Abstract
Genetic variation in the serotonin 2A receptor (HTR2A) has been associated with both schizophrenia and suicidal behavior. Our sample comprised 270 Irish high-density schizophrenia families (n=1408 subjects, including 755 with psychotic illness). Diagnoses were generated using a modified SCID. All patients who had at least one episode of psychosis were rated on the Operation Criteria Checklist for Psychotic Illness (OPCRIT). Lifetime history of suicidal ideation was determined from medical records and psychiatric interviews and was scored in the OPCRIT. Twelve SNPs were selected for study. Ten of these were tagSNPs derived from HapMap data, along with His452Tyr and T102C. We tested for association with psychotic illness as a whole, as well as stratified by the presence of suicidal ideation, using FBAT and PDTPHASE. Single-marker as well as haplotype-based tests using a “sliding window” approach were performed. We observed several 2, 3, and 4 marker haplotypes near the 3′ end of the gene that were over-transmitted to psychotic subjects (.02≤P≤.04). His452Tyr was included in these haplotypes but was not itself significant. We also observed modest over-transmission of a 2-marker haplotype that included T102C (.04≤P≤.08), which was also not itself significant in single-marker analyses. There was no significant association in the subgroup of the sample with suicidal ideation. Because of multiple testing, these results do not provide support for HTR2A as a susceptibility gene for psychotic illness, or for suicidal ideation within psychotic illness.
Keywords: serotonin 2A, polymorphism, association, schizophrenia, suicide
Introduction
In the last few years, several susceptibility genes for schizophrenia have been identified by positional cloning. Most of these occur in regions that had previously been implicated through genetic linkage(Owen et al. 2004). Naturally, these studies have examined multiple markers, as their goal was to detect association in genes about which there were few a priori hypotheses. Candidate gene studies have been proceeding simultaneously. However, possibly in part because they began at a time in which few genetic variants per gene were known, they have tended to test relatively few polymorphisms per gene. However, haplotype-based analyses, which are based on multiple markers, have been demonstrated to have more power, using both real and simulated datasets(de Bakker et al. 2005;Zhang et al. 2002).
Abnormalities in central serotonin have been implicated in studies of multiple traits and conditions, among which schizophrenia and suicidal behavior figure prominently. In schizophrenia, this evidence has included the mechanism of action of atypical antipsychotic drugs, which block 5HT receptors(Lieberman et al. 1998), as well as the mechanism of action of lysergic acid diethylamide (LSD), which is an agonist at 5HT receptors(Glennon et al. 1984). Multiple lines of evidence have implicated the role of serotonin in suicidal behavior. These include decreased levels of 5-HIAA in the CSF of suicide victims or attempters(Asberg et al. 1976;Roy et al. 1989), altered expression of HTR2A receptors in suicide and affective illness(Arango et al. 1990;Biver et al. 1997;Mann et al. 1986;Yatham et al. 2000), and the mechanism of antidepressant medications, especially SSRIs, which decrease the reuptake of serotonin, and the use of which has been associated with decreased rates of suicide(Olfson et al. 2003;Gibbons et al. 2005).
Several reports of association between HTR2A polymorphisms and schizophrenia have been published(Baritaki et al. 2004;Erdmann et al. 1996;Inayama et al. 1996;Williams et al. 1996). These have mostly examined one to three polymorphisms each, of which a T/C polymorphism at position 102 (T102C) has been most frequently studied. However, due to multiple subsequent negative studies, two recent meta-analyses demonstrated no overall effect of this polymorphism on schizophrenia risk(Abdolmaleky et al. 2004;Li et al. 2006).
In suicidal behavior, significant association with T102C has also been reported(Arias et al. 2001;Du et al. 2000). However, there have also been several negative studies(Li et al. 2006). A recent meta-analysis demonstrated no overall effect(Li et al. 2006). However, in this meta-analysis, using a pooling strategy in which suicide ideators were compared to non-ideators or healthy controls, a small effect was uncovered (p=.016). Interestingly, the same allele, T102, has had a lower frequency in cases than controls in studies of both schizophrenia and suicidal behavior.
In this study, we tested 12 HTR2A SNPs for association with psychotic illness, as well as suicidal ideation in individuals with psychotic illness, in both single-marker and haplotype-based analyses. Due to previous reports of association with both conditions, we hypothesized that there would be association with SI within individuals with psychosis. As such, we hypothesized that HTR2A belongs to a class of genes we have termed Modifier-Susceptibility genes. We have previously described these genes as genes that clearly cause susceptibility to illness; however, they preferentially cause presentations of illness with more or less specific features of illness, such as high levels of certain symptoms, poor outcome, or early age of onset (Fanous and Kendler 2005). Therefore, in a given population, these are genes not only cause illness, they also affect the distribution of clinical features in ill subjects.
Materials and Methods
Subjects and Assessment
The Irish Study of High Density Schizophrenia Families (ISHDSF) is a collaborative effort between the Medical College of Virginia of Virginia Commonwealth University, Richmond, the Queen’s University, Belfast, and the Health Research Board, Dublin. Fieldwork was done between April 1987 and November 1992, and described previously (Kendler et al. 1996). Interviews were conducted by Irish Psychiatrists and social scientists with a background in mental health or survey work after consent was obtained by using procedures approved by the ethical review panels at the Health Research Board and the Queen’s University. The original linkage sample consisted of 1425 individuals from 270 families that were ascertained on the basis of having more than one member with DSM-III-R schizophrenia or poor outcome schizoaffective disorder.
Diagnoses were generated using modified sections of the Structured Interview for DSM-III-R (SCID) for selected Axis I disorders (Spitzer et al. 1987). All relevant diagnostic information for each individual relative was reviewed, blind to pedigree assignment and marker genotypes, independently by KSK and DM. Each diagnostician made up to three best-estimate DSM-III-R diagnoses. For each subject with psychotic illness (defined as lifetime occurrence of any psychotic episode), the Operational Criteria Checklist for Psychiatric Illness (OPCRIT)(McGuffin et al. 1991) was completed by KSK (n=755, of which 722 were genotyped) based on review of hospital records and personal interviews. This is an instrument designed for use in a best-estimate procedure that codes symptom and course features as assessed over the entire course of illness. It was designed to allow the experienced clinician to integrate the relative prominence of clinical features over the entire course of illness.
In this study, we defined as affected, any subject with a history of a psychotis. Of these, we selected a group of subjects who experienced suicidal ideation. This was derived from the “suicidal ideation” item of the OPCRIT. A subject was coded as “0” if there was no lifetime history of suicidal ideation, “1” if there was suicidal ideation of at least one week duration, or “2” if there was suicidal ideation of at least two weeks duration. Subjects were considered positive for suicidal ideation if they were rated as “1” or “2” (n=127, or 16%). As would be expected, these subjects had higher levels of the OPCRIT depression factor(Fanous et al. 2005) (t=-19.82, p<.0001).
Genotyping
Ten of the 12 SNPs selected for this study were obtained from Hapmap (www.hapmap.org). These were tagSNPs derived from the Caucasian population, and identified as such by the method of Gabriel et al.(Gabriel et al. 2002), using HaploView(Barrett et al. 2005). For these SNPs, genotyping was done using the TaqMan assay (Applied Biosystems, Inc., Foster City, CA, USA)(Livak 1999) in Dr. Chen’s laboratory. Briefly, the PCR reactions were conducted in 384-well plates. To ensure the quality of genotyping, negative control samples were included in each plate. The PCRs were performed with 2 ng of genomic DNA, 0.25 μl of TaqMan assay mix and 2.5 μl of TaqMan universal PCR master mix in a total reaction volume of 5 μl. After activating the polymerase and denaturizing by heating at 95 °C for 10 minutes, 40 cycles of 92 °C for 15 seconds and 55 °C for 1 minute was performed. After the reaction, the fluorescence intensities of reporter 1 and 2 (reporter 1:VIC, exercitation = 520 ±10 nm, emission = 550 ± 10 nm; reporter 2: FAM, exercitation = 490 ±10 nm, emission = 510 ± 10 nm) were measured by the Analyst fluorescence plate reader (LJL Biosytems, Sunnyvale, CA). Based on the ratio of fluorescence intensities, genotypes were scored by a Euclidian clustering algorithm developed in our laboratory(Van Den Oord et al. 2003). Two additional SNPs had been previously genotyped in this sample based on methods described in their respective references, including T102C(Warren, Jr. et al. 1993) and His452Tyr(Ozaki et al. 1996).
Statistical Analysis
Prior to analysis, SNP data were tested for Mendelian inconsistencies using the program PEDCHECK(O’Connell and Weeks 1998). Erroneous genotypes were removed. We used two family- based methods to test for association: the Family-Based Association Test, operationalized in FBAT(Laird et al. 2000), and the Pedigree Disequilibrium Test (PDT)(Martin et al. 2000), operationalized in PDTPHASE, part of the UNPHASED package(Dudbridge 2003). Individual marker genotypes were tested for Hardy-Weinberg equilibrium, and analyzed for linkage disequilibrium relationships, in Haploview(Barrett et al. 2005). We used two programs as a means of confirming results, in an attempt to reduce the probability of false positives.
We first tested for excessive transmission of alleles at each marker separately, and then for excessive transmission of 2, 3, and 4-marker haplotypes. All haplotypes significantly associated with psychosis were followed up and tested for association with narrowly defined schizophrenia, operationalized as schizophrenia or poor-outcome schizoaffective disorder. Haplotypes were reconstructed using the HBAT command in FBAT for FBAT analyses, which utilizes the EM algorithm. In these analyses, we used the empirical variance estimator option (-e). Haplotypes were constructed for PDT analyses in PDTPHASE, also using the EM algorithm. For all PDT analyses, we used the “sum PDT” statistic, which assigns weights to pedigrees on the basis of size, and is more powerful than the “average PDT” in samples composed of pedigrees of varying sizes, such as ours(Martin et al. 2001).
Results
All markers were in Hardy-Weinberg equilibrium. Only two individual SNPs showed a significant association with psychosis at the .05 level. SNP rs659734 was significant in FBAT only, while rs2070037 was significant in PDTPHASE and reached trend significance in FBAT (p=.08). No individual SNPs were significant in the suicidal ideation group.
Using haplotype analysis, two 4-marker haplotypes comprising adjacent “sliding windows” were significant with psychosis using PDTPHASE. These consisted of markers rs3125-rs659734 as well as rs6314-rs1928042. In addition, a 2-marker haplotype in this region, consisting of rs6314 and rs1745837 was significant using FBAT (P=.02). Follow up analysis of this haplotype in narrowly defined schizophrenia was also significant (P=.04). Towards the other (5′) end of the gene, several haplotypes including rs2070037 attained a trend to significance using both programs (P<.08), while a 3-marker haplotype beginning with this marker was significant in FBAT (P=.04). All results for psychosis are presented in table 1. With the exception of rs6314-rs174837, none of the haplotoypes that were significantly associated with psychosis remained significantly associated with narrow schizophrenia.
Table 1. PDTPhase and FBAT P-values for Single Markers and Two-, Three-, and Four-marker Haplotypes.
Significance levels for association tests between HTR2A SNPs and psychotic illness in the Irish Study of High-Density Schizophrenia Families. ‘Single’ refers to the single-marker test. ‘Hap-2′, ‘Hap-3′, and ‘Hap-4′ refer to the global p-value of the haplotype block composed of the marker in question along with 2, 3, or 4 markers, 5′ to it, respectively. Two SNPs were significant in single marker analyses: rs2070037 was significant in PDTPhase and had a trend to significance in FBAT; rs659734 was significant in FBAT only. Both SNPs were part of multiple marker haplotypes that were significant using both programs. Neither of the two previously studied SNPs — rs6314 (His452Tyr) and rs6313 (T102C) — was significant. However, they were included in haplotypes that were significant.
| Phenotype: Psychotic Illness | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PDTPhase | FBAT | ||||||||||||
| SNP # |
Name | HW p- value |
|||||||||||
| Position in Gene (in bp) |
Type/ Function |
MAF | Single | Hap-2 | Hap-3 | Hap-4 | Single | Hap-2 | Hap-3 | Hap-4 | |||
| 1 | rs3125 | 0.42 | 0 | 3′ UTR | .21 | 0.12 | NS | NS | 0.04† | .14 | NS | .08 | NS |
| 2 | rs6314 (His452Tyr) |
0.93 | 183 | Coding, Nonsyn |
.43 | 0.95 | NS | NS | 0.04† | .41 | .02* | NS | NS |
| 3 | rs1745837 | 0.29 | 15961 | Intronic | .29 | 0.17 | NS | 0.02† | NS | .15 | NS | NS | NS |
| 4 | rs659734 | 0.75 | 26432 | Intronic | .08 | 0.32 | NS | NS | NS | .05† | .07 | NS | NS |
| 5 | rs1928042 | 0.14 | 28365 | Intronic | .19 | 0.22 | NS | NS | NS | .25 | NS | NS | NS |
| 6 | rs2770296 | 0.67 | 31709 | Intronic | .34 | 0.17 | NS | NS | NS | .24 | NS | NS | NS |
| 7 | rs582385 | 1.00 | 37143 | Intronic | .14 | 0.14 | NS | NS | NS | .11 | NS | NS | NS |
| 8 | rs731779 | 0.62 | 43187 | Intronic | .26 | 0.10 | NS | NS | NS | .09 | NS | NS | NS |
| 9 | rs6305 | 1.00 | 57771 | Coding, Syn |
.03 | 1.00 | 0.08 | NS | 0.06 | .54 | NS | NS | .06 |
| 10 | rs2070037 | 0.07 | 58219 | intron | .28 | 0.05† | 0.07 | 0.08 | NS | .08 | NS | .04† | NS |
| 11 | rs6313 (T102C) |
0.76 | 61089 | Coding, Syn |
.11 | 0.55 | NS | NS | NS | .50 | NS | NS | NS |
| 12 | rs1805055 | 0.23 | 61117 | Coding, Nonsyn |
.04 | 0.08 | NS | NS | NS | .09 | NS | NS | NS |
P=.04 in narrow schizophrenia.
Non-significant in narrow schizophrenia
A 3-marker haplotype beginning with rs582385 was associated with suicidal ideation (p=.05) in FBAT, while several 2- and 4-marker haplotypes containing or near this SNP trended to significance. However, none of these haplotypes were significant, even at a trend level, in PDTPHASE. Results for suicidal ideation are presented in table 2.
Table 2. PDTPhase and FBAT P-values for Single Markers and Two-, Three-, and Four-marker Haplotypes.
Significance levels for association tests between HTR2A SNPs and a subset of psychotic subjects experiencing lifetime suicidal ideation. None of the markers was individually significant. A 3-marker haplotype beginning with rs582385 was significant in FBAT only, but did not trend to significance in PDTPhase. We therefore treat this result with caution.
| Phenotype: Suicidal Ideation | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| SNP # |
Name | ||||||||
| PDTPhase | FBAT | ||||||||
| Single | Hap-2 | Hap-3 | Hap-4 | Single | Hap-2 | Hap-3 | Hap-4 | ||
| 1 | rs3125 | .48 | NS | NS | NS | .14 | NS | NS | NS |
| 2 | rs6314 (His452Tyr) |
.22 | NS | NS | NS | .12 | NS | NS | NS |
| 3 | rs1745837 | 1.00 | NS | NS | NS | .47 | NS | NS | NS |
| 4 | rs659734 | .28 | NS | NS | NS | .13 | NS | NS | NS |
| 5 | rs1928042 | .80 | NS | NS | NS | .40 | NS | NS | NS |
| 6 | rs2770296 | .92 | NS | NS | NS | .56 | NS | NS | .09 |
| 7 | rs582385 | .88 | NS | NS | NS | .28 | NS | .05 | NS |
| 8 | rs731779 | .91 | NS | NS | NS | .50 | .08 | NS | NS |
| 9 | rs6305 | 1.00 | NS | NS | NS | --* | .08 | NS | NS |
| 10 | rs2070037 | .55 | NS | NS | NS | .21 | NS | NS | NS |
| 11 | rs6313 (T102C) |
.53 | NS | NS | NS | .28 | NS | NS | NS |
| 12 | rs1805055 | 1.00 | NS | NS | NS | --* | NS | NS | NS |
There were less than 10 informative families present.
Discussion
In this study, we tested a two-part hypothesis, i.e., that genetic variation in the HTR2A receptor is associated with psychotic illness, and that furthermore, this association is greater in a subset of patients that experience suicidal ideation. This was based on prior association evidence implicating this gene in both phenotypes. We have previously termed genes that predispose to more or less specific clinical forms of illness “susceptibility-modifier” genes(Fanous and Kendler 2005). As examples of such genes, two previous studies(DeRosse et al. 2006;Fanous et al. 2005), including one in our sample(Fanous et al. 2005), reported association between dysbindin and schizophrenia with high levels of negative symptoms, while another reported association between d-amino acid oxidase activator and bipolar disorder with persecutory delusions(Schulze et al. 2005).
We observed only two SNPs achieving nominal P=.05 in the full sample of psychotic subjects. However, both of these SNPs were significant using one of the two analytic packages, but not the other. We therefore cannot infer a positive association between this gene and psychotic illness in this sample. Furthermore, as none of the SNPs reached P=.05 in the sample of patients experiencing suicidal ideation, we find no evidence of association between this gene and suicidality. These results do not support previous studies reporting association between SNPs in this gene and schizophrenia (Baritaki et al. 2004;Erdmann et al. 1996;Inayama et al. 1996;Williams et al. 1996). Although several multi-marker haplotypes were significantly associated at the .05 α level, they are very modestly significant and would not survive correction for multiple testing.
It is possible that the signals we observed in this study represent true genetic association, which this study is underpowered to detect due to a very low effect size. If this is the case, we would be able to make one additonal inference. His452Tyr nor T102C, the only two SNPs included in this study that have been repeatedly studied, was significant in single marker analyses. However, both of them were included in multiple-marker haplotypes that were themselves nominally significant. This would suggest that previous studies utilizing only individual markers may have suffered from a lack of power due to inability to adequately tag haplotypes that contain etiologically relevant variants. In family-based studies such as ours, single-marker analyses would be associated with fewer heterozygotic parents than haplotype-based analyses, and therefore less transmission information in TDT-like tests. Although His452Tyr has no known functional consequences, the latter does lead to an amino acid change and may be associated with promoter activity(Parsons et al. 2004). Nevertheless, the marginal evidence of association in previous studies, makes it unlikely that either SNP is itself causative
Meta-analysis, if performed with studies that themselves are not fully informative, may lead to false negative results. This may indeed be the case with previous studies of HTR2A. A strategy with a greater likelihood of success would be to test large samples using a dense SNP map that would allow for adequate tagging of all or most major haplotypes. An association study of HTR2A in bipolar disorder demonstrated the superior power of haplotype-based vs. single marker analyses, even in a modestly sized sample(Ranade et al. 2003). However, there are no association studies of schizophrenia or suicidal behavior that we are aware of utilizing haplotype analysis based on a SNP map designed to provide adequate coverage of haplotypic variation in HTR2A. Studies pooling multiple samples, such as a recent linkage study of bipolar disorder(McQueen et al. 2005), should test for excess transmission of informative haplotypes occurring within, or containing, genes with marginal previous evidence of association, such as HTR2A. This type of analysis may be possible in the very near future with the availability of a number of large samples genotyped using whole-genome mapping arrays, such as in the GAIN initiative(Manolio et al. 2007).
We cannot rule out that the weak association signals observed in this study are a result of LD with haplotypes in genes that are adjacent to HTR2A. Furthermore, none of the SNPs we tested has known functional consequences, although both His452Tyr as well as rs1805055 are non-synonymous coding SNPs. Lastly, we cannot rule out the possibility that our definition of suicidal ideation was too broad and therefore lacked the specificity to capture true trait suicidality, as for example, suicide attempt history might. However, this phenotype was not used in our assessments and is not an item of the OPCRIT. Furthermore, as we have previously noted, there is little consensus in what constitutes suicidality(Fanous et al. 2004).
Figure 1. Haplotype Blocks in 12 SNPs in HTR2A Determined Using the Method of Gabriel et al. in Haploview.
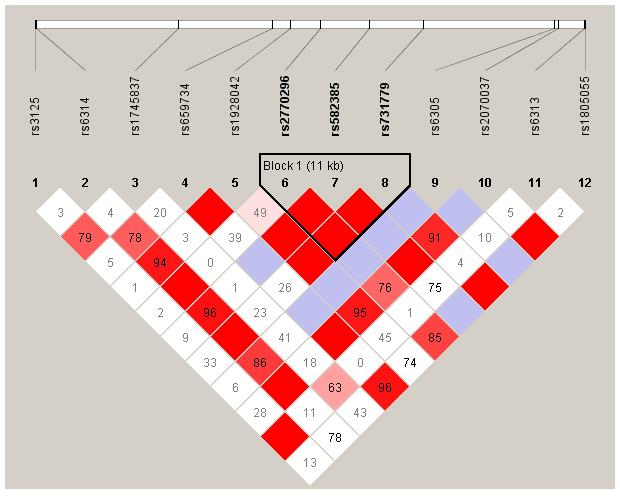
Marker to marker LD, as well as haplotype blocks, in the HTR2A gene as determined using haplotype tagging SNPs obtained from HapMap. Bright red represents D’=1 and LOD>=2, blue represents D’=1 and LOD<2, pink represents D’<1 (figure displayed) and LOD>=2, and white represents D’<1 and LOD<2.
Acknowledgements
This work was supported by NIH grants MH-41953, MH-52537, MH-45390, and IT-32 MH-20030. AF was supported by Young Investigator Award from the American Society for Suicide Prevention. Data collection was conducted under the supervision of S. Humphries, M.Healy, and A. Finnerty. Additional interviews were conducted by J. Burke, B. Murphy, F. Duke, R. Shinkwin, M. Ni Nuallain, F. McMahon, J. Downing, T. Hebron, B. Hanratty, E. Crowe, M. Doherty, J. Bray, and L. Lowry. This project would not have been possible without the cooperation of the families themselves and the staffs of the many psychiatric hospitals and units in Ireland and Northern Ireland and their efforts are greatly acknowledged. We acknowledge the contributions of Dr. Richard Straub, in whose laboratory His452Tyr and T102C were genotoyped, to previous linkage and association studies in the ISHDSF.
References
- Abdolmaleky HM, Faraone SV, Glatt SJ, Tsuang MT. Meta-analysis of association between the T102C polymorphism of the 5HT2a receptor gene and schizophrenia. Schizophr Res. 2004;67:53–62. doi: 10.1016/s0920-9964(03)00183-x. [DOI] [PubMed] [Google Scholar]
- Arango V, Ernsberger P, Marzuk PM, Chen JS, Tierney H, Stanley M, Reis DJ, Mann JJ. Autoradiographic demonstration of increased serotonin 5-HT2 and beta- adrenergic receptor binding sites in the brain of suicide victims. Arch Gen Psychiatry. 1990;47:1038–1047. doi: 10.1001/archpsyc.1990.01810230054009. [DOI] [PubMed] [Google Scholar]
- Arias B, Gasto C, Catalan R, Gutierrez B, Pintor L, Fananas L. The 5-HT(2A) receptor gene 102T/C polymorphism is associated with suicidal behavior in depressed patients. Am J Med Genet. 2001;105:801–804. doi: 10.1002/ajmg.10099. [DOI] [PubMed] [Google Scholar]
- Asberg M, Traskman L, Thoren P. 5-HIAA in the cerebrospinal fluid. A biochemical suicide predictor? Arch Gen Psychiatry. 1976;33:1193–1197. doi: 10.1001/archpsyc.1976.01770100055005. [DOI] [PubMed] [Google Scholar]
- Baritaki S, Rizos E, Zafiropoulos A, Soufla G, Katsafouros K, Gourvas V, Spandidos DA. Association between schizophrenia and DRD3 or HTR2 receptor gene variants. Eur J Hum Genet. 2004;12:535–541. doi: 10.1038/sj.ejhg.5201180. [DOI] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Biver F, Wikler D, Lotstra F, Damhaut P, Goldman S, Mendlewicz J. Serotonin 5-HT2 receptor imaging in major depression: focal changes in orbito-insular cortex. Br J Psychiatry. 1997;171:444–448. doi: 10.1192/bjp.171.5.444. [DOI] [PubMed] [Google Scholar]
- de Bakker PI, Yelensky R, Pe’er I, Gabriel SB, Daly MJ, Altshuler D. Efficiency and power in genetic association studies. Nat Genet. 2005;37:1217–1223. doi: 10.1038/ng1669. [DOI] [PubMed] [Google Scholar]
- DeRosse P, Funke B, Burdick KE, Lencz T, Ekholm JM, Kane jm, Kucherlapati R, Malhotra AK. Dysbindin genotype and negative symptoms in schizophrenia. Am J Psychiatry. 2006;163:532–534. doi: 10.1176/appi.ajp.163.3.532. [DOI] [PubMed] [Google Scholar]
- Du L, Bakish D, Lapierre YD, Ravindran AV, Hrdina PD. Association of polymorphism of serotonin 2A receptor gene with suicidal ideation in major depressive disorder. Am J Med Genet. 2000;96:56–60. doi: 10.1002/(sici)1096-8628(20000207)96:1<56::aid-ajmg12>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Dudbridge F. Pedigree disequilibrium tests for multilocus haplotypes. Genet Epidemiol. 2003;25:115–121. doi: 10.1002/gepi.10252. [DOI] [PubMed] [Google Scholar]
- Erdmann J, Shimron-Abarbanell D, Rietschel M, Albus M, Maier W, Korner J, Bondy B, Chen K, Shih JC, Knapp M, Propping P, Nothen MM. Systematic screening for mutations in the human serotonin-2A (5-HT2A) receptor gene: identification of two naturally occurring receptor variants and association analysis in schizophrenia. Hum Genet. 1996;97:614–619. doi: 10.1007/BF02281871. [DOI] [PubMed] [Google Scholar]
- Fanous AH, Kendler KS. Genetic heterogeneity, modifier genes, and quantitative phenotypes in psychiatric illness: searching for a framework. Mol Psychiatry. 2005;10:6–13. doi: 10.1038/sj.mp.4001571. [DOI] [PubMed] [Google Scholar]
- Fanous AH, Prescott CA, Kendler KS. The prediction of thoughts of death or self-harm in a population-based sample of female twins. Psychol Med. 2004;34:301–312. doi: 10.1017/s0033291703008857. [DOI] [PubMed] [Google Scholar]
- Fanous AH, Van Den Oord EJ, Riley BP, Aggen SH, Neale MC, O’Neill FA, Walsh D, Kendler KS. Relationship Between a High-Risk Haplotype in the DTNBP1 (Dysbindin) Gene and Clinical Features of Schizophrenia. Am J Psychiatry. 2005;162:1824–1832. doi: 10.1176/appi.ajp.162.10.1824. [DOI] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, Adeyemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Hur K, Bhaumik DK, Mann JJ. The relationship between antidepressant medication use and rate of suicide. Arch Gen Psychiatry. 2005;62:165–172. doi: 10.1001/archpsyc.62.2.165. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, McKenney JD. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci. 1984;35:2505–2511. doi: 10.1016/0024-3205(84)90436-3. [DOI] [PubMed] [Google Scholar]
- Inayama Y, Yoneda H, Sakai T, Ishida T, Nonomura Y, Kono Y, Takahata R, Koh J, Sakai J, Takai A, Inada Y, Asaba H. Positive association between a DNA sequence variant in the serotonin 2A receptor gene and schizophrenia. Am J Med Genet. 1996;67:103–105. doi: 10.1002/(SICI)1096-8628(19960216)67:1<103::AID-AJMG18>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Kendler KS, O’Neill FA, Burke J, Murphy B, Duke F, Straub RE, Shinkwin R, Ni NM, MacLean CJ, Walsh D. Irish study on high-density schizophrenia families: field methods and power to detect linkage. Am J Med Genet. 1996;67:179–190. doi: 10.1002/(SICI)1096-8628(19960409)67:2<179::AID-AJMG8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Laird NM, Horvath S, Xu X. Implementing a unified approach to family-based tests of association. Genet Epidemiol. 2000;19(Suppl 1):S36–S42. doi: 10.1002/1098-2272(2000)19:1+<::AID-GEPI6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Li D, Duan Y, He L. Association study of serotonin 2A receptor (5-HT2A) gene with schizophrenia and suicidal behavior using systematic meta-analysis. Biochem Biophys Res Commun. 2006;340:1006–1015. doi: 10.1016/j.bbrc.2005.12.101. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Mailman RB, Duncan G, Sikich L, Chakos M, Nichols DE, Kraus JE. Serotonergic basis of antipsychotic drug effects in schizophrenia. Biol Psychiatry. 1998;44:1099–1117. doi: 10.1016/s0006-3223(98)00187-5. [DOI] [PubMed] [Google Scholar]
- Livak KJ. Allelic discrimination using fluorogenic probes and the 5′ nuclease assay. Genet Anal. 1999;14:143–149. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Stanley M, McBride PA, McEwen BS. Increased serotonin2 and beta-adrenergic receptor binding in the frontal cortices of suicide victims. Arch Gen Psychiatry. 1986;43:954–959. doi: 10.1001/archpsyc.1986.01800100048007. [DOI] [PubMed] [Google Scholar]
- Manolio TA, Rodriguez LL, Brooks L, Abecasis G, Ballinger D, Daly M, Donnelly P, Faraone SV, Frazer K, Gabriel S, Gejman P, Guttmacher A, Harris EL, Insel T, Kelsoe JR, Lander E, McCowin N, Mailman MD, Nabel E, Ostell J, Pugh E, Sherry S, Sullivan PF, Thompson JF, Warram J, Wholley D, Milos PM, Collins FS. New models of collaboration in genome-wide association studies: the Genetic Association Information Network. Nat Genet. 2007;39:1045–1051. doi: 10.1038/ng2127. [DOI] [PubMed] [Google Scholar]
- Martin ER, Bass MP, Kaplan NL. Correcting for a potential bias in the pedigree disequilibrium test. Am J Hum Genet. 2001;68:1065–1067. doi: 10.1086/319525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin ER, Monks SA, Warren LL, Kaplan NL. A test for linkage and association in general pedigrees: the pedigree disequilibrium test. Am J Hum Genet. 2000;67:146–154. doi: 10.1086/302957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuffin P, Farmer A, Harvey I. A polydiagnostic application of operational criteria in studies of psychotic illness. Development and reliability of the OPCRIT system. Arch Gen Psychiatry. 1991;48:764–770. doi: 10.1001/archpsyc.1991.01810320088015. [DOI] [PubMed] [Google Scholar]
- McQueen MB, Devlin B, Faraone SV, Nimgaonkar VL, Sklar P, Smoller JW, Abou JR, Albus M, Bacanu SA, Baron M, Barrett TB, Berrettini W, Blacker D, Byerley W, Cichon S, Coryell W, Craddock N, Daly MJ, DePaulo JR, Edenberg HJ, Foroud T, Gill M, Gilliam TC, Hamshere M, Jones I, Jones L, Juo SH, Kelsoe JR, Lambert D, Lange C, Lerer B, Liu J, Maier W, Mackinnon JD, McInnis MG, McMahon FJ, Murphy DL, Nothen MM, Nurnberger JI, Pato CN, Pato MT, Potash JB, Propping P, Pulver AE, Rice JP, Rietschel M, Scheftner W, Schumacher J, Segurado R, Van Steen K, Xie W, Zandi PP, Laird NM. Combined analysis from eleven linkage studies of bipolar disorder provides strong evidence of susceptibility loci on chromosomes 6q and 8q. Am J Hum Genet. 2005;77:582–595. doi: 10.1086/491603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell JR, Weeks DE. PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet. 1998;63:259–266. doi: 10.1086/301904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M, Shaffer D, Marcus SC, Greenberg T. Relationship between antidepressant medication treatment and suicide in adolescents. Arch Gen Psychiatry. 2003;60:978–982. doi: 10.1001/archpsyc.60.9.978. [DOI] [PubMed] [Google Scholar]
- Owen MJ, Williams NM, O’Donovan MC. The molecular genetics of schizophrenia: new findings promise new insights. Mol Psychiatry. 2004;9:14–27. doi: 10.1038/sj.mp.4001444. [DOI] [PubMed] [Google Scholar]
- Ozaki N, Rosenthal NE, Pesonen U, Lappalainen J, Feldman-Naim S, Schwartz PJ, Turner EH, Goldman D. Two naturally occurring amino acid substitutions of the 5-HT2A receptor: similar prevalence in patients with seasonal affective disorder and controls. Biol Psychiatry. 1996;40:1267–1272. doi: 10.1016/0006-3223(95)00649-4. [DOI] [PubMed] [Google Scholar]
- Parsons MJ, D’Souza UM, Arranz MJ, Kerwin RW, Makoff AJ. The -1438A/G polymorphism in the 5-hydroxytryptamine type 2A receptor gene affects promoter activity. Biol Psychiatry. 2004;56:406–410. doi: 10.1016/j.biopsych.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Ranade SS, Mansour H, Wood J, Chowdari KV, Brar LK, Kupfer DJ, Nimgaonkar VL. Linkage and association between serotonin 2A receptor gene polymorphisms and bipolar I disorder. Am J Med Genet B Neuropsychiatr Genet. 2003;121:28–34. doi: 10.1002/ajmg.b.20070. [DOI] [PubMed] [Google Scholar]
- Roy A, De Jong J, Linnoila M. Cerebrospinal fluid monoamine metabolites and suicidal behavior in depressed patients. A 5-year follow-up study. Arch Gen Psychiatry. 1989;46:609–612. doi: 10.1001/archpsyc.1989.01810070035005. [DOI] [PubMed] [Google Scholar]
- Schulze TG, Ohlraun S, Czerski PM, Schumacher J, Kassem L, Deschner M, Gross M, Tullius M, Heidmann V, Kovalenko S, Jamra RA, Becker T, Leszczynska-Rodziewicz A, Hauser J, Illig T, Klopp N, Wellek S, Cichon S, Henn FA, McMahon FJ, Maier W, Propping P, Nothen MM, Rietschel M. Genotype-Phenotype Studies in Bipolar Disorder Showing Association Between the DAOA/G30 Locus and Persecutory Delusions: A First Step Toward a Molecular Genetic Classification of Psychiatric Phenotypes. Am J Psychiatry. 2005;162:2101–2108. doi: 10.1176/appi.ajp.162.11.2101. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon J. Structured Clinical Interview for DSM-III-R Patient Version. 1987.
- Van Den Oord EJ, Jiang Y, Riley BP, Kendler KS, Chen X.FP-TDI SNP scoring by manual and statistical procedures: a study of error rates and types Biotechniques 200334610–20., 622. [DOI] [PubMed] [Google Scholar]
- Warren JT, Jr., Peacock ML, Rodriguez LC, Fink JK. An MspI polymorphism in the hyman serotonin receptor gene (HTR2): detection by DGGE and RFLP analysis. Hum Mol Genet. 1993;2:338. doi: 10.1093/hmg/2.3.338. [DOI] [PubMed] [Google Scholar]
- Williams J, Spurlock G, McGuffin P, Mallet J, Nothen MM, Gill M, Aschauer H, Nylander PO, Macciardi F, Owen MJ. Association between schizophrenia and T102C polymorphism of the 5-hydroxytryptamine type 2a-receptor gene. European Multicentre Association Study of Schizophrenia (EMASS) Group. Lancet. 1996;347:1294–1296. doi: 10.1016/s0140-6736(96)90939-3. [DOI] [PubMed] [Google Scholar]
- Yatham LN, Liddle PF, Shiah IS, Scarrow G, Lam RW, Adam MJ, Zis AP, Ruth TJ. Brain serotonin2 receptors in major depression: a positron emission tomography study. Arch Gen Psychiatry. 2000;57:850–858. doi: 10.1001/archpsyc.57.9.850. [DOI] [PubMed] [Google Scholar]
- Zhang K, Calabrese P, Nordborg M, Sun F. Haplotype block structure and its applications to association studies: power and study designs. Am J Hum Genet. 2002;71:1386–1394. doi: 10.1086/344780. [DOI] [PMC free article] [PubMed] [Google Scholar]


