Abstract
Nicotinic cholinergic receptors mediate autonomic transmission at ganglia. However, whether different subtypes of nicotinic cholinergic receptors expressed in autonomic ganglia elicit distinct roles in mediating sympathetic and parasympathetic regulations remain to be defined. In this study, we observed that different subtypes of nicotinic receptors were responsible for the sympathetic and parasympathetic cardiovascular responses. In urethane anesthetized mice, intravenous injection with cytisine, a non-selective nicotinic agonist, induced a brief but pronounced decrease in heart rate, followed by increases in heart rate and arterial blood pressure. The bradycardic response was blocked by atropine, and the pressor response was blocked by prazosin, confirming that these responses were parasympathetic and sympathetic activities, respectively. Hexamethonium, a ganglionic blocker, blocked both sympathetic and parasympathetic responses. Pre-treatment with methyllycaconitine citrate, a selective α7 nicotinic receptor antagonist, significantly attenuated cytisine-induced sympathetic response with little effect on the parasympathetic response. In contrast, pretreatment with dihydro-β-erythroidine hydrobromide, a selective α4β2 nicotinic receptor antagonist, blocked cytisine-induced parasympathetic response but not the sympathetic response. Pretreatment with dihydro-β-erythroidine hydrobromide also blocked baroreflex associated parasympathetic bradycardic response. Moreover, treatment with nicotine induced a bradycardic response without a significant pressor response, which was also attenuated by dihydro-β-erythroidine hydrobromide. Collectively, these data suggest that different nicotinic receptors play distinct roles in sympathetic and parasympathetic ganglia. Specifically, activations of α7 and α4β2 nicotinic receptors are involved in cytisine-induced cardiovascular sympathetic and parasympathetic responses, respectively.
Keywords: nicotinic receptors, cytisine, sympathetic, parasympathetic, heart rate, ganglia
INTRODUCTION
The two branches of the autonomic nervous system, the sympathetic (SNS) and parasympathetic (PSNS) nervous systems contain pre- and post-ganglionic portions. The pre-ganglionic fibers form synapses with neurons in autonomic ganglia, whereas the post-ganglionic fibers are projected to target organs. It is evident that ganglia do not simply relay the autonomic signals but play important integrative roles in sympathetic and parasympathetic activities [1–3]. The autonomic signaling transmissions at ganglia are mediated by nicotinic cholinergic receptors (nAChRs). Therefore, the advanced functional properties of ganglia may be associated with the diversity of subtypes of nAChRs in ganglia.
There are at least 7 α subunits and 3 β subunits being cloned [4] in mammalian neuronal tissues, forming various subtypes of nAchRs due to the different combinations of these subunits as a pentameric structure. Various nAchR subunits, including α3, α4, α7, β2 and β4, express in both sympathetic and parasympathetic ganglia [5, 6]. However, the precise functional properties of nAchRs in autonomic regulation at the ganglionic level remain to be defined. A fundamental question that still needs to be better understood is whether sympathetic and parasympathetic ganglionic neurotransmissions are mediated by different subtypes of nAChRs. In this study, we report novel evidence suggesting that α7 and α4β2 nAchRs are distinctly involved in sympathetic and parasympathetic responses in heart rate and arterial blood pressure in anesthetized mice in response to ganglionic stimulation with cytisine.
METHODS
Animals
Male Swiss Webster mice (Harlan Inc., Indianapolis, IN) aged 12–14 weeks old were used in this study. Mice were maintained on commercially available normal mouse chow (Harlan) and tap water in an environment with a 12:12-h light-dark cycle and ambient temperature (22°C). All experimental procedures in the present study were approved by Institutional Animal Care and Use Committee of The University of South Dakota, and all of the procedures were in accordance with the Guide for the Care and Use of Laboratory Animals [National Institutes of Health (NIH)].
Surgery and hemodynamic measurements
Mice were anesthetized with urethane (2g/kg, ip). This anesthesia ensures a consistent stable hemodynamic baseline without cardiac inhibition. The trachea was intubated to facilitate spontaneous breathing. The right common carotid artery was catheterized with Millar pressure catheter (Model SPV-1049, Millar Inc., Houston, TX). The arterial blood pressure and heart rate were measured via the catheter which was connected to the PowerLab data-acquisition system (ADInstruments Inc., Springfield, CO). The left common jugular vein was isolated and cannulated for intravenous injections with test substances.
Treatments and responses
nAchRs agonists and antagonists used in this study include: (−)-cytisine (1390, Tocris Bioscience), a non-selective nicotinic agonist; (−)-nicotine ditartrate (N5260, Sigma-Aldrich); Hexamethonium (H0879, Sigma-Aldrich), a non-selective ganglionic nAchR blocker; Methyllycaconitine citrate (MLA, 1029, Tocris Bioscience), a selective α7 nAchR antagonist; Dihydro-β-erythroidine hydrobromide (DHβE, 2349, Tocris Bioscience), a selective antagonist to α4 containing nAchRs. All the drugs were dissolved with normal saline to appropriate concentrations to ensure the designed doses of drugs were delivered with a small volume (0.02 – 0.04 ml), to avoid significant volume expansion by multiple injections in each animal.
Before each treatment, baselines of heart rate and arterial blood pressure were recorded. Then a nAchRs agonist or antagonist was quickly injected via the jugular vein catheter and a recording was taken of the entire course of the response. The peak changes from the baseline were calculated and compared.
In order to track the responses, each injection were marked on the recording trace by manually operating the software (Chart 5.0, ADInstruments Inc). The injection marks, shown as dotted lines on the raw recording traces in the figures, indicated the relative positions of injections.
To test the role of α7 nAchRs in cytisine-induced responses, the responses to cytisine alone and the responses to cytisine following the pretreatment with MLA were measured in one animal. Briefly, after a recording of baseline, cytisine (0.1mg/kg) was injected and the entire responses were recorded. After a 15 minute-interval, MLA (1mg/kg) was injected, immediately followed by the second cytisine injection (0.1mg/kg) and the entire responses were recorded. Five mice were used for the experiment.
The same protocol was used to test the responses to cytisine alone versus cytisine following a DHβE injection (1mg/kg), and the responses to nicotine (0.1mg/kg) alone versus nicotine following a DHβE injection (1mg/kg). Five mice were used for each experiment.
Additional mice were used for the baroreflex experiment. In each mouse, the response to a bolus injection of phenylephrine (PE, 0.1mg/kg) was tested. With a 15-minute interval, DHβE was given, followed by an injection with PE. Two baroreflex responses were measured and compared.
Statistical analysis
The compiled data are expressed as means ± s.d. Comparison of data were made using one-way analysis of variance (ANOVA) followed by Student– Newman–Keuls test. Significance was accepted when P value was less than 0.05.
RESULTS
Intravenous injection with nAchR agonist cytisine (0.1mg/kg) induced a brief and pronounced decrease in heart rate, which was immediately followed by the increased heart rate and blood pressure. The decreased heart rate was abolished by atropine (0.1mg/kg), whereas the pressor response was blocked by prazosin (0.05mg/kg) (Figure 1), confirming that these responses were parasympathetic and sympathetic activities, respectively. Moreover, intravenous pre-administration of ganglionic blocker, hexamethonium (0.1mg/kg), largely blocked both parasympathetic and sympathetic responses induced by two nicotinic agonists, suggesting that these responses were caused by the stimulation of nAchRs in ganglia (Figure 1).
Figure 1.
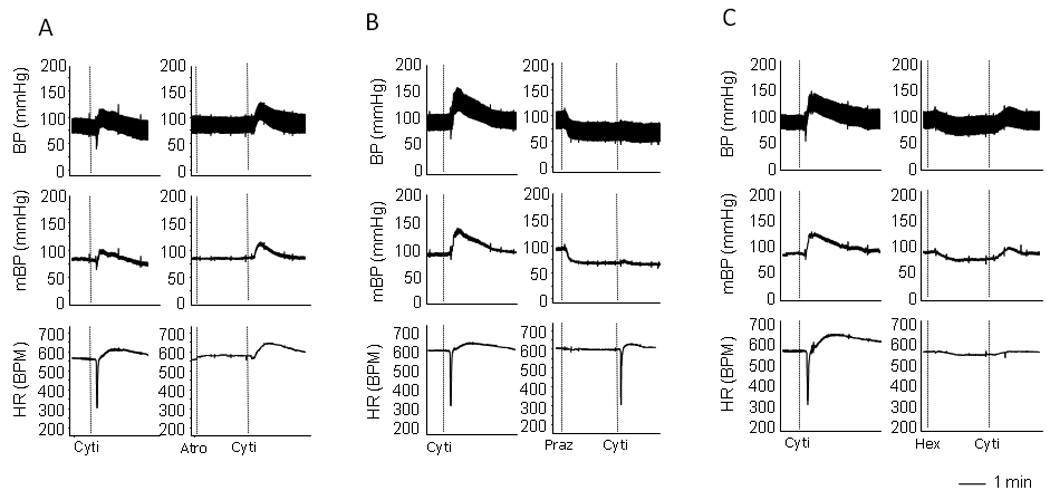
Raw recordings showing the cytisine-induced sympathetic and parasympathetic responses. A. Cytisine induced a quick decrease in heart rate, followed by an increase in heart rate and blood pressure. Pre-administration of atropine blocked the bradycardic response with no effect on pressor response. B. Pre-administration of prazosine eliminated the cytisine-induced pressor response. C. Pre-administration of ganglionic blocker hexamethonium largely blocked all components of the responses induced by cytisine. The vertical dotted lines in the recording traces showed the relative positions of injections.
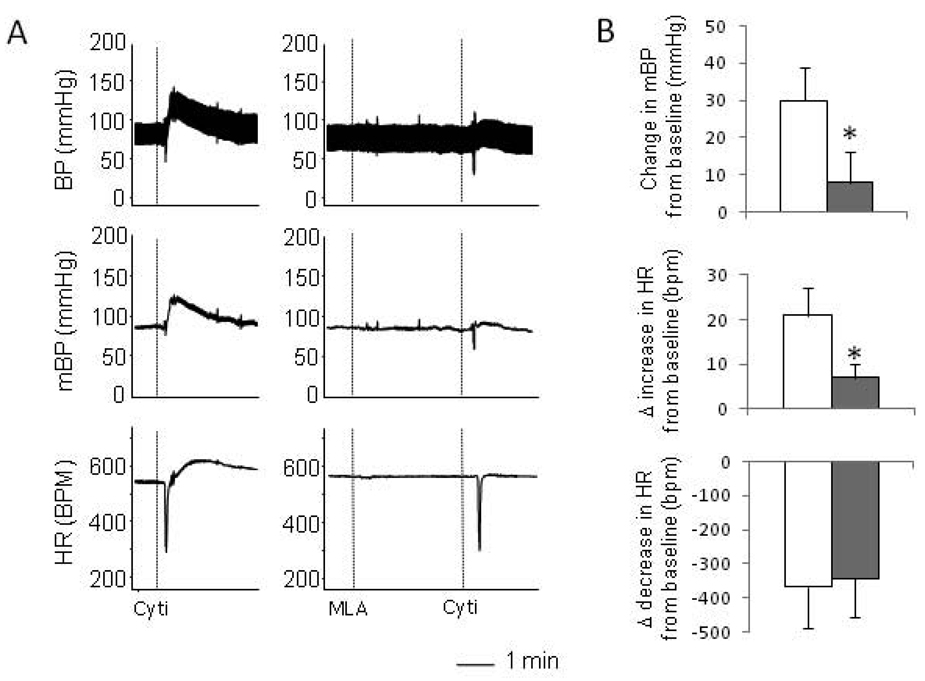
α7 nAchRs are expressed in ganglia [3, 7] and are involved in mediating autonomic activity [8]. We wanted to test whether the activation of α7 nAchRs are involved in the sympathetic and parasympathetic responses induced by cytisine. Pretreatment with MLA (1mg/kg), a selective α7 antagonist, significantly attenuated the pressor response induced by cytisine but elicited little effect on the parasympathetic bradycardic response (Figure 2). This result suggests that the activation of α7 nAchRs is involved in the sympathetic response induced by cytisine.
Figure 2.
Effects of α7 nAchRs antagonist MLA on the cytisine-induced responses. A. Raw recordings showing that cytisine-induced parasympathetic and sympathetic responses (left). Pre-administration of MLA largely blocked the sympathetic responses with no effect on the parasympathetic response (right). B. Mean data showing that compared with the treatment with cytisine alone (open bars), pre-administration of MLA (solid bars) significantly abolished the cytisine induced pressor response (top) and the increase in HR (middle) with no effect on the decrease in HR (bottom). The vertical dotted lines in the recording traces showed the relative positions of injections. “*” indicate P<0.05 compared with the cytisine only group. n=5
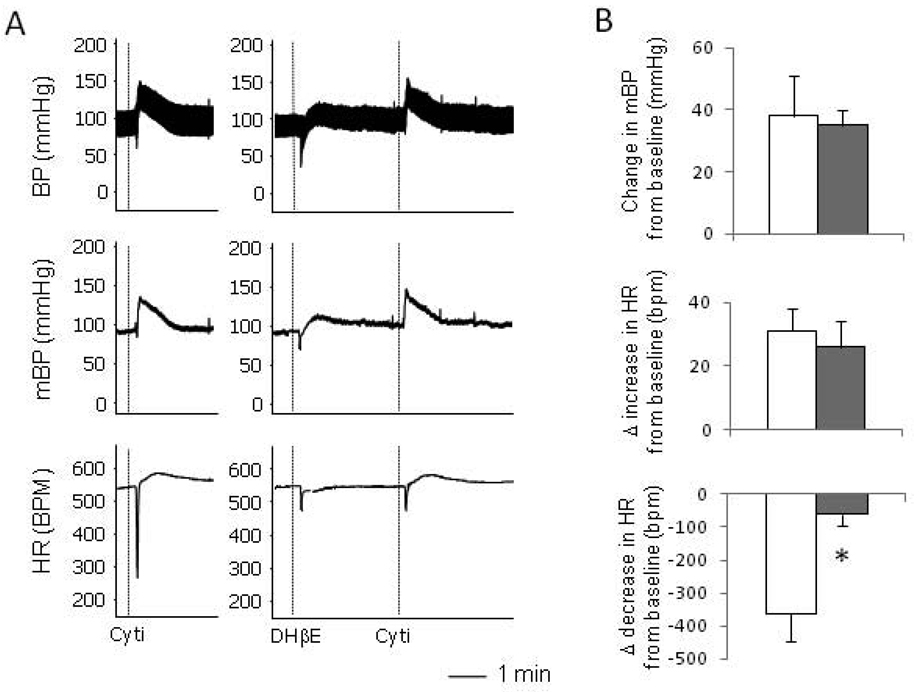
It is known that α4β2 nAchRs also express in ganglia [5, 9] and that cytisine can agonize α4β2 nAchRs [10]. We thus tested if the activation of this subtype of nAchRs is involved in the responses induced by cytisine. In contrast to MLA, the pretreatment with DHβE (1mg/kg), a selective α4β2 nAchR antagonist, largely blocked the bradycardic response, but not the pressor response, induced by cytisine (Figure 3). Moreover, pre-administration of DHβE also attenuated the baroreflex parasympathetic bradycardic response induced by the bolus injection of PE (0.1mg/kg) (Figure 4). These data suggest that the activation of α4β2 nAchRs is involved in the parasympathetic response in heart rate.
Figure 3.
Effects of α4β2 nAchRs antagonist DHβE on the cytisine-induced responses. A. Raw recordings showing that cytisine-induced parasympathetic and sympathetic responses (left). Pre-administration of DHβE largely blocked the parasympathetic response with no effect on the sympathetic pressor response (right). B. Mean data showing that, compared with the cytisine alone (open bars), pre-administration of DHβE (solid bars) significantly attenuated the cytisine induced decrease in heart rate (bottom) with no effect on the pressor response (top) or the increase in HR (middle). The vertical dotted lines in the recording traces showed the relative positions of injections. “*” indicate P<0.05 compared with the cytisine only group. n=5
Figure 4.
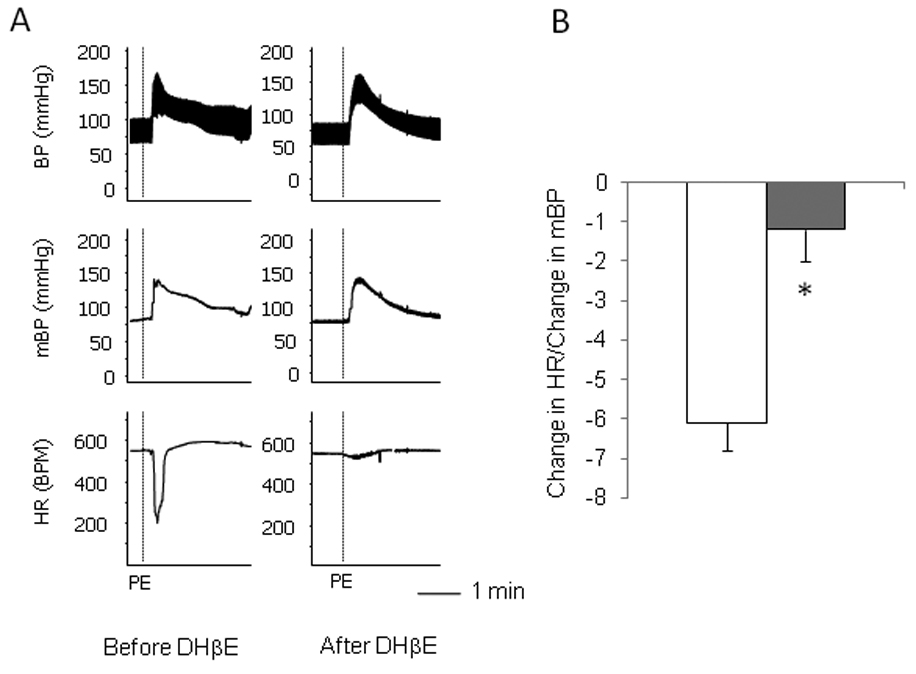
Effects of α4β2 nAchRs antagonist DHβE on the baroreflex-induced parasympathetic response. A. Raw recordings showing that intravenous injection of phenylephrine (PE) induced pressor and bradycardic responses (left). Pre-administration of DHβE largely blocked the bradycardic response (right). B. Mean data of the ratio of the decrease in heart rate versus the increase in mean blood pressure, showing that compared with the PE alone (open bars), pre-administration of DHβE (solid bars) significantly attenuated the decrease in heart rate induced by the baroreflex. The vertical dotted lines in the recording traces showed the relative positions of injections. “*” indicate P<0.05 compared with the PE only group. n=4
We also tested the responses to nicotine, a non-selective nicotinic agonist. The injection with nicotine induced a brief and pronounced bradycardic response, which was similar to that induced by cytisine. Interestingly, the sympathetic components, increases in heart rate and blood pressure, as seen in the responses to cytisine, were largely absent in the responses to nicotine (Figure 5). Furthermore, the bradycardic response induced by nicotine was also attenuated by pre-administration of DHβE (Figure 5), further suggesting that the activation of α4β2 nAchRs is involved in the response.
Figure 5.
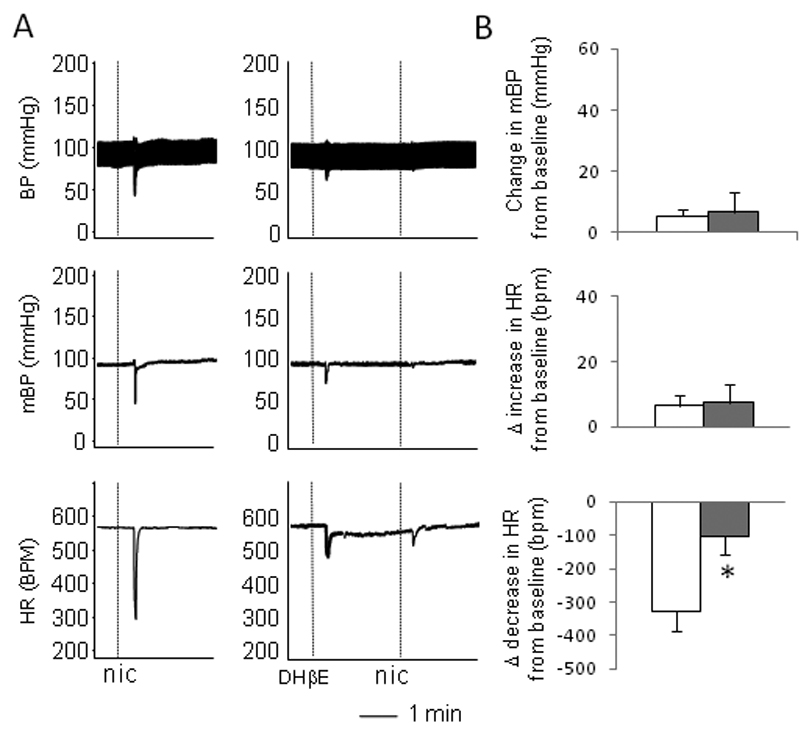
The effects of α4β2 nAchRs antagonist DHβE on nicotine-induced responses. A. Raw recordings of responses induced by nicotine, showing a prominent parasympathetic bradycardic response with little sympathetic pressor response (left), while pre-administration of DHβE largely blocked the parasympathetic bradycardic response (right). B. Mean data showing that, compared with the nicotine alone (open bars), pre-administration of DHβE (solid bars) significantly attenuated the nicotine induced decrease in heart rate (bottom) with no effect on the pressor response (top) and the increase in HR (middle). The vertical dotted lines in the recording traces showed the relative positions of injections. “*” indicate P<0.05 compared with the nicotine only group. n= 5
DISCUSSION
In this study, we found that the nicotinic agonist cytisine induced both parasympathetic and sympathetic responses in heart rate and blood pressure, reflecting the activation of the nAchRs at sympathetic and parasympathetic ganglia. While the α7 specific antagonist MLA selectively blocked the sympathetic pressor response, the specific α4β2 antagonist DHβE mainly attenuated the parasympathetic response. These data indicate that the activations of different subtypes of nAchRs at ganglia are differentially involved in the sympathetic and parasympathetic responses.
The role of α7 nAchRs in mediating autonomic activity at ganglia has been studied in vitro and in vivo. In Langendorff perfused isolated rat hearts, nicotine-induced decrease in heart rate was blocked by a α7 nAchRs antagonist, α-bungarotoxin, suggesting that α7 nAchRs may play a role in intracardiac ganglia to mediate the negative chronotropic (parasympathetic) effects [11]. However, studies using α7 nAchRs deficient mice showed that lack of α7 nAchRs did not change the baroreflex-induced [8] and vagal stimulation-induced [12] bradycardic response in vivo, suggesting that α7 nAchRs may not be important to the parasympathetic control of heart rate. In contrast, sympathetic activity was impaired in α7 deficient mice [8]. Our data showed that specific α7 antagonist MLA only attenuated the sympathetic pressor response but not the parasympathetic bradycardic response induced by cytisine. These results are consistent with the findings in α7 deficient mice, suggesting that α7 nAchRs play a role in sympathetic but not parasympathetic activity.
α4 and β2 subunits of nAchRs are also expressed in both sympathetic and parasympathetic ganglia [5, 7, 9]. However their functional roles in autonomic activity are unknown. In our study, the specific α4β2 antagonist DHβE selectively blocked the parasympathetic bradycardic response but not the sympathetic pressor response induced by cytisine. Administration of DHβE also blocked baroreflex-induced bradycardic response. These data provide novel evidence suggesting that α4β2 nAchRs may play a role in the parasympathetic regulation of the heart.
Interestingly, we found that injection of nicotine mainly induced a parasympathetic bradycardic response without significant sympathetic responses in heart rate and blood pressure, in contrast to the dual-phase responses induced by cytisine. This result seems surprising, because nicotine is known as a non-selective nicotinic agonist as cytisine is. Notably, however, nicotine has a much greater affinity at α4β2 (Ki value = 1 nM in rat brain) than at α7 (Ki = 4000nM)[13]. In comparison with nicotine and cytisine, nicotine has greater agonist potency than cytisine at α4β2 nAchRs, while cytisine has greater potency than nicotine at α7 nAchRs[14]. Therefore, it is possible that the unique bradycardic response without the following pressor response induced by nicotine may be due to its greater activity at α4β2 nAchRs but less at α7 nAchRs. Indeed, the α4β2 nAchRs selective antagonist DHβE attenuates nicotine-induced bradycardic response, further suggesting this possibility. This result provides additional evidence suggesting the notion that α4β2 nAchR may play a significant role in mediating parasympathetic control of the heart.
Notably, administration of MLA or DHβE per se did not significantly change the baselines of blood pressure and heart rate, suggesting that α7 and α4β2 subtypes of nAchRs may not be responsible for the resting sympathetic or parasympathetic tone. However, as indicated by our data, the activations of α7 or α4β2 nAchRs are indeed involved in the sympathetic or parasympathetic responses, respectively. These observations were consistent with the fact that α7 deficient mice have normal baselines of heart rate and blood pressure [12] but the sympathetic response was impaired [8]. The precise mechanisms by which α7 or α4β2 nAchRs modulate sympathetic and parasympathetic signaling transmission at ganglia require further investigation.
Collectively, our data support the notion that the sympathetic and parasympathetic pathways at the ganglionic level are mediated by different nAchRs [12]. Specifically, our data showed that the activation of α7 nAchRs are involved in the sympathetic pressor response whereas α4β2 nAchRs are important for parasympathetic negative chronotropic effect.
A better understanding of the roles of different nAchRs in sympathetic and parasympathetic ganglionic transmissions is clinically relevant. Impaired autonomic activity is a hallmark for a variety of cardiovascular diseases, including cardiac dysfunction and heart failure [15], hypertension [16], and diabetes [17, 18]. In these conditions, autonomic dysfunction is always characterized as elevated sympathetic activity and suppressed parasympathetic activity. The blockade of sympatho-excitation, such as the use of adrenergic beta receptor blockers, provides beneficial effects to a variety of cardiovascular diseases. On the other hand, increasing studies [19–21], including ours [22], suggest that enhancement of suppressed parasympathetic activity is beneficial to cardiovascular diseases. Understanding the distinct nAchR subtypes that are responsible for sympathetic and parasympathetic ganglionic transmission may provide new targets to modulate the altered sympathetic and parasympathetic activities. For example, based on our data, it is conceivable to propose that α7 nAchRs antagonism may be a new means to attenuate the increased sympathetic activity while α4β2 nAchRs agonist could enhance the suppressed parasympathetic function seen in many cardiovascular diseases.
Certainly, more studies need to be done to better understand the properties and interactions of the different nAchRs at ganglia for regulation of autonomic activities. It is also important to consider the limitation of the pharmacological approach in this study. Although MLA and DHβE are well described and widely used specific nAchRs antagonists, we could not rule out the other nAchRs based on the current data. Rather, our data invite further investigations using different approaches to confirm these findings. Nevertheless, our study provides interesting novel information to further verify the specific nAchRs in mediating sympathetic and parasympathetic signaling in ganglia.
ACKNOWLEDGEMENTS
This study was supported partially by American Heart Association SDG grant No.0835256N, NIH COBRE grants No. P20 RR01766 and No. P20 RR015567, NIH INBRE grant No.P20 RR016479 and the USD Division of BBS research bridge grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bibevski S, Dunlap ME. Ganglionic mechanisms contribute to diminished vagal control in heart failure. Circulation. 1999;99:2958–2963. doi: 10.1161/01.cir.99.22.2958. [DOI] [PubMed] [Google Scholar]
- 2.Felix B, Catalin D, Miolan JP, Niel JP. Integrative properties of the major pelvic ganglion in the rat. J Auton Nerv Syst. 1998;69:6–11. doi: 10.1016/s0165-1838(97)00133-1. [DOI] [PubMed] [Google Scholar]
- 3.Skok VI. Nicotinic acetylcholine receptors in autonomic ganglia. Auton Neurosci. 2002;97:1–11. doi: 10.1016/s1566-0702(01)00386-1. [DOI] [PubMed] [Google Scholar]
- 4.Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rust G, Burgunder JM, Lauterburg TE, Cachelin AB. Expression of neuronal nicotinic acetylcholine receptor subunit genes in the rat autonomic nervous system. Eur J Neurosci. 1994;6:478–485. doi: 10.1111/j.1460-9568.1994.tb00290.x. [DOI] [PubMed] [Google Scholar]
- 6.Mandelzys A, Pie B, Deneris ES, Cooper E. The developmental increase in ACh current densities on rat sympathetic neurons correlates with changes in nicotinic ACh receptor alpha-subunit gene expression and occurs independent of innervation. J Neurosci. 1994;14:2357–2364. doi: 10.1523/JNEUROSCI.14-04-02357.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Biasi M. Nicotinic mechanisms in the autonomic control of organ systems. J Neurobiol. 2002;53:568–579. doi: 10.1002/neu.10145. [DOI] [PubMed] [Google Scholar]
- 8.Franceschini D, Orr-Urtreger A, Yu W, Mackey LY, Bond RA, Armstrong D, et al. Altered baroreflex responses in alpha7 deficient mice. Behav Brain Res. 2000;113:3–10. doi: 10.1016/s0166-4328(00)00195-9. [DOI] [PubMed] [Google Scholar]
- 9.Listerud M, Brussaard AB, Devay P, Colman DR, Role LW. Functional contribution of neuronal AChR subunits revealed by antisense oligonucleotides. Science. 1991;254:1518–1521. doi: 10.1126/science.1720573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Steinbach JH. Cytisine binds with similar affinity to nicotinic alpha4beta2 receptors on the cell surface and in homogenates. Brain Res. 2003;959:98–102. doi: 10.1016/s0006-8993(02)03733-2. [DOI] [PubMed] [Google Scholar]
- 11.Ji S, Tosaka T, Whitfield BH, Katchman AN, Kandil A, Knollmann BC, et al. Differential rate responses to nicotine in rat heart: evidence for two classes of nicotinic receptors. J Pharmacol Exp Ther. 2002;301:893–899. doi: 10.1124/jpet.301.3.893. [DOI] [PubMed] [Google Scholar]
- 12.Deck J, Bibevski S, Gnecchi-Ruscone T, Bellina V, Montano N, Dunlap ME. Alpha7-nicotinic acetylcholine receptor subunit is not required for parasympathetic control of the heart in the mouse. Physiol Genomics. 2005;22:86–92. doi: 10.1152/physiolgenomics.00085.2004. [DOI] [PubMed] [Google Scholar]
- 13.Donnelly-Roberts DL, Puttfarcken PS, Kuntzweiler TA, Briggs CA, Anderson DJ, Campbell JE, et al. ABT-594 [(R)-5-(2-azetidinylmethoxy)-2-chloropyridine]: a novel, orally effective analgesic acting via neuronal nicotinic acetylcholine receptors: I. In vitro characterization. J Pharmacol Exp Ther. 1998;285:777–786. [PubMed] [Google Scholar]
- 14.Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (3rd edition) 2008;153 Suppl 2:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porter TR, Eckberg DL, Fritsch JM, Rea RF, Beightol LA, Schmedtje JF, Jr., et al. Autonomic pathophysiology in heart failure patients. Sympathetic-cholinergic interrelations. J Clin Invest. 1990;85:1362–1371. doi: 10.1172/JCI114580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Julius S. Autonomic nervous system dysregulation in human hypertension. Am J Cardiol. 1991;67:3B–7B. doi: 10.1016/0002-9149(91)90813-z. [DOI] [PubMed] [Google Scholar]
- 17.Frontoni S, Bracaglia D, Gigli F. Relationship between autonomic dysfunction, insulin resistance and hypertension, in diabetes. Nutr Metab Cardiovasc Dis. 2005;15:441–449. doi: 10.1016/j.numecd.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Lindmark S, Wiklund U, Bjerle P, Eriksson JW. Does the autonomic nervous system play a role in the development of insulin resistance? A study on heart rate variability in first-degree relatives of Type 2 diabetes patients and control subjects. Diabet Med. 2003;20:399–405. doi: 10.1046/j.1464-5491.2003.00920.x. [DOI] [PubMed] [Google Scholar]
- 19.Behling A, Moraes RS, Rohde LE, Ferlin EL, Nobrega AC, Ribeiro JP. Cholinergic stimulation with pyridostigmine reduces ventricular arrhythmia and enhances heart rate variability in heart failure. Am Heart J. 2003;146:494–500. doi: 10.1016/S0002-8703(03)00319-3. [DOI] [PubMed] [Google Scholar]
- 20.Olshansky B, Sabbah HN, Hauptman PJ, Colucci WS. Parasympathetic nervous system and heart failure: pathophysiology and potential implications for therapy. Circulation. 2008;118:863–871. doi: 10.1161/CIRCULATIONAHA.107.760405. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation. 2004;109:120–124. doi: 10.1161/01.CIR.0000105721.71640.DA. [DOI] [PubMed] [Google Scholar]
- 22.Freeling J, Wattier K, Lacroix C, Li YF. Neostigmine and pilocarpine attenuated TNF{alpha} expression and cardiac hypertrophy in the heart with pressure overload. Exp Physiol. 2007 doi: 10.1113/expphysiol.2007.039784. [DOI] [PubMed] [Google Scholar]