Abstract
A specific mutation (ΔE302/303) in the torsinA gene underlies most cases of dominantly inherited early-onset torsion dystonia. This mutation causes the protein to aggregate and form intracellular inclusion bodies in cultured cells and animal models. Co-expression of the wildtype and mutant proteins resulted in the redistribution of the wildtype protein from the endoplasmic reticulum to inclusion bodies in cultured HEK293 cells, and this was associated with increased interaction between the two proteins. Expression of ΔE302/303 but not wildtype torsinA in primary postnatal midbrain neurons resulted in the formation of intracellular inclusion bodies, predominantly in dopaminergic neurons. Tyrosine hydroxylase was sequestered in these inclusions and this process was mediated by increased protein-protein interaction between mutant torsinA and tyrosine hydroxylase. Analysis in an inducible neuroblastoma cell culture model demonstrated altered tyrosine hydroxylase activity in the presence of the mutant but not wildtype torsinA protein. Our results suggest that the interaction of tyrosine hydroxylase and mutant torsinA may contribute to the phenotype and reported dopaminergic dysfunction in torsinA-mediated dystonia.
Keywords: Dystonia, dopamine, catecholaminergic neurons, inclusion body
Primary dystonias are a heterogeneous group of neurologic conditions characterised by involuntary, sustained muscle contractions affecting one or more body segments, with dystonia as the sole or major symptom (Nemeth, 2002). Early-onset torsion dystonia (EOTD) is the most common and severe form of dystonia and is often associated with mutation of the DYT1 gene torsinA. In torsinA dystonia, deletion of one of a pair of glutamate residues (ΔE302/303) in the carboxy-terminal region of torsinA is associated with autosomal dominant inheritance with reduced penetrance (Ozelius et al., 1997). The neuropathology of torsinA dystonia has been described in several cases and the apparent absence of neuronal loss has led to the suggestion that torsinA dystonia is not a degenerative disease but is primarily due to neuronal dysfunction (Walker et al., 2002, Rostasy et al., 2003, McNaught et al., 2004).
The exact function of torsinA is unknown, but homology to AAA+ ATPases (Neuwald et al., 1999, Ogura and Wilkinson, 2001) and functional studies suggest a possible chaperone role (McLean, 2002, Caldwell et al., 2003, Cao et al., 2005). AAA+ proteins often self-associate and function as an oligomeric complex and torsinA has previously been shown to form higher molecular weight species and oligomerise (Kustedjo et al., 2000, Torres et al., 2004). In addition, torsinA has been shown to interact with a number of proteins of diverse function. This includes cytoskeletal-associated cytoplasmic proteins such as kinesin light chain (KLC), vimentin and actin (Kamm et al., 2004, Hewett et al., 2006). TorsinA also interacts with nuclear envelope (NE) and endoplasmic reticulum (ER) resident proteins LAP1, LULL1, nesprin-3 and printor (Goodchild and Dauer, 2005, Nery et al., 2008, Giles et al., 2009). The identification of multiple binding partners suggests that disruption of torsinA interactions might contribute to the disease phenotype. The ability to correctly oligomerise has been shown to be necessary for AAA+ ATPase function in several proteins (reviewed in (Mogk et al., 2008)). Moreover, the ΔE302/303 deletion has been demonstrated to increase interaction of the mutant protein with both itself and nesprin-3 (Torres et al., 2004, Nery et al., 2008). A feature of the ΔE302/303 mutation is the aggregation and mislocalisation of the protein to perinuclear inclusion bodies and the nuclear envelope in cell and animal models (Hewett et al., 2000, Goodchild and Dauer, 2004, Goodchild et al., 2005), suggesting a potential dominant-negative disease mechanism involving sequestration of both wildtype and interacting proteins (Torres et al., 2004).
There is considerable evidence implicating an imbalance in dopaminergic function in the aetiology of dystonia. Individuals with DYT1 dystonia demonstrated an increase in the turnover of dopamine with reduced D1 and D2 receptor binding in the striatum, suggestive of an imbalance in dopamine signaling (Augood et al., 2002, Asanuma et al., 2005). In addition, nigral dopaminergic neurons tended to be larger in size in DYT1 patients when compared to controls (Rostasy et al., 2003) and ubiquitin-positive aggregates have been identified in pigmented dopaminergic neurons of the substantia nigra pars compacta and locus ceruleus (McNaught et al., 2004). Similarly, cell and animal models have been utilized to investigate the involvement of the dopaminergic system in DYT1 dystonia and improve our understanding of disease pathophysiology. Transgenic mice over expressing human mutant torsinA have been reported to display alterations in striatal dopamine transport and/or release and turnover (Balcioglu et al., 2007, Zhao et al., 2008). Moreover, in vitro studies have also identified a role for torsinA in both dopamine transporter function (Torres et al., 2004, Cao et al., 2005) and more generally in synaptic vesicle recycling through an interaction with snapin (Granata et al., 2008). To test for the direct involvement of torsinA with the dopaminergic system, we examined whether wildtype or mutant torsinA interacted with components of the dopamine synthesis pathway, focusing on tyrosine hydroxylase (TH), the rate limiting step in dopamine synthesis.
Experimental procedures
Plasmids, antibodies and reagents
Amplification of cDNA for wildtype torsinA and mutagenesis to produce deletion mutants have been described elsewhere (O'Farrell et al., 2002). C-terminal V5 or myc tagged versions of the same constructs were generated by subcloning into pCDNA3.1V5His and pCDNA3.1Hismyc respectively. TorsinB was amplified from adult human brain cDNA using the Advantage PCR kit (both from Clontech), using the following primers (Forward 5′-CGAGGAGCGGGATGTTGCGG-3′ Reverse 5′-TCCGTGGAAATCCAGCCGCGA –3) and likewise cloned into pCDNA3.1/V5-his TOPO using the TA cloning system (Invitrogen). Primary antibodies utilized included polyclonal anti-torsinA (1:1000, torsin290, (O'Farrell et al., 2002)); monoclonal anti-V5 (1:5000, R960-25, Invitrogen); polyclonal anti-TH (1:2000, AB1542, Chemicon); polyclonal anti-V5 (Chemicon, 1:400); monoclonal anti-myc (1:1000, #2276, Cell Signaling), monoclonal anti–PDI (1:50, SPA-891, Stressgen), monoclonal anti-MAP2 (1:500, M1406, Sigma), polyclonal anti-dopamine β hydroxylase (1:1500, AB1585, Millipore), monoclonal anti-β-actin (1:2000, A5441, Sigma), monoclonal anti-nucleoporin (1:2000, 610498, BD Transduction Laboratories) and monoclonal anti-DOPA decarboxylase (1:1000, D0180, Sigma).
Cell Culture
Human embryonic kidney cells (HEK-293T) and human neuroblastoma BE(2)-M17 cells were cultured in Opti-MEM (Invitrogen) supplemented with 10% FBS, penicillin (100 units/ml) and streptomycin (100 μg/mL). Cells were plated 24 hours prior to transient transfection in 6-well culture plates at 2×105 cells per well and transfected with the various constructs using Fugene6 (Roche) according to the manufacturer's protocols. An SY5Y Tet-on cell line maintained in 400 μg/mL G418 was stably transfected with pTRE2hyg constructs containing either wildtype torsinA, ΔE302/303 torsinA or an unrelated control protein (PACRG) using Fugene6. Clonal cell lines stably expressing torsinA (isolated by limiting dilution) were maintained in 50 μg/mL hygromycin. Cell lines were induced with the addition of 2 μg/mL doxycycline daily for 4 days prior to harvesting or assaying. Primary cell cultures were prepared from post-natal mouse midbrain using methods described previously (Mena et al., 1997, Burke et al., 1998, Petrucelli et al., 2002). Briefly, midbrains containing substantia nigra (SN) and ventral tegmental area were dissected from 2 day old postnatal mouse pups using anatomical landmarks as described. Neurons from these areas were dissociated with papain and plated on top of pre-established cortical glia cell monolayers. For viral transductions, wildtype or mutant torsinA cDNAs were subcloned into the pHSVPrpUC amplicon, packaged into recombinant viral particles using 5dl1.2 helper virus and the 2-2 packaging cell line (Neve et al., 1997) and purified using sucrose gradients and titres determined as described previously (Petrucelli et al., 2002). Cells were transduced with HSV vectors for wildtype or mutant torsinA at a multiplicity of infection (MOI) of 10 and analysed 48 hours later.
Western Blot and co-immunoprecipitation analysis
Cells were harvested in extraction buffer containing 10mM Tris-HCl, pH 7.5, 2% SDS and protease inhibitors (P8340, Sigma) and protein was estimated by the BCA method (Pierce). Lysates (10 μg total protein per lane) were separated on 10% SDS-PAGE gels and transferred to PVDF membranes (Immobilon, Inc). Membranes were incubated in blocking buffer (5% skim milk in TBS-Tween) for 1 hour at room temperature. Primary antibodies were allowed to incubate overnight at 4°C. Antibody binding was revealed using peroxidase conjugated secondary antibodies donkey anti-rabbit (1:10,000 dilution, 711-035-152, Jackson) and donkey anti-mouse (1:10,000 dilution, 715-035-150, Jackson) and enhanced chemiluminescence (ECL, Amersham) according to the manufacturer's protocols. Signals were exposed to film or quantitation of protein expression was performed by capturing ECL using a CCD-camera based system (AlphaImager, Alpha Innotech Corp). For immunoprecipitation experiments, transfected cells were scraped in cold PBS and collected by centrifugation then resuspended by briefly sonicating in buffer containing 150mM NaCl, 50 mM TrisHCl (pH 8.0) 0.1% (v/v) TritonX100, 1mM PMSF and protease inhibitor cocktail (P8340, Sigma). Lysates were pre-cleared with immobilised proteinG (20398, Pierce), immunoprecipitated with primary antibody overnight at 4°C and captured with proteinG-agarose beads. After washing five times in immunoprecipitation buffer, protein was released from beads by heating in the presence of Laemmli sample buffer and immunoprecipitated complexes were analysed by western blotting as above.
Immunofluorescence
Cells were fixed 48 hours after transfection by immersion in methanol at −20°C for 10 minutes, washed with PBS and non-specific immunoreactivity was blocked with PBS containing 10% FBS and 0.1% Tween-20. Primary antibodies were diluted in PBS with 1% (wt/vol) BSA, applied and allowed to incubate overnight at 4°C. Cells were then washed twice in PBS for 5 minutes and incubated with secondary antibodies (1:1000 goat anti-rabbit AlexaFluor 488, A11034 and goat anti-mouse AlexaFluor 594, A11032, Molecular probes) for 1-2 hours at room temperature. Cells were again washed twice for five minutes before mounting under ProLong antifade medium (Molecular Probes). Primary cells were triple stained using sheep anti-TH, mouse anti-MAP2 and rabbit anti-torsinA. In some experiments we also used a monoclonal antibody to the ER marker PDI in place of MAP2. Omission of primary antibody was used to evaluate non-specific fluorescence and in all cases gave no signal. For counting of torsinA inclusions in primary neurons, the operator was blinded to the torsinA construct used (mutant vs WT) and to the neuronal cell type (TH+/-) when counts were made. Following assessment for the presence of torsinA inclusions, neurons were subsequently assigned to TH+ and TH- groups. Data shown represent at least two separate experiments where the entire neuronal (MAP2+) population of the coverslip was assessed (number of cells >200). Images were captured using an Olympus Fluoview confocal microscope using separate excitations for each channel and images were subsequently merged using Adobe Photoshop. Statistical significance was assessed by two-tailed Student's t-test, p<0.05 was considered significant.
TH activity assay
TH activity was assessed through cleavage of tyrosine-7-amino-4-methylcoumarin (Tyr-AMC, Bachem) measuring the emission at 460nm following excitation at 380nm. For each cell line assay, 48 wells of cells were plated at 10,000 cells/well in black polystyrene 96 well culture plates in medium containing hygromycin. After 48hrs growth, the cells were incubated in media containing 10ng/mL TGF-beta1 (T1654, Sigma) and allowed to differentiate for 48 hours prior to the induction of half the wells with doxycycline. Three hours prior to assay the media was replaced with serum free media and allowed to equilibrate at 37°C. Tyr-AMC (50 μM) was added to all wells and readings were taken every minute for 60 minutes by microplate spectrometry (SpectraMax Gemini, Molecular Devices). At least three different clonal cell lines were assayed for wildtype or ΔE302/303 torsinA, with parallel cultures +/- doxycycline, harvested at the time of assay and analyzed by western blot to measure induction of torsinA protein over endogenous levels. Statistical significance was evaluated using one-way ANOVA with Tukey post-hoc test for differences between subgroups with p<0.05 considered significant.
Results
Interaction of wildtype and mutant torsinA
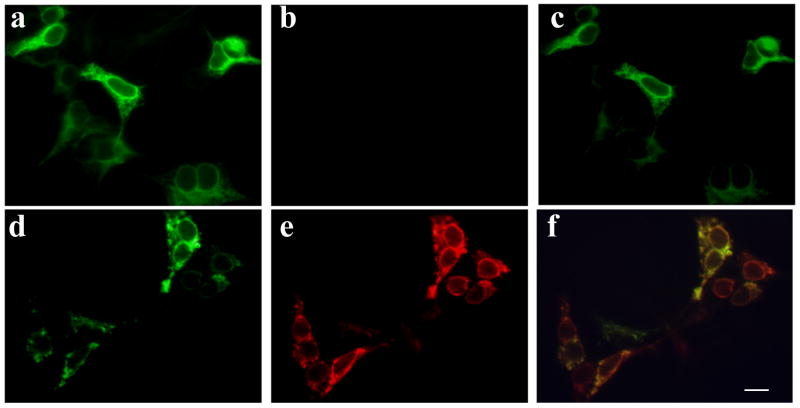
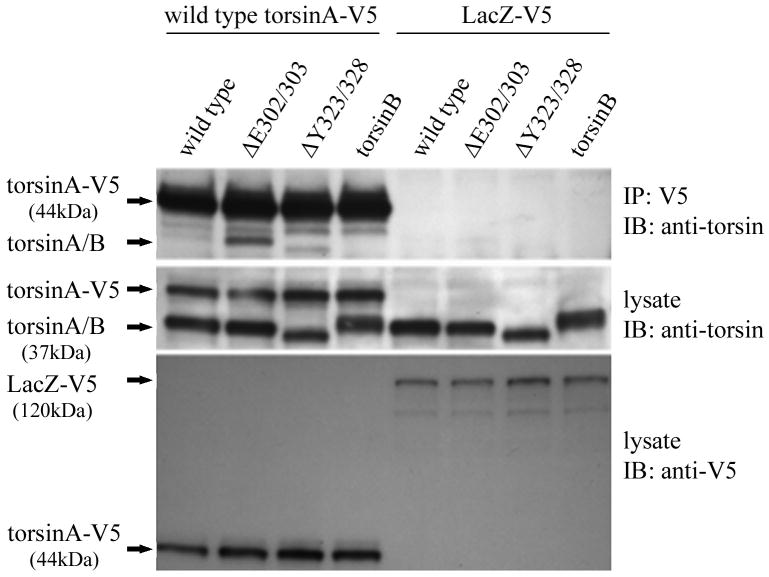
It has been previously demonstrated that wildtype torsinA resides in the lumen of the ER in cultured cells. The ΔE302/303 deletion causes the mutant torsinA protein to mislocalize to large cytoplasmic inclusion bodies when expressed in cell lines (Hewett et al., 2000, Kustedjo et al., 2000) and this was also observed in this study (Figure 1). In addition, when the wildtype protein was co-expressed with mutant torsinA, the localization of the wildtype protein was changed from the typical ER distribution, into large cytoplasmic inclusions co-localizing with the mutant protein (Figure 1). This alteration in the distribution of the wildtype protein suggests that sequestration of the normal protein was occurring. To test for possible torsinA self-interaction, we co-transfected cells with either V5 tagged wildtype torsinA or V5 tagged lacZ and various untagged constructs. Immunoprecipitation with antibodies to the V5 epitope tag and blotting for torsinA/B demonstrated an interaction between the tagged wildtype torsinA protein and untagged wildtype or ΔY323-F328 mutant but no detectable interaction with torsinB (Figure 2A). A significantly stronger interaction was observed between wildtype torsinA-V5 and the mutant ΔE302/303 torsinA in comparison to wildtype torsinA, despite similar levels of expression. The reverse immunoprecipitation with antibodies to torsinA and detection using the epitope tag also produced similar results (data not shown). No interaction of either wildtype or mutant torsinA with a second V5-HIS tagged control protein (lacZ) was observed.
Figure 1.
Mutant torsinA alters the cellular localization of wildtype torsinA. HEK-293 cells were transfected with constructs encoding either V5-tagged wildtype torsinA alone (a-c), or co-transfected with V5-tagged wildtype and myc-tagged ΔE302/303 mutant torsinA constructs (d-f). Wildtype torsinA was detected using rabbit anti-V5 antibody and AlexaFluor 488 conjugated anti-rabbit antibody. Mutant torsinA was detected using mouse anti-myc and AlexaFluor 594 conjugated anti-mouse antibody. In the presence of mutant protein, wildtype torsinA was recruited into intracellular inclusions formed by mutant torsinA, demonstrated by the overlap in signals between the two channels (c,f). Scale bar = 20μM.
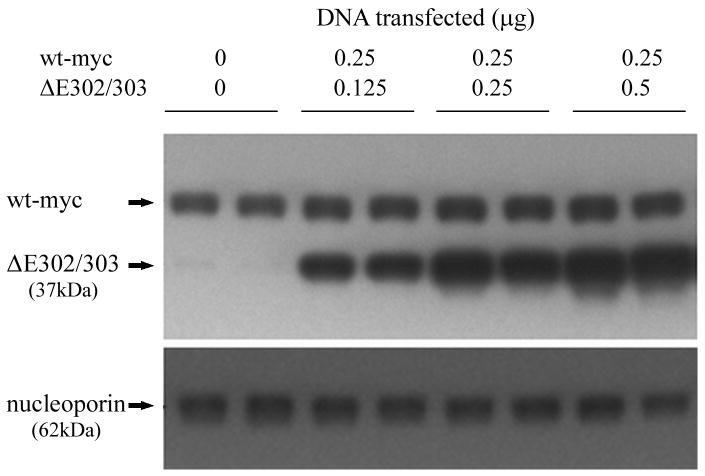
Figure 2.
Mutant torsinA interacts with wildtype torsinA in vivo. A. Self-interaction of torsinA was assessed by cotransfecting V5 tagged wildtype torsinA and various untagged torsin constructs into HEK293 cells. A construct encoding V5 tagged LacZ was used as a negative control. The upper panel shows the results of immunoprecipitation (IP) using monoclonal anti-V5 and the immunoblot (IB) probed with a polyclonal antibody that detects torsinA and torsinB (torsin290). The lower panels show the inputs (lysates, 10μg total protein per lane) probed with torsin and V5 antibodies respectively. The efficiency of the IP for the V5 tag is demonstrated by the presence of the Tor-V5 protein in the IP. B. Mutant torsinA does not alter wildtype torsinA steady-state protein levels. Cells were cotransfected with constant amounts of constructs encoding myc tagged wildtype torsinA (0.25μg plasmid per transfection) and increasing amounts of untagged mutant torsinA (0 to 0.5μg plasmid) in duplicate. Western blot analysis was performed with polyclonal anti-torsin antibody (torsin290) and monoclonal anti-nucleoporin to demonstrate equivalent loading of protein lysate.
To test if the interaction of ΔE302/303 mutant with wildtype torsinA altered the steady state protein levels of the wildtype protein, we co-transfected varying amounts of ΔE302/303 torsinA with constant amounts of tagged wildtype protein. Protein levels of wildtype torsinA-myc were unaffected by the presence of ΔE302/303 torsinA (Figure 2B). Complementary experiments where untagged wildtype protein was co-transfected with tagged mutant gave similar results (data not shown). These results suggest that one effect of the ΔE302/303 mutation in torsinA is to produce a quantitative increase in protein-protein interactions with the wildtype protein and potentially other interacting proteins.
Mutant torsinA promotes inclusion formation in catecholaminergic neurons
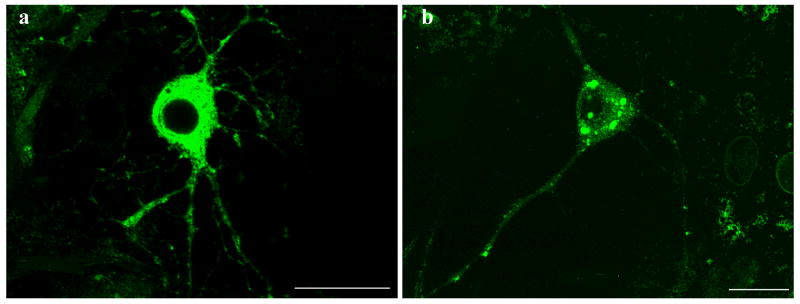
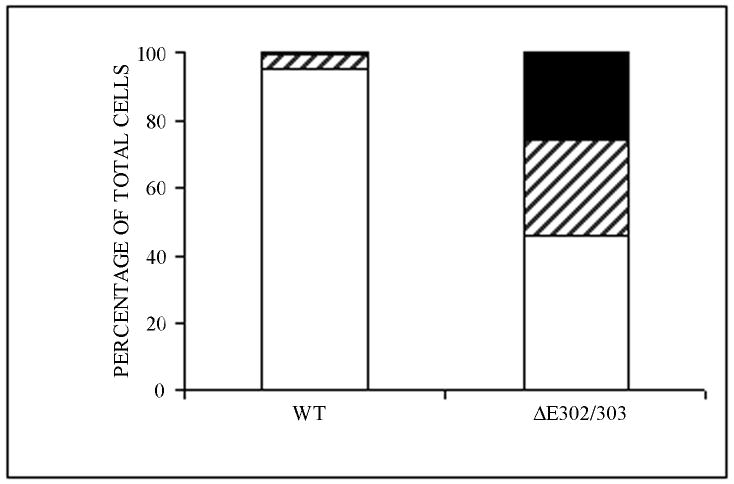
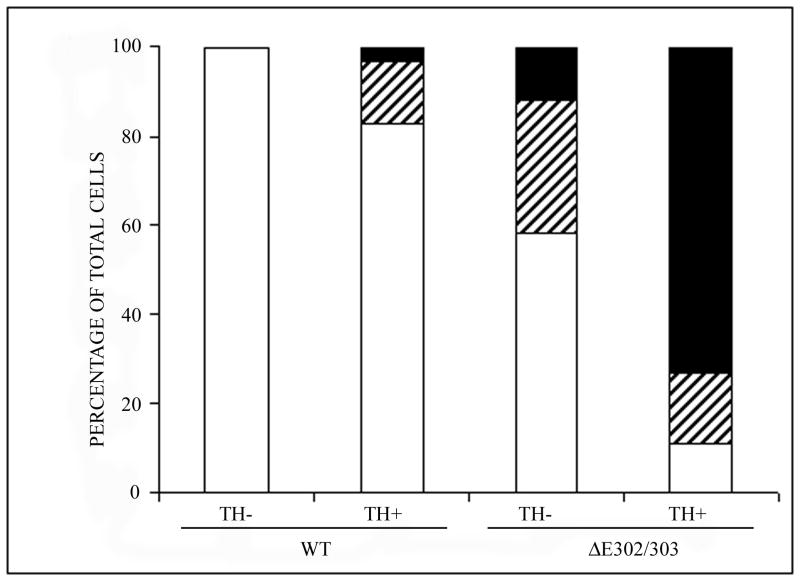
Primary neuronal cultures from the midbrain of postnatal mice were used to examine the effect of wildtype and ΔE302/303 torsinA on different neuronal populations. This model system was chosen because abnormalities in dopaminergic transmission have been hypothesized to be important in the development of the dystonia phenotype. These primary midbrain cultures contain approximately 20-30% dopaminergic neurons (Petrucelli et al., 2002) which can be distinguished from the non-dopaminergic neurons by staining for TH. We transduced the primary cultures with vectors encoding either wildtype torsinA or the ΔE302/303 mutant and examined the distribution of the proteins by immunocytochemistry. Wildtype torsinA was localized throughout the cytosol and processes of transduced neurons and inclusions were observed in less than 5% of these cells. In contrast and similar to the observations in transfected cells, approximately 50% of the neurons transduced with ΔE302/303 torsinA formed inclusions in the cytoplasm and near the nucleus (Figure 3A,B). Quantitation of inclusion numbers (Table 1) demonstrated wildtype torsinA formed inclusions in approximately 5% of neurons, whereas inclusions were observed in greater than 50% of neurons transduced with the ΔE302/303 torsinA construct (Figure 3C, p<0.0001, n=2050 neurons counted). Moreover, the propensity of ΔE302/303 torsinA to form inclusions was influenced by neuronal subtype. Mutant torsinA formed detectable inclusions in more than 80% of TH+ cells. Approximately 60% of TH+ neurons had large torsinA inclusions (>1 μM) and ∼20% contained smaller aggregates, whereas in TH- neurons, approximately 40% of cells formed inclusions which were generally smaller than 1 μM (Figure 3D, p<0.0001, n=1140 neurons counted). In contrast, wildtype torsinA formed inclusions in a small proportion of TH+ neurons (approximately 15% of the total) and these were generally small intracellular structures (<1 μM). Furthermore, such inclusions were never seen in TH negative neurons (n=910 neurons counted).
Figure 3.
Mutant torsinA promotes inclusion formation in catecholaminergic neurons. Primary midbrain cultures were transduced with HSV vectors expressing wildtype (a) or ΔE302/303 (b) mutant torsinA and stained with polyclonal anti-torsinA and anti-rabbit AlexaFluor 488. Scale bar = 20μM. C. For each experiment we counted the number of transduced cells and calculated the number of cells that had no inclusions (open bar), a few small aggregates (<1μM, hatched bar) or one or more large inclusions (>1μM, heavy shaded bar). Quantification demonstrated a significant difference in the number of cells showing inclusion formation after transduction with either the wildtype or mutant torsinA construct (wildtpe: 5±0.9% Vs ΔE302/303: 53.7±3.6%, mean±SD, p<0.0001, n=2050 neurons counted). D. Inclusion formation was influenced by neuronal subtype. Analysis as in C demonstrated wildtype torsinA did not generate inclusions in the TH- neurons but inclusions were seen in approximately 20% of TH+ neurons. In contrast, mutant torsinA formed inclusions in approximately 40% of TH- neurons but over 80% of TH+ neurons (TH-: 42.8±3.8% Vs TH+: 84.2±1.4%, mean±SD, p<0.0001, n=1140 neurons counted). Mutant torsinA formed significantly more inclusions in TH+ neurons compared to wildtype (wildtype: 20.2±8.8% Vs ΔE302/303: 84.2±1.4%, mean±SD, p=0.0002, n=567 neurons counted).
Table 1.
Primary midbrain cultures were transduced with HSV vectors expressing wildtype or ΔE302/303 mutant torsinA and co-stained with polyclonal anti-torsinA and polyclonal anti-TH, raised in rabbit and sheep respectively. For each experiment the number of transduced cells was counted and scored for no inclusions, small aggregates (<1μM) or one or more large inclusions (>1μM).
| Experiment | TH- neurons | TH+ neurons | ||||
|---|---|---|---|---|---|---|
| None | Small | Large | None | Small | Large | |
| Wildtype #1 | 311 | - | - | 112 | 16 | 3 |
| Wildtype #2 | 200 | - | - | 85 | 12 | 4 |
| Wildtype #3 | 134 | - | - | 23 | 8 | 2 |
| Δ302/303 #1 | 171 | 96 | 44 | 18 | 27 | 82 |
| Δ302/303 #2 | 114 | 64 | 29 | 11 | 18 | 38 |
| Δ302/303 #3 | 197 | 90 | 33 | 18 | 20 | 70 |
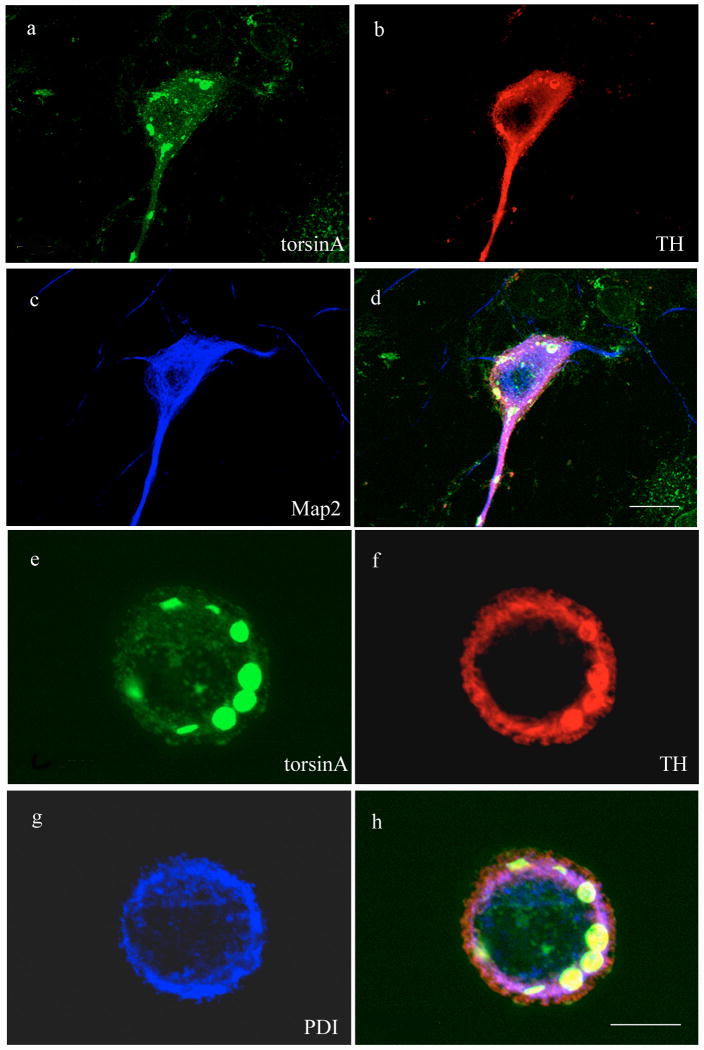
To investigate if a protein or proteins specifically expressed in dopaminergic neurons might contribute to the preferential formation of inclusion bodies in this neuronal type, we examined the cellular localization of three abundant endogenous proteins in the transduced neurons (Figure 4A-G). We found that TH co-localized with and appeared to be a significant component of the torsinA-positive intracellular inclusions. In comparison, the neuronal cytoskeletal marker MAP2 was not recruited into torsinA-positive inclusions and its distribution remained similar to that observed in untransduced neurons. Similarly, the ER marker PDI demonstrated only marginal co-localization with the inclusions, similar to previous studies performed in other cell types (Kustedjo et al., 2000, O'Farrell et al., 2002). These observations suggested that catecholaminergic midbrain neurons were vulnerable to inclusion formation mediated by mutant torsinA and there was some selectivity in the proteins incorporated into the inclusions.
Figure 4.
TH is a component of inclusion bodies formed by expression of mutant torsinA. Midbrain neurons transduced with vectors encoding ΔE302/303 were stained for torsinA (green), TH (red) and either MAP2 (blue; a-d) or PDI (blue; e-h). TH was recruited to inclusions as evidenced in the merged images (d,h), whereas neither MAP2 or PDI demonstrated significant overlap with the inclusions. Scale bar = 20μM (d) and 10μM (h) respectively.
Mutant torsinA interacts with tyrosine hydroxylase in cultured cells and alters enzyme activity
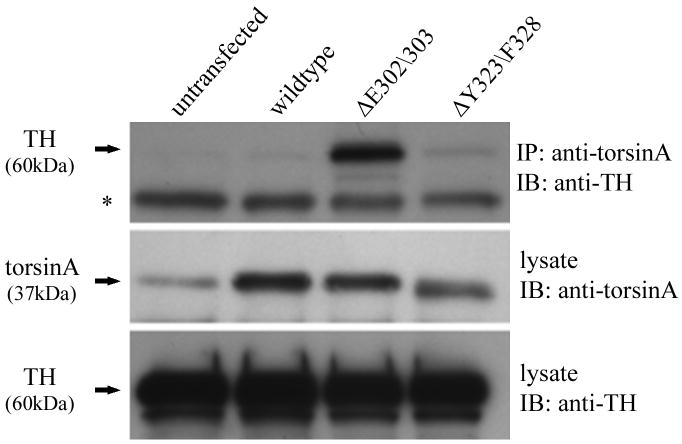
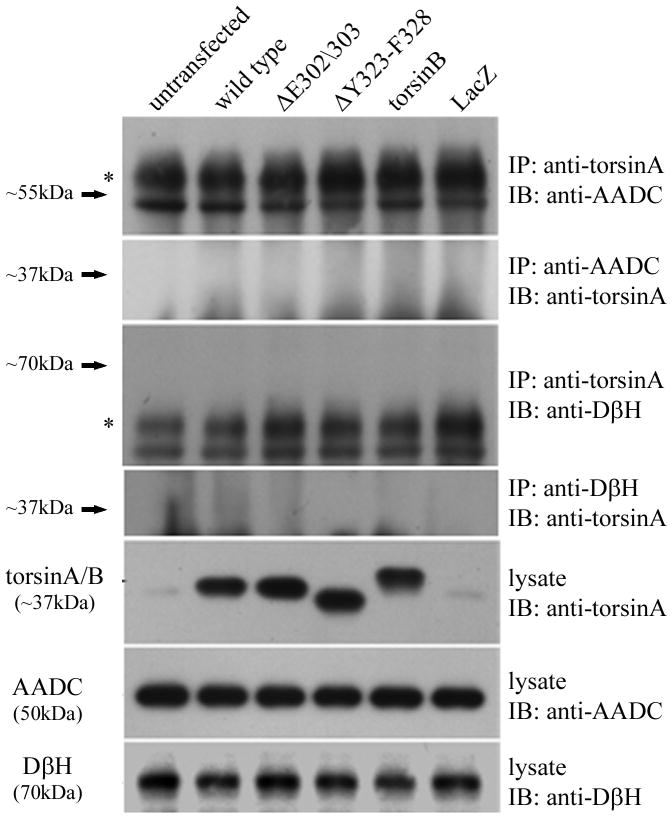
To test the possibility that mutant torsinA recruits TH into inclusion bodies by direct interaction we used the human neuroblastoma cell line BE(2)-M17. This cell line is a useful model system to investigate interactions in the dopaminergic pathway as the cells express moderate levels of endogenous TH (Ciccarone et al., 1989) and low levels of endogenous torsinA. We transfected the cells with constructs encoding untagged wildtype or mutant torsinA and performed immunoprecipitation with an anti-torsinA antibody (torsin290). Western blot analysis with an anti-TH antibody demonstrated an interaction between torsinA and TH (Figure 5A). The amount of TH co-immunoprecipitated was very low in untransfected cells, presumably reflecting the low level of endogenous torsinA. The signal was stronger when cells were transfected with wildtype or ΔY323-F328 torsinA. However, when ΔE302/303 torsinA was present a significant increase in co-immunoprecipitated TH was consistently detected in comparison to wildtype torsinA, despite similar levels of protein inputs. The interaction of mutant torsinA was shown to be specific to TH as we were unable to co-immunoprecipitate other enzymes in the dopamine synthetic pathway. There was no evidence for interaction of wildtype or mutant torsinA with either aromatic L-amino acid decarboxylase (AADC) or dopamine-β-hydroxylase (DβH), enzymes which catalyze the conversion of L-dopa to dopamine and dopamine to noradrenaline respectively (Figure 5B).
Figure 5.
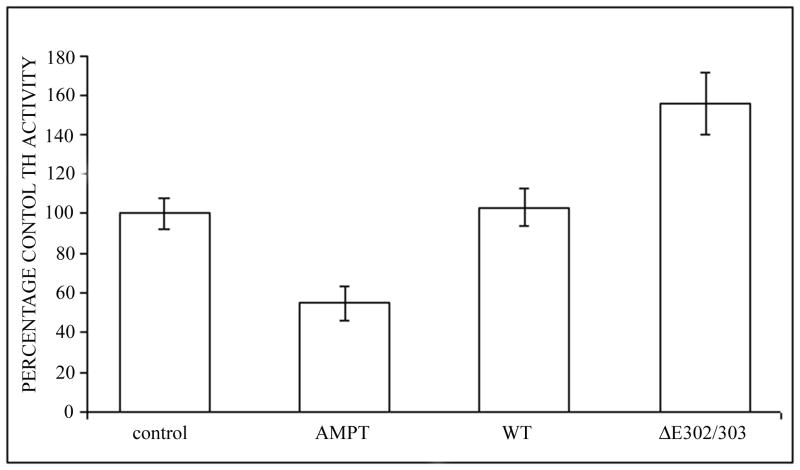
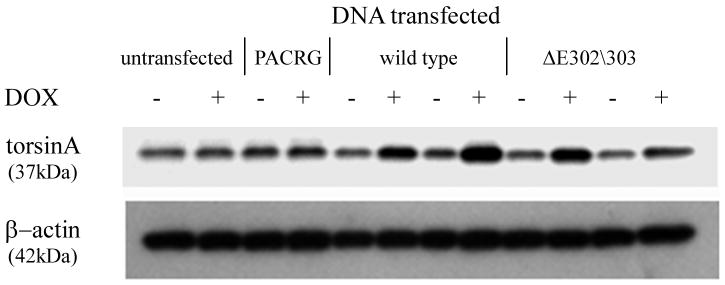
TorsinA and TH interact in vivo and is enhanced by the ΔE302/303 mutation. A. The interaction between torsinA and TH was assessed by transfecting M17 neuroblastoma cells with constructs encoding wildtype or mutant torsinA. The upper panel shows the results of immunoprecipitation (IP) using a polyclonal antibody to torsin (torsin290) and the immunoblot (IB) probed with a monoclonal antibody that detects TH. The faint TH signal seen in the untransfected and wildtype lanes, indicating interaction of TH with wildtype torsinA, was greatly enhanced in the presence of ΔE302/303 mutant torsinA. The lower panels show the inputs (lysates, 10μg total protein per lane) probed with torsin and TH antibodies respectively. The * indicates the IgG heavy chain. B. TorsinA does not interact with other enzymes involved in dopamine synthesis. M17 neuroblastoma cells were transfected with various untagged torsin constructs. The upper four panels show the results of immunoprecipitation (IP) using a polyclonal antibody to torsinA (torsin290), AADC or DβH. Neither AADC nor DβH was observed to co-immunoprecipitate with any of the torsin proteins. The expected position of immunoprecipated protein is marked by the arrow. The * indicates the IgG heavy chain. The lower panels show the inputs (lysates, 10μg total protein per lane) probed with torsin, AADC and DβH antibodies respectively. C. Mutant torsinA affects TH activity. SY5Y cells were stably transfected with torsinA or an unrelated control protein (Parkin Co-Regulated Gene) under an inducible promoter system. Clonal cell lines (n>3 for each construct) were independently assayed a minimum of five times (mean±SEM). TH activity was assessed for both the non-induced and induced state and expressed as % change compared to the non-induced cells. A significant increase in fluorescence was observed in the cells expressing ΔE302/303 torsinA but not wildtype torsinA. One-way ANOVA confirmed a difference in TH activity between the three groups [F(2,16)=7.74, p=0.004] and tukey post-hoc comparison of the 3 groups indicated a significant increase in TH activity after induction of ΔE302/303 when compared to cell lines expressing wildtype torsinA (p=0.006). D. Inducible expression of torsinA. To confirm doxycycline-induced expression of wildtype and mutant torsinA, SY5Y cells cultured in the absence or presence of 0.1μg/mL doxycycline were analysed by western blot with an anti-torsinA antibody (torsin290). Equal loading (10μg total protein per lane) was confirmed by analysis using an anti-β actin antibody.
To investigate whether the interaction of TH and mutant torsinA might affect TH activity, we used the human neuroblastoma cell line SY5Y expressing the Tet-on regulated transactivator (Clontech), to enable regulated expression of inducible torsinA constructs. TH activity was measured by the cleavage of a fluorescent coumarin derivative from tyrosine (Tyr-AMC) (Figure 5C). Generation of fluorescence depends on the sequential action of TH, the rate limiting enzyme and aromatic acid decarboxylase (AADC). One-way ANOVA confirmed a difference in TH activity between the control, wildtype and mutant TorsinA expressing cell lines [F(2,16)=7.74, p=0.004]. Tukey post-hoc comparison of the 3 groups indicated that there was a significant increase in TH activity after induction of ΔE302/303 when compared to cell lines expressing either the control construct or wildtype torsinA (p=0.006). By comparison, there was no significant difference between the control and wildtype torsinA groups (p=0.97). To confirm that the observed fluorescent signal was due to the action of TH, we added alpha-methyl-rho-tyrosine (AMPT), a specific inhibitor of TH, which reduced the signal by approximately 75%. Similarly, NSD-1015, which inhibits AADC, was also effective in preventing fluorescence generation (data not shown). Induction of expression compared to endogenous levels was confirmed by western blot analysis of parallel cultures +/- doxycycline harvested at the time of assay (Figure 5D).
Discussion
In this study we utilized cell models and biochemical analysis to investigate the functional consequences of the dystonia associated mutant ΔE302/303 torsinA and potential involvement with the dopaminergic pathway. We show that the ΔE302/303 mutation in torsinA results in an increased interaction of the mutant protein with TH, a key enzyme in the synthesis of dopamine. To date several studies have suggested that ΔE302/303 torsinA protein represents a loss of function protein and also may act to inhibit wildtype protein function through a dominant-negative mechanism (Torres et al., 2004, Goodchild and Dauer, 2005, Pham et al., 2006, Hewett et al., 2008). In addition, there is some evidence that the mutation results in an increased propensity for protein-protein interactions. For example, the ΔE302/303 mutation results in both an increased association with nesprin-3(Nery et al., 2008) and self dimerization (Torres et al., 2004) when compared to the wildtype protein. Our results extend this observation and suggest that the ΔE302/303 torsinA results in an increased propensity for protein-protein interactions between the wildtype and mutant torsinA proteins. While recent studies have suggested that ΔE302/303 torsinA has a decreased half-life and is efficiently targeted to the proteasome for degradation (Giles et al., 2008, Gordon and Gonzalez-Alegre, 2008), we did not identify any effect of increased levels of mutant torsinA on steady-state wildtype torsinA, suggesting that the interaction does not result in elevated turnover of the wildtype protein. However, consistent with several other reports in the literature, we did observe a significant alteration of the cellular localization of the wildtype protein when co-expressed with ΔE302/303 torsinA. The functional significance of this observation remains to be determined as there are conflicting results in the literature for the different mouse models and human case studies regarding protein localization and histopathological abnormalities (reviewed in (Breakefield et al., 2008)). While the development of functional assays and novel molecular tools, for example wildtype and mutant-specifc antibodies, may help to address these conflicting results, there is clear evidence from cell models that the specific subcellular localization of both the wildtype and mutant protein has significant effects on nuclear-cytoskeletal dynamics (Hewett et al., 2006, Nery et al., 2008).
In several forms of dystonia, abnormalities in the dopaminergic nigrostriatal system or mutations in the genes for dopamine synthesis cause neuronal dysfunction (Bandmann and Wood, 2002). To date, the evidence for a role for torsinA in the dopaminergic pathway has been suggestive rather than conclusive. Limited autopsy studies have suggested subtle alterations in striatal dopamine signaling and function may be present (Augood et al., 2002, Rostasy et al., 2003). Similarly, evidence from several animal models suggest alterations in dopamine signaling and metabolism may be present (reviewed in (Wichmann, 2008)), but the link between these manifestations and disease pathogenesis remain unclear. Here we present evidence for an interaction of wildtype torsinA with TH, a key component of the dopamine synthesis pathway. Using an inducible cell culture system we did not observe any effect of elevated wildtype torsinA on TH activity and we are not aware of any reports suggesting that torsinA regulates TH activity in vivo. However, it is possible that wildtype torsinA is a chaperone for TH, as other AAA+ ATPases function as chaperone proteins. TH is localized to cell bodies, axons and terminals in dopaminergic neurons within the brain (Pickel et al., 1975). While biochemical analysis suggests that torsinA is localized to the ER and adopts a type-II orientation (Liu et al., 2003) recent evidence links torsinA to the tubular-vesicular network. TorsinA immunoreactivity is associated with vesicles (Hewett et al., 2000) and studies in human and animal models have consistently demonstrated granular labeling of fibers and terminals in vivo (Shashidharan et al., 2000, Konakova and Pulst, 2001, Augood et al., 2003, Koh et al., 2004). Moreover, a proportion of TorsinA adopts a type-I membrane conformation, with residues 41-332 cytoplasmically oriented, and interacts with the microtubule motor protein Kinesin Light Chain (KLC) and the cytoskeletal intermediate filament protein vimentin (Kamm et al., 2004, Hewett et al., 2006).
A consequence of the disease-associated ΔE302/303 mutation was a significantly increased propensity for interaction between the mutant protein and TH, in comparison to wildtype torsinA. This interaction was specific for TH as co-immunoprecipitation was not observed for either wildtype or ΔE302/303 torsinA with other members of the dopamine synthetic pathway (AADC or dopamine-beta-hydroxylase). Analysis in a primary neuronal model demonstrated that ΔE302/303 torsinA mediated inclusion formation was more frequent in dopaminergic neurons than in non-dopaminergic neurons and TH was an abundant component of the intracellular inclusions. This novel observation raises the possibility that there is a selective effect of the ΔE302/303 torsinA mutation on dopaminergic neurons and potentially dopaminergic signalling. While the use of viral transduction and overexpression may have exaggerated the phenotype, there is supporting evidence in vivo. For example, multiple studies suggest a link between dystonia altered synaptic communication within the basal ganglia (reviewed in (Breakefield et al., 2008)) and neuropathology studies of human DYT1 postmortem material has identified enlarged dopaminergic cell bodies in the substantia nigra (Rostasy et al., 2003). Immunofluorescence and ultrastructural analysis of TH localization in transgenic models will help to test if there are similar changes to the distribution of the protein in vivo. In contrast, there was no evidence for the recruitment of other neuronal marker proteins, such as MAP2 or PDI, perhaps suggesting that TH plays a role in the formation and/or stabilization of intracellular inclusions, rather than simply being recruited to the inclusions as a late part of their formation. Co-expression of TH and ΔE302/303 torsinA resulted in a significant increase in TH activity, compared to wildtype torsinA or an unrelated control protein. TH activity is regulated by multiple mechanisms, including catecholamines and phosphorylation (Okuno and Fujisawa, 1985) (Fitzpatrick, 1999). It is possible that the interaction of TH with ΔE302/303 torsinA disrupted one or more of these regulatory mechanisms. Inappropriate protein-protein interactions with target enzymes and dysregulation of their activity may be a common feature of several mutant proteins that cause neurological disorders. For example, dominant mutations in the gene for huntingtin can dysregulate gene transcription by sequestering histone deacetylase and reducing its activity (Ferrante et al., 2003) or by decreasing interaction with Bmi-1, resulting in increased histone H2A monoubiquitylation and gene silencing (Kim et al., 2008). Similarly, elevated Abeta levels mediate hyperactivation of GSK3, which is associated with Alzheimer disease pathogenesis (Hu et al., 2009). DYT1 patients are not responsive to dopamine therapy and biochemical assays of postmortem material have reported normal or modest alterations in striatal dopamine levels (Furukawa et al., 2000, Augood et al., 2002). However, not all dopa-responsive dystonia patients respond to dopamine replacement therapy, suggesting that dystonia resulting from disruption of the dopaminergic pathway is more complex than a simple reduction in dopamine levels (DE Lonlay et al., 2000, Hoffmann et al., 2003).
The relevance of the intracellular ΔE302/303 torsinA inclusions observed in some cell culture and animal models, including the primary neuronal system utilized in this study, to the human condition remains unresolved. There is some evidence that inclusion formation is a consequence of high expression levels (Gonzalez-Alegre and Paulson, 2004, Goodchild and Dauer, 2004, Gordon and Gonzalez-Alegre, 2008). However, animal models have provided contradictory results, with aggregates, inclusions and blebbing reported in some strains (Dang et al., 2005, Goodchild et al., 2005, Shashidharan et al., 2005, Grundmann et al., 2007) but not others (Sharma et al., 2005, Zhao et al., 2008). Similarly, histopathological studies of brains from human DYT1 patients have yielded contradictory results. Earlier studies (Walker et al., 2002, Rostasy et al., 2003) found no evidence for torsinA positive inclusions within the brains of human DYT1 patients, however they did not discount the presence of the inclusions and suggested several reasons for the lack of detection including; antibody inefficiency, lower expression of torsinA in vivo compared to in vitro and cytoplasmic deposits of neuromelanin in dopaminergic neurons obscuring detection. Subsequently peri-nuclear inclusion bodies were detected in both cholinergic and non-cholinergic neurons of the mid brain reticular formation in brains from four known DYT1 patients by utilizing antibodies against ubiquitylated proteins and inclusion bodies (McNaught et al., 2004). Unfortunately such studies are hampered by both the limited availability of histopathological tissue samples from DYT1 patients and the absence of an antibody which distinguishes between wild type and mutant torsinA.
However, it does appear that the formation of intracellular inclusions reflects a fundamental property of the mutant protein. Our results suggest that ΔE302/303 mutation stabilizes an otherwise transient interaction between the wildtype protein and TH. The interaction and potential formation of an aggregate or inclusion could prevent TH from reaching presynaptic termini, resulting in misregulation of dopamine production and/or release. The overall increase in TH activity when co-expressed with mutant compared to wildtype torsinA suggests the interaction of the two proteins results in the misregulation rather than loss of TH activity. However, this is not incompatible with reduced dopamine availability at the synapse. The assay was performed in live whole cultured cells and the increase in TH activity is likely to be associated with TH protein within inclusions. It is possible that there is a significant decrease in cytoplasmic TH activity. This hypothesis is consistent with multiple studies in human patients and animal models suggesting that despite normal net striatal dopamine levels, alterations in dopamine turnover and release are associated with the DYT1 mutation (reviewed in (Wichmann, 2008)).
Overall, the data described here suggest that mutant torsinA is involved in inappropriate interaction with, and possibly sequestration of, a specific group of neuronal proteins that will probably include normal torsinA binding partners. The enhanced interaction with TH likely contributes, at least in part, to the dystonia phenotype in DYT1 patients. Given the apparent importance of dopamine in dystonia and other movement disorders, altered regulation or localization of TH could have profound effects on synaptic function in the nigrostriatal system.
Acknowledgments
Footnotes: This work was supported in part by National Health & Medical Research Council (Australia) project grant #436976 and by the Intramural Research Program of the NIH, National Institute on Aging. PJL is an NHMRC RD Wright Fellow (#334346) and MBD is an NHMRC Practitioner Fellow (#546452). We thank our laboratory colleagues for helpful comments and assistance.
Abbreviations
- AADC
aromatic acid decarboxylase
- AMPT
alpha-methyl-rho-tyrosine
- DBH
dopamine-β-hydroxylase
- EOTD
Early-onset torsion dystonia
- ER
endoplasmic reticulum
- HEK-293T
human embryonic kidney cells
- KLC
kinesin light chain
- MOI
multiplicity of infection
- NE
nuclear envelope
- PDI
protein disulfide isomerase
- SN
substantia nigra
- TH
tyrosine hydroxylase
References
- Asanuma K, Ma Y, Okulski J, Dhawan V, Chaly T, Carbon M, Bressman SB, Eidelberg D. Decreased striatal D2 receptor binding in non-manifesting carriers of the DYT1 dystonia mutation. Neurology. 2005;64:347–349. doi: 10.1212/01.WNL.0000149764.34953.BF. [DOI] [PubMed] [Google Scholar]
- Augood SJ, Hollingsworth Z, Albers DS, Yang L, Leung JC, Muller B, Klein C, Breakefield XO, Standaert DG. Dopamine transmission in DYT1 dystonia: A biochemical and autoradiographical study. Neurology. 2002;59:445–448. doi: 10.1212/wnl.59.3.445. [DOI] [PubMed] [Google Scholar]
- Augood SJ, Keller-McGandy CE, Siriani A, Hewett J, Ramesh V, Sapp E, DiFiglia M, Breakefield XO, Standaert DG. Distribution and ultrastructural localization of torsinA immunoreactivity in the human brain. Brain Res. 2003;986:12–21. doi: 10.1016/s0006-8993(03)03164-0. [DOI] [PubMed] [Google Scholar]
- Balcioglu A, Kim MO, Sharma N, Cha JH, Breakefield XO, Standaert DG. Dopamine release is impaired in a mouse model of DYT1 dystonia. J Neurochem. 2007;102:783–788. doi: 10.1111/j.1471-4159.2007.04590.x. [DOI] [PubMed] [Google Scholar]
- Bandmann O, Wood NW. Dopa-Responsive Dystonia - The Story so Far. Neuropediatrics. 2002;33:1–5. doi: 10.1055/s-2002-23590. [DOI] [PubMed] [Google Scholar]
- Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9:222–234. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- Burke RE, Antonelli M, Sulzer D. Glial Cell Line-Derived Neurotrophic Growth Factor Inhibits Apoptotic Death of Postnatal Substantia Nigra Dopamine Neurons in Primary Culture. J Neurochem. 1998;71:517–525. doi: 10.1046/j.1471-4159.1998.71020517.x. [DOI] [PubMed] [Google Scholar]
- Caldwell GA, Cao S, Sexton EG, Gelwix CC, Bevel JP, Caldwell KA. Suppression of polyglutamine-induced protein aggregation in Caenorhabditis elegans by torsin proteins. Hum Mol Genet. 2003;12:307–319. doi: 10.1093/hmg/ddg027. [DOI] [PubMed] [Google Scholar]
- Cao S, Gelwix CC, Caldwell KA, Caldwell GA. Torsin-Mediated Protection from Cellular Stress in the Dopaminergic Neurons of Caenorhabditis elegans. J Neurosci. 2005;25:3801–3812. doi: 10.1523/JNEUROSCI.5157-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarone V, Spengler BA, Meyers MB, Biedler JL, Ross RA. Phenotypic diversification in human neuroblastoma cells: expression of distinct neural crest lineages. Cancer Res. 1989;49:219–225. [PubMed] [Google Scholar]
- Dang MT, Yokoi F, McNaught KS, Jengelley TA, Jackson T, Li J, Li Y. Generation and characterization of Dyt1 DeltaGAG knock-in mouse as a model for early-onset dystonia. Exp Neurol. 2005;196:452–463. doi: 10.1016/j.expneurol.2005.08.025. [DOI] [PubMed] [Google Scholar]
- DE Lonlay P, Nassogne MC, van Gennip AH, van Cruchten AC, Billatte de Villemeur T, Cretz M, Stoll C, Launay JM, Steenberger-Spante GC, van den Heuvel LP, Wevers RA, Saudubray JM, Abeling NG. Tyrosine hydroxylase deficiency unresponsive to L-dopa treatment with unusual clinical and biochemical presentation. J Inherit Metab Dis. 2000;23:819–825. doi: 10.1023/a:1026760602577. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Kubilus JK, Lee J, Ryu H, Beesen A, Zucker B, Smith K, Kowall NW, Ratan RR, Luthi-Carter R, Hersch SM. Histone Deacetylase Inhibition by Sodium Butyrate Chemotherapy Ameliorates the Neurodegenerative Phenotype in Huntington's Disease Mice. J Neurosci. 2003;23:9418–9427. doi: 10.1523/JNEUROSCI.23-28-09418.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick PF. Tetrahydropterin-dependent amino acid hydroxylases. Annu Rev Biochem. 1999;68:355–381. doi: 10.1146/annurev.biochem.68.1.355. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Hornykiewicz O, Fahn S, Kish SJ. Striatal dopamine in early-onset primary torsion dystonia with the DYT1 mutation. Neurology. 2000;54:1193–1195. doi: 10.1212/wnl.54.5.1193. [DOI] [PubMed] [Google Scholar]
- Giles LM, Chen J, Li L, Chin LS. Dystonia-associated mutations cause premature degradation of torsinA protein and cell type-specific mislocalization to the nuclear envelope. Hum Mol Genet. 2008;17:2712–2722. doi: 10.1093/hmg/ddn173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles LM, Li L, Chin LS. Printor, a Novel TorsinA-interacting Protein Implicated in Dystonia Pathogenesis. J Biol Chem. 2009;284:21765–21775. doi: 10.1074/jbc.M109.004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Alegre P, Paulson HL. Aberrant Cellular Behavior of Mutant TorsinA Implicates Nuclear Envelope Dysfunction in DYT1 Dystonia. J Neurosci. 2004;24:2593–2601. doi: 10.1523/JNEUROSCI.4461-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild RE, Dauer WT. Mislocalization to the nuclear envelope: An effect of the dystonia-causing torsinA mutation. Proc Natl Acad Sci USA. 2004;101:847–852. doi: 10.1073/pnas.0304375101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild RE, Dauer WT. The AAA+ protein torsinA interacts with a conserved domain present in LAP1 and a novel ER protein. J Cell Biol. 2005;168:855–862. doi: 10.1083/jcb.200411026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild RE, Kim CE, Dauer WT. Loss of the Dystonia-Associated Protein TorsinA Selectively Disrupts the Neuronal Nuclear Envelope. Neuron. 2005;48:923–932. doi: 10.1016/j.neuron.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Gordon KL, Gonzalez-Alegre P. Consequences of the DYT1 mutation on torsinA oligomerization and degradation. Neuroscience. 2008;157:588–595. doi: 10.1016/j.neuroscience.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granata A, Watson R, Collinson LM, Schiavo G, Warner TT. The Dystonia-associated Protein TorsinA Modulates Synaptic Vesicle Recycling. J Biol Chem. 2008;283:7568–7579. doi: 10.1074/jbc.M704097200. [DOI] [PubMed] [Google Scholar]
- Grundmann K, Reischmann B, Vanhoutte G, Hübener J, Teismann P, Hauser TK, Bonin M, Wilbertz J, Horn S, Nguyen HP, Kuhn M, Chanarat S, Wolburg H, Van der Linden A, Riess O. Overexpression of human wildtype torsinA and human [Delta]GAG torsinA in a transgenic mouse model causes phenotypic abnormalities. Neurobiology of Disease. 2007;27:190–206. doi: 10.1016/j.nbd.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Hewett J, Gonzalez-Agosti C, Slater D, Ziefer P, Li S, Bergeron D, Jacoby DJ, Ozelius LJ, Ramesh V, Breakefield XO. Mutant torsinA, responsible for early-onset torsion dystonia, forms membrane inclusions in cultured neural cells. Hum Mol Genet. 2000;9:1403–1413. doi: 10.1093/hmg/9.9.1403. [DOI] [PubMed] [Google Scholar]
- Hewett JW, Nery FC, Niland B, Ge P, Tan P, Hadwiger P, Tannous BA, Sah DWY, Breakefield XO. siRNA knock-down of mutant torsinA restores processing through secretory pathway in DYT1 dystonia cells. Hum Mol Genet. 2008;17:1436–1445. doi: 10.1093/hmg/ddn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett JW, Zeng J, Niland BP, Bragg DC, Breakefield XO. Dystonia-causing mutant torsinA inhibits cell adhesion and neurite extension through interference with cytoskeletal dynamics. NeurobiolDis. 2006;22:98–111. doi: 10.1016/j.nbd.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Hoffmann GF, Assmann B, Brautigam C, Dionisi-Vici C, Haussler M, de Klerk JB, Naumann M, Steenbergen-Spanjers GC, Strassburg HM, Wevers RA. Tyrosine hydroxylase deficiency causes progressive encephalopathy and dopa-nonresponsive dystonia. Ann Neurol. 2003;54 6:S56–65. doi: 10.1002/ana.10632. [DOI] [PubMed] [Google Scholar]
- Hu S, Begum AN, Jones MR, Oh MS, Beech WK, Beech BH, Yang F, Chen P, Ubeda OJ, Kim PC, Davies P, Ma Q, Cole GM, Frautschy SA. GSK3 inhibitors show benefits in an Alzheimer's disease (AD) model of neurodegeneration but adverse effects in control animals. Neurobiol Dis. 2009;33:193–206. doi: 10.1016/j.nbd.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm C, Boston H, Hewett J, Wilbur J, Corey DP, Hanson PI, Ramesh V, Breakefield XO. The Early Onset Dystonia Protein TorsinA Interacts with Kinesin Light Chain 1. J Biol Chem. 2004;279:19882–19892. doi: 10.1074/jbc.M401332200. [DOI] [PubMed] [Google Scholar]
- Kim MO, Chawla P, Overland RP, Xia E, Sadri-Vakili G, Cha JH. Altered histone monoubiquitylation mediated by mutant huntingtin induces transcriptional dysregulation. J Neurosci. 2008;28:3947–3957. doi: 10.1523/JNEUROSCI.5667-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh YH, Rehfeld K, Ganetzky B. A Drosophila model of early onset torsion dystonia suggests impairment in TGF-{beta} signaling. Hum Mol Genet. 2004;13:2019–2030. doi: 10.1093/hmg/ddh208. [DOI] [PubMed] [Google Scholar]
- Konakova M, Pulst SM. Immunocytochemical characterization of torsin proteins in mouse brain. Brain Res. 2001;922:1–8. doi: 10.1016/s0006-8993(01)03014-1. [DOI] [PubMed] [Google Scholar]
- Kustedjo K, Bracey MH, Cravatt BF. Torsin A and Its Torsion Dystonia-associated Mutant Forms Are Lumenal Glycoproteins That Exhibit Distinct Subcellular Localizations. J Biol Chem. 2000;275:27933–27939. doi: 10.1074/jbc.M910025199. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zolkiewska A, Zolkiewski M. Characterization of human torsinA and its dystonia-associated mutant form. Biochem J. 2003;374:117–122. doi: 10.1042/BJ20030258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean PJ, Kawamata H, Shariff S, Hewett J, Sharma N, Ueda K, Breakefield XO, Hyman BT. TorsinA and heat shock proteins act as molecular chaperones: suppression of α–synuclein aggregation. J Neurochem. 2002;83:846–854. doi: 10.1046/j.1471-4159.2002.01190.x. [DOI] [PubMed] [Google Scholar]
- McNaught KS, Kapustin A, Jackson T, Jengelley TA, Jnobaptiste R, Shashidharan P, Perl DP, Pasik P, Olanow CW. Brainstem pathology in DYT1 primary torsion dystonia. Ann Neurol. 2004;56:540–547. doi: 10.1002/ana.20225. [DOI] [PubMed] [Google Scholar]
- Mena MA, Khan U, Togasaki DM, Sulzer D, Epstein CJ. Effects of Wild-Type and Mutated Copper/Zinc Superoxide Dismutase on Neuronal Survival and l-DOPA-Induced Toxicity in Postnatal Midbrain Culture. J Neurochem. 1997;69:21–33. doi: 10.1046/j.1471-4159.1997.69010021.x. [DOI] [PubMed] [Google Scholar]
- Mogk A, Haslberger T, Tessarz P, Bukau B. Common and specific mechanisms of AAA+ proteins involved in protein quality control. Biochem Soc Trans. 2008;36:120–125. doi: 10.1042/BST0360120. [DOI] [PubMed] [Google Scholar]
- Nemeth AH. The genetics of primary dystonias and related disorders. Brain. 2002;125:695–721. doi: 10.1093/brain/awf090. [DOI] [PubMed] [Google Scholar]
- Nery FC, Zeng J, Niland BP, Hewett J, Farley J, Irimia D, Li Y, Wiche G, Sonnenberg A, Breakefield XO. TorsinA binds the KASH domain of nesprins and participates in linkage between nuclear envelope and cytoskeleton. J Cell Sci. 2008;121:3476–3486. doi: 10.1242/jcs.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwald AF, Aravind L, Spouge JL, Koonin EV. AAA+: A Class of Chaperone-Like ATPases Associated with the Assembly, Operation, and Disassembly of Protein Complexes. Genome Res. 1999;9:27–43. [PubMed] [Google Scholar]
- Neve RL, Howe JR, Hong S, Kalb RG. Introduction of the glutamate receptor subunit 1 into motor neurons in vitro and in vivo using a recombinant herpes simplex virus. Neuroscience. 1997;79:435–447. doi: 10.1016/s0306-4522(96)00645-8. [DOI] [PubMed] [Google Scholar]
- O'Farrell C, Hernandez DG, Evey C, Singleton AB, Cookson MR. Normal localization of ΔF323-Y328 mutant torsinA in transfected human cells. Neurosci Lett. 2002;327:75–78. doi: 10.1016/s0304-3940(02)00400-7. [DOI] [PubMed] [Google Scholar]
- Ogura T, Wilkinson AJ. AAA+ superfamily ATPases: common structure--diverse function. Genes Cells. 2001;6:575–597. doi: 10.1046/j.1365-2443.2001.00447.x. [DOI] [PubMed] [Google Scholar]
- Okuno S, Fujisawa H. A new mechanism for regulation of tyrosine 3-monooxygenase by end product and cyclic AMP-dependent protein kinase. J Biol Chem. 1985;260:2633–2635. [PubMed] [Google Scholar]
- Ozelius LJ, Hewett JW, Page CE, Bressman SB, Kramer PL, Shalish C, de Leon D, Brin MF, Raymond D, Corey DP, Fahn S, Risch NJ, Buckler AJ, Gusella JF, Breakefield XO. The early-onset torsion dystonia gene (DYT1) encodes an ATP-binding protein. Nat Genet. 1997;17:40–48. doi: 10.1038/ng0997-40. [DOI] [PubMed] [Google Scholar]
- Petrucelli L, O'Farrell C, Lockhart PJ, Baptista M, Kehoe K, Vink L, Choi P, Wolozin B, Farrer M, Hardy J, Cookson MR. Parkin Protects against the Toxicity Associated with Mutant α-Synuclein: Proteasome Dysfunction Selectively Affects Catecholaminergic Neurons. Neuron. 2002;36:1007–1019. doi: 10.1016/s0896-6273(02)01125-x. [DOI] [PubMed] [Google Scholar]
- Pham P, Frei KO, Woo W, Truong DD. Molecular defects of the dystonia-causing torsinA mutation. Neuroreport. 2006;17:1725–1728. doi: 10.1097/WNR.0b013e3280101220. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Joh TH, Field PM, Becker CG, Reis DJ. Cellular localization of tyrosine hydroxylase by immunohistochemistry. J Histochem Cytochem. 1975;23:1–12. doi: 10.1177/23.1.234988. [DOI] [PubMed] [Google Scholar]
- Rostasy K, Augood SJ, Hewett JW, Leung JCo, Sasaki H, Ozelius LJ, Ramesh V, Standaert DG, Breakefield XO, Hedreen JC. TorsinA protein and neuropathology in early onset generalized dystonia with GAG deletion. Neurobiol Dis. 2003;12:11–24. doi: 10.1016/s0969-9961(02)00010-4. [DOI] [PubMed] [Google Scholar]
- Sharma N, Baxter MG, Petravicz J, Bragg DC, Schienda A, Standaert DG, Breakefield XO. Impaired motor learning in mice expressing torsinA with the DYT1 dystonia mutation. J Neurosci. 2005;25:5351–5355. doi: 10.1523/JNEUROSCI.0855-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashidharan P, Kramer BC, Walker RH, Olanow CW, Brin MF. Immunohistochemical localization and distribution of torsinA in normal human and rat brain. Brain Res. 2000;853:197–206. doi: 10.1016/s0006-8993(99)02232-5. [DOI] [PubMed] [Google Scholar]
- Shashidharan P, Sandu D, Potla U, Armata IA, Walker RH, McNaught KS, Weisz D, Sreenath T, Brin MF, Olanow CW. Transgenic mouse model of early-onset DYT1 dystonia. Hum Mol Genet. 2005;14:125–133. doi: 10.1093/hmg/ddi012. [DOI] [PubMed] [Google Scholar]
- Torres GE, Sweeney AL, Beaulieu JM, Shashidharan P, Caron MG. Effect of torsinA on membrane proteins reveals a loss of function and a dominant-negative phenotype of the dystonia-associated ΔE-torsinA mutant. Proc Natl Acad Sci USA. 2004;101:15650–15655. doi: 10.1073/pnas.0308088101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RH, Brin MF, Sandu D, Good PF, Shashidharan P. TorsinA immunoreactivity in brains of patients with DYT1 and non-DYT1 dystonia. Neurology. 2002;58:120–124. doi: 10.1212/wnl.58.1.120. [DOI] [PubMed] [Google Scholar]
- Wichmann T. Commentary: Dopaminergic dysfunction in DYT1 dystonia. Exp Neurol. 2008;212:242–246. doi: 10.1016/j.expneurol.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, DeCuypere M, LeDoux MS. Abnormal motor function and dopamine neurotransmission in DYT1 ΔGAG transgenic mice. Exp Neurol. 2008;210:719–730. doi: 10.1016/j.expneurol.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]