Abstract
We conducted several experiments that focused on the effect of vagal control on mucociliary clearance (MCC) in murine lungs. We hypothesized that loss of vagal control by chronic denervation (i.e. vagotomy) would reduce both basal MCC and the increase in MCC typically observed upon stimulation of capsaicin sensitive C-fibers. Vagotomy was performed on the right side of C57BL/6 mice and MCC was measured 5 days later. Mucociliary clearance was measured by gamma scintigraphy after oropharyngeal aspiration of the radioisotope 99mtechnetium and was expressed as the amount of radioactivity removed from the right lung 6 hours later. Baseline MCC was unaffected by vagotomy, averaging 6.5±4.9% and 6.8±5.8%, in 6 vagotomized and 6 non-vagotomized mice (controls), respectively. Mucociliary clearance increased significantly to 12.7±5.9% in 9 non-vagotomized mice treated with 1.6 × 10−9 M capsaicin, a vagally-mediated, nociceptor stimulus. (p=0.041). Capsaicin was admixed with 99mtechnetium and administered by oropharyngeal aspiration. In contrast, MCC was unchanged from control values in 9 vagotomized, capsaicin-treated animals, averaging 6.0±5.5% (p=0.024). These findings suggest that loss of vagal control through denervation does not affect basal MCC in C57BL/6 mice, but does appear to reduce the capacity of mice to respond to nociceptor agents that stimulate MCC. These data could have implications for patients whose lungs are denervated due to lung transplantation, since they may be at risk for an inadequate MCC response to inhaled irritants and inflammatory mediators, which are also nociceptor stimuli.
INTRODUCTION
Mucociliary clearance (MCC) is a major defense mechanism, which is essential for the protection of ciliated airway surfaces from prolonged exposure to infectious agents and inhaled irritants (Randell et al., 2006). Previously, we have demonstrated that MCC in pulmonary allografts is diminished within a few months after surgery in lung transplant patients, compared to healthy controls (Laube et al., 2007). The mechanism for this decrease in MCC is unknown.
One aspect of the lung transplantation process that could account for the decrease in MCC following surgery is denervation. This is because ciliary beat frequency (CBF) and mucus secretion, major components of the MCC apparatus, are vagally-mediated (Eljamal et al., 1994; Undem et al., 2005) and this control is severed as a result of denervation. In addition, bronchial sensory C-fibers (vagal afferent nerves) innervate the mucosa of mice and humans (Kollarik et al., 2003; Undem et al., 2005). Bronchial sensory C-fibers that conduct action potentials between 0.3–0.7 m/sec and are responsive to capsaicin, bradykinin, and ATP are classified as nociceptive C-fibers (Kollarik et al., 2003; Undem et al., 2004; Undem et al., 2005) and it has been reported that mucus secretion and MCC increase upon stimulation of capsaicin sensitive C-fibers (Kollarik et al., 2003; Undem et al., 2004; Undem et al., 2005). Stimulation with capsaicin also appears to increase CBF, thereby increasing MCC (Eljamal et al., 1994). It is likely that communication between the bronchial sensory C-fibers and the central nervous system via the vagus nerve is essential to an appropriate MCC response during exposures to irritants and autocoids that are released with tissue damage and inflammation. However, the need for such communication to elicit an appropriate MCC response has not been well studied.
We, therefore, conducted several experiments that focused on the effect of vagal control on MCC in murine lungs. We hypothesized that loss of vagal control by chronic denervation (i.e. vagotomy) would reduce both basal MCC and the increase in MCC typically observed upon stimulation of capsaicin sensitive C-fibers. Determining if denervation affects MCC in a mouse model could have important implications for understanding the mechanism (s) for the observed impairment in basal MCC in humans after lung transplantation. Results from these experiments could also provide insight into how denervation may affect the MCC response to inhaled irritants and infectious agents and this could improve our understanding of the mechanisms behind the significant morbidity and mortality due to infection as reported in humans in the first year post lung transplantation.
MATERIALS AND METHODS
Mouse Cohorts
Mucociliary clearance was compared in 8–10 week old, male C57BL/6 mice that were either vagotomized on the right side, or non-vagotomized. Animals were purchased from Jackson Laboratories (Bar Harbor, ME). Experiments were performed according to an animal protocol approved by the Animal Care Use Committee of The Johns Hopkins University School of Medicine.
Denervation
Chronic denervation was accomplished by transection of the right vagus. Prior to surgery, mice were weighed and anesthetized intraperitoneally with 100μg/g ketamine and 16 μg/g xylazine. While unconscious, a medial cervical incision was made and a 1–2 cm portion of the vagus was exposed, excised, and ligated such that a >0.5 cm gap was established between the peripheral and central nerve stumps. The nerve stumps were tightly ligated to prevent reanastomosis of the vagus during the recovery period. Mucociliary clearance was measured 4–5 days after this surgery and the gap between the nerve stumps was in effect at the time of those measurements.
Oropharyngeal Aspiration
Mucociliary clearance was quantified using a non-invasive, oropharyngeal aspiration procedure published previously (Foster et al., 2001; McGrath-Morrow et al., 2006). Mice were anesthetized by intraperitoneal injection of ketamine (100μg/g) and xylazine (16μg/g) and suspended from their upper incisors at a 45° incline. Then, two 50 μl aliquots of normal saline containing 60–80 μCi of the radioisotope 99mtechnetium-labeled sulfur colloid (99mTc-SC) were introduced separately into the distal part of the oropharynx ( McGrath-Morrow et al., 2006) and aspirated. Two aliquots were used to ensure adequate dosing for imaging. For the capsaicin experiments, two 50 μl aliquots containing 60–80 μCi of 99mTc-SC and 1.6 × 10−9 M capsaicin (Sigma-Aldrich, St. Louis, MO) were aspirated.
Gamma Scintigraphy Imaging Procedure
Mice lungs were imaged immediately after aspiration (time 0) and at 6–6.5 hours, thereafter, using an X-SPECT gamma camera (Gamma Medica, Inc. Northridge, CA) with pinhole collimation. To eliminate any possible effects of the timing of the administration of the anesthesia on mucociliary clearance, all mice (vagotomized and non-vagotomized) were anesthetized at the same time points. One dose of anesthesia (100 μg/g ketamine plus 16 μg/g xylazine) was injected intraperitoneally immediately before each of the imaging procedures. The effects of the anesthesia lasted approximately 45 min to 1 hour, so all mice were awake for most of the time between the first and subsequent imaging procedures.
We chose to acquire images over 6–6.5 hours because the first phase of mucociliary clearance of insoluble particles from the respiratory tract (clearance from ciliated airways) continues for between 20–24 hours in animals and man (Patrick et al., 1977; Pariente, 1988; Wolff, 1991; Foster et al., 2001; Hofmann et al., 2003), with half-times between 3 and 12 hours (Foster et al., 2001). Based on this information, we reasoned that we would be able to detect the greatest signal in terms of MCC from the ciliated airways by quantifying MCC over approximately 6 hours.
Quantification of Mucociliary Clearance
Mucociliary clearance was quantified as the difference between retention of radioactivity in the right lung at time 0 and at 6–6.5 hours after aspiration. Logistically, it was not possible to measure MCC at precisely 6 hours after aspiration. Retention values were background-corrected and then decay-corrected to time 0. The decay-corrected retention value was then subtracted from the actual time 0 retention value. The difference, expressed as a percentage, represented the amount of radioactivity that had been removed from the right lung by the MCC apparatus.
The left lung was not included in the analysis for several reasons. First, we have observed in unpublished experiments that MCC from the left lung can be significantly more variable between mice compared to the right lung. This may be the result of its proximity to the gastrointestinal tract, which often makes it difficult to establish a distinct region of interest for measurement of radioactive counts at the final time point. Secondly, since experiments involved vagotomy of the mouse on the right side, we reasoned that this procedure would affect MCC in the right lung to a greater extent than in the left lung and we were concerned that combining MCC measurements from both lungs could “dilute” any alterations in MCC that were the result of vagotomy.
Quantification of Aerosol Distribution in Non-Vagotomized and Vagotomized Mice
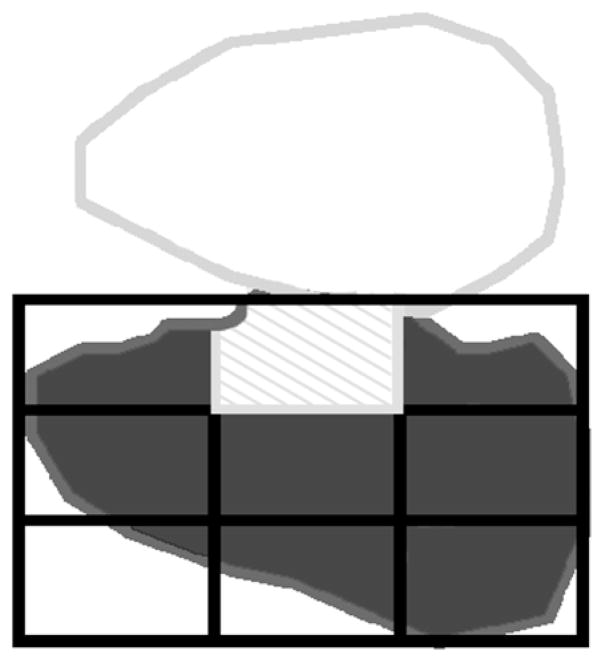
It is well known that the site of deposition of the radiotracer can affect MCC. To determine if any of the treatments resulted in differences in the distribution of the radiotracer, we analyzed the initial aerosol distribution within the right lung of all mice in this study. Distribution was quantified in terms of a central to peripheral ratio (C:P ratio). First, the borders of the right lung of each mouse were identified. Then, a nine-box grid was superimposed on the right lung border (Fig. 1). The upper middle box of the grid was categorized as central lung (based on physiologic inference) and the remaining boxes were designated as lung periphery. The central to peripheral (C:P) ratio was calculated by dividing count density in the central region by count density in the peripheral lung. One mouse from the non-vagotomized, control group was not included in this C:P analysis because its initial deposition image (i.e. time 0) was not available in the database.
Figure 1.
Line drawing showing the border of the right lung of a representative mouse from our study in dark gray, the central lung region (cross-hatched in light gray) and the lung periphery in dark gray. The number of counts and pixels in the central and peripheral regions were quantified and a count density established (counts divided by pixels). A central to peripheral (C:P) ratio was calculated to quantify the distribution of radioactivity in each mouse lung. The C:P ratio was calculated by dividing the central count density by the peripheral count density.
Data Analysis
Data are presented as mean ± standard deviation (SD) for MCC from the right lung. A one-way ANOVA was used to compare MCC and C:P ratios in the four test groups. These included non-vagotomized control mice, vagotomized mice, non-vagotomized control mice treated with capsaicin and vagotomized mice treated with capsaicin. A generalized linear model regression analysis, allowing for unequal group sizes, was used to test for differences in mean MCC and C:P ratios across the four test groups. An F-test with 3 degrees of freedom was used to determine significance between the differences in the means. A significant F value was followed by pair-wise comparisons using two-sample t-tests for the three a priori hypotheses only. Therefore, no adjustment in the significance level was made for the multiple comparisons. P-values ≤ 0.05 indicated a statistically significant difference between comparisons. The generalized linear model regression analysis and pair-wise comparisons were performed using SAS version 9.1.3 (SAS Institute, Cary, NC).
RESULTS
Distribution of Radiotracer
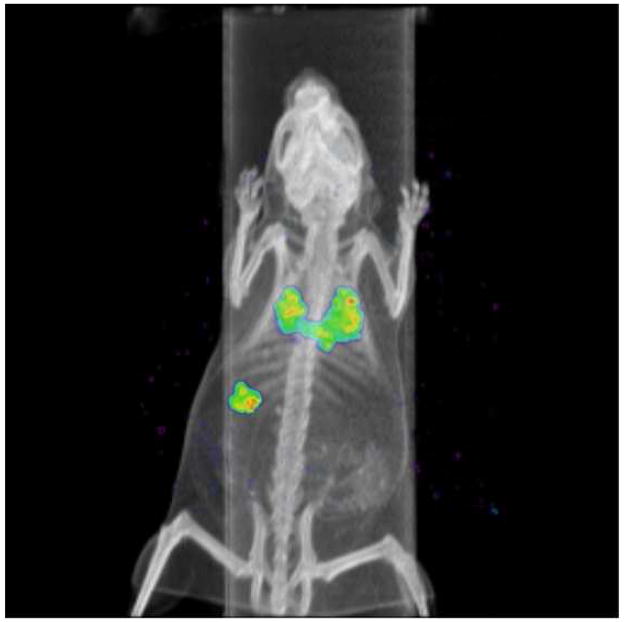
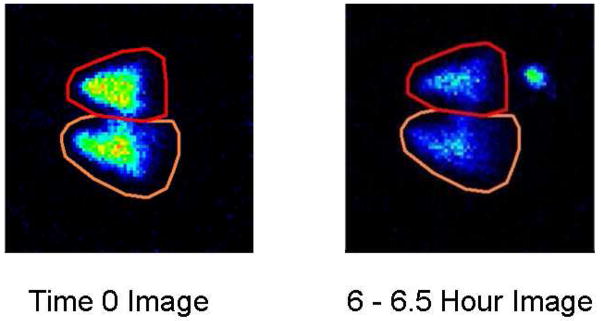
Figure 2 shows the anatomical distribution of 99mTc-SC six hours after aspiration, in one of the mice in our study. The gamma camera image is superimposed on the CT scan of the same animal. Figure 3 shows representative gamma camera images of radioactivity retained in the lungs of one mouse at time 0 and at 6–6.5 hours after aspiration.
Figure 2.
Perspective view (i.e. rendering view) of one of the C57BL/6 mice in this study, showing the radioisotopic image superimposed on the CT scan. This image was obtained approximately 6 hours after administration of the radioisotope and provides a view looking down at the animal. The two lungs and stomach appear in color. Radioactivity in the lungs resulted from oropharyngeal aspiration of 99mtechnetium-sulfur colloid. Radioactivity in the stomach resulted from removal of the radioisotope by MCC. The radioactivity was then swallowed, appearing in the stomach.
Figure 3.
Representative gamma camera images of lungs in an untreated C57BL/6 mouse at time 0 and at 6–6.5 hours after oropharyngeal aspiration of the radioaerosol. The right lung is shown at the bottom of each panel. Qualitatively, radioactivity retained in the lungs of these animals diminished over time due to a combination of radioactive decay and mucociliary clearance. The “hot spot” below the left lung (found at the top of the panel) at 6 hours post aspiration shows activity that was removed from the lungs by mucociliary clearance, swallowed and accumulated over time in the stomach.
C:P Ratios in C57BL/6 Mice
The C:P ratio averaged (± SD) 1.70±0.38 in non-vagotomized control animals (n = 5) and 2.0 ±0.46 in vagotomized mice (n = 6). The C:P ratio averaged 1.62±0.31 in non-vagotomized, capsaicin-treated mice (n = 9) and 2.11±0.71 in vagotomized, capsaicin-treated mice (n = 9). There were no statistically significant differences between mean C:P ratios for any of these groups of mice, indicating that regional deposition of the radiotracer within the right lung was unaffected by any of these treatments.
The Effect of Denervation on Mucociliary Clearance
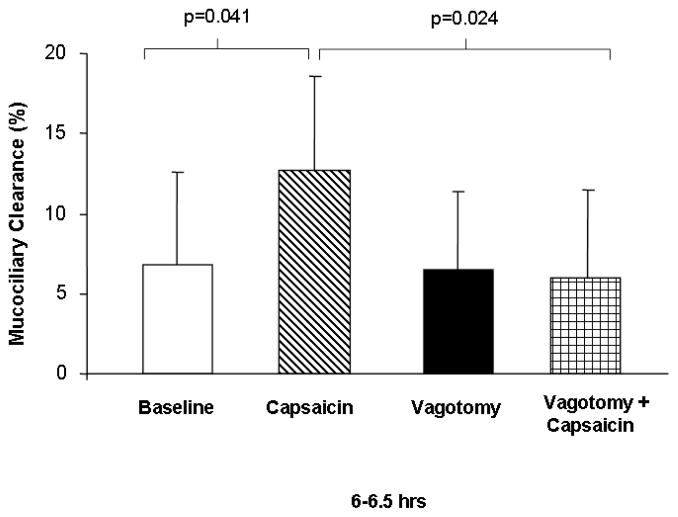
Mucociliary clearance was quantified from the right lung in 6 control mice and in another 6 vagotomized mice. At 6–6.5 hours post aspiration, MCC was not different for the two groups of mice, averaging 6.8±5.8% in the control mice and 6.5±4.9% in the vagotomized mice (Fig. 4). These results suggest that denervation does not reduce basal MCC in this murine model.
Figure 4.
Mean MCC (±SD) from the right lung between 6–6.5 hours in 6 C57BL/6 non-vagotomized control mice (white bars), 9 non-vagotomized C57BL/6 mice treated with capsaicin aerosol (striped bars), 6 vagotomized C57BL/6 mice (black bars) and 9 vagotomized C57BL/6 mice treated with capsaicin aerosol (checked bars). Between 6–6.5 hours, there was no significant difference between MCC in the control animals (white bar), compared to animals that had undergone vagotomy (black bar). However, MCC was statistically significantly greater in intact animals treated with capsaicin (striped bar), compared to controls (white bar) and animals that underwent vagotomy prior to treatment with capsaicin (checked bar).
The Effect of Capsaicin on Mucociliary Clearance
Mucociliary clearance was quantified from the right lung after the administration of capsaicin aerosol in a new cohort of 9 mice. At 6–6.5 hours post aspiration, mean MCC was significantly greater in mice treated with capsaicin, averaging 12.7±5.9%, compared to control mice, averaging 6.8±5.8% (Fig. 4) (p = 0.041). These results suggest that capsaicin significantly increases MCC at 6 hours post administration in this murine model.
The Effect of Denervation on Mucociliary Clearance Following Administration of Capsaicin
Mucociliary clearance was quantified from the right lung in a new cohort of 9 vagotomized mice after the administration of capsaicin aerosol. At 6–6.5 hours post aspiration, mean MCC in the vagotomized, capsaicin-treated mice was similar to control values, averaging 6.0±5.5%, and was significantly less than the mean MCC observed in non-vagotomized, capsaicin-treated animals (Fig. 4) (p = 0.024). These results suggest that denervation abolishes the observed increase in MCC following exposure to capsaicin, possibly because of impaired communication between nociceptive C-fibers and the central nervous system via the vagus nerve.
DISCUSSION
Mucociliary clearance can be determined in humans by measurement of the removal of inhaled radiolabeled particles that deposit in the lower airways (Anderson, 1971; Smaldone et al., 1988; Raabe et al., 1988; Ilowite et al., 1989; Mortensen et al., 1993; Bennett et al., 1996; Laube et al., 1996; Robinson et al., 1996; Oberdorster et al., 1997; Donaldson et al., 2006). A similar technique has been developed for the measurement of MCC in the mouse (Foster et al., 2001; McGrath-Morrow et al., 2006). We hypothesized that denervation would reduce basal MCC because major components of the MCC apparatus are vagally-mediated (Eljamal et al., 1994; Undem et al., 2005). However, data from our study in this murine model do not support this hypothesis, since MCC was no different in vagotomized and non-vagotomized animals.
These findings are in contrast to what Paul et al. reported in dogs (Paul et al., 1989). They observed significant decreases in MCC in autotransplanted and allotransplanted dog lungs (both involving denervation), while MCC remained normal in the sleeve-resected lung (Paul et al., 1989). One simple explanation for the differences between our results and those of Paul et al. could be the use of different species in the two studies. Another explanation for the differences between our two studies could involve differences in the site of clearance in the two studies. Paul et al. (1989) were measuring clearance from the large bronchi, whereas, because of our delivery technique, it is likely we were measuring clearance from smaller airways. It is possible that control of MCC by the vagus nerve diminishes in the more distal airways, compared to proximal airways. This could be the result of a reduced degree of innervation in the smaller airways compared to larger airways. This notion is supported by data obtained in humans by Alton and colleagues (1991). They reported no difference in in vivo measurements of lung potential difference in the denervated segmental and peripheral airways of heart-lung transplant patients, compared to non-denervated airways in non-transplant patients. These findings suggest that there is little vagal control of ion transport, and perhaps MCC, in human airways distal to the trachea and large bronchi. Other studies are needed to determine if this is the case in mice as well.
We also hypothesized that exposure to capsaicin, a nociceptor stimulus that is mediated through the vagus, would induce an increase in MCC in our murine model and this increase would be reduced by denervation of the vagus nerve. Results from our experiments support this hypothesis. Mucociliary clearance was significantly increased in non-vagotomized mice that were treated with capsaicin compared to untreated controls (p=0.041) and this increase was abolished in vagotomized mice that were also treated with capsaicin (p=0.024).
These results suggest that if the vagus nerve is cut the afferent signal from nociceptive C-fibers cannot be transmitted to the central nervous system and stimulation by a prototypical activator, such as capsaicin, fails to produce any tangible increase in MCC. This finding could have implications for the treatment outcome of patients who undergo lung transplantation since, during surgery, pulmonary innervation is severed. If the MCC response following exposure to irritants like capsaicin is significantly reduced in lung transplant patients, as it is in our murine model, it is likely that there will be a prolonged exposure to these agents and, possibly, to infectious or noxious inflammatory mediators as well. With prolonged exposure, the MCC apparatus may become permanently impaired and the lung more susceptible to damage from subsequent irritant exposures and bacterial and viral infections. An initial reduction in the MCC response to these agents in lung transplant patients could be one explanation for the significant morbidity and mortality due to infection as reported in the first year post lung transplantation (Studer et al., 2004).
An alternative explanation for our results could involve differences in the initial deposition of the radioisotope such that the marker was systematically deposited in more proximal airways in the capsaicin-treated, non-vagotomized mice, compared to control animals and capsaicin-treated, vagotomized mice. This could account for the observed faster clearance at 6 hours in the capsaicin-treated, non-vagotomized mice. However, analysis of C:P ratios in control animals, capsaicin-treated vagotomized animals, and capsaicin-treated, non-vagotomized animals showed no significant difference in initial radiotracer deposition patterns. Thus, it is unlikely that particles distributed more proximally in the non-vagotomized animals, compared to the vagotomized or control animals.
One concern regarding this mode of administration of the radiotracer is that when the liquid is presented to the back of the oropharynx of the mouse, some of it could be swallowed and deposit in the stomach rather than aspirated into the lungs. If this occurred, total radioactivity deposited within the lungs could vary between animals. However, this occurrence would not affect the quantification of mucociliary clearance in this study. This is because the time 0 count in the lungs of the mice was not based on the difference between the amount of radioactivity that was initially in the pipette tip minus the residual amount in the tip after aspiration. The time 0 count was quantified as the actual number of counts that were initially deposited in the lungs. Thus, any variation in the dose to the lungs as a result of swallowing of activity during the aspiration procedure was nullified by this normalization process. In addition, in our study, all time 0 images were analyzed for the presence of radioactive tracer in the stomach, but no measurable counts were detected. Thus, the radioisotope in this study was fully aspirated into the lungs of each of the animals, with no losses in the stomach, and there was no concern for activity that may have been swallowed.
Another explanation for our results may involve local axon reflexes, which could lead to changes in clearance by evoking the local release of sensory neuropeptides (e.g. substance P) from the nerve terminals. In our experiments, the vagus nerve was cut 4–5 days prior to measurements of MCC. After this amount of time, it is likely that the sensory nerves would have degenerated. Thus, the surgery would lead to a block of both local axon reflexes and any central parasympathetic reflex. The current study was not designed to specifically delineate the relative contributions of local axon reflexes versus central parasympathetic reflexes. To determine which is most important (axon reflex, or central reflex), we would have to carry out a future study in animals that are vagotomized hours before the MCC experiments.
The ability to measure MCC in a murine model could provide a means to dissect the role of individual components of the MCC apparatus that protect human airways and a useful tool for rapidly assessing the therapeutic potential of drugs and genetic therapies for diseases that affect MCC. Nevertheless, the murine lung does not appear to be a good model for simulating the MCC response in the proximal airways of humans because submucosal glands (a source of airway surface liquid and mucus) are plentiful in the trachea and bronchi of humans, but are found only in the proximal part of the trachea and do not extend into the bronchi in mice (Borthwick et al., 1999). In addition, proximal human airways are composed primarily of ciliated cells, while the murine trachea and bronchi are also populated with non-ciliated Clara-type cells (Pack et al., 1980). In contrast, the mouse lung may be a good model for simulating the MCC response in smaller airways in humans. This is because submucosal glands also are not abundant in human smaller airways and non-ciliated epithelial (Clara) cells predominate (McDowell et al. 1978). It should be noted that variability has been observed in the ultrastructure of non-ciliated bronchiolar epithelial cells in man versus the mouse (Plopper et al., 1980) and it is unclear how these differences may affect MCC in the two species.
In conclusion, our findings suggest that loss of vagal control does not appear to affect basal MCC in mice. It, therefore, seems unlikely that denervation alone accounts for the decrease in basal MCC observed in lung transplant patients. Nevertheless, denervation appears to reduce the capacity of mice to respond to nociceptor agents that stimulate MCC. These data suggest that denervation associated with lung transplantation may place patients at risk for an inadequate MCC response to inhaled irritants and inflammatory mediators, which are also nociceptor stimuli. Future studies are needed to test this hypothesis.
Acknowledgments
The authors wish to thank Kathryn A. Carson, Sc.M. for providing statistical assistance with the analysis of this data. This work was partially funded by the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK074480) and by the National Cancer Institute (U24 CA92871).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson WD. Thorax of Sheep and Man: An Anatomy Atlas. Dillon; Minneapolis: 1971. [Google Scholar]
- 2.Alton EWFW, Khagani A, Taylor RFH, Logan-Sinclair R, Yacoub M, Geddes DM. Effect of heart-lung transplantation on airway potential difference in patients with and without cystic fibrosis. Eur Respir J. 1991;4:5–9. [PubMed] [Google Scholar]
- 3.Bennett WD, Olivier KN, Zeman KL, Hohneker KW, Boucher RC, Knowles MR. Effect of uridine 5′-triphosphate plus amiloride on mucociliary clearance in adult cystic fibrosis. Am J Respir Crit Care Med. 1996;153:1796–1801. doi: 10.1164/ajrccm.153.6.8665037. [DOI] [PubMed] [Google Scholar]
- 4.Borthwick DW, West JD, Keighren MA, Flockhart JH, Innes BA, Dorin JR. Murine submucosal glands are clonally derived and show a cystic fibrosis gene-dependent distribution pattern. Am J Respir Cell Mol Biol. 1999;20:1181–1189. doi: 10.1165/ajrcmb.20.6.3475. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med. 2006;354:241–250. doi: 10.1056/NEJMoa043891. [DOI] [PubMed] [Google Scholar]
- 6.Eljamal M, Wong LB, Yeates DB. Capsaicin-activated bronchial- and alveolar-initiated pathways regulating tracheal ciliary beat frequency. J Appl Physiol. 1994;77:1239–1245. doi: 10.1152/jappl.1994.77.3.1239. [DOI] [PubMed] [Google Scholar]
- 7.Foster WM, Walters DM, Longphre M, Macri K, Miller LM. Methodology for the measurement of mucociliary function in the mouse by scintigraphy. J Appl Physiol. 2001;90:1111–1117. doi: 10.1152/jappl.2001.90.3.1111. [DOI] [PubMed] [Google Scholar]
- 8.Hofmann W, Asgharian B. The effect of lung structure on mucociliary clearance and particle retention in human and rat lungs. Toxicological Sciences. 2003;73:448–456. doi: 10.1093/toxsci/kfg075. [DOI] [PubMed] [Google Scholar]
- 9.Ilowite JS, Smaldone GC, Perry RJ, Bennett WD, Foster WM. Relationship between tracheobronchial particle clearance rates and sites of initial deposition in man. Arch Environ Health. 1989;44:267–273. doi: 10.1080/00039896.1989.9935893. [DOI] [PubMed] [Google Scholar]
- 10.Kollarik M, Thai Dinh Q, Fischer A, Undem BJ. Capsaicin-sensitive and -insensitive vagal bronchopulmonary C-fibres in the mouse. J Physiol. 2003;551:869–79. doi: 10.1113/jphysiol.2003.042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laube BL, Auci RM, Shields DE, et al. Effect of rhDNase on airflow obstruction and mucociliary clearance in cystic fibrosis. Am J Respir Crit Care Med. 1996;153:752–760. doi: 10.1164/ajrccm.153.2.8564129. [DOI] [PubMed] [Google Scholar]
- 12.Laube BL, Karmazyn YJ, Orens JB, Mogayzel PJ. Albuterol improves impaired mucociliary clearance after lung transplantation. J Heart Lung Transplant. 2007;26:138–44. doi: 10.1016/j.healun.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 13.McDowell EM, Barrett LA, Glavin F, Harris CC, Trump BF. The respiratory epithelium. I Human bronchus. J Nation Cancer Instit. 1978;61:539–549. [PubMed] [Google Scholar]
- 14.McGrath-Morrow S, Laube B, Tzou SC, et al. IL-12 overexpression in mice as a model for Sjogren lung disease. Am J Physiol. 2006;291:L837–846. doi: 10.1152/ajplung.00134.2006. [DOI] [PubMed] [Google Scholar]
- 15.Mortensen J, Hansen A, Falk M, Nielsen IK, Groth S. Reduced effect of inhaled beta 2-adrenergic agonists on lung mucociliary clearance in patients with cystic fibrosis. Chest. 1993;103:805–811. doi: 10.1378/chest.103.3.805. [DOI] [PubMed] [Google Scholar]
- 16.Oberdorster G, Cox C, Gelein R. Intratracheal instillation versus intratracheal inhalation of tracer particles for measuring lung clearance function. Exp Lung Res. 1997;23:17–34. doi: 10.3109/01902149709046045. [DOI] [PubMed] [Google Scholar]
- 17.Pack RJ, Al-Ugaily LH, Morris G, Widdicombe JG. The distribution and structure of cells in the tracheal epithelium of the mouse. Cell Tissue Res. 1980;208:65–84. doi: 10.1007/BF00234174. [DOI] [PubMed] [Google Scholar]
- 18.Pariente R. Therapeutic aspects of mucociliary clearance. Biomed Pharmacothe. 1988;42:521–524. [PubMed] [Google Scholar]
- 19.Patrick G, Stirling C. Measurement of mucociliary clearance from the trachea of conscious and anesthetized rats. J Appl Physiol. 1977;42:451–455. doi: 10.1152/jappl.1977.42.3.451. [DOI] [PubMed] [Google Scholar]
- 20.Paul A, Marelli D, Shennib H, et al. Mucociliary function in autotransplanted, allotransplanted, and sleeve resected lungs. J Thorac Cardiovasc Surg. 1989;98:523–8. [PubMed] [Google Scholar]
- 21.Plopper CG, Hill LH, Mairassy AT. Ultrastructure of the non-ciliated bronchiolar epithelial (Clara) cell of mammalian lung. III A study of man with comparison of 15 mammalian species. Exp Lung Res. 1980;1:171–80. doi: 10.3109/01902148009069646. [DOI] [PubMed] [Google Scholar]
- 22.Raabe OG, Al-Bayati MA, Teague SV, Rasolt A. Regional deposition of inhaled monodisperse coarse and fine aerosol particles in small laboratory animals. Ann Occup Hyg. 1988;32(Suppl1):53–63. [Google Scholar]
- 23.Randell SH, Boucher RC. Effective mucus clearance is essential for respiratory health. Am J Respir Cell Mol Bio. 2006;35:20–28. doi: 10.1165/rcmb.2006-0082SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson M, Regnis JA, Bailey DL, King M, Bautovich GJ, Bye PT. Effect of hypertonic saline, amiloride, and cough on mucociliary clearance in patients with cystic fibrosis. Am J Respir Crit Care Med. 1996;153:1503–1509. doi: 10.1164/ajrccm.153.5.8630593. [DOI] [PubMed] [Google Scholar]
- 25.Smaldone GC, Perry R, Bennett WD, Messina M, Zwang J, Ilowite J. Interpretation of 24 hr lung retention in studies of mucociliary clearance. J Aerosol Med. 1988;1:11–20. [Google Scholar]
- 26.Studer SM, Levy RD, McNeil K, Orens JB. Lung transplant outcomes: a review of survival, graft function, physiology, health-related quality of life and cost-effectiveness. Eur Respir J. 2004;24:674–685. doi: 10.1183/09031936.04.00065004. [DOI] [PubMed] [Google Scholar]
- 27.Undem BJ, Chuaychoo B, Lee MG, Weinreich D, Myers AC, Kollarik M. Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol. 2004;556:905–17. doi: 10.1113/jphysiol.2003.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Undem BJ, Kollarik M. The role of vagal afferent nerves in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:355–60. doi: 10.1513/pats.200504-033SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolff R. Mucociliary function. In: Parent RA, editor. Treatise on Pulmonary Toxicology, Volume I: Comparative Biology of the Normal Lung. CRC Press; Boca Raton: 1991. pp. 659–680. [Google Scholar]