Abstract
Introduction
This study examined the effect of vapor lock on canal debridement efficacy by testing the null hypothesis that there is no difference between a “Closed” and an “Open” system design in smear layer and debris removal using a side-vented needle for irrigant delivery.
Methods
Roots in the “Closed System” were sealed with hot glue and embedded in polyvinylsiloxane to restrict fluid flow through the apical foramen during cleaning and shaping. For the “Open System”, the apical foramen was enlarged and connected to the external environment via a channel within the polyvinylsiloxane to permit unrestricted fluid flow. Smear and debris scores were evaluated using SEM and analyzed using Cochran-Mantel-Haenszel statistic.
Results
No difference in smear scores was detected between the two systems at all canal levels. Significant differences in debris scores between the two systems were found at each canal level: coronal (p<0.001), middle (p<0 .001) and apical (p<0.001).
Conclusion
The null hypothesis was rejected; presence of an apical vapor lock effect adversely affects debridement efficacy. Thus, studies with unspecified or questionable mechanisms to restrict fluid flow through the apical foramen have to be interpreted with caution.
Keywords: Root canal, Irrigation, Vapor lock, Side-venting Syringe Delivery, Smear layer, Debris
INTRODUCTION
Thorough debridement is crucial for long-term success in root canal treatment [1–4]. The mechanical debridement efficacy of an irrigation delivery/agitation system is dependent upon its ability to deliver the irrigant to the apical and non-instrumented regions of the canal space, and to create a strong enough current to carry the debris away from the canal walls [5–9]. As the root is enclosed by the bone socket during in vivo cleaning and shaping [10–12], the canal behaves as a closed-end channel which results in gas entrainment at its closed end [13–15], producing a vapor lock effect during irrigant delivery [16,17]. Studies that were designed to simulate such a “Closed System” by embedding the root in a polyvinylsiloxane impression material (PVS) to restrict fluid flow through the apical foramen demonstrated incomplete debridement from the apical part of the canal walls with the use of a syringe delivery technique [18–20].
A “Closed System”, if not optimally designed or meticulously executed, behaves as an “Open System” that challenges the creditability of the results. For example, a hypothetical “Closed System” that consists of stabilizing the longitudinal bottom-half of a completely-demineralized root in soft silicone and covering the top-half with methyl salicylate to prevent the cleared root from opacifying, functions as an “Open System” even when the apex remains covered by silicone. This permits flow of a dye-containing irrigant through the lateral canals and apical foramen when it is delivered under positive pressure. Likewise, a hypothetical scenario that consists of post-extraction flushing of an irrigant through an unsealed apical foramen to remove blood that enters the canal space during tooth extraction bleaches the original in vivo vapor lock and revokes the goal of examining debridement efficacy in a “Closed System”.
As the debridement quality between a “Closed” versus an “Open” system design has not been evaluated simultaneously in a single study, it is dubious whether conclusions derived from studies with unspecified or ambiguous mechanism to restrict fluid flow through the apical foramen are as clinically-relevant as those which adopted a robust “Closed System” design. This study attempted to resolve this issue by testing the null hypothesis that there is no difference between a “Closed” and an “Open” system design in smear layer and debris removal using a side-vented needle for irrigant delivery.
MATERIALS AND METHODS
Twenty-eight extracted human single-rooted teeth were radiographed to ensure that each tooth contained one canal, and that an equal number of narrow (33%) and wide canals (67%) were present in the two experimental groups. Each tooth was decoronated at 17 mm from the anatomical apex. Canal patency was achieved with a size 10 K-file. Working length was established at 1 mm short of the apical foramen.
Experimental Design
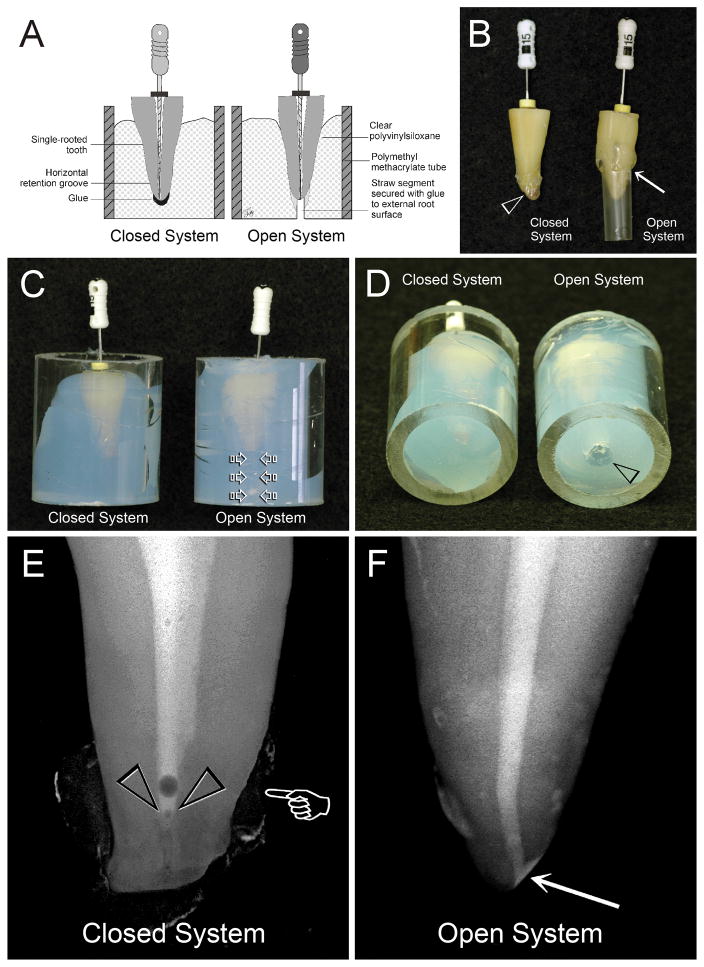
Experimental setups are depicted in Figs.1A–1D. For the “Closed System”, the cementum of each root was coated with tray adhesive. The root apex was covered with hot, flexible glue that was allowed to solidify before the root was inserted into a clear PVS-filled Plexiglas tube. This setup permitted recapitulation of canal patency but prevented fluid extrusion from the apical foramen during canal preparation. For the “Open System”, the apical foramen was enlarged by establishing apical patency to a size 30 file [21]. A straw segment was attached with glue to the external root surface to permit unrestricted communication between the apical foramen with the external environment.
Fig. 1.
A. A schematic depicting the setups for the “Closed System” and “Open System” groups. B. The apical foramen was covered with hot flexible glue for the “Closed System” group, while a straw segment was secured with glue to the external root surface (arrow) for the “Open System” control group. C. Roots shown in Figure 1B were stabilized with clear PVS in Plexiglas tubes. For the control group, a piece of cotton was placed inside the straw (open arrows) prior to the insertion of the assembly into PVS. D. The straw opening in the control group was cleared of PVS to expose the fluid escape channel (open arrowhead). E. A micro-CT snapshot of a shaped canal from the “Closed System” group following delivery of cesium chloride. Radiopaque carbon paint was applied over the solidified glue surface to enhance the contrast (pointer). A vapor lock with an air bubble on top was produced along the apical end of the canal space (open arrowheads). F. A micro-CT snapshot of a shaped canal from the “Open System” group after the canal was filled with cesium chloride. The solution was able to reach the apical 0–2 mm of the canal space when the apical foramen remained open (arrow).
Each root was instrumented to size 50/0.04 taper with a crown down approach. The canal was irrigated with 1.3% NaOCl as the initial irrigant, delivered with a 30-G Max-i-Probe needle (Dentsply-Rinn, Elgin, IL) placed to 1 mm short of working length. Each canal was filled with irrigant during instrumentation. One milliliter of 1.3% NaOCl was used to irrigate the canal between each instrument. For the “Open System”, free flow of irrigant through the straw was confirmed before using larger rotary instruments to working length.
BioPure MTAD (Dentsply-Tulsa, Tulsa, OK) was selected as the final active irrigant based on its ability to remove smear layers consistently from all regions of the canal walls without causing dentin erosion [21]. One milliliter of Biopure MTAD was delivered with the Max-i-Probe needle and left in the canal for 5 min. This was followed by irrigation of the canal with 4 mL of BioPure MTAD. Irrigants were delivered at the rate of 5 mL/min. Each canal was subsequently irrigated with 5 mL of deionized water and dried with paper points. A temporary dressing was placed over the canal orifice before the root was retrieved from the PVS.
Gas Entrapment
Two teeth from each group were prepared up to the stage shown in Fig. 1B (before insertion into the PVS). Following cleaning and shaping, a 8M cesium chloride (CsCl) contrasting medium [22] was delivered to the canal via the Max-i-Probe needle placed to 1 mm short of working length. The needle was removed and each tooth was placed inside a Skyscan 1174 micro-CT scanner (Micro Photonics, Allentown, PA). Snapshot of the liquid-filled canals were taken at 50 kV and 800 μA.
Scanning Electron Microscopy (SEM)
Ten roots each from the “Closed System” and “Open System” groups were prepared for SEM. Two longitudinal grooves were prepared in each root without perforating the canal to facilitate splitting of each root into two longitudinal halves. The root-halves were fixed in 2% glutaraldehyde, dehydrated in ascending ethanol and hexamethyldisilazane [23], sputter-coated and examined with a field emission-SEM at 5 KeV. Five representative micrographs were taken at 2,000X magnification from the apical (0–5mm), middle (5–10 mm) and coronal (11–15 mm) portions of each root-half. Only images from instrumented canal walls were taken, yielding 100 images/portion/group.
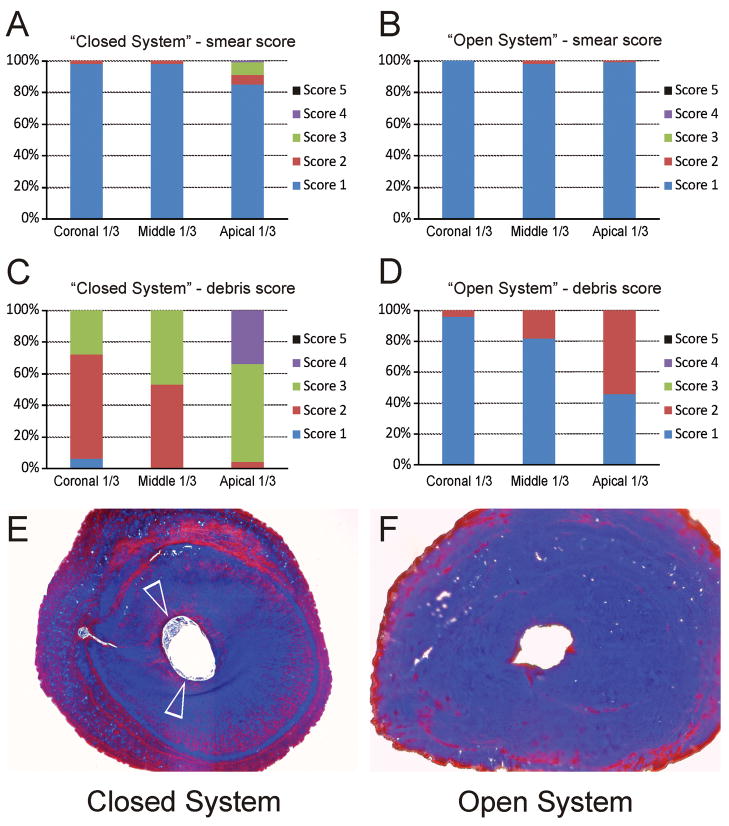
Images were examined in a blind manner by two investigators other than the one who prepared the canals. The efficacy of smear layer removal was evaluated using a 5-level scoring system based on the order of severity of smear layer retention. Canal cleanliness was evaluated using a 5-level debris scoring system based on the order of severity of debris remaining on the instrumented canal wall. Criteria for these scoring systems are listed in the figure legends of Fig. 3A (smear score) and Fig. 3C (debris score). When discrepancies exist during the course of evaluation, a “forced agreement” between the two examiners was used so that both examiners agreed on the smear and debris scores for each image taken from each canal level.
Fig. 3.
These effects could be seen from the summary of the smear score and debris score from different regions of the canal walls (Figs. A–D). A. Descriptive statistics of the distribution of smear scores from the coronal third, middle third and apical third of the canal wall in the “Closed System” group. For the apical third category, scores reflect the overall condition of the apical 0–5 mm part of the canal wall. A 5-level scoring system was used for evaluating the efficacy of smear layer removal: 1 = Smear layer is completely absent. Most tubules are patent and debris-free (coronal third and middle third), or occluded with sclerotic casts (apical third); 2 = Smear layer covering less than 25% of the canal wall and dentinal tubules; 3 = Smear layer evident in 25–50% of the canal surface and tubules; 4 = Smear layer evident in 50–75% of the canal surface and tubules; 5 = Smear layer covering 75–100% of the canal surface and tubules. B. Descriptive statistics of the distribution of smear scores in the “Open System” group. C. Descriptive statistics of the distribution of debris scores from in the “Closed System” group. For the apical third category, scores reflect the overall condition of the apical 0–5 mm part of the canal wall. A 5-level scoring system was used for evaluating the efficacy of debris removal: 1 = clean canal wall, only very few debris particles; 2 = few small conglomerations; 3 = many conglomerations, less than 50% of the canal wall covered; 4 = more than 50% of the canal wall covered with conglomerations; 5 = complete cover of the canal walls with conglomerations. D. Descriptive statistics of the distribution of debris scores in the “Open System” group. E. Masson’s trichrome-stained, light microscopy image of fixed, demineralized roots taken from 0.5–1 mm coronal to the anatomical apex. The periphery of the canal space in the “Closed System” group was filled with stained, demineralized debris (open arrowheads). F. Masson’s trichrome stained light microscopy section taken from a similar region of a root canal in the “Open System” group revealed a clean canal with no stained, demineralized debris.
Smear and debris scores were treated as ordinal data. The median was used to summarize the respective scores of the 10 micrographs taken at each level of each root in order to account for the clustered nature of the data. The Cochran-Mantel-Haenszel (CMH) method was used to test for significant differences among treatment groups (“Closed System” vs. “Open System”) separately at each canal level (coronal, middle, apical) and for all levels combined if there appeared to be no interaction between treatment group and level (α=0.05).
Light Microscopy
As the limited area from the apical 0.5–1 mm of the canal walls precluded sufficient SEM images to be taken for this region to be treated as a separate “level”, light microscopy was employed to qualitatively examine canal cleanliness (debris retention) from this region. The remaining two roots from each group were cleaned and shaped as previously described, fixed in 10% formaldehyde, completely demineralized and embedded in paraffin wax. Serial sections prepared at 0.5–1 mm coronal to the anatomical apex were stained with Masson’s trichrome and examined at 40X magnification.
RESULTS
The CsCl contrasting medium did not reach the root apex when the apical foramen was prevented from fluid and gaseous exchange with the external environment (Fig. 1E). Conversely, no vapor lock existed when the apical foramen remained open to permit fluid flow (Fig. 1F).
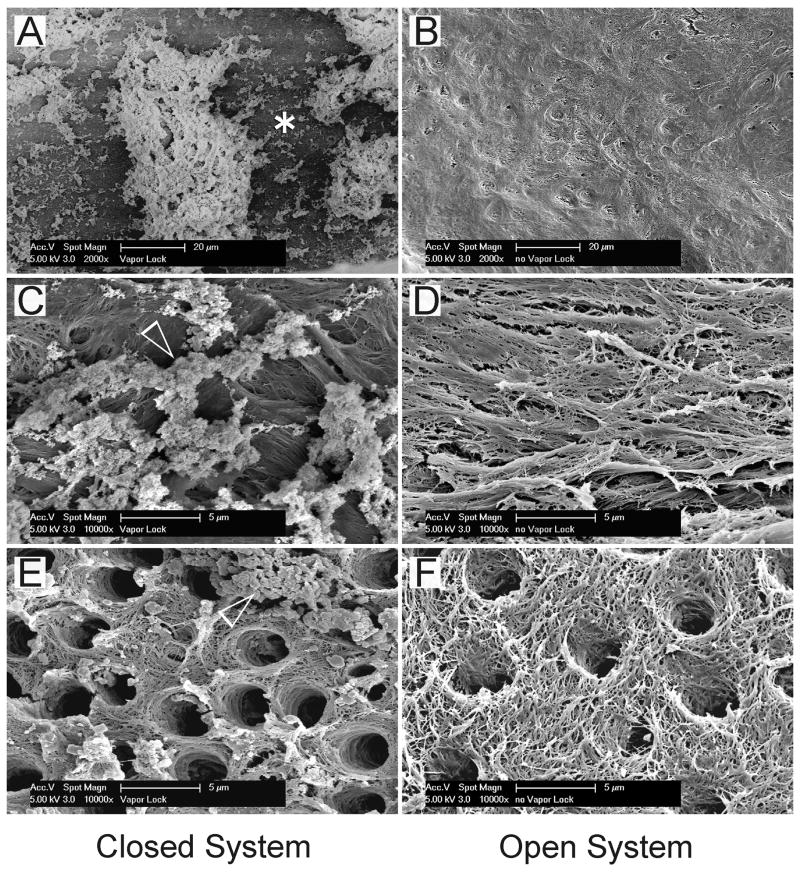
For the “Closed System”, the effect of a vapor lock was most conspicuous along the apical 0.5–1 mm of the canal, with gross retention of debris and smear layer remnants along the demineralized sclerotic dentin surface (Figs. 2A,2C). For the “Open System”, complete smear layer removal and debris clearance were seen (Figs. 2B,2D). Although dentinal tubules were mostly patent along the middle and coronal thirds of the canal walls in both the “Closed System” (Fig. 2E) and “Open System” (Fig. 2F) groups, sparsely-distributed smear layer remnants and isolated debris conglomerates were observed in the “Closed System” specimens (Fig. 2E).
Fig. 2.
Representative scanning electron micrographs taken from different parts of the cleaned and shaped canal walls. Micrographs arranged on the left (A, C and E) and right (B, D and F) sides of the plate were derived from the “Closed System” and “Open System” groups, respectively. A. Along the apical 2 mm zone, the canal wall was sclerotic with minimal tubules (asterisk). For the “Closed System”, this zone was heavily covered with loose debris and some smear layer remnants. B. For the “Open System”, the apical 2 mm zone was sclerotic but devoid of the smear layer and had minimal debris. C. A high magnification view of the region marked by the asterisk in Fig. 2A. Particulate smear layer remnants (open arrowhead) were attached to the surface of the demineralized collagen matrix. D. A high magnification view of Fig. 2B showing a clean, smear layer-free and debris-free fibrous collagen matrix. No sign of dentinal tubules could be seen in this image. E. A high magnification image representative of the middle and coronal thirds of the canal wall in the “Closed System”. The dentinal tubules were mostly patent and devoid of smear plugs. However, smear layer remnants and particulate debris conglomerates (open arrowhead) could be seen adhering to the fibrous collagen matrix. F. A high magnification image in the middle and coronal thirds of the canal wall in the control group. Tufted collagen fibrils could be identified from the surface of the smear layer-depleted, BioPure MTAD-demineralized intertubular dentin. Minimal debris was present.
Smear scores for the “Closed System” and “Open System” are shown in Figs. 3A and 3B, respectively. Examination of the 2×4 contingency tables at each canal level (not shown) indicated no interaction between treatment group and level. The contingency tables were identical at each canal level, indicating no differences in smear scores between the two systems (p=1.000). Debris scores for the “Closed System” and the “Open System” are shown in Figs. 3C and 3D, respectively. There appeared to be interaction between treatment group and canal level, particularly for the pattern of debris scores among the three levels in the “Closed System”. Therefore, separate analyses using the CMH test were performed for each level. Significant differences between the two systems were found at each canal level: coronal (p<0.001), middle (p<0 .001) and apical (p<0.001).
Stained sections from the apical 0.5–1 mm of the roots from the “Closed System” group showed that debris was incompletely cleared from the canal walls (Fig. 3E), in contrast with the clean canal space observed in the “Open System” group (Fig. 3F).
DISCUSSION
The null hypothesis has to be rejected as differences in debris debridement were detected at all canal levels between the two systems. For positive-pressure irrigation using a needle delivery system, irrigant replacement is limited to 1–1.5 mm beyond the needle tip and that a high flow rate is required to generate turbulent fluid flow for effective agitation [6–9]. The apical seat has also to be enlarged to at least size 35–40 for needle placement to within 1–2 mm of the apical seat [10,24–27]. To simplify computer-simulated evaluation of fluid dynamics, hypothetical canals were assumed to be completely filled with irrigants [8,9]. While the use of small-diameter needles and their insertion to within 1 mm of the working length appeared to be logical conclusions from those simulation studies, the contribution of the apical vapor lock to canal debridement had not been appropriately addressed.
In the “Closed System”, irrigant extrusion beyond 1–1.5 mm of a side-venting needle could have generated a liquid film along the air bubble-canal wall interface [28]. This probably accounted for the observation of demineralized sclerotic intertubular dentin at the apical 0.5–1 mm of the canals. Nevertheless, fluid stagnation in this “dead-water zone” failed to provide adequate irrigant replacement, resulting in gross debris retention in this region. Significantly more debris could also be detected from all parts of the canal walls in the “Closed System” group. Irrigation with an acidic or calcium-chelating agent created a demineralized collagen matrix on the surface of radicular dentin upon removal of the smear layer [29]. In the absence of strong turbulent fluid flow, debris particles could be trapped by this porous interlacing fibrillar network as they were displaced by the irrigant toward the canal orifice.
The results of the present study indicate that unless the use of an “Open System” was explicitly stated for the purpose of maximizing the cleaning potential of an irrigant [21], conclusions derived from studies with unspecified or questionable apical fluid movement mechanisms (e.g. reassembling a split tooth embedded in a silicone mold) have to be interpreted with caution. It must be emphasized that the current results are applicable only to side-vented needle delivery, and cannot be extrapolated to other irrigation/agitation systems [30] such as sonic [17], ultrasonic [11,31] or negative-suction devices [32–34] that have the potential to create more forceful currents. The ability of these devices to displace the apical vapor lock has to be validated in future studies that incorporate both “Closed” and “Open” system designs. It appears that dynamic mechanical agitation (the use of a well-fitting gutta-percha cone for manual agitation of an irrigant-filled canal) [27,35] has the potential to displace the apical gas entrapment from a “Closed System”. As the material cone is closely adapted to the canal, it would be of interest to see if this manual agitation technique can effectively displace debris away from the collagen matrix created by acidic/chelating irrigants, in a closed canal system that is totally sealed from apex to the cementoenamel junction.
Acknowledgments
This study was supported by the Dental Research Center, School of Dentistry, Medical College of Georgia. The authors deny any financial affiliations related to this study or its sponsors. The micro-CT scanner employed in one part of the study was acquired via Grant R21 DE019213-01 from the National Institute of Dental and Craniofacial Research (PI. Franklin R. Tay). The authors thank Mr. Thomas Bryan for SEM specimen dehydration and coating and Mrs. Michelle Burnside for secretarial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schilder H. Cleaning and shaping the root canal. Dent Clin North Am. 1974;18:269–96. [PubMed] [Google Scholar]
- 2.Sjögren U, Figdor D, Persson S, Sundqvist G. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J. 1997;30:297–306. doi: 10.1046/j.1365-2591.1997.00092.x. [DOI] [PubMed] [Google Scholar]
- 3.Nair PN. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit Rev Oral Biol Med. 2004;15:348–81. doi: 10.1177/154411130401500604. [DOI] [PubMed] [Google Scholar]
- 4.Haapasalo M, Endal U, Zandi H, Coil JM. Eradication of endodontic infection by instrumentation and irrigation solutions. Endod Topics. 2005;10:77–102. [Google Scholar]
- 5.Moser JB, Heuer MA. Forces and efficacy in endodontic irrigation systems. Oral Surg Oral Med Oral Pathol. 1982;53:425–8. doi: 10.1016/0030-4220(82)90446-7. [DOI] [PubMed] [Google Scholar]
- 6.Chow TW. Mechanical effectiveness of root canal irrigation. J Endod. 1983;9:475–9. doi: 10.1016/S0099-2399(83)80162-9. [DOI] [PubMed] [Google Scholar]
- 7.Sedgley CM, Nagel AC, Hall D, Applegate B. Influence of irrigant needle depth in removing bioluminescent bacteria inoculated into instrumented root canals using real-time imaging in vitro. Int Endod J. 2005;38:97–104. doi: 10.1111/j.1365-2591.2004.00906.x. [DOI] [PubMed] [Google Scholar]
- 8.Boutsioukis C, Lambrianidis T, Kastrinakis E. Irrigant flow within a prepared root canal using various flow rates: a Computational Fluid Dynamics study. Int Endod J. 2009;42:144–55. doi: 10.1111/j.1365-2591.2008.01503.x. [DOI] [PubMed] [Google Scholar]
- 9.Gao Y, Haapasalo M, Shen Y, Wu H, Li B, Ruse ND, Zhou X. Development and validation of a three-dimensional computational fluid dynamics model of root canal irrigation. J Endod. 2009;35:1282–7. doi: 10.1016/j.joen.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 10.Usman N, Baumgartner JC, Marshall JG. Influence of instrument size on root canal debridement. J Endod. 2004;30:110–2. doi: 10.1097/00004770-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Gutarts R, Nusstein J, Reader A, Beck M. In vivo debridement efficacy of ultrasonic irrigation following hand-rotary instrumentation in human mandibular molars. J Endod. 2005;31:166–70. doi: 10.1097/01.don.0000137651.01496.48. [DOI] [PubMed] [Google Scholar]
- 12.Burleson A, Nusstein J, Reader A, Beck M. The in vivo evaluation of hand/rotary/ultrasound instrumentation in necrotic, human mandibular molars. J Endod. 2007;33:782–7. doi: 10.1016/j.joen.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Dovgyallo GI, Migun NP, Prokhorenko PP. The complete filling of dead-end conical capillaries with liquid. J Eng Phy. 1989;56:395–7. [Google Scholar]
- 14.Migun NP, Azuni MA. Filling of one-side-closed capillaries immersed in liquids. J Coll Interf Sci. 1996;181:337–40. [Google Scholar]
- 15.Pesse AV, Warrier GR, Dhir VK. An experimental study of the gas entrapment process in closed-end microchannels. Int J Heat Mass Transfer. 2005;48:5150–65. [Google Scholar]
- 16.Senia ES, Marshall FJ, Rosen S. The solvent action of sodium hypochlorite on pulp tissue of extracted teeth. Oral Surg Oral Med Oral Pathol. 1971;31:96–103. doi: 10.1016/0030-4220(71)90040-5. [DOI] [PubMed] [Google Scholar]
- 17.de Gregorio C, Estevez R, Cisneros R, Heilborn C, Cohenca N. Effect of EDTA, sonic, and ultrasonic activation on the penetration of sodium hypochlorite into simulated lateral canals: an in vitro study. J Endod. 2009;35:891–5. doi: 10.1016/j.joen.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Baumgartner JC, Mader CL. A scanning electron microscopic evaluation of four root canal irrigation regimens. J Endod. 1987;13:147–57. doi: 10.1016/s0099-2399(87)80132-2. [DOI] [PubMed] [Google Scholar]
- 19.O’Connell MS, Morgan LA, Beeler WJ, Baumgartner JC. A comparative study of smear layer removal using different salts of EDTA. J Endod. 2000;26:739–43. doi: 10.1097/00004770-200012000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Albrecht LJ, Baumgartner JC, Marshall JG. Evaluation of apical debris removal using various sizes and tapers of ProFile GT files. J Endod. 2004;30:425–8. doi: 10.1097/00004770-200406000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Torabinejad M, Cho Y, Khademi AA, Bakland LK, Shabahang S. The effect of various concentrations of sodium hypochlorite on the ability of MTAD to remove the smear layer. J Endod. 2003;29:233–9. doi: 10.1097/00004770-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro R. A preliminary report on the use of cesium chloride in contrast radiography. Acta Radiol. 1956;46:635–9. doi: 10.3109/00016925609171456. [DOI] [PubMed] [Google Scholar]
- 23.Chissoe WF, Vezey EL, Skvarla JJ. Hexamethyldisilazane as a drying agent for pollen scanning electron microscopy. Biotech Histochem. 1994;69:192–8. doi: 10.3109/10520299409106286. [DOI] [PubMed] [Google Scholar]
- 24.Ram Z. Effectiveness of root canal irrigation. Oral Surg Oral Med Oral Pathol. 1977;44:306–12. doi: 10.1016/0030-4220(77)90285-7. [DOI] [PubMed] [Google Scholar]
- 25.Zehnder M. Root canal irrigants. J Endod. 2006;32:389–98. doi: 10.1016/j.joen.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh YD, Gau CH, Kung Wu SF, Shen EC, Hsu PW, Fu E. Dynamic recording of irrigating fluid distribution in root canals using thermal image analysis. Int Endod J. 2007;40:11–7. doi: 10.1111/j.1365-2591.2006.01168.x. [DOI] [PubMed] [Google Scholar]
- 27.Huang TY, Gulabivala K, Ng YL. A bio-molecular film ex-vivo model to evaluate the influence of canal dimensions and irrigation variables on the efficacy of irrigation. Int Endod J. 2008;41:60–71. doi: 10.1111/j.1365-2591.2007.01317.x. [DOI] [PubMed] [Google Scholar]
- 28.Liao Q, Zhao TS. Modeling of Taylor bubble rising in a vertical mini noncircular channel filled with a stagnant liquid. Int J Multiphase Flow. 2003;29:411–34. [Google Scholar]
- 29.Tay FR, Gutmann JL, Pashley DH. Microporous, demineralized collagen matrices in intact radicular dentin created by commonly used calcium-depleting endodontic irrigants. J Endod. 2007;33:1086–90. doi: 10.1016/j.joen.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 30.Gu LS, Kim JR, Ling J, Choi KK, Pashley DH, Tay FR. Review of contemporary irrigant agitation techniques and devices. J Endod. 2009;35:791–804. doi: 10.1016/j.joen.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 31.van der Sluis LW, Versluis M, Wu MK, Wesselink PR. Passive ultrasonic irrigation of the root canal: a review of the literature. Int Endod J. 2007;40:415–26. doi: 10.1111/j.1365-2591.2007.01243.x. [DOI] [PubMed] [Google Scholar]
- 32.Fukumoto Y, Kikuchi I, Yoshioka T, Kobayashi C, Suda H. An ex vivo evaluation of a new root canal irrigation technique with intracanal aspiration. Int Endod J. 2006;39:93–9. doi: 10.1111/j.1365-2591.2006.01050.x. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen BA, Baumgartner CJ. Comparison of the EndoVac system to needle irrigation of root canals. J Endod. 2007;33:611–5. doi: 10.1016/j.joen.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 34.Townsend C, Maki J. An in vitro comparison of new irrigation and agitation techniques to ultrasonic agitation in removing bacteria from a simulated root canal. J Endod. 2009;35:1040–3. doi: 10.1016/j.joen.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 35.McGill S, Gulabivala K, Mordan N, Ng YL. The efficacy of dynamic irrigation using a commercially available system (RinsEndo) determined by removal of a collagen ‘bio-molecular film’ from an ex vivo model. Int Endod J. 2008;41:602–8. doi: 10.1111/j.1365-2591.2008.01408.x. [DOI] [PubMed] [Google Scholar]