Abstract
Context
Although both genetic and environmental factors affect risk of individual personality disorders (PDs), we know little of how they contribute to the pattern of comorbidity between the PDs in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV).
Objective
To clarify the structure of the genetic and environmental risk factors for the 10 DSM-IV PDs.
Design
Assessment of PDs at personal interview and multivariate twin modeling with the Mx program.
Setting
General community.
Participants
A total of 2794 young adult members of the Norwegian Institute of Public Health Twin Panel.
Main Outcome Measure
Number of endorsed criteria for the 10 DSM-IV PDs.
Results
The best-fit multivariate twin model required 3 genetic and 3 individual-specific environmental factors and genetic and individual-specific factors unique to each PD. The first genetic factor had high loadings on PDs from all 3 clusters including paranoid, histrionic, borderline, narcissistic, dependent, and obsessive-compulsive. The second genetic factor had substantial loadings only on borderline and antisocial PD. The third genetic factor had high loadings only on schizoid and avoidant PD. Several PDs had substantial disorder-specific genetic risk factors. The first, second, and third individual-specific environmental factors had high loadings on the cluster B, A, and C PDs, respectively, with 1 exception: obsessive-compulsive PD loaded with cluster B and not cluster C PDs.
Conclusions
Genetic risk factors for DSM-IV PDs do not reflect the cluster A, B, and C typology. Rather, 1 genetic factor reflects a broad vulnerability to PD pathology and/or negative emotionality. The 2 other genetic factors are more specific and reflect high impulsivity/low agreeableness and introversion. Unexpectedly, the cluster A, B, and C typology is well reflected in the structure of environmental risk factors, suggesting that environmental experiences may be responsible for the tendency of cluster A, B, and C PDs to co-occur.
Twin studies have provided increasing evidence that genetic factors are of etiologic importance for individual personality disorders (PDs).1-5 While efforts have begun to clarify the degree to which genetic and environmental risk factors are shared across PDs, such investigations have been limited to self-report instruments that do not directly assess Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) PDs6 or to the PDs within the 3 DSM-IV PD clusters (A [“odd/eccentric”], B [“dramatic”], and C [“anxious”]).7-9
In this study, our major goal was to determine the structure of the genetic and environmental risk factors for all 10 DSM-IV10 PDs as assessed at personal interview in 2794 young adult Norwegian twins. A secondary aim was to determine whether the structure of these risk factors reflects the cluster A, B, and C typology proposed in DSM-IV.
Methods
Sample and Assessment Methods
Subjects were recruited from the Norwegian Institute of Public Health Twin Panel.11 Twins were identified through the Norwegian National Medical Birth Registry, established January 1, 1967, which receives mandatory notification of all live births. The panel began with 15 370 twins born from 1967 through 1979. Two questionnaire studies were conducted in 1992 (twins born from 1967-1974) and in 1998 (twins born from 1967-1979). Altogether, 12 700 twins received the second questionnaire, and 8045 (3334 pairs and 1377 single responders) responded after 1 reminder (response rate, 63.3%).
Data for this report utilize an interview study of Axis I and Axis II psychiatric disorders begun in 1999. To minimize the number of interviewed single twins, individuals were not approached until preliminary consent had been obtained from both members of the pair. Eligible participants were defined as the 3153 complete pairs of which both members completed the second questionnaire and agreed to cooperate in the interview study, as well as 68 pairs drawn directly from the Norwegian Institute of Public Health Twin Panel. Of these 3221 eligible pairs, 0.8% were unwilling or unable to participate, in 16.2% of pairs only 1 twin agreed to the interview, and 38.2% did not respond after 2 contacts requesting participation. The reasons for noncooperation are illustrated in the pairs of which 1 twin participated. Among the uncooperative twins, there was no response to contact in 96.0%, unknown address in 2.9%, and refusal in 1.1%. Altogether, 2794 twins (43.4% of those eligible) were interviewed. Approval was received from The Norwegian Data Inspectorate and the Regional Ethical Committee, and written informed consent was obtained from all participants after complete description of the study.
A Norwegian version of the Structured Interview for DSM-IV, Personality (SIDP-IV)12 was used to assess PDs. The DSM-III-R13 and DSM-IV versions of this interview14 had been previously used in large-scale studies in Norway. This instrument, a comprehensive semistructured diagnostic interview for the assessment of all DSM-IV PDs, contains nonpejorative questions organized into topical sections rather than by individual PD, thereby improving the interview flow. The SIDP-IV interview was conducted after an extensive interview assessing Axis I disorders. This helped distinguish longstanding behaviors from temporary states resulting from Axis I disorders.
The SIDP-IV uses the “5-year rule,” meaning that behaviors, cognitions, and feelings that predominated for most of the past 5 years are considered representative of the individual's long-term personality. Each DSM-IV criterion is scored as absent (0), subthreshold (1), present (2), or strongly present (3). To keep results parallel with other PDs, in these analyses we examined only the A criterion for antisocial PD.
Interviewers were largely senior clinical psychology students at the end of their 6-year training course (including at least 6 months of clinical practice) and psychiatric nurses with years of patient contact. They were trained by professionals with extensive previous experience with the instrument. The interviews, mostly face to face, were carried out between June 1999 and May 2004. For practical reasons, 231 interviews (8.3%) were done by telephone. Each twin in a pair was interviewed by different interviewers.
Using traditional cutoff scores, the proportion of individuals meeting full DSM-IV criteria for the 10 PDs was too low to usefully analyze.7-9 Therefore, we modeled them as ordinal counts of the number of endorsed criteria. Furthermore, defining a criterion to be present with a score of 1 or greater produced more stable results than using a minimal score of 2. This approach is justified by prior results for these 10 PDs, where, using a multiple threshold model, our group showed that the 4 response options for scoring individual PD criteria reflected varying levels of “severity” on a single continuum of liability.7-9
Very few individuals endorsed most criteria for any individual PD. Therefore, to avoid null cells, we collapsed the total criterion count into 3 to 5 categories depending on their frequencies. We have also tested the validity of this approach by examining the fit of the multiple threshold model. We asked whether the number of endorsed criteria reflected differences of severity on a single normally distributed continuum of liability. This assumption was supported for all 10 PDs examined.7-9
A standard liability-threshold model was used to estimate the genetic and environmental contributions to twin resemblance for dimensional representations of these 10 PDs. For ease of expression, we refer, in the remainder of this article, to personality disorders (PDs) in place of the more accurate but cumbersome term dimensional representations of personality disorders.
Interrater reliability was assessed by 2 raters scoring 70 audiotaped interviews. The intraclass (and polychoric) correlations for the number of endorsed criteria as defined in this study were as follows: paranoid PD, +0.92 (+ 0.94); schizoid PD, + 0.81 (+0.86); schizotypal PD, + 0.86 (+ 0.90); histrionic PD, +0.85 (+ 0.80); borderline PD, +0.93 (+0.94); antisocial PD, +0.91 (+0.94); narcissistic PD, +0.86 (+0.82); avoidant PD, +0.96 (+0.97); and dependent PD, +0.96 (+0.99). Our group has previously examined the structure of the cluster A,7 B,8 and C9 PDs in this sample.
Zygosity Diagnosis
Zygosity was initially determined by questionnaire items that correctly categorize more than 97% of pairs.11 Twenty-four microsatellite markers were then genotyped on 676 of the like-sex pairs in the sample. Results from these markers were used as dependent variables in a discriminant analysis with the questionnaire items as independent variables. Seventeen of these pairs (2.5%) were misclassified by the questionnaire data and were corrected. From these data, we estimated that, in our entire sample, zygosity misclassification occurred in 1% of pairs, a rate unlikely to substantially bias results.15
Data Analyses
Our models divide the sources of individual differences in liability to PDs into additive genetic effects (A), shared environment (C), and unique environment (E).16 The C category reflects family and community experiences that increase similarity in twins reared together; E reflects environmental experiences not shared by twins and measurement error.
Our multivariate twin models include genetic and environmental common factors that influence risk for more than 1 PD as well as disorder-specific influences. Model fitting to raw data, using the method of full information maximum likelihood, was conducted with the use of the Mx program.17 Fitting models simultaneously to 10 polychotomous variables was computationally demanding. Indeed, we had never previously succeeded in obtaining solutions to such models with more than 7 variables.18 In our initial analyses, using standard starting values, run times typically exceeded 4 weeks on our 20 dual-core node Beowulf cluster. This was impractical. Therefore, we fitted models' polychoric correlation matrixes and their corresponding diagonal asymptotic weight matrixes. Taking the best-fit solutions from these models and inputting them as starting values into the full information maximum likelihood analyses markedly reduced run times, typically to between 2 and 5 days.
We began with a model containing 3 genetic, 3 shared environmental, and 3 unique environmental common factors because of the 3-cluster structure of the PDs in DSM-IV. This model also contained genetic, shared, and unique environmental factors specific to each PD. We first compared a common pathway model, in which genetic and environmental common factors influence risk for the individual PDs via their effect on 3 latent factors, and an independent pathway model, in which genetic and environmental common factors directly influence the risk for the individual PDs (see the Figure in Kendler et al19 for more details). Taking the better fitting of these 2 types of models, we then attempted to simplify the model step by step. We did not attempt to eliminate the individual PD-specific unique environmental loadings because this made the unrealistic assumption that the PDs could be assessed without error.
When fitting models with 3 common factors, we identified the solution by arbitrarily setting to zero the loadings of 1 PD in the second factor and 2 PDs in the third factor. For the best-fit model, we submitted the resulting parameter estimates to a varimax rotation in SAS (SAS Institute Inc, Cary, North Carolina), which forced the factors to be orthogonal.20
The goal of our model fitting was to achieve the best balance of explanatory power and model simplicity. This goal was operationalized by the Akaike information criterion (AIC),21 which equals χ2 − 2 df. In model selection, we sought to minimize the AIC value.
Results
Correlation Matrixes
The polychoric correlations in monozygotic and dizygotic twin pairs within and between each of the 10 PDs are seen in Table 1. The correlations with each PD are consistently higher in monozygotic than in dizygotic pairs and generally higher than the correlations across different PDs. Consistent with the expectation that genetic factors contribute to the comorbidity between PDs, the correlations between different PDs were usually stronger in monozygotic than in dizygotic pairs.
Table 1. Pairwise Comparisons of Polychoric Correlations Within and Between the 10 DSM-IV Personality Disorders in MZ and DZ Twin Pairsa.
| TC | Paranoid | Schizoid | Schizotypal | Histrionic | Borderline | Antisocial | Narcissistic | Avoidant | Dependent | Obsessive-Compulsive | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Paranoid | MZ | 0.24 | 0.06 | 0.13 | 0.14 | 0.21 | 0.07 | 0.14 | 0.12 | 0.13 | 0.09 |
| DZ | 0.10 | ||||||||||
| Schizoid | MZ | 0.28 | 0.12 | 0.00 | 0.05 | 0.11 | 0.06 | 0.18 | 0.06 | 0.05 | |
| DZ | 0.09 | 0.14 | |||||||||
| Schizotypal | MZ | 0.26 | 0.10 | 0.15 | 0.16 | 0.06 | 0.11 | 0.15 | 0.07 | ||
| DZ | 0.08 | 0.14 | 0.14 | ||||||||
| Histrionic | MZ | 0.35 | 0.25 | 0.22 | 0.22 | 0.03 | 0.15 | 0.13 | |||
| DZ | 0.05 | 0.06 | 0.01 | 0.10 | |||||||
| Borderline | MZ | 0.36 | 0.27 | 0.18 | 0.14 | 0.21 | 0.07 | ||||
| DZ | 0.12 | 0.10 | 0.16 | 0.11 | 0.19 | ||||||
| Antisocial | MZ | 0.43 | 0.15 | 0.09 | 0.10 | −0.02 | |||||
| DZ | 0.10 | −0.01 | 0.05 | 0.14 | 0.14 | 0.10 | |||||
| Narcissistic | MZ | 0.30 | 0.10 | 0.13 | 0.13 | ||||||
| DZ | 0.08 | 0.09 | 0.11 | 0.11 | 0.12 | 0.05 | 0.10 | ||||
| Avoidant | MZ | 0.37 | 0.23 | 0.08 | |||||||
| DZ | 0.15 | 0.14 | 0.12 | 0.05 | 0.11 | 0.06 | 0.08 | 0.18 | |||
| Dependent | MZ | 0.34 | 0.04 | ||||||||
| DZ | 0.15 | 0.06 | 0.08 | 0.05 | 0.09 | 0.11 | 0.09 | 0.11 | 0.13 | ||
| Obsessive-compulsive | MZ | 0.29 | |||||||||
| DZ | 0.10 | 0.08 | 0.07 | 0.03 | 0.11 | 0.03 | 0.08 | 0.07 | 0.13 | 0.11 |
Abbreviations: DSM-IV, Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition); DZ, dizygotic; MZ, monozygotic; PD, personality disorder; TC, twin correlation.
The MZ TCs are in the shaded cells and DZ TCs in the unshaded cells. Within-PD cross-TCs are stacked in the same column.
Model Fitting
The results of our exploratory model fitting are summarized in Table 2. Model 1 was an independent pathway model containing 3 genetic, 3 shared environmental, and 3 unique environmental common factors and disorder-specific genetic, shared environmental, and unique environmental factors. Model 2 was a 3-factor common pathway model and fitted much worse than model 1 (as indicated by a more positive AIC value). Therefore, all subsequent analyses examined only independent pathway models.
Table 2. Model Fitting Results for Independent Pathway Models for the 10 DSM-IV Personality Disorders.
| Common Factors | Specifics | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | A | C | E | A | C | E | Δχ2 | Δ df | AIC |
| 1 IPa | 3 | 3 | 3 | Present | Present | Present | NAb | NAb | NAb |
| 2 CP | NAb | NAb | NAb | Present | Present | Present | 180.0 | 33 | + 114.0 |
| 3 IPc | 3 | 0 | 3 | Present | Absent | Present | 40.8 | 37 | −33.2 |
| 4 IP | 0 | 3 | 3 | Absent | Present | Present | 144.5 | 37 | + 70.5 |
| 5 IP | 2 | 0 | 3 | Present | Absent | Present | 97.9 | 45 | + 7.9 |
| 6 IP | 3 | 0 | 2 | Present | Absent | Present | 116.3 | 45 | + 26.3 |
| 7 IP | 3 | 0 | 3 | Absent | Absent | Present | 116.6 | 47 | + 22.6 |
Abbreviations: A, additive genetic effects; AIC, Akaike information criterion; C, shared environment; CP, common pathway; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition); E, unique environment; IP, independent pathway; NA, not applicable.
−2lnL = 52783.4; df = 27 783.
The common pathway model had a total of 3 common factors, each of which had individual A, C, and E influences.
Best-fit model.
Models 3 and 4 eliminated from model 1 all shared environmental and all genetic common and disorder-specific factors, respectively. Model 3 fitted considerably better than model 1 (as indicated by a more negative AIC value), while model 4 fitted much worse. We then attempted to simplify model 3 by eliminating 1 genetic common factor (model 5), then by eliminating 1 unique environmental common factor (model 6), and finally by eliminating the disorder-specific genetic effects (model 7). None of these restrictions improved the fit according to AIC. Model 3 was therefore our best-fit model.
Genetic Parameter Estimates
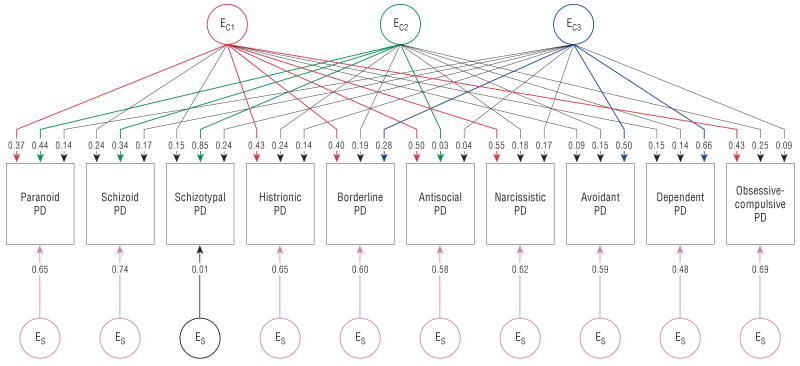
The parameter estimates for the genetic effects of the best-fit model (model 3) are depicted in Figure 1. Colored paths are those with parameter estimates of +0.28 or greater (so they explain ≥8% of phenotypic variance). Two results are noteworthy. First, the pattern of loadings on the 3 genetic factors does not correspond to the DSM-IV cluster typology. The first factor has substantial loadings on 6 PDs: 1 from cluster A (paranoid), 3 from cluster B (histrionic, borderline, and narcissistic), and 2 from cluster C (dependent and obsessive-compulsive). The second factor is much more specific, with substantial loadings on 2 cluster B PDs: borderline and antisocial. The third genetic common factor is also relatively specific, with substantial loadings only on schizoid and avoidant PD.
Figure 1.
Genetic parameter estimates from best-fitting model independent pathway model for 10 Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) personality disorders (PDs). Path estimates are standardized regression coefficients, so they must be squared to obtain the proportion of variance accounted for in the dependent variable. A represents additive genetic effects. The subscripts C and S represent common factor and disorder-specific effects, respectively. The first, second, and third genetic common factors are indicated by the subscripts C1, C2, and C3. Paths with values of +0.28 or greater (which account for ≥8% of phenotypic variance) are colored with the first, second, and third common factors, indicated by red, green, and blue, respectively; the disorder-specific factors are shown in pink. Paths not exceeding the +0.28 or greater cutoff are depicted in black.
Second, these 3 common factors do not capture a considerable proportion of the genetic risk for these 10 PDs. Indeed, loadings on the genetic paths to the individual PDs tended to be modest, with few paths accounting for more than 10% of the phenotypic variance in liability. Six of the 10 PDs had substantial disorder-specific genetic effects, including all 3 cluster A PDs as well as narcissistic, dependent, and obsessive-compulsive PD. Of note, disorder-specific genetic effects have their strongest influence on obsessive-compulsive PD.
Table 3 provides a complementary view of the genetic results of our best-fit model. The estimated heritability of the PDs varies from 20.5% for schizotypal PD to 40.9% for antisocial PD. From a genetic perspective, the 10 PDs could be tentatively classified into 4 groups as a function of whether the largest source of genetic effects originated from factor 1 (paranoid, histrionic, and narcissistic), factor 2 (borderline and antisocial), factor 3 (schizoid and avoidant), or disorder-specific genetic effects (schizotypal, dependent, and obsessive-compulsive).
Table 3. Total Heritability Estimated From the Best-Fit Model for the 10 DSM-IV Personality Disorders and the Proportion of That Heritability Deriving From the 3 Common Genetic Factors and Genetic-Specific Effectsa.
| Proportion of Heritability | |||||
|---|---|---|---|---|---|
| Personality Disorder | Total Heritability | Common Factor 1 | Common Factor 2 | Common Factor 3 | Genetic Specifics |
| Paranoid | 23.4 | 0.38 | 0.07 | 0.21 | 0.34 |
| Schizoid | 25.8 | 0.00 | 0.02 | 0.53 | 0.45 |
| Schizotypal | 20.5 | 0.06 | 0.24 | 0.24 | 0.47 |
| Histrionic | 31.3 | 0.77 | 0.17 | 0.06 | 0.00 |
| Borderline | 37.1 | 0.28 | 0.50 | 0.07 | 0.15 |
| Antisocial | 40.9 | 0.01 | 0.97 | 0.02 | 0.00 |
| Narcissistic | 25.1 | 0.49 | 0.06 | 0.02 | 0.43 |
| Avoidant | 37.3 | 0.05 | 0.01 | 0.94 | 0.00 |
| Dependent | 29.6 | 0.28 | 0.08 | 0.18 | 0.46 |
| Obsessive-compulsive | 27.3 | 0.31 | 0.01 | 0.06 | 0.62 |
Abbreviation: DSM-IV, Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition).
Proportions will not always sum to 1.00 because of rounding.
Unique Environmental Parameter Estimates
The parameter estimates for the unique environmental effects of the best-fit model 3 are depicted in Figure 2. Again, colored paths depict those with parameter estimates of + 0.28 or greater. Two results are of particular interest. First, in contrast to the pattern observed with the genetic factors, the 3 sets of environmental factor loadings identify with substantial fidelity the 3 PD DSM-IV clusters. The first unique environmental common factor has high loadings on all 4 of the cluster B PDs—histrionic, borderline, antisocial, and narcissistic—but also has strong loadings on obsessive-compulsive and paranoid PD. The second factor has substantial loadings on only the 3 cluster A PDs: paranoid, schizoid and schizotypal. The third factor has high loadings on 2 of the 3 cluster C PDs (avoidant and dependent) and a weaker loading on borderline PD.
Figure 2.
Environmental parameter estimates from best-fitting model independent pathway model for 10 Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) personality disorders (PDs). Path estimates are standardized regression coefficients, so they must be squared to obtain the proportion of variance accounted for in the dependent variable. E represents unique environmental effects. The subscripts C and S represent common factor and disorder-specific effects, respectively. The first, second, and third unique environmental common factors are indicated by the subscripts C1, C2, and C3. Paths with values of +0.28 or greater (which account for ≥8% of phenotypic variance) are colored with the first, second, and third common factors, indicated by red, green, and blue, respectively; the disorder-specific factors are shown in pink. Paths not exceeding the +0.28 or greater cutoff are depicted in black.
Second, all of the PDs had substantial disorder-specific unique environmental loadings. The sole exception was schizotypal PD, where the unusually low loading was a consistent feature of all of our models and probably resulted from the very high loading of this PD on the second unique environmental common factor.
Table 4 provides an alternative view of the unique environmental results of our best-fit model. The estimated total environmentality of the PDs varies from 59.1% for antisocial PD to 79.5% for schizotypal PD. For all but 2 of the PDs (schizotypal and dependent), the largest source of environmentality was disorder specific. This is not surprising in that this source of variance includes the effects of errors of measurement. Focusing only on the percentage of variance due to the 3 environmental common factors, the 10 PDs could be tentatively classified into 3 groups. For all cluster B PDs, the greatest proportion of variance resulted from factor 1, but this was also the case for obsessive-compulsive PD. For all cluster A PDs, the greatest proportion of variance resulted from factor 2 (although the percentage variance explained for schizoid PD was rather low). For avoidant and dependent PD, the greatest proportion of variance resulted from factor 3.
Table 4. Total Environmentality Estimated From the Best-Fit Model for the 10 DSM-IV Personality Disorders and the Proportion of That Heritability Deriving From the 3 Common Genetic Factors and Genetic-Specific Effectsa.
| Proportion of Environmentality | |||||
|---|---|---|---|---|---|
| Personality Disorder | Total Environmentality | Common Factor 1 | Common Factor 2 | Common Factor 3 | Environmental Specifics |
| Paranoid | 76.6 | 0.18 | 0.25 | 0.03 | 0.54 |
| Schizoid | 74.2 | 0.08 | 0.15 | 0.04 | 0.74 |
| Schizotypal | 79.5 | 0.03 | 0.91 | 0.07 | 0.00 |
| Histrionic | 68.7 | 0.27 | 0.08 | 0.03 | 0.61 |
| Borderline | 62.9 | 0.25 | 0.06 | 0.12 | 0.57 |
| Antisocial | 59.1 | 0.42 | 0.00 | 0.00 | 0.57 |
| Narcissistic | 74.9 | 0.40 | 0.04 | 0.04 | 0.51 |
| Avoidant | 62.7 | 0.01 | 0.04 | 0.40 | 0.55 |
| Dependent | 70.4 | 0.03 | 0.03 | 0.62 | 0.33 |
| Obsessive-compulsive | 72.7 | 0.25 | 0.09 | 0.01 | 0.65 |
Abbreviation: DSM-IV, Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition).
Proportions will not always sum to 1.00 because of rounding.
Comment
Summary of Main Findings
We sought to elucidate the underlying structure of the genetic and environmental risk factors for the 10 DSM-IV PDs. Our analyses yielded 4 findings worthy of emphasis, the first 2 of which are extensions of previous results with this sample7-9 and will not be commented on further herein. First, we found no evidence that shared environmental factors were of etiologic importance. Like the majority of common Axis I psychiatric disorders16 and normative personality traits,22,23 most familial resemblance for PDs appears to result from genetic factors. Second, the heritabilities for PDs in our sample were modest, ranging from 20% to 41%. For most PDs, our heritability estimates are lower than those reported by Torgersen et al1 from clinically ascertained twin pairs. Third, the structure of the genetic risk factors for the 10 PDs was inconsistent with that predicted by the DSM-IV PD clusters. We found no evidence of distinct genetic factors that contributed to cluster A, B, and C PDs. Instead, our first genetic factor was relatively nonspecific, with substantial loadings on PDs from all 3 clusters. Our second and third genetic factors were, by contrast, more specific. Each loaded strongly on only a pair of PDs. Fourth, in contrast to the genetic factors, the structure of the unique environmental risk factors closely resembled that predicted by the DSM-IV PD clusters. These results suggest that the tendency for PDs to have higher levels of comorbidity within than across clusters results largely from environmental factors.
The Structure of Genetic Risk Factors for PD
Our first genetic factor differed qualitatively from our second and third factors in its generality. It had substantial loadings on 6 PDs representing all 3 clusters: paranoid, histrionic, borderline, narcissistic, dependent, and obsessive-compulsive. This factor might reflect a general vulnerability to PD pathology, which has been detected in a number of previous factor analytic studies of DSM-IV PDs.24-27 Of the 6 PDs that did not have strong loadings on the more specific second and third genetic factors, 5 of them (all but schizotypal PD) had strong loadings on this factor. Our first genetic factor might also be considered from the perspective of the 5-factor model for normative personality. Behavior genetic studies have suggested that the phenotypic dimensions of the 5-factor model are influenced by 5 underlying genetic factors28 that may reflect a universal genetic structure of personality.23 The dimensions of normal and disordered personality may, in part, result from the same genetic architecture.29
Loadings on our first genetic PD factor resemble that predicted for the personality trait of neuroticism/negative emotionality. A recent meta-analysis examining the relationship between DSM-IV PDs and the 5-factor model30 found 5 PDs to be relatively strongly related to neuroticism. Of these 5, 3 had strong loadings on our first genetic factor (borderline, dependent, and paranoid PD).
Our first genetic factor can also be usefully viewed in the context of the most comparable previous study. Livesley and colleagues6 administered the self-report Dimensional Assessment of Personality Pathology, which yields scores on 18 trait scales and 4 higher-order dimensions, to 686 twin pairs from a volunteer twin registry. Although quite different statistical methods were used (oblique principal components analysis of a matrix of genetic correlations), 4 genetic factors were identified. As in our study, their first genetic factor was a very broad one with substantial loadings on 11 of their 18 traits and closely reflected the higher-order factor they termed “emotional dysregulation.” Of note, neuroticism was more strongly related to the traits of the Dimensional Assessment of Personality Pathology than any other of the dimensions of the 5-factor model.29
Our second genetic factor was quite specific, with high loadings on only borderline and antisocial PD. This factor is consistent with results from several family studies showing elevated risk for antisocial PD in relatives of probands with borderline PD. A recent review of this literature31 concludes that the results “suggest familial aggregation of borderline PD and antisocial PD … [and provide] clear evidence in support of a relationship between borderline PD and impulse spectrum disorders.”31(p12) This literature suggests that our second genetic factor may reflect genetic risk for “impulsive aggression,” a central characteristic of both of these PDs.32 Consistent with this hypothesis, a recent study reported an association between polymorphisms in the serotonin transporter gene and antisocial and borderline traits in young adults.33 They concluded that this genotype “may confer a broader endophenotype for impulsive self and other-damaging behaviors that in turn contributes to the more complex antisocial and borderline PD disorders described in DSM-IV.”33(p343)
Our second genetic factor might also be usefully viewed from the perspective of the 5-factor model for normative personality. As suggested by the recent metaanalysis,30 our second genetic factor primarily reflects genetic risk for low conscientiousness and secondarily for low agreeableness. In a hierarchical analysis of the 5-factor model, Markon et al34 suggest that the traits of conscientiousness and agreeableness—those that are indexed by our second genetic factor—are both reflections of a high-order construct they term “disinhibition.”
Of the 4 genetic factors identified by Livesley et al,6 one, which reflected their higher-order factor of dissocial behavior, had substantial similarities to our second genetic factor, with heavy loadings on the traits of “stimulus seeking,” “rejection/hostility,” “callousness,” and “conduct problems.”
Our third genetic factor, which had high loadings only on schizoid and avoidant PD, can also be interpreted in several ways. This factor might reflect genetic risk for schizophrenia spectrum pathology. In favor of this perspective is (1) evidence that both avoidant PD35-37 and schizoid PD35,38 are increased in relatives of schizophrenic probands and (2) the 2 PDs that have been more strongly associated with schizophrenia—schizotypal and paranoid—have at least modest loading (>0.20).35,39 The strongest evidence against this perspective is that the loading on this factor does not correlate well with current evidence about the degree to which various PDs reflect genetic risk for schizophrenia. Loadings are high for PDs that are probably weakly linked to schizophrenia (schizoid and avoidant) and low for PDs that are probably strongly linked to schizophrenia (schizotypal and perhaps paranoid PD).36,40-44
Viewing this second factor from the perspective of the 5-factor model of personality may be more profitable. Schizoid and avoidant PDs both reflect low extraversion, the tendency to reduce or avoid social contact. In a recent meta-analysis,30 the PD with the strongest (negative) association with the personality trait of extraversion was avoidant PD, and the third strongest association (also negative) was with schizoid PD. (Interestingly, the second strongest association with extraversion [positive] was with histrionic PD, which in our analysis had a modest negative path from the third genetic factor.) Finally, the Livesley et al twin study6 also identified a genetic factor—which reflected closely their higher-order factor of “inhibition”—that roughly paralleled our third genetic factor, having strong loadings on the 2 traits of “restricted expression” and “intimacy problems.”
Of interest, the fourth genetic factor in the Livesley et al twin study,6 which reflected their higher-order factor of “compulsivity,” had by far the strongest loading on the lower-order trait of the same name. Our results parallel this finding in that obsessive-compulsive PD had the highest disorder-specific genetic loading, ie, genetic risk factors that were specific to only this PD. These results are consistent with previous findings that obsessive-compulsive PD is only weakly related to the 3 traditional PD clusters.45-49
In a recent review of the genetics of PDs, Livesley and Jang5 concluded that 4 second-order phenotypic factors, supported by behavioral genetics analyses, underlie PD constructs. They wrote, “These secondary domains have been variously labeled a) anxious-dependency/asthenia/emotional dysregulation, b) psychopathy/antisocial/dissocial, c) social withdrawal/asocial/inhibitedness, and d) anacastic/compulsivity.”5(p255) The genetic substrate of DSM-IV PDs shown in these analyses is broadly consistent with their formulation.
Our previous multivariate twin analysis restricted to the 3 cluster A PDs suggested “empirical support for the cluster A construct.”7(p1589) This conclusion requires modification in light of the current findings. When all 10 PDs are considered together, no coherent genetic factor reflecting cluster A PDs emerged. As reviewed earlier, family studies suggest that schizotypal and paranoid and sometimes schizoid PD are found in excess in relatives of schizophrenic probands. Perhaps the genetic risk factors for schizophrenia are too rare in this epidemiologic sample to affect the genetic structure of PDs.
The Structure of Environmental Risk Factors for PD
We identified 3 common factors for environmental experiences unique to individuals that loaded relatively strongly on all of the cluster B PDs (as well as paranoid and obsessive-compulsive PD), all of the cluster A PDs, and 2 of the 3 cluster C PDs (avoidant and dependent), as well as borderline PD.
Such a pattern could also arise because of correlated errors of measurement. However, given that items in our SIDP-IV interview are not arranged by PD cluster, but instead are dispersed by thematic area across the interview, such a bias seems improbable.
Although a moderate body of literature exists on environmental risk factors for PDs (eg, the association between childhood sexual abuse and borderline PD50,51), we are unaware of any comprehensive attempt to explore the impact of a range of adverse environmental exposures on risk of all DSM-IV PDs. We are therefore unable to comment further about the degree to which our observations, based on latent environmental risk factors estimated from a multivariate twin model, can be validated by studies of specific environmental adversities.
Predictions of Sources of Comorbidity
Our best-fit model can also provide insight into the causes of the substantial comorbidity observed between PDs in both clinical52,53 and community54,55 samples. The degree to which this comorbidity arises from genetic vs environmental factors differs widely between pairs of PDs. For example, our model indicates that, within the cluster B disorders, well over half of the comorbidity between borderline and antisocial PD arises from genetic factors shared between the 2 disorders. By contrast, nearly two-thirds of the comorbidity between borderline and narcissistic PD is predicted to arise from environmental experiences that predispose to both of these PDs.
Limitations
These results should be viewed in the context of 7 methodologic limitations. First, because of their rarity, we could not analyze dichotomous DSM-IV PD diagnoses. Instead we examined a dimensional representation of these disorders. We showed previously that, for these PDs, our “criterion count” was indexing the same liability that underlay the fully syndromal diagnoses.7-9 Furthermore, PDs may be best conceptualized as dimensional constructs.56-60 However, much of our information derives from individuals with levels of PD symptoms below diagnostic threshold. Second, because twins were interviewed only once, unreliability of measurement is confounded with individual-specific environment. Although our assessments had high interrater reliability, test-retest reliability is probably considerably lower.61 Indeed, when we modeled errors of measurement for these PDs using both self-report questionnaires and personal interviews,62 considerable levels of unreliability were detected. Such unreliability attenuates heritability estimates. Third, we were unable to get models with sex-dependent genetic and environmental effects to run. Given the absence of detectable sex effects for these PDs when analyzed at the cluster level,7-9 our results are not likely to be substantially biased by our inability to estimate such effects in this study. Fourth, our exploratory models did not explicitly test the DSM-IV cluster model for PDs. Therefore, we fitted a model with 3 uncorrelated common factors for cluster A, B, and C PDs. The PDs were allowed to load only on the cluster to which they were assigned. Confirming results from our exploratory analyses, this model fitted very poorly. Fifth, our results, obtained in young adult Norwegians, may not extend to other cultural and ethnic groups. Sixth, substantial attrition was observed from the original birth registry through 3 waves of contact. Detailed analyses of the predictors of nonresponse across waves (K.T., unpublished data, September 2007) show that cooperation was strongly and consistently predicted by female sex, monozygosity, older age, and higher educational status, but not by psychiatric symptoms or psychoactive drug use. We assessed PD traits at the second questionnaire13 and weighted these items to maximize the prediction of the number of criteria endorsed for the 10 PDs at the later interview. Controlling for demographic variables, these weighted scores did not predict participation in the personal interview. It is unlikely that our sample was substantially unrepresentative with respect to PD psychopathology. Seventh, because of its complexity, it was not possible to obtain confidence intervals for our best-fit model. Although our sample size was relatively large, endorsement rates for many of the PD criteria were low. Previous estimates of confidence intervals from our cluster-level analyses7-9 suggest that most of our estimated parameters are known with only moderate levels of precision.
Acknowledgments
Funding/Support: This study was supported in part by grant MH-068643 from the National Institutes of Health and by grants from the Norwegian Research Council, the Norwegian Foundation for Health and Rehabilitation, the Norwegian Council for Mental Health, and the European Commission under the program “Quality of Life and Management of the Living Resources” of the Fifth Framework Program (No. QLG2-CT-2002-01254).
Footnotes
Financial Disclosure: None reported.
References
- 1.Torgersen S, Lygren S, Oien PA, Skre I, Onstad S, Edvardsen J, Tambs K, Kringlen E. A twin study of personality disorders. Compr Psychiatry. 2000;41(6):416–425. doi: 10.1053/comp.2000.16560. [DOI] [PubMed] [Google Scholar]
- 2.Livesley WJ, Jang KL, Jackson DN, Vernon PA. Genetic and environmental contributions to dimensions of personality disorder. Am J Psychiatry. 1993;150(12):1826–1831. doi: 10.1176/ajp.150.12.1826. [DOI] [PubMed] [Google Scholar]
- 3.Jang K, Vernon PA. Genetics. In: Livesley WJ, editor. Handbook of Personality Disorders: Theory, Research, and Treatment. New York, NY: Guilford Press; 2001. pp. 177–195. [Google Scholar]
- 4.Reichborn-Kjennerud T. Genetics of personality disorders. Psychiatr Clin North Am. 2008;31(3):421–440. doi: 10.1016/j.psc.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Livesley WJ, Jang KL. The behavioral genetics of personality disorder. Annu Rev Clin Psychol. 2008;4:247–274. doi: 10.1146/annurev.clinpsy.4.022007.141203. [DOI] [PubMed] [Google Scholar]
- 6.Livesley WJ, Jang KL, Vernon PA. Phenotypic and genetic structure of traits delineating personality disorder. Arch Gen Psychiatry. 1998;55(10):941–948. doi: 10.1001/archpsyc.55.10.941. [DOI] [PubMed] [Google Scholar]
- 7.Kendler KS, Czajkowski N, Tambs K, Torgersen S, Aggen SH, Neale MC, Reichborn-Kjennerud T. Dimensional representation of DSM-IV cluster A personality disorders in a population-based sample of Norwegian twins: a multivariate study. Psychol Med. 2006;36(11):1583–1591. doi: 10.1017/S0033291706008609. [DOI] [PubMed] [Google Scholar]
- 8.Torgersen S, Czajkowski N, Jacobson K, Reichborn-Kjennerud T, Røysamb E, Neale MC, Kendler KS. Dimensional representations of DSM-IV cluster B personality disorders in a population-based sample of Norwegian twins: a multivariate study. Psychol Med. doi: 10.1017/S0033291708002924. published online ahead of print February 14, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Reichborn-Kjennerud T, Czajkowski N, Neale MC, Ørstavik RE, Torgersen S, Tambs K, Røysamb E, Harris JR, Kendler KS. Genetic and environmental influences on dimensional representations of DSM-IV cluster C personality disorders: a population-based multivariate twin study. Psychol Med. 2007;37(5):645–653. doi: 10.1017/S0033291706009548. [DOI] [PubMed] [Google Scholar]
- 10.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 11.Harris JR, Magnus P, Tambs K. The Norwegian Institute of Public Health twin panel: a description of the sample and program of research. Twin Res. 2002;5(5):415–423. doi: 10.1375/136905202320906192. [DOI] [PubMed] [Google Scholar]
- 12.Pfohl B, Blum N, Zimmerman M. Structured Interview for DSM-IV Personality (SIDP-IV) Iowa City: University of Iowa, Dept of Psychiatry; 1995. [Google Scholar]
- 13.Torgersen S, Kringlen E, Cramer V. The prevalence of personality disorders in a community sample. Arch Gen Psychiatry. 2001;58(6):590–596. doi: 10.1001/archpsyc.58.6.590. [DOI] [PubMed] [Google Scholar]
- 14.Helgeland MI, Kjelsberg E, Torgersen S. Continuities between emotional and disruptive behavior disorders in adolescence and personality disorders in adulthood. Am J Psychiatry. 2005;162(10):1941–1947. doi: 10.1176/appi.ajp.162.10.1941. [DOI] [PubMed] [Google Scholar]
- 15.Neale MC. A finite mixture distribution model for data collected from twins. Twin Res. 2003;6(3):235–239. doi: 10.1375/136905203765693898. [DOI] [PubMed] [Google Scholar]
- 16.Kendler KS, Prescott CA. Genes, Environment, and Psychopathology: Understanding the Causes of Psychiatric and Substance Use Disorders. New York, NY: Guilford Press; 2006. [Google Scholar]
- 17.Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6th. Richmond, VA: Dept of Psychiatry, Virginia Commonwealth University Medical School; 2003. [Google Scholar]
- 18.Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60(9):929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 19.Kendler KS, Heath AC, Martin NG, Eaves LJ. Symptoms of anxiety and symptoms of depression: same genes, different environments? Arch Gen Psychiatry. 1987;44(5):451–457. doi: 10.1001/archpsyc.1987.01800170073010. [DOI] [PubMed] [Google Scholar]
- 20.SAS OnlineDoc Version 9.1.3. Cary, NC: SAS Institute Inc; 2005. [Google Scholar]
- 21.Akaike H. Factor analysis and AIC. Psychometrika. 1987;52(3):317–332. [Google Scholar]
- 22.Bouchard TJ, Jr, Loehlin JC. Genes, evolution, and personality. Behav Genet. 2001;31(3):243–273. doi: 10.1023/a:1012294324713. [DOI] [PubMed] [Google Scholar]
- 23.Yamagata S, Suzuki A, Ando J, Ono Y, Kijima N, Yoshimura K, Ostendorf F, Angleitner A, Riemann R, Spinath FM, Livesley WJ, Jang KL. Is the genetic structure of human personality universal? a cross-cultural twin study from North America, Europe, and Asia. J Pers Soc Psychol. 2006;90(6):987–998. doi: 10.1037/0022-3514.90.6.987. [DOI] [PubMed] [Google Scholar]
- 24.Cox BJ, Sareen J, Enns MW, Clara I, Grant BF. The fundamental structure of axis II personality disorders assessed in the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2007;68(12):1913–1920. doi: 10.4088/jcp.v68n1212. [DOI] [PubMed] [Google Scholar]
- 25.Nestadt G, Hsu FC, Samuels J, Bienvenu OJ, Reti I, Costa PT, Jr, Eaton WW. Latent structure of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition personality disorder criteria. Compr Psychiatry. 2006;47(1):54–62. doi: 10.1016/j.comppsych.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Austin EJ, Deary IJ. The “four As”: a common framework for normal and abnormal personality? Pers Individ Dif. 2000;28(5):977–995. [Google Scholar]
- 27.Rodebaugh TL, Chambless DL, Renneberg B, Fydrich T. The factor structure of the DSM-III-R personality disorders: an evaluation of competing models. Int J Methods Psychiatr Res. 2005;14(1):43–55. doi: 10.1002/mpr.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCrae RR, Jang KL, Livesley WJ, Riemann R, Angleitner A. Sources of structure: genetic, environmental, and artifactual influences on the covariation of personality traits. J Pers. 2001;69(4):511–535. doi: 10.1111/1467-6494.694154. [DOI] [PubMed] [Google Scholar]
- 29.Jang KL, Livesley WJ. Why do measures of normal and disordered personality correlate? a study of genetic comorbidity. J Personal Disord. 1999;13(1):10–17. doi: 10.1521/pedi.1999.13.1.10. [DOI] [PubMed] [Google Scholar]
- 30.Saulsman LM, Page AC. The five-factor model and personality disorder empirical literature: a meta-analytic review. Clin Psychol Rev. 2004;23(8):1055–1085. doi: 10.1016/j.cpr.2002.09.001. [DOI] [PubMed] [Google Scholar]
- 31.White CN, Gunderson JG, Zanarini MC, Hudson JI. Family studies of borderline personality disorder: a review. Harv Rev Psychiatry. 2003;11(1):8–19. doi: 10.1080/10673220303937. [DOI] [PubMed] [Google Scholar]
- 32.Siever LJ, Torgersen S, Gunderson JG, Livesley WJ, Kendler KS. The borderline diagnosis III: identifying endophenotypes for genetic studies. Biol Psychiatry. 2002;51(12):964–968. doi: 10.1016/s0006-3223(02)01326-4. [DOI] [PubMed] [Google Scholar]
- 33.Lyons-Ruth K, Holmesa BM, Sasvari-Szekely M, Ronai Z, Nemoda Z, Pauls D. Serotonin transporter polymorphism and borderline or antisocial traits among low-income young adults. Psychiatr Genet. 2007;17(6):339–343. doi: 10.1097/YPG.0b013e3281ac237e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Markon KE, Krueger RF, Watson D. Delineating the structure of normal and abnormal personality: an integrative hierarchical approach. J Pers Soc Psychol. 2005;88(1):139–157. doi: 10.1037/0022-3514.88.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kendler KS, McGuire M, Gruenberg AM, O'Hare A, Spellman M, Walsh D. The Roscommon Family Study, III: schizophrenia-related personality disorders in relatives. Arch Gen Psychiatry. 1993;50(10):781–788. doi: 10.1001/archpsyc.1993.01820220033004. [DOI] [PubMed] [Google Scholar]
- 36.Asarnow RF, Nuechterlein KH, Fogelson D, Subotnik KL, Payne DA, Russell AT, Asamen J, Kuppinger H, Kendler KS. Schizophrenia and schizophrenia-spectrum personality disorders in the first-degree relatives of children with schizophrenia: the UCLA Family Study. Arch Gen Psychiatry. 2001;58(6):581–588. doi: 10.1001/archpsyc.58.6.581. [DOI] [PubMed] [Google Scholar]
- 37.Fogelson DL, Nuechterlein KH, Asarnow RA, Payne DL, Subotnik KL, Jacobson KC, Neale MC, Kendler KS. Avoidant personality disorder is a separable schizophrenia-spectrum personality disorder even when controlling for the presence of paranoid and schizotypal personality disorders: the UCLA Family Study. Schizophr Res. 2007;91(13):192–199. doi: 10.1016/j.schres.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parnas J, Cannon TD, Jacobsen B, Schulsinger H, Schulsinger F, Mednick SA. Lifetime DSM-III-R diagnostic outcomes in the offspring of schizophrenic mothers: results from the Copenhagen High-Risk Study. Arch Gen Psychiatry. 1993;50(9):707–714. doi: 10.1001/archpsyc.1993.01820210041005. [DOI] [PubMed] [Google Scholar]
- 39.Parnas J, Licht D, Bovet P. Cluster A personality disorders: a review. In: Maj M, Akiskal H, Mezzich JE, Okasha A, editors. Personality Disorders. New York, NY: John Wiley & Sons Ltd; 2005. pp. 1–124. [Google Scholar]
- 40.Kety SS, Wender PH, Jacobsen B, Ingraham LJ, Jansson L, Faber B, Kinney DK. Mental illness in the biological and adoptive relatives of schizophrenic adoptees: replication of the Copenhagen Study in the rest of Denmark. Arch Gen Psychiatry. 1994;51(6):442–455. doi: 10.1001/archpsyc.1994.03950060006001. [DOI] [PubMed] [Google Scholar]
- 41.Onstad S, Skre I, Edvardsen J, Torgersen S, Kringlen E. Mental disorders in first-degree relatives of schizophrenics. Acta Psychiatr Scand. 1991;83(6):463–467. doi: 10.1111/j.1600-0447.1991.tb05577.x. [DOI] [PubMed] [Google Scholar]
- 42.Tienari P, Wynne LC, Laksy K, Moring J, Nieminen P, Sorri A, Lahti I, Wahlberg KE. Genetic boundaries of the schizophrenia spectrum: evidence from the Finnish adoptive family study of schizophrenia. Am J Psychiatry. 2003;160(9):1587–1594. doi: 10.1176/appi.ajp.160.9.1587. [DOI] [PubMed] [Google Scholar]
- 43.Torgersen S, Onstad S, Skre I, Edvardsen J, Kringlen E. “True” schizotypal personality disorder: a study of co-twins and relatives of schizophrenic probands. Am J Psychiatry. 1993;150(11):1661–1667. doi: 10.1176/ajp.150.11.1661. [DOI] [PubMed] [Google Scholar]
- 44.Baron M, Gruen R, Rainer JD, Kane J, Asnis L, Lord S. A family study of schizophrenic and normal control probands: implications for the spectrum concept of schizophrenia. Am J Psychiatry. 1985;142(4):447–455. doi: 10.1176/ajp.142.4.447. [DOI] [PubMed] [Google Scholar]
- 45.Hyler SE, Lyons M. Factor analysis of the DSM-III personality disorder clusters: a replication. Compr Psychiatry. 1988;29(3):304–308. doi: 10.1016/0010-440x(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 46.Kass F, Skodol AE, Charles E, Spitzer RL, Williams JB. Scaled ratings of DSM-III personality disorders. Am J Psychiatry. 1985;142(5):627–630. doi: 10.1176/ajp.142.5.627. [DOI] [PubMed] [Google Scholar]
- 47.Nestadt G, Eaton WW, Romanoski AJ, Garrison R, Folstein MF, McHugh PR. Assessment of DSM-III personality structure in a general-population survey. Compr Psychiatry. 1994;35(1):54–63. doi: 10.1016/0010-440x(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 48.Dyce JA, O'Connor BP. Personality disorders and the five-factor model: a test of facet-level predictions. J Personal Disord. 1998;12(1):31–45. doi: 10.1521/pedi.1998.12.1.31. [DOI] [PubMed] [Google Scholar]
- 49.Sanislow CA, Morey LC, Grilo CM, Gunderson JG, Shea MT, Skodol AE, Stout RL, Zanarini MC, McGlashan TH. Confirmatory factor analysis of DSM-IV borderline, schizotypal, avoidant and obsessive-compulsive personality disorders: findings from the Collaborative Longitudinal Personality Disorders Study. Acta Psychiatr Scand. 2002;105(1):28–36. doi: 10.1034/j.1600-0447.2002.0_479.x. [DOI] [PubMed] [Google Scholar]
- 50.Zanarini MC. Childhood experiences associated with the development of borderline personality disorder. Psychiatr Clin North Am. 2000;23(1):89–101. doi: 10.1016/s0193-953x(05)70145-3. [DOI] [PubMed] [Google Scholar]
- 51.Lieb K, Zanarini MC, Schmahl C, Linehan MM, Bohus M. Borderline personality disorder. Lancet. 2004;364(9432):453–461. doi: 10.1016/S0140-6736(04)16770-6. [DOI] [PubMed] [Google Scholar]
- 52.Oldham JM, Skodol AE, Kellman HD, Hyler SE, Rosnick L, Davies M. Diagnosis of DSM-III-R personality disorders by two structured interviews: patterns of comorbidity. Am J Psychiatry. 1992;149(2):213–220. doi: 10.1176/ajp.149.2.213. [DOI] [PubMed] [Google Scholar]
- 53.Pilkonis PA, Heape CL, Proietti JM, Clark SW, McDavid JD, Pitts TE. The reliability and validity of two structured diagnostic interviews for personality disorders. Arch Gen Psychiatry. 1995;52(12):1025–1033. doi: 10.1001/archpsyc.1995.03950240043009. [DOI] [PubMed] [Google Scholar]
- 54.Coid J, Yang M, Tyrer P, Roberts A, Ullrich S. Prevalence and correlates of personality disorder in Great Britain. Br J Psychiatry. 2006;188:423–431. doi: 10.1192/bjp.188.5.423. [DOI] [PubMed] [Google Scholar]
- 55.Lenzenweger MF, Lane MC, Loranger AW, Kessler RC. DSM-IV personality disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;62(6):553–564. doi: 10.1016/j.biopsych.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oldham JM, Skodol AE. Charting the future of axis II. J Personal Disord. 2000;14(1):17–29. doi: 10.1521/pedi.2000.14.1.17. [DOI] [PubMed] [Google Scholar]
- 57.Skodol AE, Oldham JM, Bender DS, Dyck IR, Stout RL, Morey LC, Shea MT, Zanarini MC, Sanislow CA, Grilo CM, McGlashan TH, Gunderson JG. Dimensional representations of DSM-IV personality disorders: relationships to functional impairment. Am J Psychiatry. 2005;162(10):1919–1925. doi: 10.1176/appi.ajp.162.10.1919. [DOI] [PubMed] [Google Scholar]
- 58.Widiger TA, Simonsen E. Alternative dimensional models of personality disorder: finding a common ground. J Personal Disord. 2005;19(2):110–130. doi: 10.1521/pedi.19.2.110.62628. [DOI] [PubMed] [Google Scholar]
- 59.Widiger TA, Samuel DB. Diagnostic categories or dimensions: a question for the Diagnostic and Statistical Manual of Mental Disorders–fifth edition. J Abnorm Psychol. 2005;114(4):494–504. doi: 10.1037/0021-843X.114.4.494. [DOI] [PubMed] [Google Scholar]
- 60.Cramer V, Torgersen S, Kringlen E. Personality disorders and quality of life: a population study. Compr Psychiatry. 2006;47(3):178–184. doi: 10.1016/j.comppsych.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 61.McGlashan TH, Grilo CM, Sanislow CA, Ralevski E, Morey LC, Gunderson JG, Skodol AE, Shea MT, Zanarini MC, Bender D, Stout RL, Yen S, Pagano M. Two-year prevalence and stability of individual DSM-IV criteria for schizotypal, borderline, avoi dant, and obsessive-compulsive personality disorders: toward a hybrid model of axis II disorders. Am J Psychiatry. 2005;162(5):883–889. doi: 10.1176/appi.ajp.162.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kendler KS, Myers J, Torgersen S, Neale MC, Reichborn-Kjennerud T. The heritability of cluster A personality disorders assessed by both personal interview and questionnaire. Psychol Med. 2007;37(5):655–665. doi: 10.1017/S0033291706009755. [DOI] [PubMed] [Google Scholar]