Summary
Sudden unexpected death in epilepsy (SUDEP) is a significant cause of mortality in people with epilepsy. Two postulated causes for SUDEP, cardiac and respiratory depression, can both be explained by over-stimulation of adenosine receptors. We hypothesized that SUDEP is a consequence of a surge in adenosine as a result of prolonged seizures combined with deficient adenosine clearance; consequently, blockade of adenosine receptors should prevent SUDEP. Here we induced impaired adenosine clearance in adult mice by pharmacological inhibition of the adenosine removing enzymes, adenosine kinase and deaminase. Combination of impaired adenosine clearance with kainic acid-induced seizures triggered sudden death in all animals. Most importantly, the adenosine receptor antagonist caffeine when given after seizure onset increased survival from 23.75± 1.35 min to 54.86 ± 6.59 min (p<0.01). Our data indicate that SUDEP is due to over-activation of adenosine receptors and that caffeine treatment after seizure onset might be beneficial.
Keywords: adenosine, adenosine clearance, SUDEP, kainic acid, seizure, caffeine
Introduction
Sudden unexpected death in epilepsy (SUDEP) occurs at an incidence of 1 to 2.5 per 1000 patient years (Mohanraj et al., 2006), however the mechanisms underlying SUDEP remain unclear, and there is no effective preventative therapy (Brodie and Holmes, 2008; So, 2008). Both cardiac, as well as respiratory mechanisms, particularly ictal bradycardia and apnea, have been proposed as prime causal mechanisms for SUDEP (So, 2008). On the other hand, both cardiac and respiratory dysfunction can be explained by over-stimulation of both adenosine A1 receptors (A1Rs), as well as A2ARs in brainstem leading to respiratory and cardiovascular collapse (Tseng et al., 1988; Barraco et al., 1990; McCarley, 2007).
Release of the endogenous anticonvulsant adenosine is a physiological consequence of seizures and a mechanism of the brain for seizure termination (During and Spencer, 1992). Likewise, therapeutic adenosine augmentation is an effective strategy to suppress seizures (Boison, 2007). Under normal conditions adenosine concentrations are kept in the range of 25 to 250 nM by phosphorylation via adenosine kinase (ADK) into 5’-adenosine monophosphate (AMP), or deamination into inosine by adenosine deaminase (ADA) (Boison, 2006). Under conditions of extreme metabolic stress, as occurs during prolonged seizures or during traumatic brain injury, a surge of micromolar levels of adenosine results (Clark et al., 1997) that is far above the affinity of the adenosine A1 receptor (A1R) (~70 nM) or the A2AR (~150 nM) for adenosine. Thus, any combination of prolonged or excessive seizure activity with impaired adenosine clearance is likely to result in extended over-activation of ARs.
The objective of this study was to test the hypothesis that SUDEP is a consequence of a surge in adenosine as a result of prolonged seizures combined with deficient adenosine clearance. We present a new model of SUDEP in mice that is based on kainic acid-induced seizures in combination with pharmacologically impaired adenosine clearance and demonstrate that the adenosine receptor antagonist caffeine can extend survival time. This study is of significance to understand a putative new molecular mechanism for SUDEP, and suggests that caffeine, when given after seizure onset improves survival.
Methods
Animals and Drug Treatments
All animal procedures were conducted in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) in accordance with protocols approved by the Institutional Animal Care and Use Committee and the principles outlined in the NIH Guide for the Care and Use of Laboratory Animals. Male C57BL/6 mice (Charles River, Tacoma, WA, weight, 25–30 g) were habituated to the testing environment 4 days prior to behavioral testing.
Mice were treated with combinations of the following drugs by intraperitoneal injection in a volume of 0.1 ml 0.9% saline / 10 g body weight: (i) erythro-9-(2-hydroxy-3-nonyl)-adenine hydrochloride (EHNA, Sigma-Aldrich): 10 mg/kg; (ii) 5-iodotubercidin (ITU, RBI): 3.1 mg/kg; (iii) kainic acid (KA, Sigma-Aldrich): 35 or 40 mg/kg; (iv) caffeine (Sigma-Aldrich): 40 mg/kg.
Mouse model for SUDEP
Inhibition of the two major adenosine removing enzymes adenosine deaminase (ADA) and adenosine kinase (ADK) with their respective inhibitors EHNA (10mg/kg, i.p.) and ITU (3.1mg/kg, i.p.) was performed 15 min prior to induction of acute seizures with KA (35mg/kg, i.p., Figure 1A). Mice were randomly divided into two groups, (i) EI-K (pretreatment with EHNA and ITU followed by kainic acid), and (ii) SAL-K (pretreatment with 0.9% saline followed by kainic acid).
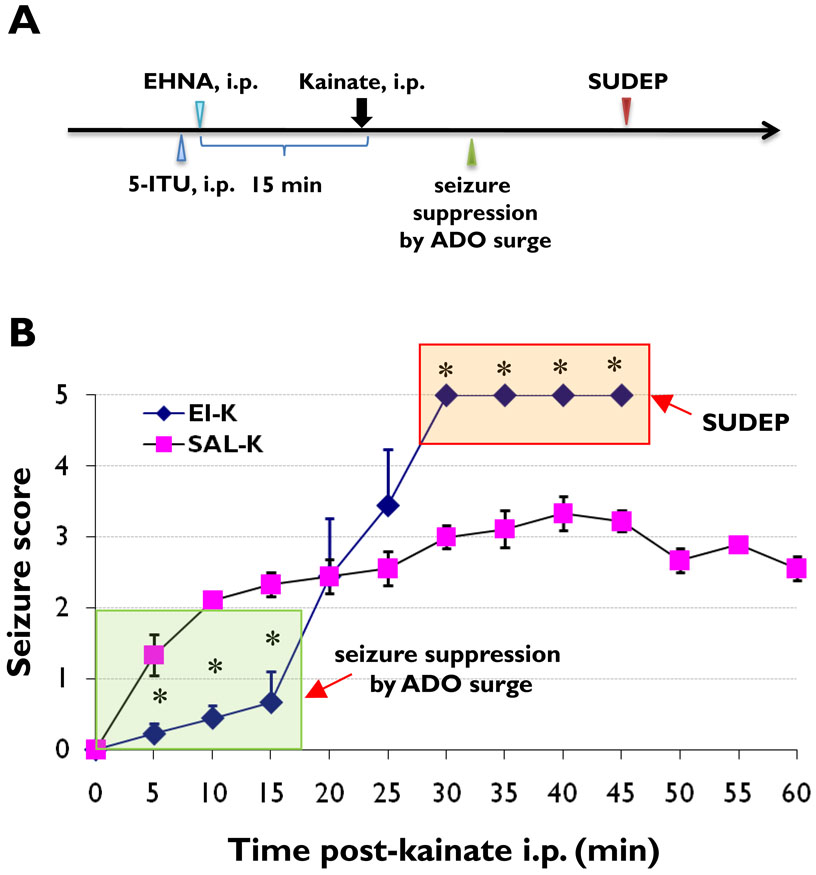
Figure 1. Injection paradigm and seizure scores of SUDEP model.
(A) Injection paradigm of SUDEP model. (B) Averaged seizure scores are shown of animals with pretreatment of EHNA (10 mg/kg, i.p.) and ITU (3.1 mg/kg, i.p.) (EI-K, n=9), or saline (SAL-K, n=9) followed by kainic acid injection (35mg/kg, i.p.). * P<0.01 (one-way ANOVA). The green box indicates initially enhanced seizure suppression by potentiation of the kainic acid-induced adenosine (ADO) surge; the red box indicates the SUDEP phase. All mice in the EI-K group died within 30 to 45 minutes after kainic acid injection.
To explore the effects of adenosine receptor blockade in our SUDEP model, two groups of animals (SUDEP+CAF and SUDEP+SAL) were subjected to treatment with caffeine immediately after seizure onset (Figure 2A). Mice received pretreatment with EHNA (10 mg/kg, i.p.) and 5-ITU (3.1 mg/kg, i.p.), 15 min prior to injection of kainic acid (40 mg/kg, i.p.). 30 sec after seizure onset (at first appearance of a seizure score of 5) animals from the SUDEP+CAF group received caffeine (40mg/kg, i.p.) whereas animals from the SUDEP+SAL group received a saline injection. The survival time following kainic acid injection was recorded in each animal.
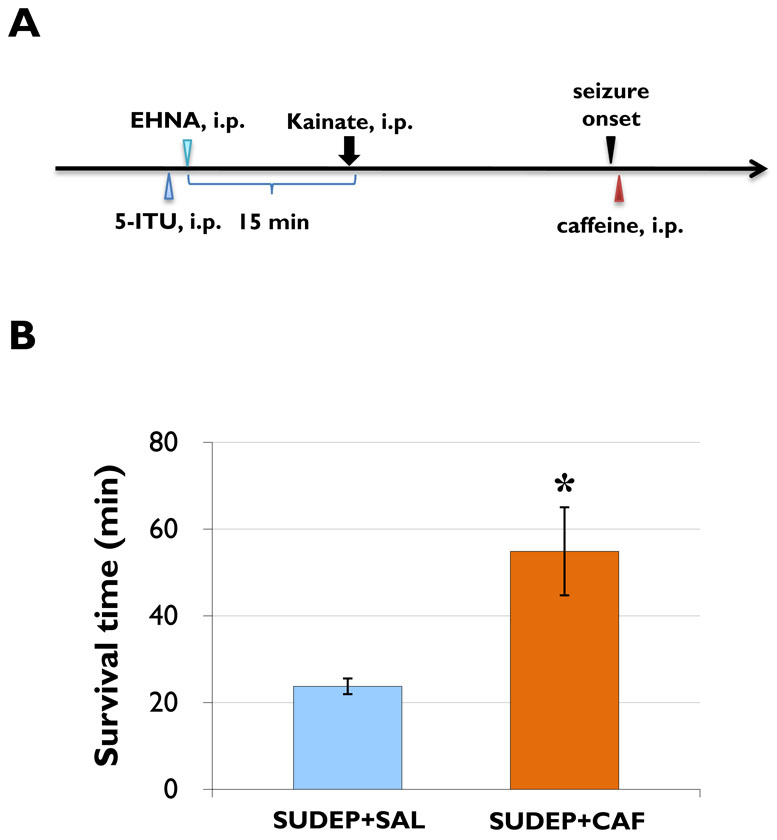
Figure 2. Caffeine prolongs survival time of SUDEP mice.
(A) Injection paradigm of caffeine-rescue of SUDEP. (B) The average survival time in caffeine-treated group (SUDEP+CAF, n=8) is significantly longer than that of saline-treated mice (SUDEP+SAL, n=8), * P<0.01 (one-way ANOVA). The longest survival time in the SUDEP+CAF group was 91 min.
Assessment of seizure activity
Following kainic acid administration, seizure activity was scored according to the Racine scale in 5-min bins: stage 1, mouth and facial twitches; stage 2, clonic head movements; stage 3, unilateral forelimb clonus followed by contralateral clonus; stage 4, clonic rearing; and stage 5, loss of postural control and falling.
Statistical analyses
Differences among groups were analyzed using one-way ANOVA followed by Bonferroni post hoc test. P < 0.05 was determined as significant. Values are expressed as means ± standard deviation.
Results
To evaluate whether seizure-induced adenosine release in combination with deficient adenosine clearance might be a cause for SUDEP, we treated mice with the inhibitors (EHNA and ITU) of the two major adenosine degradation pathways 15 min prior to the induction of acute seizures with 35 mg/kg KA (Figure 1A). KA-injected control animals pretreated with saline instead of EHNA and ITU (SAL-K group, n = 9) developed seizures of progressive intensity within 5 minutes after KA-injection. Seizures in SAL-K control animals reached a severity score of about 3 and continued for > 60 min; none of these animals died as a consequence of the acute seizures. In contrast, animals from the EHNA/ITU-treated group (EI-K group, n = 9) were initially (first 15 min) protected from seizures, presumably due to pharmacological enhancement of the anticonvulsant adenosine. However, as a likely consequence of seizure induced adenosine-release and deficient adenosine clearance all animals progressed to stage 5 seizures at 30 minutes and died within 20 minutes after the onset of stage 5 seizures (Figure 1B). These data suggest a novel model of SUDEP that is related to deficient adenosine clearance.
If impaired adenosine clearance in our model is the cause for SUDEP, then adenosine receptor blockade by caffeine should delay or prevent SUDEP. To further increase seizure severity in our SUDEP group, we increased the dose of KA from 35 to 40 mg/kg. All animals were treated with EHNA and ITU 15 min prior to the injection of 40 mg/kg KA. 30 sec after the onset of stage 5 seizures the animals received either an injection of saline (SUDEP+SAL group, n = 8) or 40 mg/kg caffeine (SUDEP+CAF group, n = 8) (Figure 2A). The average survival time of caffeine treated mice (54.86 ± 6.59 min) was significantly longer than that of the control animals (23.75± 1.35 min, P<0.01) (Figure 2B). Caffeine extended survival to up to 91 min. These data demonstrate (i) that SUDEP in our model is linked to adenosine, and (ii) that caffeine, when given after seizure onset, promotes survival of the animals.
Discussion
Here we propose an adenosine-based mechanism for SUDEP
seizure-induced adenosine release (During and Spencer, 1992) combined with deficient adenosine-clearance – induced here pharmacologically with ITU and EHNA – is likely to result in excessive levels of adenosine, which, by overstimulation of adenosine receptors in brainstem might trigger prolonged central apnea and suppression of cardiac functions. Although these physiological downstream effects were not directly assessed here, we demonstrate that SUDEP in our model can be reversed by antagonizing the effects of adenosine with caffeine. Several aspects of this study warrant further discussion.
Biphasic seizure response in SUDEP model
our model is characterized by initial seizure suppression followed by eventual seizure exacerbation and sudden death. Both effects can be triggered by deficient adenosine clearance. Pharmacological inhibition of adenosine clearance before seizure induction with KA is likely to delay seizure onset by activation of brain A1Rs (Etherington et al., 2009). In line with increased A1R activation, ITU/EHNA treatment reduced activity levels in our experimental animals. The phenomenon of “quiescent waking”, a combination of decreased spontaneous locomotor activity with decreased sleep in electroencephalogram recordings has been described as a consequence of impaired adenosine clearance (Mendelson et al., 1983). In contrast, a surge of high levels of adenosine, triggered after delayed seizure onset and combination with impaired adenosine clearance, may (i) exacerbate seizures, and (ii) may affect the caudal nucleus tractus solitarius – the central cardiorespiratory center in brainstem – and cause cardiopulmonary arrest, a presumed cause for SUDEP. Indeed, during the SUDEP phase our animals displayed periods of irregular breathing, prolonged apnea, and respiratory secretions, which indicate the suppression of respiratory functions (So, 2008). Future studies need to address cardio-respiratory outcome parameters in more detail.
Effects of caffeine
Under conditions of impaired adenosine clearance and excessive levels of adenosine, adenosine-based seizure suppression mechanisms likely fail (by over-activation of pro-convulsive adenosine A2ARs, or by heterologous desensitization of A1Rs) as is suggested by the exaggerated seizure-response during the SUDEP-phase in the EI-K group (Figure 1). Caffeine, which modulates seizure susceptibility by non-selective blockade of all subtypes of adenosine receptors, is not expected to exacerbate seizures under these conditions (Fredholm et al., 1999; El Yacoubi et al., 2008); indeed chronic caffeine was demonstrated to decrease the susceptibility to convulsants in mice, an effect that involved A2ARs (El Yacoubi et al., 2008). Thus, by its ability to block A2A receptors, caffeine is ideally suited to limit the proconvulsant activity of A2ARs and thus to counteract pro-convulsant effects triggered by excessive adenosine. On the other hand, a proconvulsive effect of caffeine acting on A1Rs, which have been demonstrated to limit seizure spread (Fedele et al., 2006), cannot be excluded. Our results, however, suggest that, under the experimental conditions employed here, the beneficial A2AR-mediated effects of caffeine on seizures seem to dominate. In addition, acute caffeine may have direct positive effects on respiratory functions. As outlined above, caffeine – by blocking A1Rs as well as A2ARs in brainstem – may directly prevent apnea and the suppression of cardiopulmonary functions and thus promote survival of the SUDEP-animals.
Consistent with these considerations we demonstrate that caffeine can significantly prolong the survival time in seizing animals under conditions of impaired adenosine clearance. These data are of importance for two reasons: (i) the beneficial effect of caffeine demonstrated here indicates that SUDEP is due to excessive adenosine, as generated in our model by impaired adenosine clearance. (ii) The prolongation of the survival time by caffeine after seizure onset may open a therapeutic window of opportunity to prevent SUDEP, e.g. by cardio-respiratory support or aggressive seizure control. The adenosine-based mechanism of SUDEP explored here certainly warrants further investigation; most importantly, we demonstrate novel beneficial effects of caffeine, when given after seizure onset.
Acknowledgments
This study was supported by grant NS061844 from the National Institute of Neurological Disorders and Stroke (NINDS).
Footnotes
Conflict of interest
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. None of the authors has any conflicts of interest to disclose.
References
- Barraco RA, Janusz CA, Schoener EP, Simpson LL. Cardiorespiratory function is altered by picomole injections of 5'-N-ethylcarboxamidoadenosine into the nucleus tractus solitarius of rats. Brain Res. 1990;507:234–246. doi: 10.1016/0006-8993(90)90277-i. [DOI] [PubMed] [Google Scholar]
- Boison D. Adenosine kinase, epilepsy and stroke: mechanisms and therapies. Trends Pharmacol Sci. 2006;27:652–658. doi: 10.1016/j.tips.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Boison D. Adenosine-based cell therapy approaches for pharmacoresistant epilepsies. Neurodegener Dis. 2007;4:28–33. doi: 10.1159/000100356. [DOI] [PubMed] [Google Scholar]
- Brodie MJ, Holmes GL. Should all patients be told about sudden unexpected death in epilepsy (SUDEP)? Pros and Cons. Epilepsia. 2008;49 Suppl 9:99–101. doi: 10.1111/j.1528-1167.2008.01933.x. [DOI] [PubMed] [Google Scholar]
- Clark RS, Carcillo JA, Kochanek PM, Obrist WD, Jackson EK, Mi Z, Wisneiwski SR, Bell MJ, Marion DW. Cerebrospinal fluid adenosine concentration and uncoupling of cerebral blood flow and oxidative metabolism after severe head injury in humans. Neurosurgery. 1997;41:1284–1292. doi: 10.1097/00006123-199712000-00010. discussion 1292-1283. [DOI] [PubMed] [Google Scholar]
- During MJ, Spencer DD. Adenosine: a potential mediator of seizure arrest and postictal refractoriness. Ann Neurol. 1992;32:618–624. doi: 10.1002/ana.410320504. [DOI] [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Parmentier M, Costentin J, Vaugeois JM. Evidence for the involvement of the adenosine A(2A) receptor in the lowered susceptibility to pentylenetetrazol-induced seizures produced in mice by long-term treatment with caffeine. Neuropharmacology. 2008;55:35–40. doi: 10.1016/j.neuropharm.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Etherington LA, Patterson GE, Meechan L, Boison D, Irving AJ, Dale N, Frenguelli B. Astrocytic adenosine kinase regulates basal synaptic adenosine levels and seizure activity but not activity-dependent adenosine release in the hippocampus. Neuropharmacology. 2009;56:429–437. doi: 10.1016/j.neuropharm.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedele DE, Li T, Lan JQ, Fredholm BB, Boison D. Adenosine A1 receptors are crucial in keeping an epileptic focus localized. Exp Neurol. 2006;200:184–190. doi: 10.1016/j.expneurol.2006.02.133. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- McCarley RW. Neurobiology of REM and NREM sleep. Sleep Med. 2007;8:302–330. doi: 10.1016/j.sleep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Mendelson WB, Kuruvilla A, Watlington T, Goehl K, Paul SM, Skolnick P. Sedative and electroencephalographic actions of erythro-9-(2-hydroxy-3-nonyl)-adenine (EHNA): relationship to inhibition of brain adenosine deaminase. Psychopharmacology (Berl) 1983;79:126–129. doi: 10.1007/BF00427798. [DOI] [PubMed] [Google Scholar]
- Mohanraj R, Norrie J, Stephen LJ, Kelly K, Hitiris N, Brodie MJ. Mortality in adults with newly diagnosed and chronic epilepsy: a retrospective comparative study. Lancet Neurol. 2006;5:481–487. doi: 10.1016/S1474-4422(06)70448-3. [DOI] [PubMed] [Google Scholar]
- So EL. What is known about the mechanisms underlying SUDEP? Epilepsia. 2008;49 Suppl 9:93–98. doi: 10.1111/j.1528-1167.2008.01932.x. [DOI] [PubMed] [Google Scholar]
- Tseng CJ, Biaggioni I, Appalsamy M, Robertson D. Purinergic receptors in the brainstem mediate hypotension and bradycardia. Hypertension. 1988;11:191–197. doi: 10.1161/01.hyp.11.2.191. [DOI] [PubMed] [Google Scholar]