, et al. Peptide Based Cancer Vaccines. Georgetown, TX and Austin, TX: Landes Biosciences; 2000. Mutant oncogene and tumor suppressor gene products and fusion proteins created by chromosomal translocations as targets for cancer vaccines; pp. 17–39. [Google Scholar]
- 30.Radvanyi L. Discovery and immunologic validation of new antigens for therapeutic cancer vaccines. Int Arch Allergy Immunol. 2004 Feb;133(2):179–197. doi: 10.1159/000076625. [DOI] [PubMed] [Google Scholar]
- 31.Roskrow MA, Suzuki N, Gan Y, et al. Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes for the treatment of patients with EBV-positive relapsed Hodgkin's disease. Blood. 1998 Apr 15;91(8):2925–2934. [PubMed] [Google Scholar]
- 32.Bollard CM, Straathof KC, Huls MH, et al. The generation and characterization of LMP2-specific CTLs for use as adoptive transfer from patients with relapsed EBV-positive Hodgkin disease. J Immunother. 2004 Jul–Aug;27(4):317–327. doi: 10.1097/00002371-200407000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004 Sep;10(9):909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dazzi F, Szydlo RM, Goldman JM. Donor lymphocyte infusions for relapse of chronic myeloid leukemia after allogeneic stem cell transplant: where we now stand. Exp Hematol. 1999 Oct;27(10):1477–1486. doi: 10.1016/s0301-472x(99)00096-x. [DOI] [PubMed] [Google Scholar]
- 35.Finke LH, Wentworth K, Blumenstein B, Rudolph NS, Levitsky H, Hoos A. Lessons from randomized phase III studies with active cancer immunotherapies--outcomes from the 2006 meeting of the Cancer Vaccine Consortium (CVC) Vaccine. 2007 Sep 27;25 Suppl 2:B97–B109. doi: 10.1016/j.vaccine.2007.06.067. [DOI] [PubMed] [Google Scholar]
- 36.Kennedy-Nasser AA, Brenner MK. T-cell therapy after hematopoietic stem cell transplantation. Curr Opin Hematol. 2007 Nov;14(6):616–624. doi: 10.1097/MOH.0b013e3282ef615a. [DOI] [PubMed] [Google Scholar]
- 37.Feuchtinger T, Matthes-Martin S, Richard C, et al. Safe adoptive transfer of virus-specific T-cell immunity for the treatment of systemic adenovirus infection after allogeneic stem cell transplantation. Br J Haematol. 2006 Jul;134(1):64–76. doi: 10.1111/j.1365-2141.2006.06108.x. [DOI] [PubMed] [Google Scholar]
- 38.Gerdemann U, Christin AS, Vera JF, et al. Nucleofection of DCs to generate Multivirus-specific T cells for prevention or treatment of viral infections in the immunocompromised host. Mol Ther. 2009 Sep;17(9):1616–1625. doi: 10.1038/mt.2009.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heslop HE. How I treat EBV lymphoproliferation. Blood. 2009 Sep 1; doi: 10.1182/blood-2009-07-143545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leen AM, Christin A, Myers GD, et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplant. Blood. 2009 Aug 21; doi: 10.1182/blood-2009-07-232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leen AM, Myers GD, Sili U, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006 Oct;12(10):1160–1166. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 42.Perruccio K, Tosti A, Burchielli E, et al. Transferring functional immune responses to pathogens after haploidentical hematopoietic transplantation. Blood. 2005 Dec 15;106(13):4397–4406. doi: 10.1182/blood-2005-05-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995 Oct 19;333(16):1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 44.Heslop HESK, Pule MA, et al. Long term outcome of EBV specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. In press. 2009 doi: 10.1182/blood-2009-08-239186. Blood. 2009. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Einsele H, Roosnek E, Rufer N, et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood. 2002 Jun 1;99(11):3916–3922. doi: 10.1182/blood.v99.11.3916. [DOI] [PubMed] [Google Scholar]
- 46.Peggs KS, Verfuerth S, Pizzey A, et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003 Oct 25;362(9393):1375–1377. doi: 10.1016/S0140-6736(03)14634-X. [DOI] [PubMed] [Google Scholar]
- 47.Cobbold M, Khan N, Pourgheysari B, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med. 2005 Aug 1;202(3):379–386. doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haque T, Wilkie GM, Jones MM, et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood. 2007 Aug 15;110(4):1123–1131. doi: 10.1182/blood-2006-12-063008. [DOI] [PubMed] [Google Scholar]
- 49.Jedema I, Meij P, Steeneveld E, et al. Early detection and rapid isolation of leukemia-reactive donor T cells for adoptive transfer using the IFN-gamma secretion assay. Clin Cancer Res. 2007 Jan 15;13(2 Pt 1):636–643. doi: 10.1158/1078-0432.CCR-06-2093. [DOI] [PubMed] [Google Scholar]
- 50.Rooney CM, Smith CA, Ng CY, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998 Sep 1;92(5):1549–1555. [PubMed] [Google Scholar]
- 51.Bollard CM, Aguilar L, Straathof KC, et al. Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin's disease. J Exp Med. 2004 Dec 20;200(12):1623–1633. doi: 10.1084/jem.20040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Louis CU, Straathof K, Bollard CM, et al. Enhancing the in vivo expansion of adoptively transferred EBV-specific CTL with lymphodepleting CD45 monoclonal antibodies in NPC patients. Blood. 2009 Mar 12;113(11):2442–2450. doi: 10.1182/blood-2008-05-157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bollard CM, Gottschalk S, Leen AM. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007 Oct 15;110(8):2838–2845. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Falkenburg JH, Wafelman AR, Joosten P, et al. Complete remission of accelerated phase chronic myeloid leukemia by treatment with leukemia-reactive cytotoxic T lymphocytes. Blood. 1999 Aug 15;94(4):1201–1208. [PubMed] [Google Scholar]
- 55.Porter DL, Levine BL, Bunin N, et al. A phase 1 trial of donor lymphocyte infusions expanded and activated ex vivo via CD3/CD28 costimulation. Blood. 2006 Feb 15;107(4):1325–1331. doi: 10.1182/blood-2005-08-3373. [DOI] [PubMed] [Google Scholar]
- 56.Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003 Jan;3(1):35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 57.Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahmed N, Ratnayake M, Savoldo B, et al. Regression of experimental medulloblastoma following transfer of HER2-specific T cells. Cancer Res. 2007 Jun 15;67(12):5957–5964. doi: 10.1158/0008-5472.CAN-06-4309. [DOI] [PubMed] [Google Scholar]
- 59.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008 Nov;14(11):1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Till BG, Jensen MC, Wang J, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008 Sep 15;112(6):2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klebanoff CA, Gattinoni L, Torabi-Parizi P, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005 Jul 5;102(27):9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haynes BF, Sempowski GD, Wells AF, Hale LP. The human thymus during aging. Immunol Res. 2000;22(2–3):253–261. doi: 10.1385/IR:22:2-3:253. [DOI] [PubMed] [Google Scholar]
- 63.Mackall CL, Fleisher TA, Brown MR, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995 Jan 19;332(3):143–149. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 64.Hakim FT, Memon SA, Cepeda R, et al. Age-dependent incidence, time course, and consequences of thymic renewal in adults. J Clin Invest. 2005 Apr;115(4):930–939. doi: 10.1172/JCI22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weinberg K, Blazar BR, Wagner JE, et al. Factors affecting thymic function after allogeneic hematopoietic stem cell transplantation. Blood. 2001 Mar 1;97(5):1458–1466. doi: 10.1182/blood.v97.5.1458. [DOI] [PubMed] [Google Scholar]
- 66.Mackall CL, Bare CV, Granger LA, Sharrow SO, Titus JA, Gress RE. Thymic-independent T cell regeneration occurs via antigen-driven expansion of peripheral T cells resulting in a repertoire that is limited in diversity and prone to skewing. J Immunol. 1996 Jun 15;156(12):4609–4616. [PubMed] [Google Scholar]
- 67.Surh CD, Sprent J. Regulation of mature T cell homeostasis. Semin Immunol. 2005 Jun;17(3):183–191. doi: 10.1016/j.smim.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 68.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005 Jun 1;174(11):6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 69.Guimond M, Veenstra RG, Grindler DJ, et al. Interleukin 7 signaling in dendritic cells regulates the homeostatic proliferation and niche size of CD4+ T cells. Nat Immunol. 2009 Feb;10(2):149–157. doi: 10.1038/ni.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sportes C, Hakim FT, Memon SA, et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008 Jul 7;205(7):1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cui Y, Zhang H, Meadors J, Poon R, Guimond M, Mackall CL. Harnessing the physiology of lymphopenia to support adoptive immunotherapy in lymphoreplete hosts. Blood. 2009 Aug 24; doi: 10.1182/blood-2009-03-212134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mackall CL, Rhee EH, Read EJ, et al. A pilot study of consolidative immunotherapy in patients with high-risk pediatric sarcomas. Clin Cancer Res. 2008 Aug 1;14(15):4850–4858. doi: 10.1158/1078-0432.CCR-07-4065. [DOI] [PMC free article] [PubMed] [Google Scholar]