Abstract
Cloning of MAO (monoamine oxidase) A and B has demonstrated unequivocally that these enzymes are made up of different polypeptides, and our understanding of MAO structure, regulation, and function has been significantly advanced by studies using their cDNA. MAO A and B genes are located on the X-chromosome (Xp11.23) and comprise 15 exons with identical intron-exon organization, which suggests that they are derived from the same ancestral gene. MAO A and B knockout mice exhibit distinct differences in neurotransmitter metabolism and behavior. MAO A knock-out mice have elevated brain levels of serotonin, norephinephrine, and dopamine and manifest aggressive behavior similar to human males with a deletion of MAO A. In contrast, MAO B knock-out mice do not exhibit aggression and only levels of phenylethylamine are increased. Mice lacking MAO B are resistant to the Parkinsongenic neurotoxin, 1-methyl-4-phenyl-1,2,3,6-tetra-hydropyridine. Both MAO A and B knock-out mice show increased reactivity to stress. These knock-out mice are valuable models for investigating the role of monoamines in psychoses and neurodegenerative and stress-related disorders.
Keywords: aggression, characterization, cloning, knock-out mice, neurotransmitter
INTRODUCTION AND BACKGROUND
Monoamine oxidase (MAO) (monoamine; oxygen oxidoreductase, deaminating, EC 1.4.3.4) catalyzes the oxidative deamination of a number of biogenic amines in the brain and peripheral tissues by the production of hydrogen peroxide (H2O2) (Shih 1991, Thorpe et al 1987). On the basis of substrate selectivity and inhibitor sensitivity, two forms of MAO were proposed and designated MAO A and B (Johnston 1968, Knoll & Magyar 1972). MAO A has higher affinity for the substrates serotonin (5-HT), norepinephrine (NE), dopamine (DA), and the inhibitor clorgyline, whereas MAO B has higher affinity for phenylethylamine (PEA), benzylamine, and the inhibitor deprenyl. Both MAO A and B are located throughout the brain in the outer membrane of mitochondria (Green & Youdim 1975) and are encoded by different genes (Bach et al 1988, Grimsby et al 1991). Eight males from a Dutch family with a MAO A deficiency due to a point mutation in the gene encoding MAO A manifest abnormal aggressiveness (Brunner et al 1993a,b), and alterations in MAO B activity have been implicated in Parkinson’s disease (Hotamisligil et al 1994, Sano et al 1997). A new function of MAO that is related to imidazole binding has recently been suggested (Raddatz & Lanier 1997, Raddatz et al 1995). In this review, we discuss the progress that has been made in determining the structure and function of MAO A and B genes, their involvement in behavior and diseases, and future research directions using knock-out (KO) mice.
MAO A and B Nomenclature
Over two decades ago, Johnston defined two subtypes of MAO based on the observation that one form (A) but not the other (B) was sensitive to the irreversible inhibitor, clorgyline (Johnston 1968). Additional studies demonstrated that MAO B is more sensitive than MAO A to pargyline and deprenyl (Knoll & Magyar 1972). Furthermore, MAO A preferentially oxidizes 5-HT and NE whereas MAO B preferentially oxidizes PEA and benzylamine (Fowler et al 1982). Although in humans DA is oxidized by MAO B (Glover et al 1977) and in rodents it is oxidized by MAO A (Johnston 1968, Neff & Yang 1974), in most species DA can be oxidized by both forms of the enzymes (O’Carroll et al 1983). There are, however, numerous exceptions to this rule because the specificity of MAO for its substrate depends on the concentration, affinity, and turnover rate of the substrate and the concentration of enzyme (Tipton et al 1987). For example, 10 μM PEA is oxidized by MAO B, but 1 mM PEA is a common substrate for both forms of MAO in rodent, human, bovine, and guinea pig brain (Suzuki et al 1981). In addition, although 5-HT is considered the preferred substrate for MAO A, in rat (Green & Youdim 1975, Mitra & Guha 1980), bovine (Achee & Gabay 1977), and pig brain (Ekstedt & Oreland 1976), some 5-HT is oxidized by MAO B.
Localization and Development of MAO
MAO has been localized in rodent, cat, primate, and human brain by a variety of techniques, including immunohistochemistry (Levitt et al 1982, Westlund 1994, Westlund et al 1985, Westlund et al 1988, Westlund et al 1993, Willoughby et al 1988), enzyme autoradiography (Saura et al 1996, Saura et al 1992), and in situ hybridization (Jahng et al 1997, Saura et al 1996). The distribution of MAO A and B in the brain shows little species variation. MAO A is predominantly found in catecholaminergic neurons, and MAO B is the form most abundant in serotonergic and histaminergic neurons and glial cells (Jahng et al 1997, Luque et al 1995, Saura et al 1994a, Saura et al 1994b, Westlund et al 1988, Willoughby et al 1988). In the brain, the highest concentration of MAO A is found in the locus coerulus, and the highest concentration of MAO B is found in the raphe nuclei (Jahng et al 1997, Saura et al 1994, Willoughby et al 1988).
The distribution of MAO in the periphery varies within the same organism. Some tissues (human platelets and bovine liver and kidney) mainly contain MAO B (Denny et al 1982, Erwin & Hellerman 1967, Minamiura & Yasunobu 1978) and others (human placenta and bovine thyroid) predominantly contain MAO A (Masini-Repiso et al 1986, Weyler & Salach 1985). Rat skeletal muscle contains negligible levels of both forms (Saura et al 1992).
The presence of MAO in most tissues appears to reflect a functional need. However, in some regions of the brain MAO and its substrate are not found in the same neuron. For example, MAO A prefers to metabolize 5-HT but it is not found in serotonergic neurons, and MAO B prefers to metabolize PEA but it is present in serotonergic neurons and glial cells (Table 1). The role of MAO in these regions may be to protect neurons from stimulation by oxidizing extraneous amines that can act as false neurotransmitters (Saura et al 1996, Westlund et al 1988). This, however, is not consistent with pharmacological data where clorgyline inhibits 5-HT degradation (Blier et al 1986, Twist et al 1990), which suggests that MAO A may oxidize 5-HT outside the serotonergic neuron or the antibody did not detect the 5-HT neuron.
Table 1.
Levels of monoamine oxidase (MAO) A and B in rodent brain measured by immunohistochemistry, enzyme autoradiography, and in situ hybridizationa
| MAO A |
MAO B |
|||||
|---|---|---|---|---|---|---|
| Neuron and region | Neuron stainingb | Proteinc | mRNAd | Neuron stainingb | Proteinc | mRNAd |
| Serotonergic neurons in raphe nucleus | − | − | + | ++ | ++ | ++ |
| Catecholaminergic neurons in locus coerulus | ++ | ++ | ++ | − | − | − |
++, High concentration; +, moderate concentration; −, undetectable.
From Willoughby et al (1988).
From Saura et al (1994).
From Jahng et al (1997).
In humans and rodents, MAO A is present before MAO B in most tissues. MAO A is almost at adult levels at birth whereas MAO B activity increases several-fold with aging (Diez & Maderdrut 1977, Lewinsohn et al 1980, Saura et al 1994, Tsang et al 1986). Because MAO B is predominantly located in glial cells (Levitt et al 1982, Westlund et al 1985), the large increase in MAO B with aging may be attributable to the proliferation of these cells. An increase in MAO B activity with development is consistent with the finding that pups deficient in MAO A show higher levels of 5-HT and 5-HT immunoreactivity compared with adults (Cases et al 1995). It has been suggested that altered 5-HT levels may affect brain development in utero (Brunner 1995). Indeed, there is evidence that increased 5-HT levels in MAO A KO pup brains causes cytoarchitectural alterations in the somatosensory cortex, since administration of parachlorophenylalanine, an inhibitor of 5-HT synthesis, reverses the changes (Cases et al 1996). Thus, developmental changes of MAO may underlie reduced 5-HT metabolism in MAO A KO pups.
MAO GENES
Cloning of Human Liver MAO A and B
The discovery in 1968 of the two forms of MAO (Johnston 1968) raised a question: Are MAO A and B different proteins or the same protein differentially modified posttranslationally by carbohydrates or lipids? In 1988, in collaboration with Peter Seeburg, two full-length cDNAs encoding for human liver MAO A and B were cloned (Bach et al 1988). A comparison of the deduced amino acid sequences showed that the A and B forms have subunit molecular weights of 59,700 and 58,000, respectively, and have 70% amino acid sequence identity. Subsequently, we successfully transfected MAO A and B cDNA in mammalian cells and demonstrated that they exhibit substrate and inhibitor specificity similar, respectively, to endogenous MAO A and B (Lan et al 1989a). This work has demonstrated unequivocally that these two isoenzymes are made up of two different polypeptides and are not the same protein differentially modified.
Chromosomal Location of MAO and B
Preliminary studies with antibodies suggested that the genes for MAO were located on the X-chromosome in a number of mammalian species (Kochersperger et al 1986, Pintar et al 1981). In 1989, human (Lan et al 1989b, Levy et al 1989) and mouse (Derry et al 1989) MAO A and B genes were mapped to adjacent sites at Xp11.23 and were deleted in patients with Norrie disease (Lan et al 1989b, Sims et al 1989). Norrie disease is an X-linked recessive neurologic disorder characterized by blindness, hearing loss, and mental retardation (Brunner et al 1993a,b; Collins et al 1992).
Organization of Human MAO A and B Genes
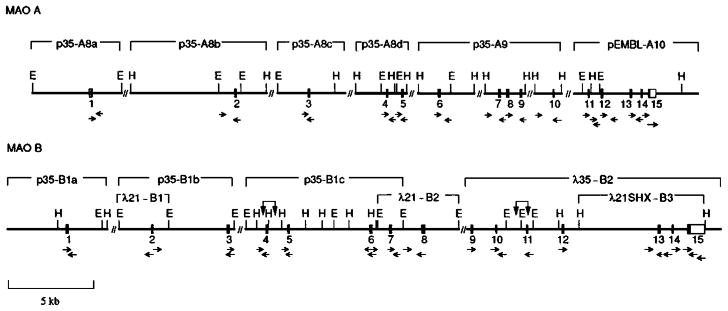
Using MAO A and B cDNA specific fragments, human MAO A and B genes were isolated from X-chromosome–specific libraries spanning at least 60 kb (Grimsby et al 1991). These two genes (Figure 1) were found to consist of 15 exons and had identical exon-intron organization (Grimsby et al 1991), which suggests that MAO A and B are derived from the duplication of a common ancestral gene. Interestingly, the MAO A and B exon 12 products share 93.9% amino acid identity (Grimsby et al 1991). The amino acid sequence of MAO A from humans (Bach et al 1988, Hsu et al 1988), bovines (Powell et al 1989), and rats (Kuwahara et al 1990) are highly conserved (>87% identity). The amino acid sequences of MAO B from humans (Bach et al 1988) and rats (Ito et al 1988) are also highly conserved (88.3% identity). The strong conservation of the amino acid sequence of each isoenzyme among mammalian species may reflect evolutionary pressure to maintain the specific physiological function of each MAO.
Figure 1.
Partial structural map of the monoamine oxidase (MAO) A and B genes showing the location of exons. (Filled bars) Coding regions; (unfilled bars) untranslated regions of the exons. Exon numbers are below the bars. (horizontal arrows) The regions sequenced; (vertical arrows) equivocal assignment of an exon to either restriction fragment. The prefix “λ,” λ bacteriophage clones; the prefix “p,” pUC19 subclones of λ phage DNA; E, EcoRI restriction site; H, HindIII restriction site;//, intron gap. (From Grimsby et al 1991.)
Organization of MAO A and B Promoters
Using a series of 5′ flanking sequences linked to a human growth hormone gene, the DNA sequence responsible for the transcription activation of MAO A and B genes were identified. When these constructs were transfected into NIH3T3, SHSY-5Y, and COS7 cells, the maximal promoter activity for MAO A and B was found in a 0.14-kb Pvull/Drall and 0.15-kb Pstl/Nael fragment, respectively (Zhu et al 1992). Both fragments are GC-rich, contain potential Sp1 binding sites, and share approximately 60% sequence identity. However, the organization of the transcription elements is distinctly different between these two promoters (Zhu et al 1992). The MAO A 0.14-kb fragment lacks a TATA box, consists of three Sp1 elements (Denney et al 1994, Zhu et al 1992), and exhibits bidirectional promoter activity (Zhu et al 1994). The MAO B core promoter 0.15-kb fragment consists of two clusters of overlapping Sp1 sites separated by a CACCC element (Zhu et al 1992). The different promoter organization of MAO A and B genes may underlie their different tissue- and cell-specific expression. An upstream 5′ sequence of the human MAO A gene down-regulates the MAO A promoter in the presence or absence of initiator-like protein (Zhu & Shih 1997). Preliminary data suggests that two novel factors (F and M) and Sp1 may be important for transcriptional regulation of the MAO B gene (Zhu et al 1994). The role of factors F and M and Sp1 in tissue- and cell-specific expression can be addressed after they are cloned.
Structural Requirements of MAO A and B That Are Essential for Their Catalytic Activity
Using site-specific mutagenesis and chimeric enzyme constructs of MAO A and B cDNAs transiently expressed into mammalian cells or transformed into yeast cells, considerable progress has been made in the understanding of the active site and the domains that confer the substrate and inhibitor specificities of MAO. Between mammalian MAO isoenzymes and trout MAO (Chen et al 1994) there are four highly conserved regions. In MAO B, these regions include (a) an ADP binding β-α-β unit (residues 6–43); (b) a putative substrate-binding domain (residues 178–221); (c) a site of flavin adenine dinucleotide (FAD) covalent attachment site (residues 380–458); and (d) a C terminus region (residues 491–511) predicted to form a transmembrane-associated α-helix (Bach et al 1988, Chen et al 1994, Hsu et al 1988).
Each of these regions has been studied to further elucidate the structure and function of MAO. Exchanging the N terminus ADP-binding β-α-β unit between MAO A and B has no effect on substrate or inhibitor selectivity compared with wild-type (Chen et al 1996b, Gottowik et al 1995). Mutagenesis of a highly conserved glutamic acid residue (Glu-34) and tyrosine residue (Tyr-44) located in the ADP binding domain of human MAO B drastically reduced catalytic activity (Kwan et al 1995, Zhou et al 1995). The Glu-34 is also critical for the initial noncovalent binding and delivering of FAD to the covalent attachment site at Cys-397 (Wu et al 1993, Zhou et al 1995). Exchanging a portion of the FAD-binding region to the COOH terminus of MAO A (residues 402–527) with the corresponding region of MAO B produced no change in substrate or inhibitor selectivity compared with wild-type MAO A (Chen et al 1996b). However, the reciprocal chimera of MAO B was inactive, which suggests that this region was critical for MAO B catalytic activity. However, more recent studies with human MAO A (Weyler 1994) and trout MAO (Chen et al 1994) suggest that this region is not bound to the mitochondria by a simple C-terminal membrane anchor (Mitoma & Ito 1992).
A detailed examination of 18 chimeric MAO forms, made by progressively moving the junction of the N terminus of one form with the C terminus of the other form, has demonstrated that two sequences in MAO B (residues 62–103 and 146–220) constitute the binding site of MAO B (Gottowik et al 1995). Replacement of the internal segment 161–375 of human MAO A with the corresponding MAO B segment converts the substrate and inhibitor selectivity of MAO A to that of MAO B (Grimsby et al 1996). A parallel experiment using rat MAOs has demonstrated that the region between residues 120–220 is responsible for the substrate specificities of MAO A and MAO B (Tsugeno et al 1995). Further mutagenesis studies have found that substituting Phe-208 in rat MAO A with the corresponding Ile in MAO B was sufficient to convert the A-type substrate selectivity to that of MAO B and the B-type substrate selectivity to that of MAO A. Phe at this position was replaceable with Tyr for the A-type specificity, and Ile was replaceable with Val and Ala for the B-type specificity. Thus, aromatic and aliphatic residues seem to contribute to substrate selectivity of MAO A and MAO B, respectively (Tsugeno & Ito 1997).
Nine cysteine residues exist in the deduced amino acid sequences of both MAO A and B in human liver. By mutating each of these cysteines to serine it was demonstrated that two cysteines (Cys-374 and –406) in MAO A and three cysteines (Cys-156, –365, and –397) in MAO B are important for catalytic activity (Wu et al 1993). These cysteines may be crucial because when they are mutated to serine in COS cells, catalytic activity was not observed (Wu et al 1993). The loss of catalytic activity in MAO A Ser-406 and MAO B Ser-397 mutants may be due to the prevention of covalent binding of the enzyme to the cofactor FAD through a 8α-methyl-S-cysteinyl bond (Kearney et al 1971). When the FAD-binding cysteine of rat liver MAO A was mutated to Ala, the mutant enzyme still retained catalytic activity. This suggests that the covalent attachment of FAD may not be required for catalytic activity but may function as a structural core for the active conformation in the membrane (Hiro et al 1996).
The structural features of the active site of human MAO B were investigated by affinity labeling and site-directed mutagenesis. The labeled putative active-site tryptic peptides were isolated and found to contain the FAD-modified Cys-397. Substitution of His-382 of MAO B with an Arg greatly reduced the enzymatic activity, which suggests that this residue may represent a nucleophile relevant for MAO B catalytic activity (Cesura et al 1996). Cesura et al (1996) also demonstrated that a MAO B Thr-158 to Ala mutation resulted in a dramatic loss of enzymatic activity. This finding is consistent with the possible role of Cys-156 in MAO B. Interestingly, substitution of Cys-389 by Ala completely abolished the MAO B activity (Cesura et al 1996), and substitution of the same residue to Ser does not appear to change the enzymatic activity (Wu et al 1993). Inactivation of beef liver MAO B with N-cyclopropyl-α-methylbenzylamine, Lys-C digestion, and high-pressure liquid chromatography separation of the peptides isolated a modified octapeptide and identified a modified Cys-365. This is consistent with the results from the site-directed mutagenesis study and further demonstrates that Cys-365 is the substrate binding site (Zhong & Silverman 1997).
Recently, the quaternary structure and subunit composition of bovine liver MAO B was investigated using size-exclusion chromatography, sucrose gradient centrifugation, and electron microscopy. Data suggest that MAO B preferentially functions as hexamers that contain three-fold rotational symmetry. The hexamers may be composed of a trimer of MAO B homodimers (Shiloff et al 1996).
Cloning of MAO cDNA from Trout Liver
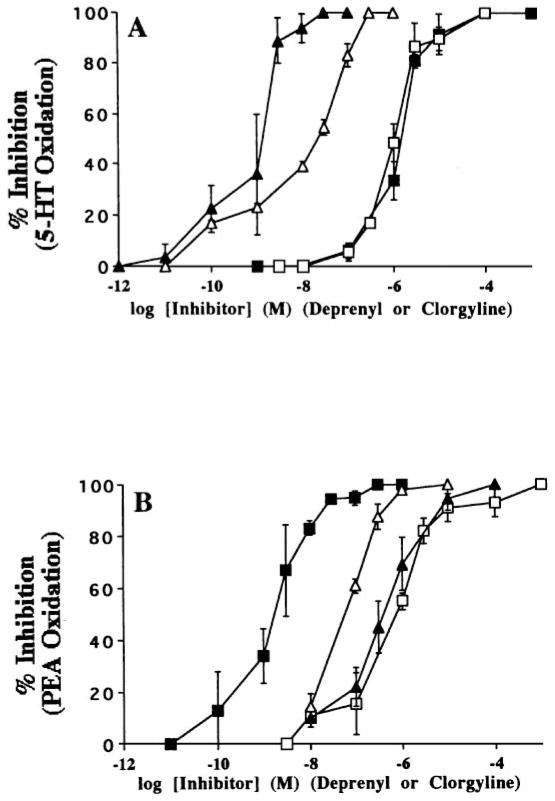
Although the MAO A and B are well defined, evidence suggests that other forms are present in various tissue. To search for other forms of MAO, a human MAO A cDNA probe was used to screen a trout liver cDNA library. A trout liver MAO cDNA encoding 499 amino acids was cloned (Chen et al 1994). MAO A and B consist of 529 and 520 amino acids, respectively. The deduced amino acid sequence of trout MAO shows 70% and 71% identity with human MAO A and B, respectively. Transient expression of the cDNA in COS-7 cells shows that unlike human MAO A and B, which only oxidize 5-HT and PEA, respectively, trout MAO A and B both oxidize 5-HT and PEA. For trout MAO, the Michaelis constant values for 5-HT and PEA were similar to human MAO A and B, respectively. When 5-HT is used as a substrate, both trout MAO and human MAO A are more sensitive to clorgyline than to deprenyl but trout MAO is less sensitive to clorgyline (Figure 2). When PEA is used as a substrate, both trout MAO and human MAO B are more sensitive to deprenyl (Chen et al 1994). These results suggest that trout MAO is a novel form of MAO that displays substrate and inhibitor selectivities that are not identical to either MAO A or B. The structure of trout MAO will provide insights into the substrate and inhibitor selectivities of the MAOs.
Figure 2.
Clorgyline and deprenyl inhibition of (A) trout monoamine oxidase (T-MAO) (open triangles, open squares) and human (H) MAO (closed triangles, closed squares) activity expressed in COS cells using serotonin (5-HT) (100 mM) as the substrate, and of (B) T-MAO (open triangles, open squares) and H-MAO (closed triangles, closed squares) activity expressed in COS cells using phenylethylamine (PEA) (10 mM) as the substrate. (Triangles) Clorgyline; (squares) deprenyl. (From Chen et al 1994.)
Monoamine Metabolism in MAO A- and B-Deficient Mice
Current gene KO techniques have provided a useful tool for understanding the function of enzymes in vivo. In collaboration with Isabelle Seif and Edward De Maeyer at the Centre National de la Recherche Scientifique in France, a line of transgenic mice has been generated in which the gene that encodes MAO A (Cases et al 1995) is disrupted. In addition, mice deficient in MAO B have been generated by homologous recombination (Grimsby et al 1997).
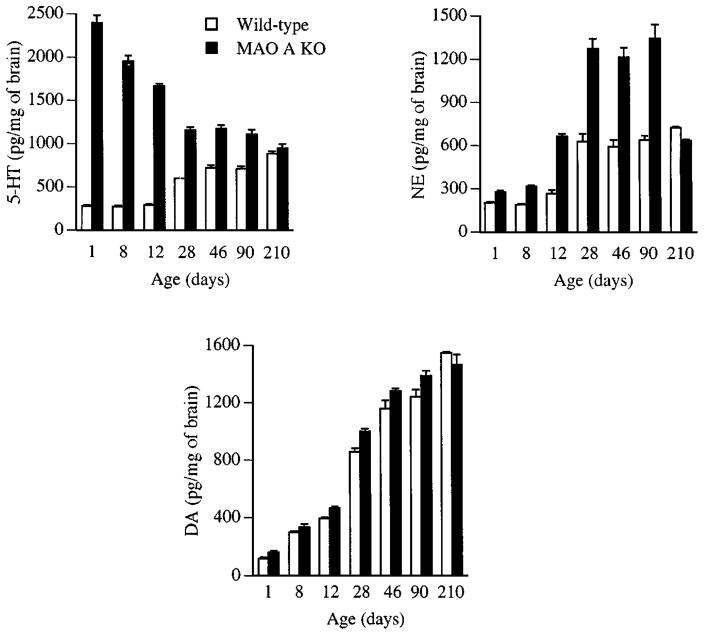
In brains of MAO A KO pups, 5-HT concentrations were increased up to nine-fold compared with wild-type mice. In adult brains, the 5-HT levels were only increased two-fold because of the development of MAO B (Figure 3). In brains of MAO A KO pups and adults, NE concentrations were increased up to two-fold, and a small increase in DA levels was observed in pup brains (Cases et al 1995). Conversely, only levels of PEA were increased in MAO B KO mice: 1.70 ± 0.17 (wild-type) and 14.1 ± 2.81 (MAO B KO) (Grimsby et al 1997).
Figure 3.
Concentration of serotonin (5-HT), norepinephrine (NE), and dopamine (DA) in whole brains from wild-type and monoamine oxidase (MAO) A–deficient mice. Concentrations are in picomoles per milligram (wet weight) of brain and represent the mean plus or minus the standard error of the mean. For 5-HT, n = 4 in wild-type and n = 5 in MAO A knock-out (KO) mice; NE, n = 2 in wild-type and n = 2 in MAO A KO mice; DA, n = 4 in wild-type and MAO A KO mice. (From Cases et al 1995.)
MAO AND BEHAVIOR
MAO A and B in Parkinson’s Disease
MAO B activity increases with aging in the human brain (Fowler et al 1980). Because MAO B is predominantly located in glial cells (Levitt et al 1982, Westlund et al 1985) the large increase in MAO B may be attributable to the proliferation of these cells. Increased oxidation of DA by MAO B in the elderly may be associated with the loss of dopaminergic neurones in the substantia nigra, which underlies Parkinson’s disease. Indeed, patients with Parkinson’s disease have elevated MAO B activity in the substantia nigra (Riederer & Jellinger 1983), and the MAO B inhibitor, deprenyl, delays the progression of symptoms (Knoll 1988, Sano et al 1997). However, it has been suggested that deprenyl may have neuroprotective effects independent of MAO B inhibition (Tatton & Chalmers-Redman 1996). Increased oxidation of DA by MAO B in the brains of sufferers of Parkinson’s disease may produce levels of oxygen radicals that are sufficient to trigger oxidative damage of nigrostriatal neurons (Cohen 1990, Gerlach et al 1996a). This is consistent with oxidative damage to mitochondrial DNA by H2O2 generated during MAO-catalyzed oxidation of DA (Hauptmann et al 1996) and the preservation of nigrostriatal neurons by prolonged deprenyl treatment (Knoll 1989).
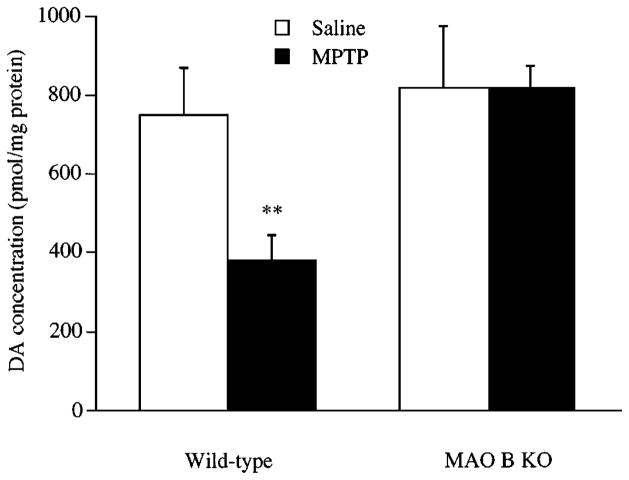
MAO B converts 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) to the toxic metabolite 1-methyl-4-phenylpyridine (MPP+), which selectively destroys nigrostriatal neurons (Gerlach et al 1996b). The neurodegeneration induced by MPTP is similar to the neuronal damage in Parkinson’s disease and is prevented by the MAO B inhibitor deprenyl (Fuller & Hemrick-Luecke 1984, Heikkila et al 1984, Langston et al 1984). Thus, MAO B appears to be involved in the pathogenesis of MPTP-induced Parkinsonism. This is consistent with our finding that mice deficient in MAO B did not sustain damage to the dopaminergic terminals of the striatum after MPTP injection (Grimsby et al 1997). These data are shown in Figure 4. This study has proved clearly that MAO B is required for MPTP toxicity. MAO B may promote the aging process in the brain either by bioactivating exogenous/endogenous neurotoxins or by increasing the levels of toxic H2O2.
Figure 4.
Histogram showing the level of dopamine (DA) in the striatum of wild-type and monoamine oxidase (MAO) B knock-out (KO) pups after subcutaneous administration of 60 mg of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) per kg. Values shown are the mean plus or minus the standard deviation. Saline-treated mice, n = 10; MPTP-treated mice, n = 4. P < 0.01 level (t-test). (From Grimsby et al 1997.)
A gene responsible for Parkinsonism has been identified in four unrelated families (Polymeropoulos et al 1997), indicating that familial Parkinson’s disease may be inherited. In scattered cases of Parkinson’s disease, a genetic component may increase the vulnerability of dopaminergic neurons in the substantia nigra to environmental factors. Because changes in MAO activity can affect neurotransmitters or neurochemistry, hereditary variations in the genes that control the levels of MAO activity may influence susceptibility to Parkinson’s disease. This is consistent with the different overall distribution of alleles of MAO B between patients with Parkinson’s disease and control subjects (Hotamisligil et al 1994). Indeed, the presence of allele B4 (Nanko et al 1996) and allele 1 of MAO B (Kurth et al 1993) may increase the risk of developing Parkinson’s disease.
A highly polymorphic (GT)n repeat sequence has been identified in intron 2 of the human MAO B gene (Grimsby et al 1992, Konradi et al 1992). If the levels of MAO are controlled by the genes that encode them, then variations in alleles for these genes may underlie the genetic predisposition to Parkinson’s disease. This is consistent with a polymorphism of a single base (adenine or guanine) of the MAO B gene in intron 13, which is more frequent in patients with Parkinson’s disease (Kurth et al 1993). However, the levels of MAO B will be unaffected by this polymorphism because intron sequences do not encode proteins. No association has been found between Parkinson’s disease and MAO A gene polymorphisms (Haney et al 1990, Hotamisligil et al 1994, Nanko et al 1996).
MAO A and B in Smoking and Alcoholism
Several lines of evidence suggest a link between cigarette smoke and MAO inhibition. Cigarette smokers have reduced brain levels of MAO A (Fowler et al 1996a) and B (Fowler et al 1996b). Likewise, the activities of MAO A and B are decreased in animals exposed to cigarette smoke (Carr & Rowell 1990) and in vitro (Yu & Boulton 1987), and heavy smokers have reduced levels of MAO in peripheral tissues (Berlin et al 1995, Carr & Basham 1991).
Despite the evidence that smoking inhibits MAO, the mechanism of MAO inhibition by cigarette smoke is not known. Two components of cigarette smoke, hydrazine and phenylpyridine, do not inhibit MAO in vivo (Carr & Basham 1991). However, formaldehyde and cyanide in cigarette smoke form adducts with the reactive amino groups of the MAO protein (Boulton et al 1988). This may reflect a decrease in MAO catalytic activity because cigarette smoke can induce conformational changes of amine/smoke adducts (Boulton et al 1988). Although nicotine is the main pharmacologically active compound in tobacco, its effects on MAO catalytic activity are unclear. At physiological concentrations, nicotine does not affect cerebral MAO A and B activity (Carr & Basham 1991) or MAO B activity in platelets (Oreland et al 1981). CaCO2 cells develop morphological characteristics and marker enzyme activities of normal enterocytes after reaching confluence in culture and exhibit a high level of MAO B but a low level of MAO A. Using CaCO2 cells, we have shown that nicotine increases MAO B activity (Chen et al 1996a). Thus, the effects of nicotine on MAO B activity are complex and the molecular action of nicotine on the regulation of MAO B may be studied further using CaCO2 cells.
MAO may also be involved in alcoholism because lower levels of MAO B activity are present in alcoholics (Devor et al 1993, Faraj et al 1994). Furthermore, MAO A mutations may underlie the susceptibility of individuals to alcoholism because MAO A alleles have been associated with alcoholism among Euro-Americans (Parsian et al 1995, Vanyukov et al 1995) and Han Chinese (Hsu et al 1996).
MAO A and B in Stress-Related Disorders
MAO inhibitors benefit patients with posttraumatic stress syndrome and patients with panic attacks (Liebowitz et al 1990), which suggests that these compounds have direct effects on stress and fear. Although MAO inhibitors can clinically treat stress, a direct link between MAO and stress has only recently been established. Doyle et al (1996) demonstrated that an increase in salivary MAO A and B activities is correlated with stress. Both MAO A–deficient mice (Cases et al 1995) and MAO B–deficient mice (Grimsby et al 1997) show an increased reactivity to stress in the forced-swim test. As NE and DA mediate the stress response and their action is potentiated by PEA (Berry et al 1994 Dyck et al 1993, Juorio et al 1988, Paterson et al 1990, Scarr et al 1994, Yu et al 1994), these findings are consistent with elevated brain levels of NE and DA in MAO A KO mice (Cases et al 1995) and PEA in MAO B KO mice (Grimsby et al 1997).
Aggressive Behavior in MAO A–Deficient Mice
Several lines of evidence suggest that 5-HT may be required for aggressive behavior (Korte et al 1996, Popova et al 1996, Saudou et al 1994). Mice lacking 5-HT1B receptors show enhanced offensive aggression (Saudou et al 1994), and changes in 5-HT1A and 5-HT2A receptor expression have been reported in aggressive mice (Korte et al 1996, Popova et al 1996). Furthermore, 5-HT1A (Molina et al 1987, Olivier & Mos 1992, Sanchez et al 1993), 5-HT1B/C (Olivier & Mos 1992), and 5-HT2 (Olivier & Mos 1992) receptor agonists and antagonists (Sanchez et al 1993, Sorensen et al 1993) reduce many forms of offensive aggression. MAO A KO pups have elevated brain levels of 5-HT (Cases et al 1995), and a distinct behavioral syndrome, including enhanced aggression, is manifested by adult males (Cases et al 1995). Thus, elevated levels of 5-HT in MAO A KO pups may underlie aggression in adult MAO A KO mice. Similarily, elevated levels of 5-HT may be important in the enhanced emotional learning that adult MAO A KO mice exhibit (Kim et al 1997). It is possible that aggression associated with MAO A deficiency may be related to structural changes to the somatosensory cortex (Cases et al 1996) in response to elevated cortical levels of 5-HT in MAO A KO mice. The enhanced aggressive behavior exhibited by MAO A KO mice is consistent with the abnormal aggression reported in males from a Dutch family with a complete MAO A deficiency due to a point deletion in the gene encoding MAO A (Brunner et al 1993a). Thus, MAO A deficiency may render mice more susceptible to the effects of environmental substances, drugs, or biogenic amines.
CONCLUDING REMARKS AND FUTURE DIRECTIONS
This is an exciting period for MAO research and its effects on behavior. Following the cloning of human MAO A and B, molecular biology studies using the cDNA have significantly advanced our understanding of the regulation, active site composition, and function of MAO. Studies of MAO A and B KO mice have clearly shown that MAO A and B have distinct functions in neurotransmitter metabolism and behavior.
It is puzzling that in some regions of the brain MAO and its preferred substrate are not found in the same neuron, the localization of MAO A in MAO B KO mice and MAO B in MAO A KO mice can now be determined and correlated with the brain levels of MAO substrates. Thus, using KO mice the differences in MAO and preferred substrate localization can now be reconciled.
KO mice can be used to study new functions of MAO and the relationship between MAO and other proteins. MAO B can metabolize the main metabolite of histamine, tele-histamine (Hough & Domino 1979). Studies with MAO A and B KO mice may clarify the physiological role of MAO B in histamine catabolism. Imidazole binding compounds (IBS) bind to a site that may be associated with MAO A and B proteins. By using KO mice, the location of the IBS binding site on MAO can be determined. Because the imidazoline binding site may be connected to blood pressure regulation (Raddatz & Lanier 1997, Raddatz et al 1995), MAO may have new functions in addition to neurotransmitter oxidation and H2O2 production.
By use of tissue-specific gene KO techniques, it is possible to delete the MAO A and B genes within specific brain regions instead of in the whole animal. Thus, the function of MAO in a specific region can be studied, which will help to identify the brain region responsible for aggressive behavior. This ultimately will lead to an understanding of the molecular basis of aggression and to the development of novel antipsychotics.
The MAO B inhibitor, deprenyl, delays the progression of the symptoms of Parkinson’s disease (Knoll 1988, Sano et al 1997), and H2O2 generated during MAO-catalyzed oxidation of neurotransmitters may cause damage to mitochondrial DNA (Hauptmann et al 1996). This effect may have implications for aging and neurodegenerative processes. Therefore, MAO B KO mice may be useful in studying the molecular basis of oxidative stress and aging.
In addition, multiple genes might be involved in the regulation of MAO activity in vivo. These include structural genes for MAO, genes that posttranslationally modify MAO, genes that control the microenvironment of MAO, and genes encoding regulatory factors that may modulate levels of MAO transcription. The regulation of MAO A and B gene expression is of fundamental importance. To clone and to understand the functions of MAO transcription factors are essential prerequisites for understanding the mechanism of MAO gene expression. When these are cloned, it will be possible to determine whether the tissue-specific expression is due to the availability of these factors. Following that, other questions can then be addressed: Do these factors regulate both MAO A and B expression? Are the concentrations of these factors related to increased MAO B activity in aging? Do these factors affect other genes related to MAO? Are these factors related to diseases with altered MAO B activity? Are there any polymorphisms in their DNA binding sequences in diseases associated with altered MAO B catalytic activity?
After studying the crystal structure of the enzyme, an ultimate understanding of the active site and the domains that confer substrate and inhibitor specificities of MAO A and B will be reached. Once the substrate site of MAO is determined, more effective inhibitors can be produced. This may lead to more effective treatments of neurodegenerative disorders such as Parkinson’s disease.
In summary, the regulation of gene expression and behavioral effects of MAO are largely unexplored. With the newly available MAO A and B KO mice, many unresolved questions about MAO can be answered, including where the localization and oxidation sites are, what its relationship to other proteins is, and what additional functions it serves. Furthermore, because MAO A and B can oxidize each other’s preferred substrates when the substrate or enzyme concentration is changed (Cases et al 1995, Hsu & Shih 1988), MAO A and B double-KO mice could be generated to clearly define the role of these two isoenzymes.
Acknowledgments
This study has been supported by grants from the National Institute for Mental Health (R37 MH39085, MERIT Award; K05 MH00795, Research Scientist Award; and RO1MH37020, the Boyd and Elsie Welin Professorship Award).
Literature Cited
- Achee FM, Gabay S. Studies of mono-amine oxidases. Inhibition of bovine brain MAO in intact mitochondria by selective inhibitors. Biochem Pharmacol. 1977;26(17):1637–44. doi: 10.1016/0006-2952(77)90081-8. [DOI] [PubMed] [Google Scholar]
- Bach AW, Lan NC, Johnson DL, Abell CW, Bembenek ME, et al. cDNA cloning of human liver monoamine oxidase A and B: molecular basis of differences in enzymatic properties. Proc Natl Acad Sci USA. 1988;85:4934–38. doi: 10.1073/pnas.85.13.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin I, Said S, Spreux-Varoquaux O, Olivares R, Launay JM, et al. Monoamine oxidase A and B activities in heavy smokers. Biol Psychiatr. 1995;38(11):756–61. doi: 10.1016/0006-3223(95)00084-4. [DOI] [PubMed] [Google Scholar]
- Berry MD, Scarr E, Zhu MY, Paterson IA, Juorio AV. The effects of administration of monoamine oxidase-B inhibitors on rat striatal neurone responses to dopamine. Br J Pharmacol. 1994;113(4):1159–66. doi: 10.1111/j.1476-5381.1994.tb17119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P, De Montigny C, Azzaro AJ. Modification of serotonergic and noradrenergic neurotransmissions by repeated administration of monoamine oxidase inhibitors: electrophysiological studies in the rat central nervous system. J Pharmacol Exp Ther. 1986;237(3):987–94. [PubMed] [Google Scholar]
- Boulton AA, Yu PH, Tipton KF. Biogenic amine adducts, monoamine oxidase inhibitors, and smoking. Lancet. 1988;1(8577):114–15. doi: 10.1016/s0140-6736(88)90308-x. (Letter) [DOI] [PubMed] [Google Scholar]
- Brunner HG. Monoamine oxidase and behavior. Ann Med. 1995;27(4):431–32. doi: 10.3109/07853899509002449. (Editorial) [DOI] [PubMed] [Google Scholar]
- Brunner HG, Nelen M, Breakefield XO, Ropers HH, Van Oost BA. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993a;262:578–80. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- Brunner HG, Nelen MR, Van Zandvoort P, Abeling NGGM, Van Gennip AH, et al. X-linked borderline mental retardation with prominent behavioral disturbance: phenotype, genetic localization, and evidence for disturbed monoamine metabolism. Am J Hum Genet. 1993b;52:1032–39. [PMC free article] [PubMed] [Google Scholar]
- Carr LA, Basham JK. Effects of tobacco smoke constituents on MPTP-induced toxicity and monoamine oxidase activity in the mouse brain. Life Sci. 1991;48(12):1173–77. doi: 10.1016/0024-3205(91)90455-k. [DOI] [PubMed] [Google Scholar]
- Carr LA, Rowell PP. Attenuation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity by tobacco smoke. Neuropharmacology. 1990;29(3):311–14. doi: 10.1016/0028-3908(90)90019-n. [DOI] [PubMed] [Google Scholar]
- Cases O, Seif I, Grimsby J, Gaspar P, Chen K, et al. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAO A. Science. 1995;268(5218):1763–66. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cases O, Vitalis T, Seif I, De Maeyer E, Sotelo C, et al. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficient mice: role of a serotonin excess during the critical period. Neuron. 1996;16:297–307. doi: 10.1016/s0896-6273(00)80048-3. [DOI] [PubMed] [Google Scholar]
- Cesura AM, Gottowik J, Lahm HW, Lang G, Imhof R, et al. Investigation on the structure of the active site of monoamine oxidase-B by affinity labeling with the selective inhibitor lazabemide and by site-directed mutagenesis. Eur J Biochem. 1996;236(3):996–1002. doi: 10.1111/j.1432-1033.1996.00996.x. [DOI] [PubMed] [Google Scholar]
- Chen K, Liao W, Shen WC, Shih JC. Differential expression of monoamine oxidase (MAO) A and B in CaCO2 cells. Presented at 26th Annu. Meet. Neurosci; Washington, DC. 1996a. [Google Scholar]
- Chen K, Wu HF, Grimsby J, Shih JC. Cloning of a novel monoamine oxidase cDNA from trout liver. Mol Pharmacol. 1994;46(6):1226–33. [PubMed] [Google Scholar]
- Chen K, Wu HF, Shih JC. Influence of C terminus on monoamine oxidase A and B catalytic activity. J Neurochem. 1996b;66(2):797–803. doi: 10.1046/j.1471-4159.1996.66020797.x. [DOI] [PubMed] [Google Scholar]
- Cohen G. Monoamine oxidase and oxidative stress at dopaminergic synapses. J Neural Transm. 1990;32(Suppl):229–38. doi: 10.1007/978-3-7091-9113-2_33. [DOI] [PubMed] [Google Scholar]
- Collins FA, Murphy DL, Reiss AL, Sims KB, Lewis JG, et al. Clinical, biochemical, and neuropsychiatric evaluation of a patient with a contiguous gene syndrome due to a microdeletion Xp11.3 including the Norrie disease locus and monoamine oxidase (MAO A and MAO B) genes. Am J Med Genet. 1992;42(1):127–34. doi: 10.1002/ajmg.1320420126. [DOI] [PubMed] [Google Scholar]
- Denney RM, Fritz RR, Patel NT, Abell CW. Human liver MAO-A and MAO-B separated by immunoaffinity chromatography with MAO-B specific monoclonal antibody. Science. 1982;215:1400–3. doi: 10.1126/science.7063850. [DOI] [PubMed] [Google Scholar]
- Denney RM, Sharma A, Dave SK, Waguespack A. A new look at the promoter of the human monoamine oxidase A gene: mapping transcription initiation sites and capacity to drive luciferase expression. J Neurochem. 1994;63:843–56. doi: 10.1046/j.1471-4159.1994.63030843.x. [DOI] [PubMed] [Google Scholar]
- Derry JM, Lan NC, Shih JC, Barnard EA, Barnard PJ. Localization of mono-amine oxidase A and B genes on the mouse X chromosome. Nucleic Acids Res. 1989;17:8403. doi: 10.1093/nar/17.20.8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor EJ, Cloninger CR, Hoffman PL, Tabakoff B. Association of monoamine oxidase (MAO) activity with alcoholism and alcoholic subjects. Am J Med Genet. 1993;48:209–13. doi: 10.1002/ajmg.1320480407. [DOI] [PubMed] [Google Scholar]
- Diez JA, Maderdrut JL. Development of multiple forms of mouse brain monoamine oxidase in vivo and in vitro. Brain Res. 1977;128(1):187–92. doi: 10.1016/0006-8993(77)90249-9. [DOI] [PubMed] [Google Scholar]
- Doyle A, Hucklebridge F, Evans P, Clow A. Salivary monoamine oxidase A and B inhibitory activities correlate with stress. Life Sci. 1996;59(16):1357–62. doi: 10.1016/0024-3205(96)00461-4. [DOI] [PubMed] [Google Scholar]
- Dyck LE, Durden DA, Boulton AA. Effects of monoamine oxidase inhibitors on the acid metabolites of some trace amines and of dopamine in the rat striatum. Biochem Pharmacol. 1993;45(6):1317–22. doi: 10.1016/0006-2952(93)90285-5. [DOI] [PubMed] [Google Scholar]
- Ekstedt B, Oreland L. Heterogeneity of pig liver and pig brain mitochondrial monoamine oxidase. Arch Int Pharmacodyn Ther. 1976;222(1):157–65. [PubMed] [Google Scholar]
- Erwin VG, Hellerman L. Mitochondrial monoamine oxidase I. Purification and characterization of the bovine kidney enzyme. J Biol Chem. 1967;242:4230–38. [PubMed] [Google Scholar]
- Faraj BA, Davis DC, Camp VM, Mooney AJ, Holloway T, et al. Platelet monoamine oxidase activity in alcoholics, alcoholics with drug dependence, and cocaine addicts. Alcohol Clin Exp Res. 1994;18:1114–20. doi: 10.1111/j.1530-0277.1994.tb00090.x. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Mantle TJ, Tipton KF. The nature of the inhibition of rat liver monoamine oxidase types A and B by the acetylenic inhibitors clorgyline, l-deprenyl and pargyline. Biochem Pharmacol. 1982;31:3555–61. doi: 10.1016/0006-2952(82)90575-5. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Wiberg A, Oreland L, Marcusson J, Winblad B. The effect of age on the activity and molecular properties of human brain monoamine oxidase. J Neural Transm. 1980;49(1–2):1–20. doi: 10.1007/BF01249185. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, et al. Inhibition of monoamine oxidase B in the brains of smokers. Nature. 1996a;379(6567):733–36. doi: 10.1038/379733a0. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, et al. Brain monoamine oxidase A inhibition in cigarette smokers. Proc Natl Acad Sci USA. 1996b;93(24):14065–69. doi: 10.1073/pnas.93.24.14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller RW, Hemrick-Luecke SK. Deprenyl protection against striatal dopamine depletion by 1-methyl-4-phenyl-1,2,3,6-tetra-hydropyridine in mice. Res Commun Subst Abuse. 1984;5:241–46. [Google Scholar]
- Gerlach M, Riederer P, Youdim MBH. Molecular mechanisms for neurodegeneration: synergism between reactive oxygen species, calcium and excitotoxic amino acids. Adv Neurol. 1996a;69:177–94. [PubMed] [Google Scholar]
- Gerlach M, Youdim MBH, Riederer P. Pharmacology of selegiline. Neurology. 1996b;47(3):S137–45. doi: 10.1212/wnl.47.6_suppl_3.137s. [DOI] [PubMed] [Google Scholar]
- Glover V, Sandler M, Owen F, Riley GJ. Dopamine is a monoamine oxidase B substrate in man. Nature. 1977;265(5589):80–81. doi: 10.1038/265080a0. [DOI] [PubMed] [Google Scholar]
- Gottowik J, Malherbe P, Jang G, Da Prada M, Cesura AM. Structure/function relationships of mitochondrial monoamine oxidase A and B chimeric forms. Eur J Biochem. 1995;230:934–42. doi: 10.1111/j.1432-1033.1995.tb20639.x. [DOI] [PubMed] [Google Scholar]
- Green AR, Youdim MBH. Effects of monoamine oxidase inhibition by clorgyline, deprenyl or tranycypromine on 5-hydroxy-tryptamine concentrations in rat brain and hyperactivity following subsequent tryptophan administration. Br J Pharmacol. 1975;55:415–22. doi: 10.1111/j.1476-5381.1975.tb06946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsby J, Chen K, Devor EJ, Cloninger CR, Shih JC. Dinucleotide repeat (TG)23 polymorphism in the MAO B gene. Nucleic Acids Res. 1992;20(4):924. doi: 10.1093/nar/20.4.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsby J, Chen K, Wang LJ, Lan NC, Shih JC. Human monoamine oxidase A and B genes exhibit identical exon-intron organization. Proc Natl Acad Sci USA. 1991;88(9):3637–41. doi: 10.1073/pnas.88.9.3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsby J, Toth M, Chen K, Kumazawa T, Klaidman L, et al. Increased stress response and β-phenylethylamine in MAO B-deficient mice. Nature Genet. 1997;17:1–5. doi: 10.1038/ng1097-206. [DOI] [PubMed] [Google Scholar]
- Grimsby J, Zentner M, Shih JC. Identification of a region important for human monoamine oxidase B substrate and inhibitor selectivity. Life Sci. 1996;58(9):777–87. doi: 10.1016/0024-3205(95)02356-9. [DOI] [PubMed] [Google Scholar]
- Haney M, Noda K, Kream R, Miczek KA. Regional serotonin and dopamine activity: sensitivity to amphetamine and aggressive behavior in mice. Aggress Behav. 1990;16:259–70. [Google Scholar]
- Hauptmann N, Grimsby J, Shih JC, Cadenas E. The metabolism of tyramine by monoamine oxidase A/B causes oxidative damage to mitochondrial DNA. Arch Biochem Biophys. 1996;335(2):295–304. doi: 10.1006/abbi.1996.0510. [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Manzino L, Cabbat FS, Duvoisin RC. Protection against the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine by monoamine oxidase inhibitors. Nature. 1984;311(5985):467–69. doi: 10.1038/311467a0. [DOI] [PubMed] [Google Scholar]
- Hiro I, Tsugeno Y, Hirashiki I, Ogata F, Ito A. Characterization of wild-type and mutant forms of human monoamine oxidase A and B expressed in a mammalian cell line. J Biochem. 1996;124(4):759–65. doi: 10.1093/oxfordjournals.jbchem.a021476. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Girmen AS, Fink JS, Tivol E, Shalish C, et al. Hereditary variations in monoamine oxidase as a risk factor for Parkinson’s disease. Mov Disord. 1994;9(3):305–10. doi: 10.1002/mds.870090304. [DOI] [PubMed] [Google Scholar]
- Hough LB, Domino EF. Tele-methylhistamine oxidation by type B monoamine oxidase. J Pharmacol Exp Ther. 1979;208:422–28. [PubMed] [Google Scholar]
- Hsu MC, Shih JC. Photoaffinity labeling of human placenta monoamine oxidase A by 4-fluoro-3-nitrophenylazide. Mol Pharmacol. 1988;33:237–41. [PubMed] [Google Scholar]
- Hsu YP, Weyler W, Chen S, Sims KB, Rinehart WB, et al. Structural features of human monoamine oxidase A elucidated from cDNA and peptide sequences. J Neurochem. 1988;51(4):1321–24. doi: 10.1111/j.1471-4159.1988.tb03105.x. [DOI] [PubMed] [Google Scholar]
- Hsu Y-PP, Loh EW, Chen WJ, Chen C-C, Yu J-M, et al. Association of monoamine oxidase A alleles with alcoholism among male Chinese in Taiwan. Am J Psychiatr. 1996;153:1209–11. doi: 10.1176/ajp.153.9.1209. [DOI] [PubMed] [Google Scholar]
- Ito A, Kuwahara T, Inadome S, Sagara Y. Molecular cloning of a cDNA for rat liver monoamine oxidase B. Biochem Biophys Res Commun. 1988;157:970–76. doi: 10.1016/s0006-291x(88)80969-0. [DOI] [PubMed] [Google Scholar]
- Jahng JW, Houpt TA, Wessel TC, Chen K, Shih JC, et al. Localization of monoamine oxidase A and B mRNA in the rat brain by in situ hybridization. Synapse. 1997;25(1):30–36. doi: 10.1002/(SICI)1098-2396(199701)25:1<30::AID-SYN4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Johnston JP. Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem Pharmacol. 1968;17(7):1285–97. doi: 10.1016/0006-2952(68)90066-x. [DOI] [PubMed] [Google Scholar]
- Juorio AV, Greenshaw AJ, Wishart TB. Reciprocal changes in striatal dopamine and β-phenylethylamine induced by reserpine in the presence of monoamine oxidase inhibitors. Naunyn-Schmiedeberg’s Arch Pharmacol. 1988;338(6):644–48. doi: 10.1007/BF00165628. [DOI] [PubMed] [Google Scholar]
- Kearney EB, Salach JL, Walker WH, Seng RL, Singer TP. The covalently bound flavin of hepatic monoamine oxidase. 1 Isolation and sequence of a flavin peptide and evidence for binding at the 8a position. Eur J Biochem. 1971;24:321–27. doi: 10.1111/j.1432-1033.1971.tb19689.x. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Shih JC, Chen K, Chen L, Bao S, et al. Selective enhancement of emotional, but not motor, learning in monoamine oxidase A-deficient mice. Proc Natl Acad Sci USA. 1997;94:5929–33. doi: 10.1073/pnas.94.11.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll J. Extension of life span of rats by long-term (−)deprenyl treatment. Mt Sinai J Med. 1988;55(1):67–74. [PubMed] [Google Scholar]
- Knoll J. The pharamcology of selegiline ((−)deprenyl) New aspects Acta Neurol Scand Suppl. 1989;126:83–91. doi: 10.1111/j.1600-0404.1989.tb01787.x. [DOI] [PubMed] [Google Scholar]
- Knoll J, Magyar K. Some puzzling pharmacological effects of monoamine oxidase inhibitors. Adv Biochem Psychopharmacol. 1972;5:393–408. [PubMed] [Google Scholar]
- Kochersperger LM, Parker EL, Siciliano M, Darlington GJ, Denney RM. Assignment of genes for human monoamine oxidases A and B to the X chromosome. J Neurosci Res. 1986;16:601–16. doi: 10.1002/jnr.490160403. [DOI] [PubMed] [Google Scholar]
- Konradi C, Ozelius L, Breakefield XO. Highly polymorphic (GT)n repeat sequence in intron II of the human MAO B gene. Genomics. 1992;12(1):176–77. doi: 10.1016/0888-7543(92)90426-s. [DOI] [PubMed] [Google Scholar]
- Korte SM, Meijer OC, De Kloet RE, Buwalda B, Keijser J, et al. Enhanced 5-HT1A receptor expression in forebrain regions of aggressive house mice. Brain Res. 1996;736(1–2):338–43. doi: 10.1016/0006-8993(96)00723-8. [DOI] [PubMed] [Google Scholar]
- Kurth JH, Kurth MC, Poduslo SE, Schwankhaus JD. Association of a monoamine oxidase B allele with Parkinson’s disease. Ann Neurol. 1993;33:368–72. doi: 10.1002/ana.410330406. [DOI] [PubMed] [Google Scholar]
- Kuwahara T, Takamoto S, Ito A. Primary structure of rat monoamine oxidase A deduced from cDNA and its expression in rat tissues. Agric Biol Chem. 1990;(54):253–57. [PubMed] [Google Scholar]
- Kwan SW, Lewis DA, Zhou BP, Abell CW. Characterization of a dinucleotide-binding site in monoamine oxidase B by site-directed mutagenesis. Arch Biochem Biophys. 1995;316(1):385–91. doi: 10.1006/abbi.1995.1051. [DOI] [PubMed] [Google Scholar]
- Lan NC, Chen CH, Shih JC. Expression of functional human monoamine oxidase A and B cDNAs in mammalian cells. J Neurochem. 1989a;52:1652–54. doi: 10.1111/j.1471-4159.1989.tb09223.x. [DOI] [PubMed] [Google Scholar]
- Lan NC, Heinzmann C, Gal A, Klisak I, Orth U, et al. Human monoamine oxidase A and B genes map to Xp 11.23 and are deleted in a patient with Norrie disease. Genomics. 1989b;4(4):552–59. doi: 10.1016/0888-7543(89)90279-6. [DOI] [PubMed] [Google Scholar]
- Langston JW, Forno LS, Rebert CS, Irwin I. Selective nigral toxicity after systemic administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in the squirrel monkey. Brain Res. 1984;292:390–94. doi: 10.1016/0006-8993(84)90777-7. [DOI] [PubMed] [Google Scholar]
- Levitt P, Pintar JE, Breakefield XO. Immunocytochemical demonstration of monoamine oxidase B in brain astrocytes and serotonergic neurons. Proc Natl Acad Sci USA. 1982;79(20):6385–89. doi: 10.1073/pnas.79.20.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy ER, Powell JF, Buckle VJ, Hsu YP, Breakefield XO, et al. Localization of human monoamine oxidase-A gene to Xp11.23–11.4 by in situ hybridization: implications for Norrie disease. Genomics. 1989;5:368–70. doi: 10.1016/0888-7543(89)90072-4. [DOI] [PubMed] [Google Scholar]
- Lewinsohn R, Glover V, Sandler M. Development of benzylamine oxidase and mono-amine oxidase A and B in man. Biochem Pharmacol. 1980;29(9):1221–30. doi: 10.1016/0006-2952(80)90278-6. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR, Hollander E, Schneier F, Campeas K, Welkowitz L, et al. Reversible and irreversible monoamine oxidase inhibitors in other psychiatric disorders. Acta Psychiatr Scand Suppl. 1990;360:29–34. doi: 10.1111/j.1600-0447.1990.tb05321.x. [DOI] [PubMed] [Google Scholar]
- Luque JM, Kwan S-W, Abell CW, Da Prada M, Richards GJ. Cellular expression of mRNAs encoding monoamine oxidases A and B in the rat central nervous system. J Comp Neurol. 1995;363:665–80. doi: 10.1002/cne.903630410. [DOI] [PubMed] [Google Scholar]
- Masini-Repiso AM, Cabanillas AM, Andrada MC, Coleoni AH. Monoamine oxidase in bovine thyroid tissue. Horm Metabol Res. 1986;18:750–53. doi: 10.1055/s-2007-1012426. [DOI] [PubMed] [Google Scholar]
- Minamiura N, Yasunobu KT. Bovine liver monoamine oxidase. A modified purification procedure and preliminary evidence for two subunits and one FAD. Arch Biochem Biophys. 1978;189:481–89. doi: 10.1016/0003-9861(78)90237-0. [DOI] [PubMed] [Google Scholar]
- Mitoma J, Ito A. Mitochondrial targeting signal of rat liver monoamine oxidase B is located at its carboxy terminus. J Biochem. 1992;111(1):20–24. doi: 10.1093/oxfordjournals.jbchem.a123712. [DOI] [PubMed] [Google Scholar]
- Mitra C, Guha SR. Serotonin oxidation by type B MAO of rat brain. Biochem Pharmacol. 1980;29:1213–16. doi: 10.1016/0006-2952(80)90276-2. [DOI] [PubMed] [Google Scholar]
- Molina V, Ciesielski L, Gobaille S, Isel F, Mandel P. Inhibition of mouse-killing behavior by serotonin-mimetic drugs: effects of partial alterations of serotonin neurotransmission. Pharmacol Biochem Behav. 1987;27:123–31. doi: 10.1016/0091-3057(87)90486-2. [DOI] [PubMed] [Google Scholar]
- Nanko S, Ueki A, Hattori M. No association between Parkinson’s disease and monoamine oxidase A and B gene polymorphism. Neurosci Lett. 1996;204:125–27. doi: 10.1016/0304-3940(95)12298-2. [DOI] [PubMed] [Google Scholar]
- Neff NH, Yang HY. Another look at the monoamine oxidases and the monoamine oxidase inhibitor drugs. Life Sci. 1974;14(11):2061–74. doi: 10.1016/0024-3205(74)90089-7. [DOI] [PubMed] [Google Scholar]
- O’Carroll AM, Fowler CJ, Phillips JP, Tobbia I, Tipton KF. The deamination of dopamine by human brain monoamine oxidase. Specificity for the two enzyme forms in seven brain regions. Naunyn-Schmiedeberg’s Arch Pharmacol. 1983;322(3):198–202. doi: 10.1007/BF00500765. [DOI] [PubMed] [Google Scholar]
- Olivier B, Mos J. Rodent models of aggressive behavior and serotonergic drugs. Prog Neuro-Psychopharmacol Biol Psychiatr. 1992;16:847–70. doi: 10.1016/0278-5846(92)90104-m. [DOI] [PubMed] [Google Scholar]
- Oreland L, Fowler CJ, Schalling D. Low platelet monoamine oxidase activity in cigarette smokers. Life Sci. 1981;29(24):2511–18. doi: 10.1016/0024-3205(81)90706-2. [DOI] [PubMed] [Google Scholar]
- Parsian A, Suarez BK, Tabakoff B, Ovchinnikova L, Fisher L, et al. Monoamine oxidases and alcoholism. I. Studies in unrelated alcoholics and normal controls. Am J Med Genet Neuropsychiatr Genet. 1995;60:409–16. doi: 10.1002/ajmg.1320600511. [DOI] [PubMed] [Google Scholar]
- Paterson IA, Juorio AV, Boulton AA. Possible mechanism of action of deprenyl in Parkinsonism. Lancet. 1990;336(8708):183. doi: 10.1016/0140-6736(90)91709-j. (Letter) [DOI] [PubMed] [Google Scholar]
- Pintar JE, Barbosa J, Francke U, Castiglione CM, Hawkins M, et al. Gene for monoamine oxidase type A assigned to the human X chromosome. J Neurosci. 1981;1:166–75. doi: 10.1523/JNEUROSCI.01-02-00166.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, et al. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–47. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Popova NK, Kulikov AV, Avgustinovich DE, Shigantsov SN. The characteristics of the brain serotonin system and anxiety in the C57BL and CBA mouse strains. Zh Vyss Nerv Deyat im I P Pavlova. 1996;46(2):348–54. [PubMed] [Google Scholar]
- Powell JF, Hsu YP, Weyler W, Chen SA, Salach J, et al. The primary structure of bovine monoamine oxidase type A. Comparison with peptide sequences of bovine monoamine oxidase type B and other flavoenzymes. J Biochem. 1989;259:407–13. doi: 10.1042/bj2590407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raddatz R, Lanier SM. Relationship between imidazoline/guanidinium receptive sites and monoamine oxidase A and B. Neurochem Int. 1997;30:109–17. doi: 10.1016/s0197-0186(96)00036-8. [DOI] [PubMed] [Google Scholar]
- Raddatz R, Parini A, Lanier SM. Imidazoline/guanidinium binding domains on monoamine oxidases. Relationship to subtypes of imidazoline-binding proteins and tissue-specific interaction of imidazoline ligands with monoamine oxidase B. J Biol Chem. 1995;270:27961–68. doi: 10.1074/jbc.270.46.27961. [DOI] [PubMed] [Google Scholar]
- Riederer P, Jellinger K. Neurochemical insights into monoamine oxidase inhibitors, with special reference to deprenyl (selegiline) Acta Neurol Scand Suppl. 1983;95:43–55. doi: 10.1111/j.1600-0404.1983.tb01516.x. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Arnt J, Hyttel J, Moltzen EK. The role of serotonergic mechanisms in inhibition of isolation-induced aggression in male mice. Psychopharmacology. 1993;110:53–59. doi: 10.1007/BF02246950. [DOI] [PubMed] [Google Scholar]
- Sano M, Ernesto C, Ronald MS, Thomas RG, Klauber MR, et al. A controlled trial of selegiline or α-tocopherol, or both as treatment for Alzheimer’s disease. N Engl J Med. 1997;336(17):1216–22. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- Saudou F, Amara DA, Dierich A, LeMeur M, Rarnboz S, et al. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science. 1994;265:1875–78. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- Saura J, Bleuel Z, Ulrich J, Mendelowitsch A, Chen K, et al. Molecular neuroanatomy of human monoamine oxidases A and B revealed by quantitative enzyme radioautography and in situ hybridization. Neuroscience. 1996;70(3):755–74. doi: 10.1016/s0306-4522(96)83013-2. [DOI] [PubMed] [Google Scholar]
- Saura J, Kettler R, Da Prada M, Richards JG. Quantitative enzyme radioautography with 3H-Ro 41-1049 and 3H-Ro 19-6327 in vitro: localization and abundance of MAO-A and MAO-B in rat CNS, peripheral organs, and human brain. J Neurosci. 1992;12(5):1977–99. doi: 10.1523/JNEUROSCI.12-05-01977.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura J, Richards JG, Mahy N. Age-related changes in MAO in Bl/C57 mouse tissues: a quantitative radioautographic study. J Neural Transm. 1994a;41:89–94. doi: 10.1007/978-3-7091-9324-2_11. [DOI] [PubMed] [Google Scholar]
- Saura J, Richards JG, Mahy N. Differential age-related changes of MAO-A and MAO-B in mouse brain and peripheral organs. Neurobiol Aging. 1994b;15(4):399–408. doi: 10.1016/0197-4580(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Scarr E, Wingerchuk DM, Juorio AV, Paterson IA. The effects of monoamine oxidase B inhibition on dopamine metabolism in rats with nigro-striatal lesions. Neurol Res. 1994;19(2):153–59. doi: 10.1007/BF00966810. [DOI] [PubMed] [Google Scholar]
- Shih JC. Molecular basis of human MAO A and B. Neuropsychopharmacology. 1991;4(1):1–7. [PubMed] [Google Scholar]
- Shiloff BA, Behrens PQ, Kwan SW, Lee JH, Abell CW. Monoamine oxidase B isolated from bovine liver exists as large oligomeric complexes in vitro. Eur J Biochem. 1996;242(1):41–50. doi: 10.1111/j.1432-1033.1996.0041r.x. [DOI] [PubMed] [Google Scholar]
- Sims KB, de la Chapelle A, Norio R, Sankila EM, Hsu Y-PP, et al. Monoamine oxidase deficiency in males with an X chromosome deletion. Neuron. 1989;2:1069–76. doi: 10.1016/0896-6273(89)90231-6. [DOI] [PubMed] [Google Scholar]
- Sorensen SM, Kehne JH, Fadayel GM, Humphreys TM, Ketteler HJ, et al. Characterization of the 5-HT2 receptor antagonist MDL 100907 as a putative atypical antipsychotic: behavioral, electrophysiological and neurochemical studies. J Pharmacol Exp Ther. 1993;266(2):684–91. [PubMed] [Google Scholar]
- Suzuki O, Katsumata Y, Oya M. Oxidation of β-phenylethylamine by both types of monoamine oxidase: examination of enzymes in brain and liver mitochondria of eight species. J Neurochem. 1981;36(3):1298–301. doi: 10.1111/j.1471-4159.1981.tb01734.x. [DOI] [PubMed] [Google Scholar]
- Tatton WG, Chalmers-Redman RME. Modulation of gene expression rather than monoamine oxidase inhibition: (−)-deprenyl-related compounds in controlling neuro-degeneration. Neurology. 1996;47(Suppl 3):S171–83. doi: 10.1212/wnl.47.6_suppl_3.171s. [DOI] [PubMed] [Google Scholar]
- Thorpe LW, Westlund KN, Kochersperger LM, Abell CW, Denney RM. Immunocytochemical localization of monoamine oxidases A and B in human peripheral tissues and brain. J Histochem Cytochem. 1987;35(1):23–32. doi: 10.1177/35.1.3025289. [DOI] [PubMed] [Google Scholar]
- Tipton KF, O’Carroll AM, McCrodden JM. The catalytic behavior of monoamine oxidase. J Neural Transm. 1987;23(Suppl):25–35. doi: 10.1007/978-3-7091-8901-6_2. [DOI] [PubMed] [Google Scholar]
- Tsang D, Ho KP, Wen HL. Ontogenesis of multiple forms of monoamine oxidase in rat brain regions and liver. Dev Neurosci. 1986;8(4):243–50. doi: 10.1159/000112258. [DOI] [PubMed] [Google Scholar]
- Tsugeno Y, Hirashiki I, Ogata F, Ito A. Regions of the molecule responsible for substrate specificity of monoamine oxidase A and B: a chimeric enzyme analysis. J Biochem. 1995;118(5):974–80. doi: 10.1093/jb/118.5.974. [DOI] [PubMed] [Google Scholar]
- Tsugeno Y, Ito A. A key amino acid responsible for substrate selectivity of mono-amine oxidase A and B. J Biol Chem. 1997;272(22):14033–36. doi: 10.1074/jbc.272.22.14033. [DOI] [PubMed] [Google Scholar]
- Twist EC, Brammer MJ, Stephenson JD, Corn TH, Campbell IC. Effect of chronic ritanserin and clorgyline administration on 5-HT2 receptor linked inositol phospholipid hydrolysis. Biochem Pharmacol. 1990;40(9):2111–16. doi: 10.1016/0006-2952(90)90242-d. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Moss HB, Yu LM, Tarter RE, Deka R. Preliminary evidence for an association of a dinucleotide repeat polymorphism at the MAO A gene with early onset alcoholism/substance abuse. Am J Med Genet Neuropsychiatr Genet. 1995;60:122–26. doi: 10.1002/ajmg.1320600207. [DOI] [PubMed] [Google Scholar]
- Westlund KN. The distribution of monoamine oxidases A and B in normal human brain. In: Lieberman A, Olanow CW, Youdim MBH, Tipton K, editors. Monoamine Oxidase Inhibitors in Neurological Diseases. New York: Dekker; 1994. pp. 1–19. [Google Scholar]
- Westlund KN, Denney RM, Kochersperger LM, Rose RM, Abell CW. Distinct monoamine oxidase A and B populations in primate brain. Science. 1985;230(4722):181–83. doi: 10.1126/science.3875898. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Denney RM, Rose RM, Abell CW. Localization of distinct monoamine oxidase A and monoamine oxidase B cell populations in human brainstem. Neuroscience. 1988;25(2):439–56. doi: 10.1016/0306-4522(88)90250-3. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Krakower TJ, Kwan SW, Abell CW. Intracellular distribution of monoamine oxidase A in selected regions of rat and monkey brain and spinal cord. Brain Res. 1993;612(1–2):221–30. doi: 10.1016/0006-8993(93)91664-e. [DOI] [PubMed] [Google Scholar]
- Weyler W. Functional expression of C-terminally truncated human monoamine oxidase type A in Saccharomyces cerevisiae. J Neural Transm. 1994;41(Suppl):3–15. doi: 10.1007/978-3-7091-9324-2_1. [DOI] [PubMed] [Google Scholar]
- Weyler W, Salach JI. Purification and properties of mitochondrial monoamine oxidase type A from human placenta. J Biol Chem. 1985;260:13199–207. [PubMed] [Google Scholar]
- Willoughby J, Glover V, Sandler M. Histochemical localization of monoamine oxidase A and B in rat brain. J Neural Transm. 1988;74(1):29–42. doi: 10.1007/BF01243573. [DOI] [PubMed] [Google Scholar]
- Wu HF, Chen K, Shih JC. Site-directed mutagenesis of monoamine oxidase A and B: role of cysteines. Mol Pharmacol. 1993;43(6):888–93. [PubMed] [Google Scholar]
- Yu PH, Boulton AA. Irreversible inhibition of monoamine oxidase by some components of cigarette smoke. Life Sci. 1987;41(6):675–82. doi: 10.1016/0024-3205(87)90446-2. [DOI] [PubMed] [Google Scholar]
- Yu PH, Davis BA, Durden DA, Barber A, Terleckyj I, et al. Neurochemical and neuroprotective effects of some aliphatic propargylamines: new selective nonamphetamine-like monoamine oxidase B inhibitors. J Neurochem. 1994;62(2):697–704. doi: 10.1046/j.1471-4159.1994.62020697.x. [DOI] [PubMed] [Google Scholar]
- Zhong B, Silverman RB. Identification of the active site cysteine in bovine liver monoamine oxidase B. J Am Chem Soc. 1997;119:6690–91. [Google Scholar]
- Zhou BP, Lewis DA, Kwan SW, Kirksey TJ, Abell CW. Mutagenesis at a highly conserved tyrosine monoamine oxidase B affects FAD incorporation and catalytic activity. Biochemistry. 1995;34(29):9526–33. doi: 10.1021/bi00029a029. [DOI] [PubMed] [Google Scholar]
- Zhu QS, Chen K, Shih JC. Bidirectional promoter of human monoamine oxidase A (MAO A) controlled by transcription factor Sp1. J Neurosci. 1994;14(12):7393–403. doi: 10.1523/JNEUROSCI.14-12-07393.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu QS, Grimsby J, Chen K, Shih JC. Promoter organization and activity of human monoamine oxidase (MAO) A and B genes. J Neurosci. 1992;12(11):4437–46. doi: 10.1523/JNEUROSCI.12-11-04437.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu QS, Shih JC. An extensive repeat structure down-regulates human monoamine oxidase A promoter activity independent of an initiator-like sequence. J Neurochem. 1997;69:1368–73. doi: 10.1046/j.1471-4159.1997.69041368.x. [DOI] [PubMed] [Google Scholar]