Abstract
Context
While both environmental and genetic factors are important in the etiology of psychoactive substance use (PSU), we know little of how these influences differ through development.
Objective
To clarify the changing role of genes and environment in PSU from early adolescence through middle adulthood.
Design
Retrospective assessment by life history calendar, with univariate and bivariate structural modeling.
Setting
General community.
Participants
A total of 1796 members of male-male pairs from the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders.
Main Outcome Measures
Levels of use of alcohol, caffeine, cannabis, and nicotine recorded for every year of the respondent's life.
Results
For nicotine, alcohol, and cannabis, familial environmental factors were critical in influencing use in early adolescence and gradually declined in importance through young adulthood. Genetic factors, by contrast, had little or no influence on PSU in early adolescence and gradually increased in their effect with increasing age. The sources of individual differences in caffeine use changed much more modestly over time. Substantial correlations were seen among levels of cannabis, nicotine, and alcohol use and specifically between caffeine and nicotine. In adolescence, those correlations were strongly influenced by shared effects from the familial environment. However, as individuals aged, more and more of the correlation in PSU resulted from genetic factors that influenced use of both substances.
Conclusions
These results support an etiologic model for individual differences in PSU in which initiation and early patterns of use are strongly influenced by social and familial environmental factors while later levels of use are strongly influenced by genetic factors. The substantial correlations seen in levels of PSU across substances are largely the result of social environmental factors in adolescence, with genetic factors becoming progressively more important through early and middle adulthood.
While a Critical role for genetic factors in the etiology of psychoactive substance use (PSU) has been demonstrated in many studies,1-4 a range of social environmental variables including peer drug use, poor quality of family bonds, low neighborhood cohesion, and high levels of drug availability also predict PSU.5
The relative importance of social and genetic factors in the etiology of PSU may vary across the life span. Prior research suggests that the initiation of drug use is strongly influenced by social environmental factors, whereas the progression from first use to heavy use and from heavy use to abuse or dependence is more associated with neurobiological factors.6
This hypothesis has been supported by genetically informative studies, which have recently shown that from adolescence to early adulthood, the importance of genetic factors in the etiology of PSU typically increases; during the same period, shared environmental factors (which reflect family, peer, and community influences) decrease in importance.7-10 These results are supported by 2 meta-analyses of genetically informative studies of antisocial or externalizing behaviors—which are strongly correlated with PSU—showing, with increasing age, declining shared environmental influences11 and increasing heritability.12
Prior genetically informative longitudinal studies of drug use have been restricted to a small number of measurements (typically≤3), thereby preventing detailed analyses of developmental changes.12 In our most recent interview with male-male twin pairs from the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders (VATSPSUD), we used a life history calendar to assess participants' average use of alcohol, caffeine, nicotine, and cannabis for each year of their lives up to their age at interview.
In this article, we compare the similarity of PSU in monozygotic (MZ) and dizygotic (DZ) pairs at each year of age. We seek to address 3 questions. First, what is the developmental pattern of genetic and environmental influences on PSU? Second, do the resulting patterns differ across various substances? Finally, how do genetic and environmental factors contribute to the correlations in use of different psychoactive substances, and does this pattern change over development?
Methods
Sample
This article uses data collected in the third wave of interviews in white, adult, male twins born between 1940 and 1974 from the VATSPSUD.13 All of the subjects for the VATSPSUD were ascertained from the Virginia Twin Registry, a population-based register formed from a systematic review of birth certificates in the Commonwealth of Virginia. Response rates for the first (1993-1996) and second (1994-1998) waves of interviews were 72% and 83%, respectively. The third interview wave, restricted to male-male twins, was completed in 1998 to 2004 by 1796 male twins (75%) who had participated in the second interview, including both members of 469 MZ and 287 DZ pairs. Subjects were aged 24 to 62 years (mean [SD] age, 40.3 [9.0] years). Most subjects were interviewed by telephone. After an explanation of the research protocol, signed informed consent was obtained for face-to-face interviews and verbal consent was obtained for telephone interviews. This project was approved by the Office of Research Subjects Protection at Virginia Commonwealth University. Members of a twin pair were always interviewed by different interviewers. Zygosity was assigned by a combination of self-report measures, photographs, and DNA polymorphisms.13
Assessment
To improve recall accuracy, we used a life history calendar.14 Following methods by Cohen et al,15 the calendar contained columns for each year of the subject's life. The first rows, completed early in the interview, documented key changes in living situation as well as major educational, employment, and interpersonal milestones. Toward the end of the interview, after completion of drug sections assessing standard questions about age at first use, maximal lifetime use, and symptoms of abuse and dependence, we returned to the calendar. For tobacco, the interviewer then asked, “You said that you began smoking when you were [age previously stated for first used cigarettes]? How many cigarettes per day did you smoke then? For how many years did you smoke at this rate?” The interviewers then obtained a year-by-year history of average daily cigarette use with suggested questions such as the following: what happened then, did you smoke more or less, did you ever stop smoking for a full year, and which years? If necessary, the interviewers would use other memory prompts from the information previously recorded on the calendar to “cue” the respondent into the relevant “memory files.” For caffeine, we inquired about average daily consumption of caffeine-containing drinks, that is, caffeinated soft drinks, tea, and coffee. For alcohol, we inquired separately about the average number of days per month on which the subject consumed alcoholic beverages and the average number of drinks consumed per day when drinking. We defined a drink as 1 bottle of beer, 1 glass of wine, or 1 shot of liquor. For cannabis, we inquired about the average number of times used per month. The instruction manual to the interviewers read, “By ‘times’ we mean individual doses. Thus, if the respondent smoked 3 joints every day, you would rate 90 [per month], not 30.”
Statistical Analyses
Because of skewed distributions of our drug consumption data, for our analyses we divided information on frequency of drug use into 6 to 8 categories. For caffeine, we used number of caffeinated drinks consumed per day: 0, 1 to 2, 3, 4, 5, 6, 7 to 10, and more than 10. For nicotine, we used cigarettes per day: 0, 1 to 5, 6 to 10, 11 to 20, 21 to 30, 31 to 40, and more than 40. For alcohol, we used drinks per month: 0, 1 to 5, 6 to 15, 16 to 30, 31 to 60, 61 to 200, and more than 200. For cannabis, we used units (“joints”) per month: 0, 1 to 10, 11 to 20, 21 to 50, 51 to 100, and more than 100. The similarity of use in members of twin pairs over age was assessed by polychoric correlation.
To evaluate in an unconfounded manner our assumption of the unidimensionality of our PSU measures, we assessed the fit of multiple threshold models by the root mean squared error of approximation (RMSEA). Good models have RMSEA values of 0.05 or less.16 In the Mx program,17 we ran models separately for MZ and DZ twin pairs, for each of the 4 substances, and for the 11 years from ages 25 to 35 years. Three of the 88 models would not converge. Of the remaining 85, only 3 produced RMSEA values greater than 0.05.
Using the Mx program, we then fitted to the raw data a full or “ACE” univariate twin model to the results for each year for which there were sufficient data. These models decompose the sources of variation into those arising from additive genetic effects (A), familial or common environmental effects (C) (which would include all environmental exposures shared by the twins such as in the home of origin, school, community, and church), and unique or individual-specific environment and measurement error (E).
Finally, also in Mx, we fit full bivariate models for each year to selected pairs of substances. We applied a full model and then decomposed the correlation in liability between level of use of the 2 substances into that due to A, C, and E. For example, if the heritabilities of nicotine and caffeine use in a particular year were and , respectively, the genetic correlation between them was ra, and the phenotypic correlation was rp, then the proportion of that correlation due to genetic effects would be equal to [(anac) × ra]/rp.
To maximize the comparability of results across years, we present parameter estimates only from the full or ACE model. Furthermore, with our moderate sample size, these results are likely to best approximate the true sources of individual differences.18
Our data are right censored as individuals could report substance use only up to their age at interview. Although we want to understand the causes of substance use well into adulthood, the sample size at older ages became progressively smaller and our estimates less accurate. As a compromise, we present results up to age 35 years (at which point we had data from 1215 participants [68%] in our sample) for our univariate analyses of caffeine, nicotine, and cannabis and all of our bivariate analyses. For alcohol only—to clarify the persistence of an apparent change in sources of individual differences in the mid 30s—we present data up to age 40 years (at which point we had data from 904 participants [50%] in our sample). At young ages, PSU was too infrequent to produce stable results. We defined the lowest age for which to present results for each substance as the first age when statistically significant parameter estimates for a2 or c2 emerged from our ACE models. This was age 9 years for caffeine, age 13 years for nicotine, and age 14 years for both alcohol and cannabis.
Results
Frequency of Use Over Age and Reliability
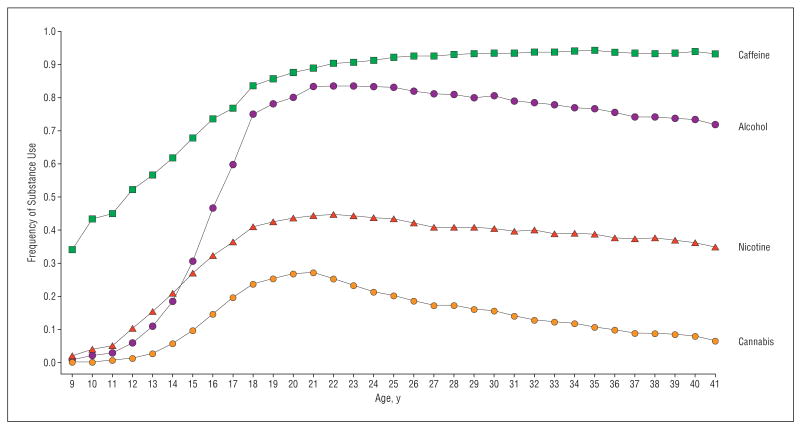
Figure 1 illustrates the proportion of subjects who reported any use of caffeine, nicotine, alcohol, and cannabis in each year from ages 9 to 40 years. Use of caffeine begins earliest and is eventually consumed by more than 90% of subjects. Nicotine use begins next and reaches a maximal consumption rate of 45%. Use of alcohol begins slightly later than nicotine but quickly overtakes it, achieving maximal use by more than 80% of subjects. Cannabis use begins the latest and is the rarest, never being used by more than 27% of the sample in any one year. Maximal use of nicotine, alcohol, and cannabis all occur in the early 20s.
Figure 1.
The frequency of any use of caffeine, alcohol, nicotine, and cannabis by year from ages 9 to 41 years.
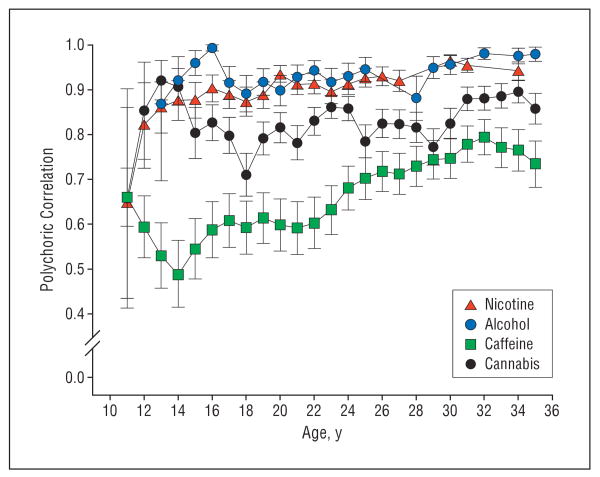
We assessed the reliability of our PSU measures in 3 ways. First, short-term test-retest data were available from 142 subjects interviewed an average of 29 days apart. Polychoric correlations for the quantity consumed from ages 13 to 35 years are seen in Figure 2. For nicotine and alcohol, the patterns of results were similar, with correlations starting at around +0.65 at age 13 years and increasing quickly to +0.90 or higher. Reliability for cannabis use was lower, with most estimates around +0.80. Caffeine consumption was least reliably assessed, with most correlations ranging from +0.60 to +0.70. Second, long-term test-retest polychoric correlations were available from the entire sample for lifetime heaviest use assessed at our second interview, conducted a mean (SD) of 51 (21) months earlier for caffeine (+0.68), nicotine (+0.94), alcohol (+0.81), and cannabis (+0.80). Third, we recorded the last year's average use of alcohol at both the first (mean [SD], 68 [22] months earlier) and second interviews and of caffeine and nicotine at the second interview. Polychoric correlations between those contemporary measures and the retrospective use reported at the third interview were +0.84, +0.93, and +0.66 for alcohol, nicotine, and caffeine, respectively, reported at the second interview and +0.81 for alcohol reported at the first interview.
Figure 2.
Test-retest reliability for level of nicotine, alcohol, caffeine, and cannabis use for ages 11 to 35 years (n=142) as assessed by polychoric correlations. Error bars indicate ±1 SE.
Univariate Analyses
Caffeine
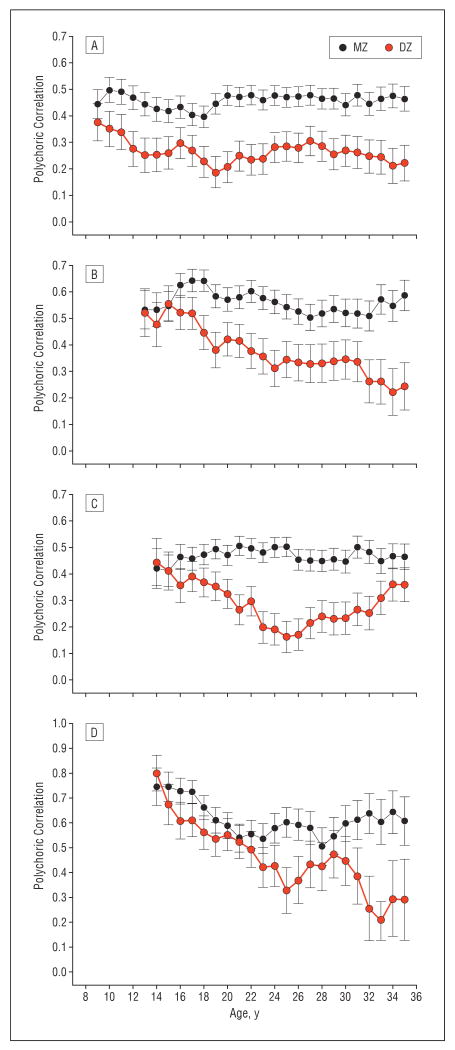
Figure 3A depicts the polychoric correlation± 1 SE in pair similarity for the average number of caffeinated beverages consumed per day from ages 9 to 35 years. At age 9 years, resemblance for caffeine consumption is only slightly lower in DZ twins (red line) than in MZ twins (black line). However, starting at age 10 years the curves diverge, with the difference between them gradually increasing until age 20 years. From ages 20 to 35 years, the curves are broadly stable (with a bit of “wobble”), with the correlations in MZ pairs (approximately 0.45) substantially exceeding those in DZ pairs (approximately 0.25).
Figure 3.
Polychoric correlations±1 SE in monozygotic (MZ) twins and dizygotic (DZ) twins for the average daily number of caffeine-containing drinks for ages 9 to 35 years (A), the average daily number of cigarettes for ages 13 to 35 years (B), the average number of alcoholic drinks consumed per month for ages 14 to 35 years (C), and the average number of units of cannabis consumed per month for ages 14 to 35 years (D).
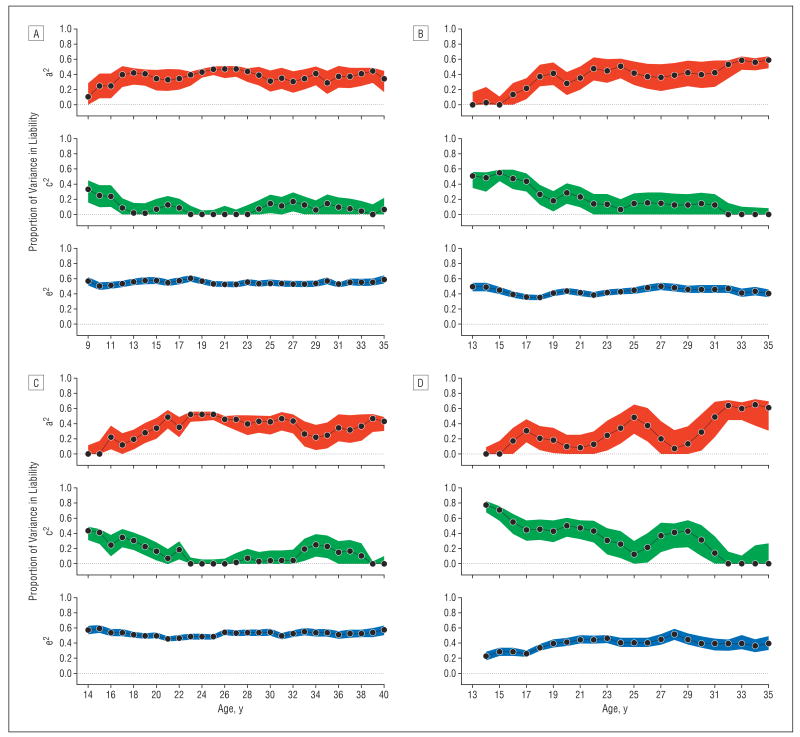
Figure 4A shows the parameter estimates from twin modeling±1 SE. At age 9 years, twin resemblance for caffeine use results largely from the family environment, which accounts for 33% of the variance. However, from ages 10 to 18 years, familial environmental influences gradually decline. Genetic effects on caffeine consumption rise from age 9 years to ages 13 to 14 years and then remain relatively stable at between 30% and 45% heritability.
Figure 4.
Parameter estimates ± 1 SE for the contributions to variation in liability to psychoactive drug use of additive genetic effects (a2), familial environmental factors (c2), and the individual-specific environment (e2) by year for the average daily number of caffeine-containing drinks for ages 9 to 35 years (A), the average daily number of cigarettes for ages 13 to 35 years (B), the average number of alcoholic drinks consumed per month for ages 14 to 40 years (C), and the average number of units of cannabis consumed per month for ages 14 to 35 years (D). The actual parameter estimates are depicted by the black lines, and the colored regions represent the possible range of estimates ± 1 SE.
Nicotine
Figure 3B shows the polychoric correlation in average daily number of cigarettes consumed from ages 13 to 35 years. Between ages 13 and 15 years, twin resemblance for daily smoking is quite high and similar in MZ and DZ twins. However, at age 16 years, the curves begin to diverge systematically. Resemblance for nicotine consumption climbs slightly in MZ twins and remains between +0.50 and +0.60 until age 35 years. By contrast, in DZ pairs, the correlation in nicotine use declines steadily from ages 16 to 35 years, with the exception of a stable period from ages 25 to 31 years.
Figure 4B shows twin modeling results for nicotine use. At age 13 years, shared environmental influences on cigarette use are strong, accounting for 50% of the variance in liability. The importance of the shared environment declines rapidly from ages 13 to 22 years and is responsible for approximately 10% of the variance for most of the 20s. Then, early in the 30s, shared environmental influences on nicotine consumption disappear.
Genetic factors have no influence on the degree of cigarette use at age 13 years. From ages 15 to 19 years, the importance of genetic influences rises rapidly followed by a slower and somewhat irregular increase that stabilizes at nearly 60% heritability by the early 30s.
Alcohol
Figure 3C depicts the polychoric correlation in monthly alcohol consumption from ages 14 to 35 years. At ages 14 and 15 years, twin resemblance for alcohol consumption is very similar in MZ and DZ twins. However, at age 16 years, the curves start to diverge, with resemblance for alcohol consumption in MZ twins remaining stable between +0.40 and +0.50 until age 35 years. By contrast, the correlation in alcohol use declines in DZ twins until around age 26 years, at which point it increases again, only to decline again in the late 30s (data not shown).
Twin modeling results for alcohol consumption are seen in Figure 4C for ages 14 to 40 years. At age 14 years, familial resemblance for alcohol consumption results entirely from familial environmental influences, accounting for 40% of the variance. The importance of the familial environment declines steadily until age 23 years. From then until age 35 years, familial environmental influences on alcohol consumption are modest except for a brief “burst” in the mid 30s that disappears by age 40 years.
At age 14 years, genetic factors have no influence on the level of alcohol consumption. From ages 15 to 23 years, we see a steady rise in the importance of genetic influences, which reach a heritability level of approximately 40% through age 35 years with the exception of a “dip” in the mid 30s that reverses itself by age 40 years.
Cannabis
Figure 3D depicts the polychoric correlation for monthly cannabis consumption for ages 14 to 35 years. These curves are noisier than those seen with other substances—with wider confidence intervals and more year-to-year fluctuations. At age 14 years, twin resemblance for cannabis use is similar in MZ and DZ twins and differs only slightly from ages 14 to 21 years. At age 22 years, the MZ twin resemblance begins to modestly and consistently exceed that seen in DZ twins, although the 2 curves reapproach each other in the late 20s. These 2 curves strongly diverge only at age 30 years, at which point resemblance in the DZ curve declines sharply.
Figure 4D depicts the results of twin modeling for cannabis use. At age 14 years, familial environmental influences on cannabis use are very strong, accounting for nearly 80% of the variance. Shared environmental influences remain important for cannabis use until well into the late 20s. By contrast, the result of additive genetic effects is only evident at age 16 years and is modest in the late teens. There is a peak in genetic influences in the mid 20s and then a decline followed by a rise. By age 32 years, virtually all of the twin resemblance in cannabis use results from genetic effects.
Bivariate Analyses
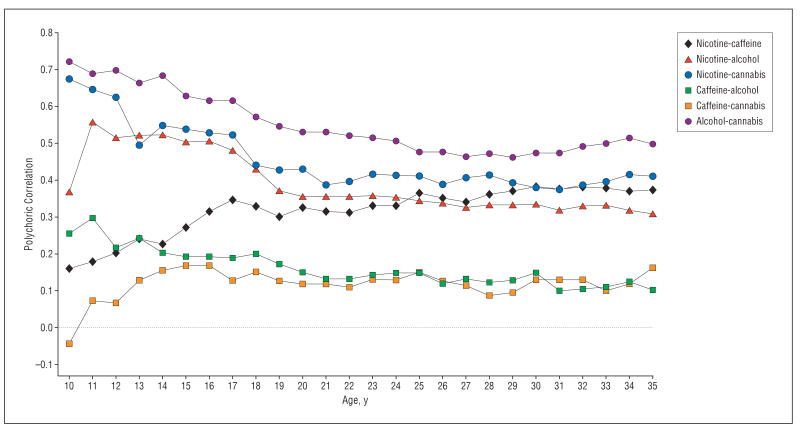
Figure 5 shows the polychoric correlation between ages 10 and 35 years for levels of PSU for each pairwise combination of our 4 substances. For alcohol-cannabis, nicotine-cannabis, and nicotine-alcohol, correlations start at relatively high levels (ie, generally>+0.50) and slowly decline over age. By contrast, caffeine consumption is generally not correlated with the use of other substances. The exception is for nicotine-caffeine, which begins at low levels in early adolescence and gradually increases with age.
Figure 5.
The polychoric correlation in individual twins between different forms of psychoactive drug use from ages 10 to 35 years.
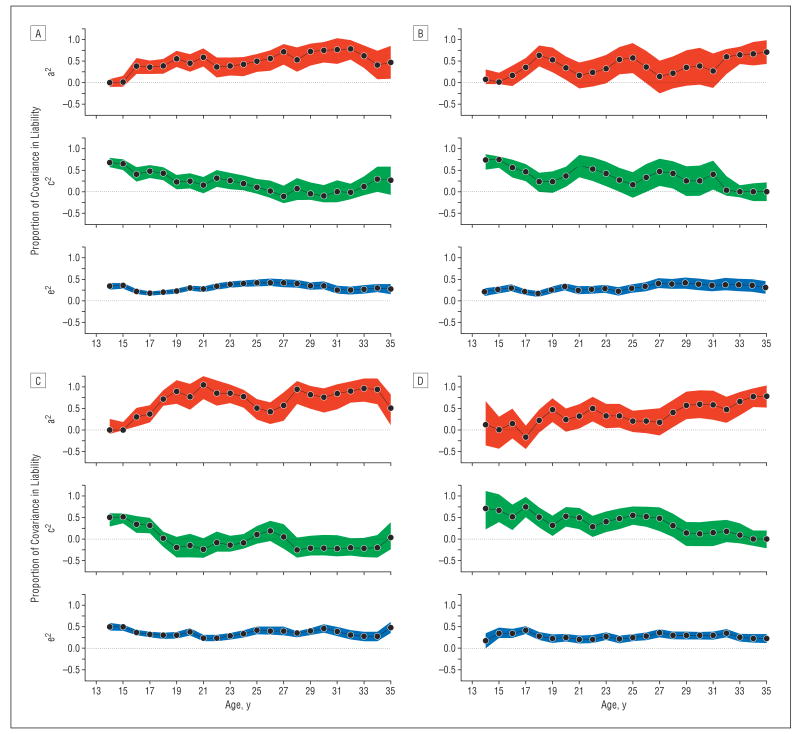
In Figure 6A-D, we show bivariate twin modeling results for 4 of these combinations: alcohol-cannabis, nicotine-cannabis, nicotine-alcohol, and nicotine-caffeine. These depict the proportion of the observed correlation resulting from genetic, familial environmental, and individual-specific environmental influences shared between the 2 substances.
Figure 6.
Parameter estimates ± 1 SE for the contributions to covariation in liability to pairs of psychoactive drug use of additive genetic effects (a2), familial environmental factors (c2), and the individual-specific environment (e2) by year for ages 14 to 35 years for alcohol-cannabis (A), nicotine-cannabis (B), nicotine-alcohol (C), and nicotine-caffeine (D). The actual parameter estimates are depicted by the black lines, and the colored regions represent the possible range of estimates ± 1 SE.
For alcohol and cannabis (Figure 6A), the substantial correlation observed at age 14 years results entirely from the effect of environmental factors, largely those shared by members of the same family. However, during the next 21 years, the genetic contribution to this correlation becomes generally stronger, whereas the effect of familial environmental factors becomes relatively weaker. The pattern for nicotine and cannabis (Figure 6B) is similar to that seen for alcohol and cannabis except that shared environmental effects persist longer. The pattern for nicotine and alcohol (Figure 6C) differs from these patterns in the more rapid decline of familial environmental effects and the parallel brisk rise of strong genetic effects in the 20s and 30s. The pattern of sources of the nicotine-caffeine correlation (Figure 6D) most closely resembles that seen for alcohol and cannabis, with gradually decreasing familial environmental effects and gradually increasing genetic effects from adolescence into middle adulthood.
Comment
The first 2 goals of these analyses were to clarify in a fine-grained manner the developmental pattern of genetic and environmental influences on PSU and to determine whether these patterns differed across substance classes.
For nicotine, alcohol, and cannabis, our univariate analyses produced a qualitatively similar pattern. From the point in early adolescence at which we had statistically meaningful results, familial environmental influences on PSU were quite strong. As individuals aged, however, these effects generally declined in importance; they had disappeared by age 35 years for nicotine and cannabis and by age 40 years for alcohol. The reverse picture was seen for genetic factors. Genes were without influence on PSU in early adolescence but gradually grew in importance as individuals aged. Of interest, the magnitude and duration of the familial environmental influences differed across substances, being least prominent for alcohol, intermediate for nicotine, and most marked for cannabis.
These results for nicotine, alcohol, and cannabis can be usefully viewed from an alternative perspective. The influence of familial factors (a2 or c2) on these forms of PSU was relatively constant over age. However, over development, familial environmental influences on PSU were replaced by genetic influences.
The unique feature of the developmental pattern for caffeine was the very short interval in late childhood (ages 9-12 years) with strong familial environmental influences. This largely disappeared by age 13 years, and from then on, no systematic changes in genetic and environmental influences on caffeine use were seen.
These results are broadly comparable to those of prior studies7-12 that have examined the development of PSU or associated externalizing behaviors in genetically informative populations. For example, in twins from the Australian Twin Registry at ages 13 to 18 years, familial environmental and genetic factors were responsible for approximately 45% and 25%, respectively, of individual differences in smoking.8 When followed up over 2 subsequent waves, the role of familial environment declined and that of genetic factors increased.8
Familial environmental effects on PSU in our study persist long after most twins have left home and do not disappear until the early 30s for nicotine, cannabis, and perhaps alcohol. Familial environmental influences on PSU are therefore not solely attributable to the immediate effect of parental, community, school, or peer influences or to differing degrees of drug availability shared by adolescent twins. Rather, familial environmental effects on PSU are at least partially mediated by more enduring processes. One such mechanism might be attitudes toward substance use, including those related to religious beliefs,19 that are acquired in the home or community. Our results suggest that such attitudes have their most potent and enduring effects on cannabis, intermediate effects on alcohol and nicotine, and the least effects on caffeine.
What is responsible for the increasing genetic influences on PSU with age? Although our data provide no direct answer to this question, we would suggest for further consideration a biological hypothesis and a socially mediated hypothesis. The biological hypothesis posits that genes influencing PSU are expressed once exposed to sufficient quantities of drug over an adequate period. This hypothesis is consistent with the substantial body of research showing unique genetic effects on the transition from drug use to drug dependence—that is, genetic factors whose phenotypic effect only becomes manifest after initial exposure.20,21 The socially mediated hypothesis is based on increasing evidence that as we develop from childhood through adolescence to adulthood, our genes play an increasing role in shaping our own social environment.22 This hypothesis suggests that genes affect PSU indirectly by influencing the selection of environments that actively either discourage or encourage drug use. We would speculate that each of these mechanisms explains part of the expanding developmental role of genes in PSU.
The third goal of this study was to explore how genetic and environmental factors contribute to the correlations in use of different psychoactive substances. Our results suggested dynamic developmental changes in the sources of correlations in drug use that mirror those seen for individual substances. For the drug pairs we explored in detail (alcohol-cannabis, nicotine-cannabis, nicotine-alcohol, and nicotine-caffeine), a broadly similar picture emerged. In adolescence, the correlations in different forms of substance use are driven largely by shared environmental factors. However, as individuals age, the genetic contribution to the correlations in drug use becomes progressively stronger, whereas the effect of familial environmental factors becomes gradually weaker. These results are consistent with prior studies showing that in adolescence, both shared genetic and environmental factors contribute to the observed correlation for use of the common psychoactive substances,7,23 whereas in adulthood, shared environmental factors play little apparent role.24
These results suggest a substantial degree of nonspecificity in the shared environmental risk factors for PSU. Whether it be the immediate effects of parental substance use, substance availability, or learned attitudes about drug use that constitute the familial environmental influences on PSU, our results show that these influences often affect use of multiple psychoactive substances rather than just 1 substance alone. These analyses also illustrate that sources of covariation in PSU can be as developmentally dynamic as sources of variation.
These results should be interpreted in the context of 7 potential methodological limitations. First, this sample is restricted to white male twins born in Virginia. Although the patterns of PSU in our twin cohort are quite similar to those reported from comparable nationally representative samples of white individuals in the United States,25 these findings may not be generalizable to females or to other ethnic groups. We examined whether this sample was representative of the larger study from which it was derived by predicting cooperation from information collected in the previous wave of the last year's caffeine, nicotine, and alcohol use and lifetime maximal cannabis use. Controlling for age, education, and the twin structure of the data, completing this interview was significantly but very modestly predicted by higher levels of daily caffeine use (odds ratio=1.03; z=2.15; P=.03), but it was not statistically associated with alcohol, nicotine, or cannabis use. Participation in this study appears to be largely unrelated to prior PSU.
Second, given the low rates of PSU at some ages and especially with cannabis, our results were somewhat statistically noisy. Presenting the results in Figures 3, 4, and 6 with standard errors helps to avoid overinterpretation of small “bumps” and “dips” in the curves that are unlikely to be reliable. While we could have smoothed our curves by lumping individual years into larger units, this might have obscured important developmental processes, especially those occurring in early adolescence.
Third, these models assume that excess similarity for PSU in MZ vs DZ twins did not result from greater similarity in MZ twins in their exposure to relevant environmental factors—particularly their peer group. We examined this assumption previously in this sample and found it to be supported.4,26 We asked the twins, only for the ages of 12 to 14 years, 15 to 17 years, 18 to 21 years, and 22 to 25 years, “how often did you and your twin have the same friends?” At each age, MZ pairs shared more of their peer group than did DZ pairs (Table). As they grew older, sharing of friends declined in both twin groups, with the difference between MZ and DZ pairs first increasing slightly and then declining as most of the twins left home. The Table also includes as an example the average correlation in nicotine use in these age groups in MZ and DZ pairs. Two trends are noteworthy, both of which are inconsistent with the hypothesis that our results arise from more similar social environments for MZ twins than DZ twins. In MZ pairs, similarity for smoking rises dramatically during this period while peer sharing declines. Furthermore, from the ages of 15 to 25 years, the difference in the degree of friend sharing in MZ vs DZ twins decreases while the difference in the degree of their similarity for smoking increases. Because rising heritabilities are seen for nicotine, alcohol, and cannabis use from ages 15 to 25 years (as well as stable or increasing MZ correlations for all substances except cannabis), it is unlikely that our results are substantially biased by differential peer group similarities in MZ vs DZ twins.
Table. Similarity of the Peer Group and of Nicotine Use in Monozygotic and Dizygotic Twins Over Development.
| Frequency of Same Friends, Meana | Correlation in Nicotine Use | |||||
|---|---|---|---|---|---|---|
| Age, y | MZ | DZ | MZ-DZ | MZ | DZ | MZ-DZ |
| 12-14 | 3.19 | 2.66 | 0.53 | 0.34 | 0.34 | 0.00 |
| 15-17 | 2.89 | 2.31 | 0.58 | 0.52 | 0.47 | 0.07 |
| 18-21 | 2.39 | 1.89 | 0.50 | 0.55 | 0.37 | 0.18 |
| 22-25 | 2.16 | 1.76 | 0.40 | 0.55 | 0.31 | 0.24 |
Abbreviations: DZ, dizygotic; MZ, monozygotic.
1 indicates never; 2, sometimes; 3, usually; and 4, always.
Fourth, because our information on PSU was collected retrospectively from adults, our findings could result from retrospective recall bias that systematically differed in MZ and DZ twins. However, it is difficult to imagine a pattern of biased recall that would artifactually produce the observed results. We tested the reliability of our assessments in 3 ways, all of which except for caffeine use demonstrated excellent reliability. (The lower reliability for caffeine use may explain the reduced MZ twin correlations observed for this form of PSU because unreliability attenuates such correlations and reduces estimates of genetic and shared environmental effects.) Furthermore, we used a life history calendar in our assessment of substance use. This method, which reflects the structure of autobiographical memory and promotes sequential retrieval within memory networks, has been shown to substantially improve the completeness and accuracy of retrospective reports.14,27-29
Fifth, our measures of PSU were not ideal. In assessing nicotine use, we did not include pipes, cigars, or smokeless tobacco. For caffeine, we did not account for differences in caffeine content between different caffeinated beverages.
Sixth, because our sample was born over a 34-year period, the pattern of our results could differ meaningfully across age cohorts (although we previously found no cohort differences in the genetic and environmental influences on PSU in this sample30). To address this issue, we divided our sample at the median age at interview and then compared the fit of a model that constrained our ACE parameter estimates to equality in the older and younger cohorts vs allowed separate estimates for the 2 groups for PSU from ages 14 to 35 years. Of the 88 individual tests (4 substances×22 years), only 7 (8%) were statistically significant at the 5% level, a result not different from chance expectations.31
Lastly, the goal of these analyses was to provide a fine-grained looked at developmental trends in sources of individual differences in vulnerability to PSU. We have not been able to address other intriguing questions such as variation in individual drug use trajectories or the degree of cross-time continuity in genetic and environmental risk factors for PSU. We hope to address these and other interesting issues in future studies.
Acknowledgments
Funding/Support: This work was supported in part by grants DA-011287 and AA-011408 from the National Institutes of Health.
Footnotes
Financial Disclosure: None reported.
Additional Contributions: Patricia Cohen, PhD, provided permission to use and adapt the life calendar assessment.
References
- 1.Pedersen N. Twin similarity for usage of common drugs. In: Gedda L, Parisi P, Nance WE, editors. Twin Research 3: Epidemiological and Clinical Studies. New York, NY: Alan R. Liss Inc; 1981. pp. 53–59. [PubMed] [Google Scholar]
- 2.Tsuang MT, Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, Meyer JM, Toomey R, Faraone SV, Eaves L. Genetic influences on DSM-III-R drug abuse and dependence: a study of 3372 twin pairs. Am J Med Genet. 1996;67(5):473–477. doi: 10.1002/(SICI)1096-8628(19960920)67:5<473::AID-AJMG6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 3.van den Bree MB, Johnson EO, Neale MC, Pickens RW. Genetic and environmental influences on drug use and abuse/dependence in male and female twins. Drug Alcohol Depend. 1998;52(3):231–241. doi: 10.1016/s0376-8716(98)00101-x. [DOI] [PubMed] [Google Scholar]
- 4.Kendler KS, Karkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Arch Gen Psychiatry. 2000;57(3):261–269. doi: 10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- 5.Petraitis J, Flay BR, Miller TQ, Torpy EJ, Greiner B. Illicit substance use among adolescents: a matrix of prospective predictors. Subst Use Misuse. 1998;33(13):2561–2604. doi: 10.3109/10826089809059341. [DOI] [PubMed] [Google Scholar]
- 6.Glantz M, Pickens R. Vulnerability to Drug Abuse. Washington, DC: American Psychological Association; 1992. [Google Scholar]
- 7.Koopmans JR, van Doornen LJ, Boomsma DI. Association between alcohol use and smoking in adolescent and young adult twins: a bivariate genetic analysis. Alcohol Clin Exp Res. 1997;21(3):537–546. [PubMed] [Google Scholar]
- 8.White VM, Hopper JL, Wearing AJ, Hill DJ. The role of genes in tobacco smoking during adolescence and young adulthood: a multivariate behaviour genetic investigation. Addiction. 2003;98(8):1087–1100. doi: 10.1046/j.1360-0443.2003.00427.x. [DOI] [PubMed] [Google Scholar]
- 9.Viken RJ, Kaprio J, Koskenvuo M, Rose RJ. Longitudinal analyses of the determinants of drinking and of drinking to intoxication in adolescent twins. Behav Genet. 1999;29(6):455–461. doi: 10.1023/a:1021631122461. [DOI] [PubMed] [Google Scholar]
- 10.Malone SM, Taylor J, Marmorstein NR, McGue M, Iacono WG. Genetic and environmental influences on antisocial behavior and alcohol dependence from adolescence to early adulthood. Dev Psychopathol. 2004;16(4):943–966. doi: 10.1017/s0954579404040088. [DOI] [PubMed] [Google Scholar]
- 11.Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: a meta-analysis of twin and adoption studies. Psychol Bull. 2002;128(3):490–529. [PubMed] [Google Scholar]
- 12.Bergen SE, Gardner CO, Kendler KS. Age-related changes in heritability of behavioral phenotypes over adolescence and young adulthood: a meta-analysis. Twin Res Hum Genet. 2007;10(3):423–433. doi: 10.1375/twin.10.3.423. [DOI] [PubMed] [Google Scholar]
- 13.Kendler KS, Prescott CA. Genes, Environment, and Psychopathology: Understanding the Causes of Psychiatric and Substance Use Disorders. New York, NY: Guilford Press; 2006. [Google Scholar]
- 14.Freedman D, Thornton A, Camburn D, Alwin D, Young-DeMarco L. The life history calendar: a technique for collecting retrospective data. Sociol Methodol. 1988;18(2):37–68. [PubMed] [Google Scholar]
- 15.Cohen P, Kasen S, Chen H, Hartmark C, Gordon K. Variations in patterns of developmental transitions in the emerging adulthood period. Dev Psychol. 2003;39(4):657–669. doi: 10.1037/0012-1649.39.4.657. [DOI] [PubMed] [Google Scholar]
- 16.Steiger JH. Structural model evaluation and modification: an interval estimation approach. Multivariate Behav Res. 1990;25(2):173–180. doi: 10.1207/s15327906mbr2502_4. [DOI] [PubMed] [Google Scholar]
- 17.Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6th. Richmond: Dept of Psychiatry, Virginia Commonwealth University Medical School; 2003. [Google Scholar]
- 18.Sullivan PF, Eaves LJ. Evaluation of analyses of univariate discrete twin data. Behav Genet. 2002;32(3):221–227. doi: 10.1023/a:1016025229858. [DOI] [PubMed] [Google Scholar]
- 19.Larson DB, Swyers JP, McCullough ME. Scientific Research on Spirituality and Health: A Consensus Report. Rockville, MD: National Institute for Healthcare Research; 1998. [Google Scholar]
- 20.Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychol Med. 1999;29(2):299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- 21.Agrawal A, Neale MC, Jacobson KC, Prescott CA, Kendler KS. Illicit drug use and abuse/dependence: modeling of two-stage variables using the CCC approach. Addict Behav. 2005;30(5):1043–1048. doi: 10.1016/j.addbeh.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Kendler KS, Jacobson KC, Gardner CO, Gillespie NA, Aggen SH, Prescott CA. Creating a social world: a developmental study of peer deviance. Arch Gen Psychiatry. 2007;64(8):958–965. doi: 10.1001/archpsyc.64.8.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han C, McGue MK, Iacono WG. Lifetime tobacco, alcohol and other substance use in adolescent Minnesota twins: univariate and multivariate behavioral genetic analyses. Addiction. 1999;94(7):981–993. doi: 10.1046/j.1360-0443.1999.9479814.x. [DOI] [PubMed] [Google Scholar]
- 24.Hettema JM, Corey LA, Kendler KS. A multivariate genetic analysis of the use of tobacco, alcohol, and caffeine in a population based sample of male and female twins. Drug Alcohol Depend. 1999;57(1):69–78. doi: 10.1016/s0376-8716(99)00053-8. [DOI] [PubMed] [Google Scholar]
- 25.Substance Abuse and Mental Health Services Administration, Office of Applied Studies. National Household Survey on Drug Abuse: Population Estimates, 1997. Rockville, MD: Substance Abuse and Mental Health Services Administration; 1998. [Google Scholar]
- 26.Kendler KS, Prescott CA. Cannabis use, abuse, and dependence in a population-based sample of female twins. Am J Psychiatry. 1998;155(8):1016–1022. doi: 10.1176/ajp.155.8.1016. [DOI] [PubMed] [Google Scholar]
- 27.Yoshihama M, Clum K, Crampton A, Gillespie B. Measuring the lifetime experience of domestic violence: application of the life history calendar method. Violence Vict. 2002;17(3):297–317. doi: 10.1891/vivi.17.3.297.33663. [DOI] [PubMed] [Google Scholar]
- 28.Cook LS, White JL, Stuart GC, Magliocco AM. The reliability of telephone interviews compared with in-person interviews using memory aids. Ann Epidemiol. 2003;13(7):495–501. doi: 10.1016/s1047-2797(03)00039-5. [DOI] [PubMed] [Google Scholar]
- 29.Belli RF. The structure of autobiographical memory and the event history calendar: potential improvements in the quality of retrospective reports in surveys. Memory. 1998;6(4):383–406. doi: 10.1080/741942610. [DOI] [PubMed] [Google Scholar]
- 30.Kendler KS, Gardner C, Jacobson KC, Neale MC, Prescott CA. Genetic and environmental influences on illicit drug use and tobacco use across birth cohorts. Psychol Med. 2005;35(9):1349–1356. doi: 10.1017/S0033291705004964. [DOI] [PubMed] [Google Scholar]
- 31.Feild HS, Armenakis AA. On use of multiple tests of significance in psychological research. Psychol Rep. 1974;35:427–431. [Google Scholar]