Abstract
Background
Major depression and alcohol dependence co-occur within individuals and families to a higher than expected degree. This study investigated whether mood-related drinking motives mediate the association between major depression and alcohol dependence, and what the genetic and environmental bases are for this relationship.
Methods
The sample included 5,181 individuals from the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders, aged 30 and older. Participants completed a clinical interview which assessed lifetime major depression, alcohol dependence, and mood-related drinking motives.
Results
Mood-related drinking motives significantly explained the depression-alcohol dependence relationship at both the phenotypic and familial levels. Results from twin analyses indicated that for both males and females, the familial factors underlying mood-related drinking motives accounted for virtually all of the familial variance that overlaps between depression and alcohol dependence.
Conclusions
The results are consistent with an indirect role for mood-related drinking motives in the etiology of depression and alcohol dependence, and suggest that mood-related drinking motives may be a useful index of vulnerability for these conditions.
Keywords: Major Depression, Alcohol Dependence, Drinking Motives, Genetics, Twins
Excessive Alcohol Consumption is the third leading cause of preventable death in the United States (Mokdad et al., 2004), and alcohol use disorders, including alcohol dependence (AD), can result in functional disability and serious immediate and long-term health problems. Similarly, major depression (MD) has a marked effect on quality of life and is associated with substantial impairment in psychosocial and occupational functioning. In the National Comorbidity Study Replication, a nationally representative household survey of 9,282 individuals, 16.6% of the sample met lifetime criteria for MD and 13.2 and 5.4% met lifetime criteria for alcohol abuse and AD (Kessler et al., 2005a). Nationally representative surveys typically find higher prevalence of MD, and lower prevalence of AD, in women compared to men (e.g., Hasin et al., 2005; Kessler et al., 2005a).
MD and AD co-occur within individuals at higher rates than expected by chance in both treatment and community-based studies (Kessler et al., 2005b; Merikangas and Gelernter, 1990; Ross et al., 1988). Nationally representative studies in the United States typically find a 2- to 4-fold increased risk of MD or AD given the occurrence of the other (Grant and Harford, 1995; Kessler et al., 1997; Regier et al., 1990). Although the higher than expected association between AD and MD is widely recognized, there is little agreement about the basis for the relationship between the 2 disorders (for reviews see Davidson and Ritson, 1993; Swendsen and Merikangas, 2000).
Mediation of Negative Affect and AD by Drinking Motives
Cognitive schema, such as drinking motives, is one possible explanation for the association between MD and AD. Drinking motives are important predictors of problem drinking, and 4 key motives for alcohol consumption have been described in the literature: drinking to cope with negative feelings, for psychological or physiological enhancement, for social facilitation, and for conformity (Cooper, 1994; Cooper et al., 1992b). Motivational models of alcohol use (Cooper et al., 1992a, 1995) propose that negative emotions lead to the use of alcohol to cope with negative mood, and the reduction of negative affect is commonly cited as a motive for using alcohol (Peirce et al., 1994). Findings concerning the role of gender in mood-related drinking motives suggest that males typically report stronger mood-related drinking motives and alcohol expectancies than females (Nolen-Hoeksema, 2004).
Previous studies indicate an association between mood-related drinking motives and the occurrence of multiple negative drinking outcomes (Carey and Correia, 1997; Carpenter and Hasin, 1998a,b, 1999; Cooper et al., 1992b; Fromme et al., 1993; Holahan et al., 2003; Kassel et al., 2000; Peirce et al., 1994; Prescott et al., 2004). In addition, mood-related drinking motives and alcohol problems are associated even after adjusting for drinking levels, indicating that these drinking motives contribute additional risk for problematic drinking beyond their influence on alcohol consumption (e.g., Cooper et al., 1988; Kassel et al., 2000).
Several studies have reported that mood-related drinking motives mediate the relationship between negative affect and alcohol-related problems (Cooper et al., 1995; Gaher et al., 2006; Holahan et al., 2004; Peirce et al., 1994), suggesting that individuals with higher levels of negative emotions are at an increased risk of drinking to self-medicate (Colder, 2001). Zack and colleagues (1999) found that among individuals with drinking problems, those with high psychiatric distress demonstrated stronger cognitive associations between negative affect and alcohol concepts. In a prospective study, Holahan and colleagues (2003) found that higher levels of drinking to cope with negative mood at a baseline assessment predicted heavier alcohol consumption at 1-, 4-, and 10-year follow-ups, and alcohol problems at the 1- and 4-year follow-ups. In a Puerto Rican sample, Johnson and Gurin (1994) reported that drinking and negative affect were more likely to co-occur among individuals who believed that alcohol would improve their mood. The available evidence suggests that drinking to regulate affect may partially explain the relationship between depressed mood and alcohol consumption within individuals.
Familial Transmission of MD and AD
Family and twin studies are used to assess possible mechanisms for the association between AD and MD. One proposed explanation that has been extensively evaluated is that the disorders have a shared etiology, such as overlapping genetic or environmental risk factors. The genetic influences on risk for developing MD and AD are well established (Prescott et al., 2006; Sullivan et al., 2000), and several family and adoption studies support the hypothesis of a common familial liability underlying both disorders (Finn et al., 1990; Ingraham and Wender, 1992). For example, Grant and colleagues (1996) and Dawson and Grant (1996) reported that relatives of individuals with both AD and MD had a higher prevalence of AD than relatives of individuals with AD only. In addition, individuals with MD without AD were more likely to have alcoholic relatives compared with individuals without either disorder, suggesting that AD and MD have a shared underlying liability.
Twin studies also provide evidence for overlapping genetic and environmental influences for the association of MD and AD. In an earlier study with the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders (VATSPSUD) sample, Prescott and colleagues (2000) found that 9 to 14% of the variation in liability to AD overlapped with MD, with 50 to 60% of this overlap due to genetic factors and the remainder to specific environmental influences. In a study of Australian twin pairs, Knopik and colleagues (2004) found that a history of MD was an important genetic correlate of risk for alcoholism, but the heritability of alcoholism was still large after adjusting for MD and other psychiatric and personality measures. Lyons and colleagues (2006), in a sample of males from the Vietnam Era Veteran Twin Registry, could not distinguish between the overlapping causes model and a causal model in which MD leads to AD and vice versa. In summary, these results suggest that there are genetic and environmental factors that are overlapping between MD and AD, as well as specific genetic and environmental factors that influence the liability to MD or to AD.
Mediation of MD–AD Overlapping Liability by Drinking Motives
The underlying genetic and environmental factors that contribute to this overlapping liability to MD and AD remain unclear. The phenotypic association between cognitive schema and AD raises the possibility of drinking motives as a potential explanation. Although there is relatively little research on familial influences on drinking motives, the existing research suggests that there is a moderate familial resemblance (possibly genetic) for mood-related drinking motives. Studies have shown that twins have similar beliefs about alcohol and some of this similarity can be attributed to shared genetic factors (Perry, 1973; Prescott et al., 2004; Vernon et al., 1996). Using a sample from VATSPSUD, Prescott and colleagues (2004) found that approximately 40% of variation in mood-related drinking motives was attributed to genetic factors. Agrawal and colleagues (2008) reported that genetic factors contributed to 18% of the total variance in mood-related drinking motives in a sample of young adult same-sex female twins. Results from an adolescent sample in Finland suggest strong genetic effects on positive expectancies and weaker genetic effects on negative expectancies (Merrill et al., 1999; Viken et al., 2002). In addition, multiple family studies indicate that a family history of alcoholism is associated with more positive expectancies and stronger motives for drinking (Brown et al., 1987; Chalder et al., 2006; Mann et al., 1987; Sher et al., 1991; Worobec et al., 1990). Prior research with the current sample suggests that mood-related drinking motives mediate the genetic contribution to AD (Prescott et al., 2004), with genetic factors underlying mood-related drinking motives accounting for virtually all of the genetic risk for AD in males, and about two-thirds of the genetic risk in females.
Given the evidence that the overlap between MD and AD, and the overlap between AD and mood-related drinking motives are partially familial, it is possible that mood-related drinking motives contribute to the familial association between MD and AD. One potential mechanism is that there is a genetic basis underlying positive hedonic response to alcohol, which would increase likelihood of drinking in response to negative mood, and indirectly increase risk for AD. Alternatively, there may be an inherited sensitivity to negative affect, which could contribute to drinking to manage mood in response to psychological distress, increasing risk for AD and/or the development of MD. It is also possible that mood-related drinking motives are transmitted through the family environment. Individuals reared in an environment where alcohol is used as a means to cope with MD may learn to manage negative mood states in the same way.
In summary, mood-related drinking motives are associated with negative mood and multiple indices of problem drinking. Prior studies have examined the contribution of mood-related drinking to depression and alcohol use symptomatology, but to the best of our knowledge this is the first study examining whether mood-related drinking motives mediate the familial association of MD and AD diagnoses. We evaluated 2 hypotheses: First, that mood-related drinking motives partially mediate the association between lifetime MD and AD. Second, that mood-related drinking motives have a substantial familial overlap with MD and AD and partially mediate the overlapping risk for lifetime MD and AD.
Material and Methods
Subjects
The sample comprised individuals from the VATSPSUD, a longitudinal study of psychopathology in 2 samples of adult twins. The samples were originally identified through the Virginia Twin Registry, and include Caucasian twins born in Virginia between 1934 and 1974. Data for this study come from wave 4 of data collection in female twin pairs (FF4), a telephone interview conducted 1995 to 1997. Of 2,295 women eligible to participate in FF4, 1,939 (84.5%) were interviewed. This study also includes data from wave 2 of data collection in male–male and male–female (MM/MF2) twin pairs, an in-person interview conducted 1995 to 1998. Of 6,812 males and females eligible to participate in MM/MF2, 5,621 individuals (82.5%) were interviewed. (See Kendler and Prescott, 2006 for more details on recruitment and nonparticipation.) The MM/MF2 and FF4 assessments were chosen for this study because the AD assessment was most comparable across the 2 samples and these provide the most recent information about lifetime MD and AD. Participants were informed about the purpose of the study and gave verbal informed consent before telephone interviews and written informed consent before in-person interviews.
For this study, we limited the sample to individuals aged 30 and older to exclude participants who were not yet past the primary risk period for developing either disorder. This resulted in excluding 2,029 participants from the original sample. Although limiting our sample reduces our power, it is expected to increase the accuracy of our results. The ages of onset for MD and AD differ across gender (Beckman, 1975; Piccinelli and Wilkinson, 2000), and it is especially important that our sample consists of individuals who have already passed through the highest risk period to make inferences about sex differences. This way, estimates of twin-pair resemblance in the opposite-sex pairs are not biased in a different way than might exist for same-sex pairs. Age 30 was used as the cut-off age because previous research with this sample shows incidence of MD and AD levels off in males and females after age 30 (Kuo et al., 2006).
Unlike alcohol expectancies, drinking motives are based on personal experience with drinking alcohol, and motives for drinking are not valid to measure in lifetime abstainers and very infrequent drinkers. Consequently, 325 individuals who reported that they had never used alcohol or had never consumed 12 or more drinks within a year were not administered the motives questionnaire and are excluded from these analyses. Another 25 individuals missing AD data are excluded from these analyses. An additional 686 individuals did not return the motives questionnaire and are subsequently missing on drinking motives; however, the MD and AD data for these individuals are included in the analyses and we examine the effects of this incomplete data in the Results.
The analyses for this study are thus based on data from a total of 5,181 individuals aged 30 and older who completed the clinical interview, including 1,321 females from FF4 and 932 females and 2,928 males from MM/MF2. The sample includes 3,908 individuals from complete pairs and 1,273 individuals whose cotwin did not participate or were excluded for insufficient drinking history. The mean age of the sample is 40.6 (SD = 7.0, range 30–62).
Measures
Lifetime AD and MD diagnoses were made using DSM-IV criteria based on a structured diagnostic interview assessment, adapted from the Structured Clinical Interview for DSM Disorders (Spitzer and Williams, 1985) and administered by clinically trained interviewers. Use of lifetime AD and MD, rather than current diagnosis, allows for the assessment of liability to these disorders. Although participants who have not had AD or MD may still develop these disorders in their 30 seconds or later, the twin pairs are matched for age and are thus at the same place in the distribution of risk. Test–retest reliability among randomly selected participants re-interviewed 2 to 8 weeks after their original interview was κ = 0.66 (95% CI= 0.58–0.74) for MD (N = 375) and κ = 0.72 (95% CI = 0.61–0.82) for AD (N = 382) (Kendler and Prescott, 2006).
Drinking motives were assessed using 4 scales from the Alcohol Use Inventory (AUI), a self-report measure developed in alcoholism treatment centers (Horn and Wanberg, 1983). This study utilizes the Mood Management scale (abbreviated MOT for motives). Respondents answered 7 items that assess mood-related drinking motives (e.g., Do you drink to change your mood? Do you start drinking to get over being depressed?). The time frame for measurement for the MOT scale is not specified in the AUI instructions, except that individuals who are not current drinkers are instructed to answer for the period when they were drinking. The internal consistency estimate (Cronbach alpha) for the MOT scale was 0.82 and the test–retest correlation based on 256 subjects assessed twice over a 8-week interval was r = 0.85 (Prescott et al., 2004). A principal components analysis of MOT items yielded 1 large factor (eigenvalue = 3.5), that accounted for 50% of the total variance in the 7 items. Factor loadings from the 1-factor solution ranged from 0.48 to 0.81. MOT scores were calculated by adding the item scores (0 = no, 1 = yes) and dividing by the number of items (1 item was coded 0 to 2) to get an average score. The distribution of scores was positively skewed, so scores were log-transformed prior to analysis.
In the Results, we report analyses that evaluated several covariates that could affect the association between AD and MD, including gender, age, education, and family income. We also examined the evidence for biases associated with incomplete data and sample attrition.
Twin Model Estimation and Assumptions
Twin models were fit directly to the raw data and included individuals from both complete and incomplete pairs. Standard liability threshold models were used to estimate the genetic and environmental contributions to twin-pair resemblance for their liability to MD and AD. Liability is an inferred trait that is assumed to be continuous and normally distributed. Individual differences in liability arise from 3 sources: additive genetic variation (A) from genes whose allelic effects combine additively; common environment (C) includes all shared environments that make family members more similar, and specific environment (E) which includes all remaining environmental factors and measurement error. Monozygotic (MZ) twins are similar because they share all of their genetic and shared environmental factors, whereas dizygotic (DZ) twins resemble each other because they share on average half of their genetic variation and all of their shared environment. Comparing the resemblance of MZ and DZ twins allows for the estimation of each source's contribution to individual differences in liability to a disorder, or to the covariance between disorders.
Twin studies have several assumptions including: random mating with respect to genetic liability, equal environment assumption (EEA), and additivity and independence of genetic and environmental components. Although there is evidence of phenotypic nonrandom mating for MD and AD (e.g., Maes et al., 1998), it does not seem to be a large enough effect to substantially bias the estimates in the current analyses. The EEA is that the shared environment of MZ pairs is equally similar to that of DZ pairs. Previous studies of MD and AD in the VATSPSUD are consistent with the validity of the EEA (Kendler and Gardner, 1998). In a traditional twin design (without information on other relatives), the assumptions of additivity and independence cannot be evaluated. However, studies of alcohol consumption in extended families suggest gene–environment correlations contribute only a small amount of variance (Eaves et al., 1989).
Statistical Analyses
Analyses included all participants with lifetime MD and AD information regardless of whether their co-twins participated in the study. Analyses were conducted in 3 steps. First, biases due to attrition and incomplete data were tested by comparing group means using SAS™ software version 9.1 (SAS Institute, 2004).
Second, the mediating role of MOT (Hypothesis 1) was investigated by structural modeling analyses of the phenotypic data. The correlation between MD and AD was estimated before and after partialling the variance in each disorder attributable to MOT. Standard errors and statistical tests were adjusted to account for correlated observations by allowing twins within pairs to be correlated (but the correlations were otherwise unstructured).
Third, evidence for Hypothesis 2, mediation at the familial level was investigated by structural models fit to the twin-pair data. These models are the biometric version of the analyses conducted for step 2. Several trivariate twin models were fit to partition the covariance among MOT, MD, and AD into genetic and environmental components.1
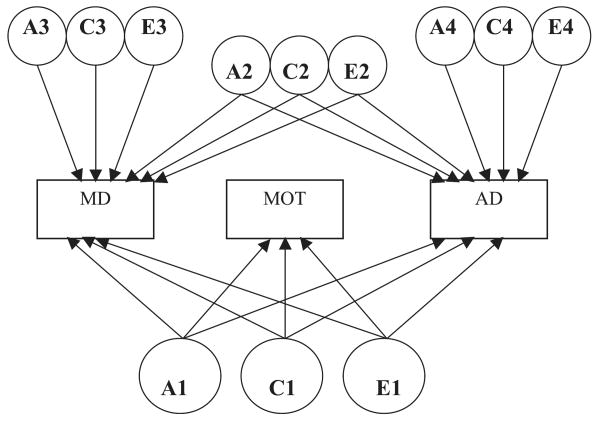
Model I is a variation of a full Cholesky decomposition model (Neale and Cardon, 1992) that assesses indirect mediation (see Fig. 1). In the Cholesky model, the contribution of an independent variable is assessed after adjusting for its shared variance with other independent variables. In the current multivariate model, the first set of factors (A1, C1, and E1) contributes to MOT, MD, and AD. The second set of factors is shared only between MD and AD (A2, C2, and E2), the third set of factors is unique to MD (A3, C3, and E3), and fourth set of factors is unique to AD (A4, C4, and E4).2 Mediation is estimated by examining how much overlapping variance remains between MD and AD after partialling the variance in common with MOT.
Fig. 1.
Path diagram for indirect mediation model of the alcohol dependence (AD)–major depression (MD) relationship by drinking to manage mood (MOT). A, additive genetic factors; C, common environmental factors; E, individual-specific environmental factors. Model shown for 1 twin in a pair. MOT indirectly explains the overlap between MD and AD if the loadings on A2, C2, and/or E2 paths are significantly reduced with the inclusion of the factors underlying MOT. For model identification, the corresponding MD and AD loadings from A2, C2, and E2 are equated. Thresholds and mean values were estimated but not shown.
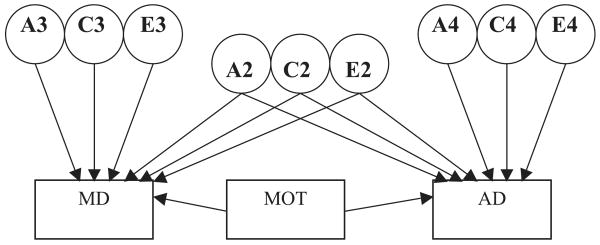
The fit of this baseline model (Model 1, Table 4) was compared to 3 models representing alternative mechanisms for mediation of the MD–AD association: (1) complete mediation (that the MD–AD association is completely explained by the familial and environmental factors overlapping with MOT) was tested with a model that fixed the genetic, common environment, and individual specific environment paths from the MD–AD common factor (A2, C2, and E2) to zero; (2) familial mediation was tested with a model that fixed the genetic and common environment paths from the MD–AD common factor (A2 and C2) to zero; and (3) a direct mediation model was tested that directly regressed AD and MD on MOT (see Fig. 2).
Table 4.
Goodness-of-Fit Results From Twin Models for Associations Among Lifetime Alcohol Dependence (AD), Major Depression (MD), and Drinking to Manage Mood (MOT)
| Difference test | ||||||
|---|---|---|---|---|---|---|
| Model | Model description | χ2* (param) | Comp | ΔParam | Δχ2 | p-Value |
| Indirect mediation models | ||||||
| I | Full unconstrained model | 73.6 (38) | – | – | – | – |
| II | Reduced model | 79.2 (26) | I | 12 | 7.6 | 0.58 |
| III | Complete mediation model | 90.3 (22) | II | 4 | 23.6 | 0.001 |
| IV | Familial mediation | 79.8 (24) | II | 2 | 0.7 | 0.71 |
| Direct mediation model | ||||||
| V | Full unconstrained direct mediation model | 95.0 (30) | I | 8 | 28.7 | 0.001 |
N = 5,181 twins from 3,904 pairs.
χ2*, Mplus WLSMV chi-square values are approximate, but chi-square difference test values are exact; Param, number of free parameters; Comp, comparison model; Reduced model, no common environment for males or females.
Fig. 2.
Path diagram for direct mediation model of the alcohol dependence (AD)–major depression (MD) relationship by drinking to manage mood (MOT). MOT directly explains the overlap between MD and AD if the genetic (A2) and/or environmental paths (C2, E2) contributing to MD and AD are significantly reduced with the inclusion of MOT in the model.
All structural models were fit using Mplus™ software version 4.2 (Muthen and Muthen, 2006) using the WLSMV estimation option (See Prescott, 2004, for details of twin modeling with Mplus). The goodness-of-fit of alternative models was evaluated using chi-square difference tests (with p < 0.05 indicating worse fit). All model comparisons were between nested models, so the model fits could be compared directly. Standard likelihood-based calculations using the Mplus MISSING command were used to incorporate information from the 625 nonabstaining individuals missing MOT scores. Essentially, this estimates model parameters adjusting for missing data using the missing at random assumption. Including information from people without MOT provides more data to test the basis for the association of MD with AD and also provides correct estimates for the MD–AD association if it varies between those with and without MOT data.
In prior research with this sample, males had a higher prevalence of AD and higher MOT scores, and females had a higher prevalence of MD. Consequently, the phenotypic analyses were fit allowing gender differences in prevalence, regressions and correlations. Unless stated otherwise, all twin models were fit allowing the means, thresholds, variances, and covariances to vary across males and females.
Results
Descriptive statistics by sex for AD, MD, MOT, and demographic variables are listed in Table 1. There were significant sex differences for all 3 measures. Consistent with prior findings in the larger sample from which these participants were drawn, compared to females, males had higher prevalence of lifetime AD (26.2% vs. 10.1%) and MOT scores (.20 vs. .16), and lower prevalence of lifetime MD (29.9% vs. 36.6%). Correlations of MD, AD, and MOT by sex and zygosity are shown in Table 2. AD was significantly correlated with MD and MOT for both males and females. MZ pair correlations were greater than DZ pair correlations for all main variables except MD in males, consistent with genetic influences on MOT and AD in both sexes, and MD in females. The observation that DZ male pairs were nearly as correlated for MD as MZ pairs is consistent with familial environmental effects, but combined with the opposite-sex DZ pair information indicates a combination of genetic and family environment.
Table 1.
Descriptive Statistics for Alcohol Dependence (AD), Major Depression (MD), Mood Drinking Motives (MOT), and Demographic Variables
| Males | Females | |||
|---|---|---|---|---|
| Variable | M (SD) | Range | M (SD) | Range |
| Age (years) | 41.1 (7.1) | 30–58 | 39.5 (6.8) | 30–62 |
| Education (years) | 13.4 (2.8) | 2–20 | 13.9 (2.4) | 3–20 |
| Family income ($1,000s) | 56.8 (36.9) | 0–230 | 56.5 (37.4) | 0–230 |
| MOT | 0.20 (0.21) | 0–0.76 | 0.16 (0.19) | 0–0.77 |
| Lifetime MDa (%) | 29.9 | 36.6 | ||
| Lifetime ADa (%) | 26.2 | 10.1 | ||
| MD and AD (%) | 11.8 | 6.9 | ||
Males (N = 2,928) females (N = 2,253). MOT is log of the average Mood score.
Includes individuals who have both MD and AD.
Table 2.
Within-Person and Cross-Twin Correlations for Lifetime DSM-IV Alcohol Dependence (AD), Major Depression (MD), and Mood-Related Drinking Motives (MOT)
| Within-person correlations | Cross-twin correlations | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Males | Females | Male MZ pairs | Male DZ pairs | Female MZ pairs | Female DZ pairs | Opposite-sex | DZ pairs |
| N | 2,928 | 2,253 | 426 | 332 | 318 | 227 | 649 | |
| MOT | – | – | 0.38 (0.002) | 0.13 (0.002) | 0.44 (0.002) | 0.08 (0.002) | 0.15 (0.002) | |
| MD | – | – | 0.25 (0.08) | 0.23 (0.09) | 0.45 (0.08) | 0.08 (0.11) | 0.16 (0.05) | |
| AD | – | – | 0.60 (0.07) | 0.34 (0. 09) | 0.63 (0.10) | 0.18 (0.18) | 0.16 (0.09) | |
| MOT–MD | 0.34 (0.02) | 0.23 (0.03) | 0.19 (0.01) | 0.12 (0.01) | 0.14 (0.01) | 0.03 (0.01) | 0.10a (0.01) | 0.11b (0.01) |
| MOT–AD | 0.54 (0.03) | 0.53 (0.03) | 0.37 (0.01) | 0.19 (0.01) | 0.43 (0.01) | 0.11 (0.02) | 0.07c (0.01)) | 0.08d (0.01 |
| MD–AD | 0.32 (0.03) | 0.43 (0.03) | 0.20 (0.06) | 0.21 (0.07) | 0.20 (0.09) | 0.02 (0.10) | 0.12e (0.08) | 0.19f (0.07) |
Values in parentheses are standard errors.
MZ, monozygotic; DZ, dizygotic.
Model based estimates from Mplus. Number of complete pairs with MOT: Male MZ = 355; Male DZ = 282; Female MZ = 207; Female DZ = 143; Opp. Sex = 539.
Male MOT with female MD.
Female MOT with male MD.
Male MOT with female AD.
Female MOT with male AD.
Male MD with female AD.
Female MD with male AD.
Testing for Biases From Incomplete Data
Possible biases due to incomplete data were tested in several ways. Demographic variables, MD and AD were used to predict the presence or absence of MOT scores using multiple logistic regression. The only finding significant at p < 0.05 was that females with lower education were more likely to be missing MOT scores (p < 0.01). However, the effect size was small (OR = 1.08, per year of schooling) and not of practical significance. There were no significant differences between individuals missing MOT and those not missing MOT on any of the demographic variables or AD in either gender. Females missing MOT were significantly less likely to have MD (32.3% compared to 37.6%, p < 0.05).
Next, bias from incomplete twin pairs was evaluated by comparing means of MOT scores and prevalence of diagnoses of participants from complete versus incomplete pairs. The groups did not differ significantly on AD, MD, or MOT scores. We also found no evidence that the AD–MOT relationship differed by completion status. In a logistic regression analysis of AD on MOT, MD, completion status, and the interaction of completion by MOT, there was no significant interaction for completion × MOT.
In prior analyses with the complete sample, younger age, lower education, and lower family income were associated with higher risk for AD. We therefore investigated the potential impact of these characteristics on our analyses. We tested whether the MD–AD association was attenuated by accounting for covariates (i.e., mediation) as well as whether it varied across levels of covariates (i.e., moderation). Regressions of AD on MD and cross twin MD–AD correlations were computed with and without adjusting for the demographic covariates. The effect of covariates on the regression of AD on MD or on the cross-twin MD–AD correlations was negligible (reduced in value by <0.02) in males and females. In addition, there were no significant interactions of MD with the demographic variables in predicting AD in either gender.
In sum, the results suggest little evidence of bias from missing MOT data or incomplete twin pairs. Given the trivial influence of demographic covariates on the AD–MD correlations, and the complexity of including them in the twin models, phenotypic and twin-pair analyses were conducted without covariates.
Phenotypic Mediation Analyses
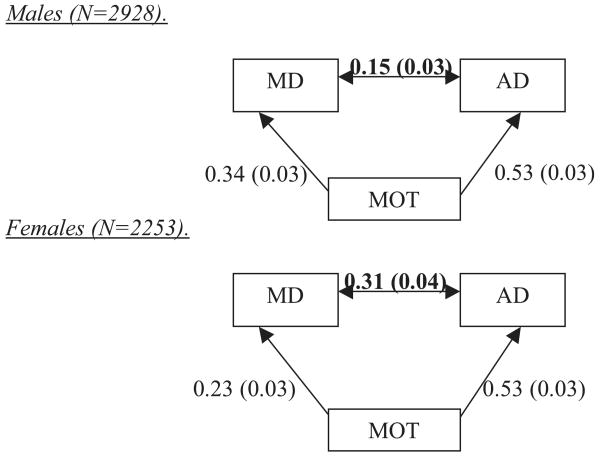
To test the mediation hypothesis, the phenotypic relationships among MD, AD, and MOT were investigated. A preliminary model equating the correlation estimates among MD, AD, and MOT across males and females produced a poor fit to the data [Δχ2(3) = 13.3, p < 0.01], so all models were fit allowing gender differences. The estimates from a baseline model correlating MOT, MD, and AD (Model I, Table 3), were compared to those of a partial mediation model that regressed AD on MOT, MD on MOT, and estimated the residual correlation between AD and MD (Model II, Table 3). Comparing the MD–AD estimates from the baseline and partial mediation model indicates how much the MD–AD relationship changes when the overlap due to MOT is partialled (Fig. 3). There was a significant reduction in the correlation between AD and MD after including the regressions of AD on MOT and MD on MOT. In males, the MD–AD correlation was reduced from r = 0.32 to 0.15 (p < 0.01). In females, the reduction was somewhat less, from 0.43 to 0.31 (p < 0.01).
Table 3.
Goodness-of-Fit Results From Phenotypic Analyses Testing the Mediation of the Association of Alcohol Dependence (AD) and Major Depression (MD) by Drinking Motives (MOT)
| Difference test | ||||||
|---|---|---|---|---|---|---|
| Model | Model description | χ2 (param) | Comp | ΔParam | Δχ2 | p-Value |
| I | Baseline correlational model | 66.6 (44) | – | – | ||
| II | Partial mediation model | 67.3 (44) | – | – | ||
| III | Complete mediation | 111.7 (42) | II | 2 | 96.5 | 0.001 |
N = 5,181; Param, number of free parameters; Comp, comparison model.
Bold entries indicate that estimates are shown in Fig. 3.
Fig. 3.
Standardized estimates from mediation analyses of the alcohol dependence (AD)–major depression (MD) relationship by drinking to manage mood (MOT). Estimates from Model II in Table 3.
A third model was also fit, in which the MD–AD residual correlation was fixed at zero. This resulted in significantly worse model fit [Δχ2(2) = 96.5, p < 0.01], indicating that the association between MD and AD cannot be fully accounted for by MOT (Model III, Table 3).
Twin Models
Several trivariate twin models were fit to partition the covariance among MOT, MD, and AD into genetic and environmental components. First, a full trivariate model equating all the unique and common parameters across males and females, produced a poor fit to the data [Δχ2(18) = 39.9, p < 0.01], so all subsequent models were fit allowing gender differences. Next, a full trivariate model that estimated all possible unique and common parameters served as the baseline model (Model I, Table 4, also see Fig. 1). Based on the obtained estimates from Model I, a reduced model was fit that fixed nonsignificant paths in Model I to zero (Model II, Table 4). All common environmental influences for females and males (i.e., all loadings on C1, C2, C3, and C4) could be fixed to zero without a significance worsening in fit [Δχ2(12) = 7.6, p < 0.58].
Model III (Table 4) tests the hypothesis of complete mediation: that MD and AD are associated only through the familial and environmental effects they share with MOT. This was fit by altering Model II so that the loadings of AD and MD on A2 and E2 were fixed at zero. This model fit significantly worse than Model II [Δχ2(4) = 23.6, p < 0.001], indicating that there is not complete mediation.
In Model II, the path from the genetic factor underlying MD and AD (A2) was not significantly different from zero for males or females. We thus fit a reduced model with these paths fixed to zero, representing the hypothesis of no familial overlap of MD and AD other than what is shared with MOT. This model (IV in Table 4) does not fit significantly worse than Model II [Δχ2(2) = 0.7, p < 0.71], consistent with complete mediation of the familial MD–AD overlap.
Finally, a direct mediation model was tested to represent the hypothesis that drinking to manage mood directly mediates the association between MD and AD (see Fig. 2 and Model V, Table 4). This model fit significantly worse than the baseline indirect mediation model [Δχ2(8) = 28.7, p < 0.001], so further reductions of this model were not conducted.
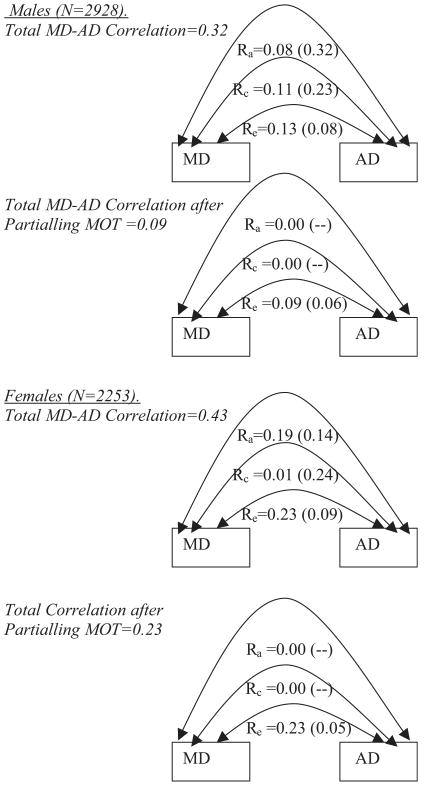
Based on these results, the familial mediation model (IV) was selected as the best-fitting model for representing the basis for the associations among MOT, AD, and MD. Table 5 provides standardized parameter estimates and standard errors from Model IV. Figure 4 shows how the MD–AD association is partitioned into genetic (ra), common environment (rc), and individual-specific environment (re) components in the full bivariate model (i.e., before partialling MOT) and after partialling MOT (Model IV). For males, ra is reduced from 0.08 to 0.0, rc is reduced from 0.11 to 0.0, and re is reduced from 0.13 to 0.09. For females, ra is reduced from 0.19 to 0.0, rc is reduced from 0.01 to 0.0, and re remains the same (0.23).
Table 5.
Standardized Parameter Estimates and Standard Errors From Best Fitting Indirect Twin Model (Model IV, Table 4) for Alcohol Dependence (AD), Major Depression (MD), and Drinking to Manage Mood (MOT)
| Variable | Common factor 1 | Residual MD–AD factor 2 | MD-specific factor 3 | AD-specific factor 4 | Total % variance |
|---|---|---|---|---|---|
| Males | A1 | A2 | A3 | A4 | a2 |
| MOT | 0.59 (0.09) | 35% | |||
| MD | 0.32 (0.06) | 0.00 (−) | 0.41 (0.08) | 27% | |
| AD | 0.61 (0.06) | 0.00 (−) | 0.47 (0.07) | 59% | |
| E1 | E2 | E3 | e2 | ||
| MOT | 0.81 (0.06) | 65% | |||
| MD | 0.19 (0.05) | 0.30 (0.06) | 0.78 (0.06) | 73% | |
| AD | 0.22 (0.05) | 0.30 (0.06) | 0.52 (0.06) | 41% | |
| Females | A1 | A2 | A3 | A4 | a2 |
| MOT | 0.59 (0.09) | 35% | |||
| MD | 0.31 (0.08) | 0.00 (−) | 0.57 (0.09) | 41% | |
| AD | 0.60 (0.09) | 0.00 (−) | 0.46 (0.12) | 57% | |
| E1 | E2 | E3 | e2 | ||
| MOT | 0.81 (0.06) | 65% | |||
| MD | 0.06 (0.06) | 0.49 (0.06) | 0.59 (0.09) | 59% | |
| AD | 0.23 (0.07) | 0.49 (0.06) | 0.38 (0.12) | 43% | |
Estimates are from Model IV in Table 4. The first set of factors (A1, E1) are factors shared among all 3 variables. A2 and E2 are shared by MD and AD; A3 and E3 are specific to MD; A4 and E4 are specific to AD.
a2, total genetic influences from all sources; e2, total individual specific environment.
All common environmental loadings were estimated at or could be fixed to 0. Values in parentheses are standard errors. (−)parameter fixed to zero.
Fig. 4.
Major depression (MD)–alcohol dependence (AD) correlations before and after partialling drinking to manage mood (MOT). For each gender, estimates for upper figures are based on full bivariate model (see text). Those for the lower figures are from Table 5.
Discussion
Although prior research indicates MD and mood-related drinking motives each share familial risk with AD, no prior study has examined the role of mood-related drinking motives as a mediator of the familial association between MD and AD. We first hypothesized that mood-related drinking motives would partially explain the MD–AD relationship in both males and females. The results of this study support this hypothesis.
Given that individual differences in MD, AD, and mood-related drinking motives are partially heritable, we also hypothesized that mood-related drinking motives would have a substantial familial overlap with MD and AD, and partially explain some of the familial overlapping risk for lifetime MD and AD. The results were consistent with this hypothesis: In both males and females, the familial factors underlying drinking to manage mood accounted for essentially all of the genetic and common environmental overlap of MD and AD.
Gender Differences in Mediation
Our results indicate stronger mood-related drinking motives, and a greater association between MD and mood-related drinking motives, in males compared to females. These findings are consistent with results from a comprehensive review of gender differences in risk factors for alcoholism, indicating that males consistently report stronger mood-related drinking motives and expectancies than females (Nolen-Hoeksema, 2004). Although the familial MD–AD association is entirely explained by mood-related drinking motives in both genders, the remaining MD–AD overlap, due to individual-specific factors, is larger in females compared to males.
There are several potential mechanisms behind the gender differences in mediation. The degree to which an individual drinks alcohol to regulate mood is partially determined by the availability of alterative emotion regulation strategies. Because seeking treatment and social support for MD may be perceived as more socially acceptable for females (Moller-Leimkuhler, 2002), males may be more likely than females to drink to regulate mood rather than seek treatment or help from others. Some evidence indicates males are more likely to respond to negative mood states by taking action or distracting themselves, whereas females are more likely to engage in passive rumination as a coping strategy (Nolen-Hoeksema and Girgus, 1994). This gender difference in strategy potentially explains the gender difference in residual MD–AD overlap after accounting for mood-related drinking motives. Research also suggests that females perceive greater social sanctions for drinking and are more nurturant toward others than males, factors which may protect them from the development of alcohol-related problems (Nolen-Hoeksema and Girgus, 1994) and possibly decrease risk for drinking in response to negative mood.
Mechanisms for Overlapping Vulnerability
Our results shed light on several possible explanations for the etiology of the familial overlap between MD, AD, and mood-related drinking motives.
Overlapping dysregulations in stress and reward pathways probably contribute to a common biological pathway to both MD and AD (e.g., Markou et al., 1998; Rao, 2006), and individuals with a genetic predisposition to neurochemical dysfunction may be at an increased risk for MD, mood-related drinking motives, and AD (e.g., Lotrich and Pollock, 2004; Nellissery et al., 2003; Samochowiec et al., 2006).
Another explanation for how mood-related drinking motives might explain the overlapping MD–AD genetic variance is that genetic variation underlies the degree to which individuals experience alcohol as mood enhancing (Koob and Le Moal, 2001). If positive hedonic response to alcohol is genetically influenced, and promotes drinking to manage mood in response to MD, this could create shared genetic variation among MD, mood-related drinking motives, and AD.
It is also possible that mood-related drinking motives, MD, and AD are all influenced by some other, genetically influenced variable. If the personality trait of neuroticism is associated with high stress reactivity and leads to increased mood-related drinking, it could create shared genetic variance for MD and AD. This explanation is supported by evidence that neuroticism accounts for approximately one-third of the comorbidity between MD and AD (Khan et al., 2005).
Clinical Implications
These findings have implications for prevention and intervention for alcohol use disorders. Some prevention strategies are aimed at challenging and changing alcohol expectancies and drinking motives. However, changes in expectancies and motives do not always lead to reductions in alcohol consumption (Jones et al., 2001). Our results are not consistent with mood-related drinking motives acting as a direct cause of the association between MD and AD. Instead, drinking to manage mood may be a useful index of vulnerability for risk to develop MD and AD. This suggests that interventions that target drinking motives may not necessarily directly lead to long-term changes in risk for AD. Interestingly, these results differ from our findings for anxiety and heavy drinking, which are more consistent with anxiety-reduction motives acting as a direct mediator between social anxiety and heavy drinking (Jajodia et al., 2008).
These findings do not preclude the possibility that MD can directly lead to AD, and vice versa, and it is likely that there are multiple mechanisms of overlapping vulnerability. However, the current findings are informative about the mechanisms underlying the familial association between MD and AD even without knowing the direction of effect.
Some of these interpretations are tentative and await replication. Longitudinal, genetically informative studies that assess both positive and negative motives for drinking and their associations with depressive symptoms and alcohol use are needed to clarify these explanations.
Limitations and Strengths
One limitation to this study is the use of Caucasian Virginia-born twins. Although Virginia is culturally and geographically diverse, more research is needed to determine whether the results from our study can be generalized to individuals from other ethnic and regional backgrounds.
Another potential limitation is recall bias and accuracy of self-reported lifetime MD and AD symptoms based on one measurement occasion. However, test–retest reliability in this sample is good and supports the reliability of the interview procedures.
Our emphasis on clinical diagnoses overlooks individuals with sub-threshold symptoms. We chose to use DSM-IV diagnoses for this study because they are clinically informative and have not been addressed in prior studies that assessed the contribution of drinking motives to the relationship between mood and drinking.
Another conceptual difficulty is the lack of measured drinking motives for lifetime abstainers. Because abstainers are not at risk for developing AD, their information would not be informative for predicting familial risk for overlapping MD and AD.
Because this study was cross-sectional and drinking to manage mood probably reflects current drinking motives, the results cannot be used to evaluate the temporal association between MD and AD. However, given that the familial overlap between MD and AD is completely accounted for by familial factors shared with mood-related drinking motives, this study is still informative about the mechanisms underlying the association between MD and AD.
There are several strengths to this study. This is the first study to assess the role of drinking to manage mood as a mediator of the association between clinically assessed MD and AD. The use of a large sample of community-ascertained twins is especially instructive because it provides data to test explicit hypotheses about the importance of genetic and environmental risk factors underlying mood-related drinking motives in contributing to overlapping liability to MD and AD. Limiting the sample to individuals past the primary risk period for developing MD and AD is expected to increase the accuracy of the results, especially given the different distributions of onset age of MD and AD across gender.
This study contributes to understanding the mechanisms underlying the co-occurrence of lifetime MD and AD. Prospective twin studies that assess the role of drinking motives in the development of AD and MD at multiple time points are needed to clarify the temporal nature of the relationships among these variables. Future studies should also incorporate the role of measured genes in the development of MD and AD over time.
Acknowledgments
Data collection supported by grants MH/AA-49492, AA/DA-09095, and AA-00236 from the National Institutes of Health and funds from the Carman Trust and the WM Keck, John Templeton, and Robert Wood Johnson Foundations. Analyses supported by an Individual Investigator award from the National Alliance for Research on Schizophrenia and Depression (to CAP) and a Kellerman Fellowship in Clinical Psychology (to KYW). Patsy Waring, Frank Butera, Sarah Woltz, Barbara Brooke, and Lisa Halberstadt supervised data collection, Indrani Ray and Steven Aggen provided programming and database management. Linda Corey and the staff of the Mid-Atlantic Twin Registry assisted with subject identification and recruitment. We thank Archana Jajodia, Margaret Gatz, Lewina Lee, Susan Luczak, and Richard Viken for helpful suggestions about the analyses. Preliminary versions of these analyses were presented at the 2008 annual meetings of the Research Society on Alcoholism and the Behavior Genetics Association.
Footnotes
Past research using the VATSPSUD sample found the genetic correlation for AD in opposite sex pairs to be less than the expected 0.5 (i.e., genetic factors are not completely the same in males and females). In this sample, the genetic correlation of AD in opposite sex twins (ra) was estimated at 0.29, but was not significantly different from 0.50. Therefore, all models fixed ra at the assumed value of 0.5.
This model is mathematically equivalent to a full Cholesky model, but estimates residual factors for both MD and AD. To identify this model, we equated the loadings of A2, C2, and E2 to be equivalent for MD and for AD. Doing this clarifies the interpretation of the estimates for the factors contributing to the MD–AD overlap.
References
- Agrawal A, Dick DM, Bucholz KK, Madden PAF, Cooper ML, Sher KJ, Heath AC. Drinking expectancies and motives: a genetic study of young adult women. Addiction. 2008;103:194–204. doi: 10.1111/j.1360-0443.2007.02074.x. [DOI] [PubMed] [Google Scholar]
- Beckman LJ. Women alcoholics. A review of social and psychological studies. J Stud Alcohol. 1975;36:797–824. doi: 10.15288/jsa.1975.36.797. [DOI] [PubMed] [Google Scholar]
- Brown SA, Christiansen BA, Goldman MS. Alcohol expectancy questionnaire: an instrument for the assessment of adolescent and adult alcohol expectancies. J Stud Alcohol. 1987;48:483–491. doi: 10.15288/jsa.1987.48.483. [DOI] [PubMed] [Google Scholar]
- Carey KB, Correia CJ. Drinking motives predict alcohol-related problems in college students. J Stud Alcohol. 1997;58:100–105. doi: 10.15288/jsa.1997.58.100. [DOI] [PubMed] [Google Scholar]
- Carpenter KM, Hasin DS. Reasons for drinking alcohol: relationships with DSM-IV alcohol diagnoses and alcohol consumption in a community sample. Psychol Addict Behav. 1998a;12:168–184. [Google Scholar]
- Carpenter KM, Hasin DS. A prospective evaluation of the relationship between reasons for drinking and DSM-IV alcohol use disorders. Addict Behav. 1998b;23:41–46. doi: 10.1016/s0306-4603(97)00015-4. [DOI] [PubMed] [Google Scholar]
- Carpenter KM, Hasin DS. Drinking to cope with negative affect and DSM-IV alcohol use disorders: a test of three alternative explanations. J Consult Clin Psychol. 1999;51:93–99. doi: 10.15288/jsa.1999.60.694. [DOI] [PubMed] [Google Scholar]
- Chalder M, Elgar FJ, Bennett P. Drinking and motivations to drink among adolescent children of parents with alcohol problems. Alcohol Alcohol. 2006;41:107–113. doi: 10.1093/alcalc/agh215. [DOI] [PubMed] [Google Scholar]
- Colder CR. Life stress, physiological and subjective indexes of negative emotionality, and coping reasons for drinking: is there evidence for a self-medication model of alcohol use? Psychol Addict Behav. 2001;15:237–245. [PubMed] [Google Scholar]
- Cooper ML. Motivations for alcohol use among adolescents: development and validation of a four-factor model. Psychol Assess. 1994;6:117–128. [Google Scholar]
- Cooper ML, Frone MR, Russell M, Mudar P. Drinking to regulate positive and negative emotions: a motivational model of alcohol use. J Pers Soc Psychol. 1995;69:990–1005. doi: 10.1037//0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Russell M, George WH. Coping, expectancies, and alcohol abuse: a test of social learning formulations. J Abnorm Psychol. 1988;97:218–230. doi: 10.1037//0021-843x.97.2.218. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Russell M, Skinner JB, Frone MR, Mudar P. Stress and alcohol use: moderating effects of gender, coping, and alcohol expectancies. J Abnorm Psychol. 1992a;101:139–152. doi: 10.1037//0021-843x.101.1.139. [DOI] [PubMed] [Google Scholar]
- Cooper ML, Russell M, Skinner JB, Windle M. Development and validation of a three-dimensional measure of drinking motives. Psychol Assess. 1992b;4:123–132. [Google Scholar]
- Davidson KM, Ritson BE. The relationship between alcohol dependence and depression. Alcohol Alcohol. 1993;28:147–155. [PubMed] [Google Scholar]
- Dawson DA, Grant BF. Family history of alcoholism and gender: their combined effects on DSM-IV alcohol dependence and major depression. J Stud Alcohol. 1996;59:97–106. doi: 10.15288/jsa.1998.59.97. [DOI] [PubMed] [Google Scholar]
- Eaves LJ, Eysenck HJ, Martin NG. Genes, Culture and Personality: An Empirical Approach. Academic Press; San Diego: 1989. [Google Scholar]
- Finn PR, Kleinman I, Pihl RO. The lifetime prevalence of psychopathology in men with multigenerational family histories of alcoholism. J Nerv Ment Dis. 1990;178:500–504. [PubMed] [Google Scholar]
- Fromme K, Stroot E, Kaplan D. Comprehensive effects of alcohol: development and psychometric analysis of a new alcohol expectancy questionnaire. Psychol Assess. 1993;5:19–26. [Google Scholar]
- Gaher RM, Simons JS, Jacobs GA, Meyer D, Johnson-Jimenez E. Coping motives and trait negative affect: testing mediation and moderation models of alcohol problems among American Red Cross disaster workers who responded to the September 11, 2001 terrorist attacks. Addict Behav. 2006;31:1319–1330. doi: 10.1016/j.addbeh.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: results of a national survey. Drug Alcohol Depend. 1995;39:197–206. doi: 10.1016/0376-8716(95)01160-4. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Dawson DA. The relationship between DSM-IV alcohol use disorders and DSM-IV major depression: examination of the primary-secondary distinction in a general population sample. J Affect Disord. 1996;38:113–128. doi: 10.1016/0165-0327(96)00002-x. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the national epidemiologic survey on alcoholism and related conditions. Arch Gen Psychiatry. 2005;62:1097–1106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- Holahan CJ, Moos RH, Holahan CK, Cronkite RC, Randall PK. Drinking to cope and alcohol use and abuse in unipolar depression: a 10-year model. J Abnorm Psychol. 2003;112:159–165. [PubMed] [Google Scholar]
- Holahan CJ, Moos RH, Holahan CK, Cronkite RC, Randall PK. Unipolar depression, life context vulnerabilities, and drinking to cope. J Consult Clin Psychol. 2004;72:269–275. doi: 10.1037/0022-006X.72.2.269. [DOI] [PubMed] [Google Scholar]
- Horn JL, Wanberg KW. Assessment of alcohol use with multidimensional concepts and measures. Am Psychol. 1983;38:1055–1069. doi: 10.1037//0003-066x.38.10.1055. [DOI] [PubMed] [Google Scholar]
- Ingraham LJ, Wender PH. Risk for affective disorder and alcohol and other drug abuse in the relatives of affectively ill adoptees. J Affect Disord. 1992;26:45–52. doi: 10.1016/0165-0327(92)90033-3. [DOI] [PubMed] [Google Scholar]
- Jajodia A, Kendler KS, Prescott CA. Mediation of the genetic link between social anxiety and heavy drinking by drinking motives. Alcohol Clin Exp Res. 2008;32(Suppl):238a. [Google Scholar]
- Johnson PB, Gurin G. Negative affect, alcohol expectancies and alcohol-related problems. Addiction. 1994;89:581–586. doi: 10.1111/j.1360-0443.1994.tb03334.x. [DOI] [PubMed] [Google Scholar]
- Jones BT, Corbin W, Fromme K. A review of expectancy theory and alcohol consumption. Addiction. 2001;96:57–72. doi: 10.1046/j.1360-0443.2001.961575.x. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Jackson SI, Unrod M. Generalized expectancies for negative mood regulation and problem drinking among college students. J Stud Alcohol. 2000;61:332–340. doi: 10.15288/jsa.2000.61.332. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO. Twin studies of adult psychiatric and substance dependence disorders: are they biased by differences in the environmental experiences of monozygotic and dizygotic twins in childhood and adolescence? Psychol Med. 1998;28:625–633. doi: 10.1017/s0033291798006643. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Genes, Environment, and Psychopathology. Guilford; New York: 2006. [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005a;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005b;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Crum R, Warner LA, Nelson CB, Schulenberg H, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Khan AA, Jacobson KC, Gardner CO, Prescott CO, Kendler KS. Personality and comorbidity of common psychiatric disorders. Br J Psychiatry. 2005;186:190–196. doi: 10.1192/bjp.186.3.190. [DOI] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Madden PF, Bucholz KK, Slutske WS, Nelson EC, Statham D, Whitfield JB, Martin NG. Genetic effects on alcohol dependence risk: re-evaluating the importance of psychiatric and other heritable risk factors. Psychol Med. 2004;34:1519–1530. doi: 10.1017/s0033291704002922. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychcopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Kuo P, Gardner CO, Kendler KS, Prescott CA. The temporal relationship of alcohol dependence and major depression: using a genetically informative study design. Psychol Med. 2006;36:1153–1162. doi: 10.1017/S0033291706007860. [DOI] [PubMed] [Google Scholar]
- Lotrich FE, Pollock BG. Meta-analysis of serotonin transporter polymorphisms and affective disorders. Psychiatr Genet. 2004;14:121–129. doi: 10.1097/00041444-200409000-00001. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, Schultz MS, Neale MN, Brady K, Eisen S, Toomey R, Rhein A, Faraone S, Tsuang M. Specificity of famililial vulnerability for alcoholism versus major depression in men. J Nerv Ment Dis. 2006;194:809–817. doi: 10.1097/01.nmd.0000244480.78431.49. [DOI] [PubMed] [Google Scholar]
- Maes HH, Neale MC, Kendler KS, Hewitt JK, Silberg JL, Foley DL, Meyer JM, Rutter M, Simononoff E, Pickles A, Eaves LJ. Assortative mating for major psychiatric diagnoses in two population-based samples. Psychol Med. 1998;28:1389–1401. doi: 10.1017/s0033291798007326. [DOI] [PubMed] [Google Scholar]
- Mann LM, Chassin L, Sher KJ. Alcohol expectancies and the risk for alcoholism. J Consult Clin Psychol. 1987;55:411–417. doi: 10.1037//0022-006x.55.3.411. [DOI] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Gelernter CS. Comorbidity for alcoholism and depression. Psychiatr Clin North Am. 1990;13:13–631. [PubMed] [Google Scholar]
- Merrill KA, Viken RJ, Kaprio J, Rose RJ. Thinking about drinking: genetic and environmental influences on alcohol expectancies. Paper presented at: The Annual Meeting of the Behavior Genetics Association; July 1999; Vancouver, Canada. 1999. [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Moller-Leimkuhler AM. Barriers to help-seeking by men: a review of sociocultural and clinical literature with particular reference to depression. J Affect Disord. 2002;71:1–9. doi: 10.1016/s0165-0327(01)00379-2. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus User's Guide. 4th. Muthen and Muthen; Los Angeles: 2006. [Google Scholar]
- Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families (NATO ASI Series D: Behavioral and Social Sciences, Vol. 67) Kluwer Academic; Dordrecht, The Netherlands: 1992. [Google Scholar]
- Nellissery M, Feinn RS, Covault J, Gelernter J, Anton RF, Pettinati H, Moak D, Mueller T, Kranzler HR. Alleles of a functional serotonin transporter promoter polymorphism are associated with major depression in alcoholics. Alcohol Clin Exp Res. 2003;27:1402–1408. doi: 10.1097/01.ALC.0000085588.11073.BB. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. Gender differences in risk factors and consequences for alcohol use and problems. Clin Psychol Rev. 2004;24:981–1010. doi: 10.1016/j.cpr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychol Bull. 1994;115:424–443. doi: 10.1037/0033-2909.115.3.424. [DOI] [PubMed] [Google Scholar]
- Peirce RS, Frone MR, Russell M, Cooper ML. Relationship of financial strain and psychosocial resources to alcohol use and abuse: the mediating role of negative affect and drinking motives. J Health Soc Behav. 1994;35:291–308. [PubMed] [Google Scholar]
- Perry A. The effect of heredity on attitudes toward alcohol, cigarettes and coffee. J Appl Psychol. 1973;58:275–277. [Google Scholar]
- Piccinelli M, Wilkinson G. Gender differences in depression. Br J Psychiatry. 2000;177:486–492. doi: 10.1192/bjp.177.6.486. [DOI] [PubMed] [Google Scholar]
- Prescott CA. Using the Mplus computer program to estimate models for continuous and categorical data from twins. Behav Genet. 2004;34:17–40. doi: 10.1023/B:BEGE.0000009474.97649.2f. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Aggen SH, Kendler KS. Sex-specific genetic influcenes on the comorbidity of alcoholism and major depression in a population-based sample of U.S. twins. Arch Gen Psychiatry. 2000;57:803–811. doi: 10.1001/archpsyc.57.8.803. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Cross RJ, Kuhn JW, Horn JL, Kendler KS. Is risk for alcoholism mediated by individual differences in drinking motivations? Alcohol Clin Exp Res. 2004;28:29–39. doi: 10.1097/01.ALC.0000106302.75766.F0. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Madden PA, Stallings MC. Challenges in genetic studies of the etiology of substance use and substance use disorders: introduction to the special issue. Behav Genet. 2006;36:473–492. doi: 10.1007/s10519-006-9072-9. [DOI] [PubMed] [Google Scholar]
- Rao U. Links between depression and substance abuse in adolescents. Am J Prev Med. 2006;31:S161–S174. doi: 10.1016/j.amepre.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Lock B, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse: results from the Epidemiologic Catchment Area Study. JAMA. 1990;264:2511–2519. [PubMed] [Google Scholar]
- Ross HE, Glaser FB, Germanson T. The prevalence of psychiatric disorders in patients with alcohol and other drug problems. Arch Gen Psychiatry. 1988;45:1023–1031. doi: 10.1001/archpsyc.1988.01800350057008. [DOI] [PubMed] [Google Scholar]
- Samochowiec J, Kucharska-Mazur J, Grzywacz A, Jablonski M, Rommelspacher H, Samochowiec A, Sznabowicz M, Horodnicki H, Sagan L, Pelka-Wysiecka J. Family-based and case-control study of DRD2, DAT, 5HTT, COMT genes polymorphisms in alcohol dependence. Neurosci Lett. 2006;410:1–5. doi: 10.1016/j.neulet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc [SAS] Version 9.1.3. SAS Institute Inc.; Cary, NC: 2004. [Google Scholar]
- Sher KJ, Walitzer KS, Wood PK, Brent EE. Characteristics of children of alcoholics: putative risk factors and substance use and abuse, and psychopathology. J Abnorm Psychol. 1991;100:427–448. doi: 10.1037//0021-843x.100.4.427. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-III-R (SCID) Biometrics Research Department, New York State Psychiatric Institute; New York: 1985. [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. The genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Swendsen JD, Merikangas KR. The comorbidity of depression and substance use disorders. Clin Psychol Rev. 2000;20:173–189. doi: 10.1016/s0272-7358(99)00026-4. [DOI] [PubMed] [Google Scholar]
- Vernon PA, Lee D, Harris JA, Jang KL. Genetic and environmental contributions to individual differences in alcohol expectancies. Pers Individ Dif. 1996;21:183–187. [Google Scholar]
- Viken RJ, Johnson JK, Kaprio J, Rose RJ. Genetic and environmental contributions to variation in alcohol expectancies and to the covariation between expectancies and related phenotypes. Paper presented at: The Annual Meeting of the Behavior Genetics Association; July 2002; Keystone, CO. 2002. [Google Scholar]
- Worobec TG, Winston MT, O'Farrell TJ, Cutter HS, Bayog RD, Tsuang MT. Alcohol use by alcoholics with and without a history of parental alcoholism. Alcohol Clin Exp Res. 1990;14:887–892. doi: 10.1111/j.1530-0277.1990.tb01832.x. [DOI] [PubMed] [Google Scholar]
- Zack M, Toneatto T, MacLeod CM. Implicit activation of alcohol concepts by negative affective cues distinguishes between problem drinkers with high and low psychiatric distress. J Abnorm Psychol. 1999;108:518–531. doi: 10.1037//0021-843x.108.3.518. [DOI] [PubMed] [Google Scholar]