Introduction
Arachidonic acid (AA) is liberated from membrane phospholipid pools by phospholipase A2 (PLA2) and subsequently metabolized by cyclooxygenase (COX), lipoxygenase (LOX) and cytochrome P450 (CYP) epoxygenase and hydroxylase enzymes to form a group of metabolites collectively termed ‘eicosanoids’ (Figure 1) [1; 2]. Epoxyeicosatrienoic acids (EETs), epoxide metabolites of CYP epoxygenases, have garnered increasing attention since their initial identification in the liver in the early 1980s. Interest in EETs signaling has centered predominantly upon their role in the regulation of renal and cardiovascular function, particularly in their potent vasodilator actions. EETs signaling has been the subject of numerous research articles, and their effects on cellular function have been investigated in different tissues, including heart, lung, kidney, gastro-intestinal tract and brain. The vasomotor actions of EETs have been studied in the renal, coronary, pulmonary, skeletal muscle, sub-cutaneous, carotid, mesenteric, and cerebral vascular beds. These studies have yielded much valuable insight into the biochemical mechanisms of EETs synthesis, action, and metabolism [3; 4].
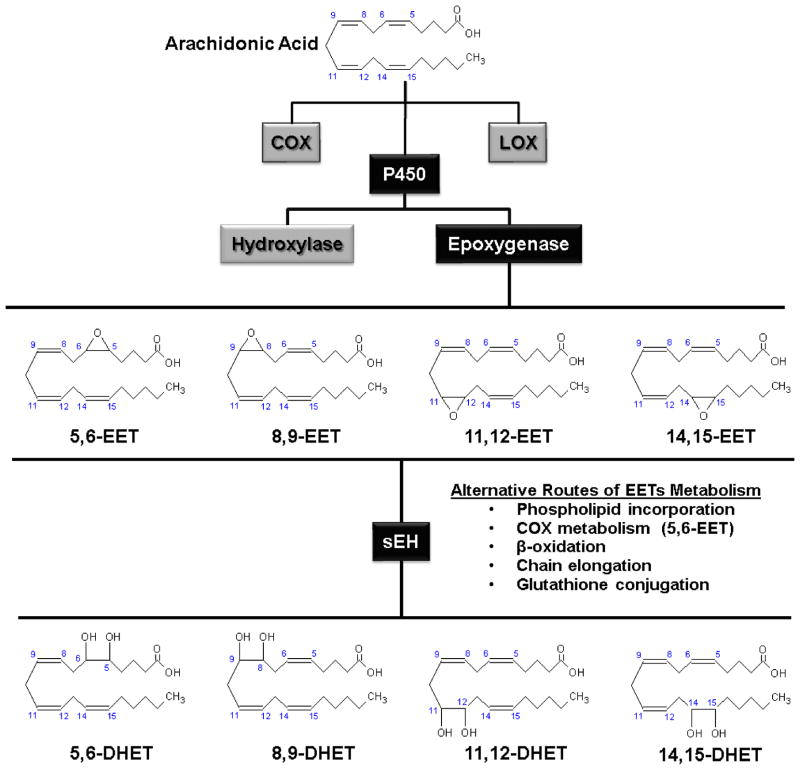
Figure 1.
Pathways of epoxyeicosatrienoic acid (EETs) synthesis and metabolism. EETs are synthesized from arachidonic acid by cytochrome P450 epoxygenase enzymes, producing four distinct regio-isomers. EETs are metabolized predominantly through the activity of soluble epoxide hydrolase (sEH) which catalyzes their hydrolysis to corresponding dihydroxyeicosatrienoic acids (DHETs). COX: cyclooxygenase; LOX: lipoxygenase.
At first glance, the role of EETs in the brain and broader central nervous system (CNS) appears to closely parallel functions described in other peripheral tissues, including a key role in the regulation of the cerebral vasculature [1; 3]. A more detailed review of the defined functions of EETs in the CNS, however, suggests that EETs signaling may play an important and distinct role in CNS function compared to that of peripheral tissues. Indeed, based upon expression data, EETs production and metabolism in the brain spans many regions and extends to peripheral and central neurons, astroglia and oligodendrocytes, vascular endothelium and vascular smooth muscle (VSM) (for references, see Table 1). In terms of cellular actions, EETs signaling in the CNS is importantly involved in processes that are specific to CNS function. Furthermore, EETs often appear to specifically mediate processes in which communication is integrated across multiple cell types. EETs’ role in the regulation of cerebral blood flow (CBF) extends beyond that of an endothelium-derived hyperpolarizing factor (EDHF) as described in peripheral circulatory beds, and includes the astrocyte-mediated coupling of cortical neuronal activity to cerebral blood supply as well as the regulation of the cerebral surface vasculature by perivascular nerve fibers [5–8]. EETs modulate neuronal pain processing in the brainstem [9] and the CYP epoxygenase metabolic pathway interacts with the neuro-active endocannabinoid pathway at a number of mechanistic levels [10–14]. Indeed, the long-established and often overlooked role for EETs in regulating neurohormone release from neuroendocrine regions of the brain [15; 16] in addition to very recent data implicating EETs in the neurogenic regulation of cerebral blood flow suggest that EETs may be key regulators of synaptic transmission, a function distinct to CNS function. Lastly, during conditions of stress or injury such as cerebral ischemia, the EETs signaling pathway is actively up-regulated and exerts a concerted protective action upon the many interacting cellular components of the brain, including neurons, glia, vascular and inflammatory cells [17].
Table 1.
Expression of P450 epoxygenase and sEH enzymes in CNS tissue.
| Species | Isoform | Brain Region | Cell Type | Detection Method | EETs Profile | References |
|---|---|---|---|---|---|---|
| Human | CYP-4X1 | W, Cb | PCR | 8,9:14,15 = 1:2 | [23] | |
| Human | sEH | Co, BG, BS, Th, HTh, CP, V | N, Ast, O, EP, VSM, Endo | IHC | [48] | |
| Mouse | CYP-2C29 | W | PCR | 8,9–6%; 11,12–12%; 14,15–82% | [32] | |
| Mouse | CYP-2C37 | W | PCR | 8,9–24%; 11,12–61%; 14,15–15% | [32] | |
| Mouse | CYP-2C38 | W | PCR | 8,9–9%; 11,12–65%; 14,15–26% | [32] | |
| Mouse | CYP-2C40 | W | PCR | 8,9–16%; 11,12–11%; 14,15–40% | [32] | |
| Mouse | CYP-4X1 | Co, Hip, Cb, BS | N | WB, IHC, NB | [36] | |
| Mouse | sEH | W, Co, BG, Hip,Th, HTh, BS, V | N, Ast | WB, IHC, MS, PCR | [42–44; 141] | |
| Rat | CYP-2C11 | Co, OB | Ast | WB, IHC, PCR, SB, ISH | 8,9–25%; 11,12–40%; 14,15–35% | [19; 31; 33; 35; 138] |
| Rat | CYP-2C12 | W, OB | PCR | [31] | ||
| Rat | CYP-2C23 | OB | PCR | 8,9–26%; 11,12–51%; 14,15–23% | [31; 139] | |
| Rat | CYP-2C6 | OB | PCR | [31] | ||
| Rat | CYP-2C7 | W | WB, PCR | [31; 35] | ||
| Rat | CYP-2D18 | W | PCR | 8,9–10%; 11,12–59%; 14,15–31% | [22; 22; 34] | |
| Rat | CYP-2J3 | SPG, TG | N | WB, IHC, PCR | 8,9–29%; 11,12–28%; 14,15–43% | [8; 140] |
| Rat | CYP-2J4 | SPG, TG | N | WB, IHC, PCR | [8] | |
| Rat | CYP-4X1 | Co, Hip, Cb, BS | N | NB, ISH | [37] | |
| Rat | sEH | W, Co, SPG, TG, V | N, Ast, VSM, O | WB, IHC, PCR | [8; 46; 47] |
W: whole brain; Co: cerebral cortex; BG: basal ganglia; Cb: cerebellum; Hip: hippocampus; BS: brain stem; OB: olfactory bulb; Th: thalamus; HTh: hypothalamus; SPG: sphenopalatine ganglia; TG: trigeminal ganglia; V: vasculature;; CP: chorioid plexus. N: neurons, Ast: astrocytes; VSM: vascular smooth muscle; Endo: endothelium; EP: ependymal cells; O: oligodendrocytes. WB: Western blot; IHC: immunohistochemistry; MS: mass spectroscopy; PCR: polymerase chain reaction (RT or real time); NB: Northern blot; SB: Southern blot; ISH: in situ hybridization. 5,6: 5,6-EET; 8,9: 8,9-EET; 11,12: 11,12-EET; 14,15: 14,15-EET.
The emerging involvement of EETs signaling in CNS-specific processes suggests that epoxyeicosanoid signaling in the CNS is in many ways distinct from EETs’ actions in other peripheral tissues. The common biochemical mechanisms governing the EETs pathway ensure that many mechanistic insights into EETs signaling in the CNS will be gained from studies in the periphery. However, the distinct actions of EETs in the CNS argue that epoxyeicosanoid signaling in this system is best considered independently within the specific framework of CNS function and disease. Towards this end, the present review will outline the well-established role for the epoxyeicosanoid pathway in cerebrovascular regulation and the targeting of this pathway in the treatment of cerebral ischemia. We will then critically evaluate the evidence for the broader involvement of epoxyeicosanoid signaling in CNS function and disease.
Brain epoxyeicosatrienoic acids (EETs) synthesis and metabolism
Identification of EETs production in the brain followed very closely on the heels of the initial discovery of these novel CYP-derived epoxyeicosanoids [18]. In the 1990s, studies reporting the specific synthesis of EETs first by astrocytes and then by the vascular endothelium helped to secure EETs’ place as key astrocyte- and endothelium-derived regulators cerebrovascular function [19–21]. At the time, these findings appeared to fit well with results from peripheral circulatory beds indicating that EETs functioned as an EDHF and were key regulators of vascular function [3]. More recent studies, however have characterized both the expression of CYP epoxygenases and the function of CYP-derived EETs in cell types throughout many brain regions (Table 1). The result of these studies has been the demonstration that enzymes capable of EETs synthesis and metabolism, CYP epoxygenases and soluble epoxide hydrolase (sEH), are dominantly expressed in non-vascular cells throughout the CNS including both neuronal and glial cells. These findings suggest that the role of EETs signaling in the CNS may extend beyond simply that of vascular regulation and may subserve functions that are specific to the activity of the CNS, including those processes involving the concerted action of multiple CNS cell types.
EETs production in the brain
Cytochrome P450 enzymes are members of the hemoprotein superfamily, important players in the cellular adaptation to stress. The CYP enzymes are membrane-bound mixed function oxidases that utilize molecular oxygen and several co-factors including P450 reductase and reduced nicotinamide adenine dinucleotide phosphate (NADPH) to catalyze the oxidation of substrates through the transfer of one oxygen atom to the substrate and the other to water. Two classes of CYP enzymes metabolize AA: hydroxylases, which add a hydroxyl group to AA to form hydroxyeicosatetraenoic acids (HETEs) and epoxygenases, which add an oxygen atom to a double bond of AA to form an epoxide (Figure 1) [2; 3]. This latter pathway forms the subject of the present review. Depending upon which of the four double bonds of AA that is replaced by an epoxide, four distinct regio-isomers may be produced. As illustrated in Figure 1, AA (5,8,11,14-Eicosatetraenoic acid) has four double bonds situated between carbon atoms 5 and 6, 8 and 9, 11 and 12, and 14 and 15, which give rise to 5,6-EET, 8,9-EET, 11,12-EET and 14,15-EET, respectively. In addition, each regio-isomer has two stereo-isomers (R,S and S,R), resulting in eight chemically distinct EETs enantiomers [2; 3].
Of the many CYP enzymes, the members of the CYP-2C and CYP-2J classes are most commonly noted for their epoxygenase activity and for their role in the synthesis of EETs. This includes the human CYP-2C and CYP-2J isoforms, CYP-2C8, CYP-2C9, CYP-2C19 and CYP-2J2 [3]. In addition to members of the CYP-2C and CYP-2J classes, however, other CYP isoforms exhibit epoxygenase activity and the ability to catalyze the synthesis of EETs, including rat CYP-2D18 [22] and the human orphan isoform CYP-4X1 [23]. It is important to highlight that the synthesis of EETs from AA is catalyzed by many different CYP isoforms and that the regio- and stereo-selectivity of these enzymes varies widely [2; 3]. Human CYP-2C8 produces 11,12-EET and 14,15-EET in a 1:1.25 ratio, yet produces no 8,9-EET. By contrast, CYP-2C9 produces 8,9-EET, 11,12-EET and 14,15-EET in the ratio of 2.3:1.0:0.5. In a similar manner, the stereo-selectivity of these enzymes varies, with CYP-2C8 producing the (S,R) and (R,S) 11,12-EET stereo-isomers in a 20:80 ratio, while CYP-2C9 produces them in a 70:30 ratio [24].
P450 enzymes are conventionally associated with liver physiology where high expression levels reflect their participation in the oxidative metabolism of endogenous and xenobiotic molecules [2]. As their role in the synthesis of AA metabolites, including those that participate in cell signaling pathways in the CNS, has been elucidated, the expression of CYP epoxygenase enzymes has been characterized both in the brain and the associated vasculature [1; 3; 4; 25]. This has been done implicitly through the detection of epoxyeicosanoid production in CNS tissue and explicitly through molecular methods such as RT-PCR, in situ hybridization, and immunocytochemistry. A summary of CYP epoxygenases specifically identified in the brain is presented in Table 1.
Early studies on EETs signaling pathways centered upon their stimulatory effects upon neurohormone release from the hypothalamus and pituitary [15; 16], a function that will be discussed in detail below. These first studies identified native brain epoxygenase activity through the detection of EETs production following incubation of brain microsomes with radio-labeled AA. As early as 1984, Capdevila et al. reported such EETs production in isolated rat pituitary microsomes [26]. The authors analyzed the relative abundance of the four EETs regio-isomers and their respective dihydroxyeicosatrienoic acid (DHET) metabolites (Figure 1) and found that 5,6-, 11,12-, and 14,15-EETs and DHETs accounted for nearly 30% of AA metabolism in the microsomal preparation. In a later study, Junier et al. detected endogenous production of 8,9-EET, 11,12-EET, 14,15-EET in extracts from male rat hypothalamus [27]. In this study, the authors estimated the hypothalamic EETs concentration to be 120 ng/g in wet tissue. While these early studies identifying EETs as endogenous stimulators of neurohormone secretion in the hypothalamus and pituitary have been largely overshadowed by the emerging appreciation of EETs’ role in cardiovascular [3] and cerebrovascular function and disease [1; 25], they provided the earliest indication that epoxyeicosanoids were produced endogenously within the CNS and associated structures.
In the early 1990s, EETs release from forebrain structures was first reported. Utilizing gas chromatography mass spectroscopy (GC-MS), conversion of radio-labeled AA into both 5,6- and 14,15-EET was reported from mouse whole brain slices [20]. In a subsequent study, the authors reported that homogenate from primary cultured rat hippocampal astrocytes produced both 5,6-, 14,15-EET, and their corresponding DHET metabolites when incubated with AA; thus identifying astrocytes as one potential site of endogenous EETs production in the CNS [28]. These findings were confirmed in a study by Gebremedhin et al. in which the authors demonstrated using high pressure liquid chromatography (HPLC) the production of all four EETs region-isomers in cat cerebral microsomes incubated with AA [29]. In a subsequent study, Alkayed et al. confirmed EETs’ production in cultured rat cortical astrocytes [19]. This work was later extended with the finding that EETs production by and release from cultured rat cortical astrocytes was increased in response to stimulation with the excitatory neurotransmitter glutamate [30]. This last study provided the first concrete evidence that EETs might represent a releasable astrocyte-derived signaling molecule in the CNS.
Limited experimental evidence also suggests that cerebral vascular endothelium exhibits epoxygenase activity, as demonstrated by the detection of EETs in primary cerebral vascular endothelial cells [21]. Utilizing a fluorescence-based HPLC method for quantification, 5,6-, 11,12- and 14,15-EET production was identified from the cerebral endothelium. In contrast, very little endothelial production of the 8,9-EET regio-isomer was detected.
CYP epoxygenase expression in the brain
Despite the repeated identification of epoxygenase activity and EETs production within brain tissue, less is known concerning the expression and activity of specific CYP epoxygenases in the brain. RT-PCR screens have been conducted upon whole brain homogenate to identify the expression of CYP epoxygenase message in brain. This has included the identification of rat CYP-2C6, CYP-2C7, CYP-2C11, CYP-2C12, CYP-2C23, CYP-2D18, mouse CYP-2C27, CYP-2C37, CYP-2C38, and CYP-2C40 in brain tissue [19; 31–34]. In only two cases, rat CYP-2C11 and CYP-2C7 [35], was protein expression confirmed by Western blot from whole brain microsomal extract. For two isoforms, rat CYP-2C11 and human, mouse and rat CYP-4X1, extensive analysis of expression and epoxygenase activity was conducted within the brain [19; 23; 33; 36; 37]. In cultured rat primary cortical astrocytes, Alkayed et al. demonstrated by RT-PCR, Western blot and immunocytochemistry the specific expression of the CYP-2C11 epoxygenase in cortical astrocytes, suggesting that this isoform is likely the astrocytic ‘EETs-synthase’ postulated by earlier work in these glial cells [19]. This work was later corroborated in vivo by the localization of CYP-2C11 to cortical astrocytes by in situ hybridization [33] and immunohistochemistry (Figure 2A).
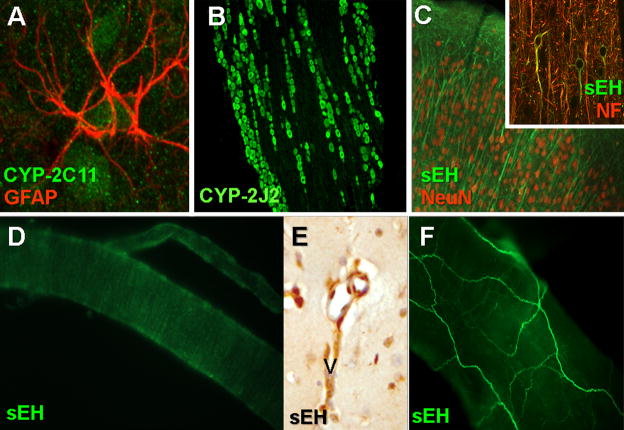
Figure 2.
EETs biosynthetic and metabolizing enzymes in the brain. (A) sEH-IR (green) in rat cortical slices co-localizes with the neuronal markers NeuN (red) and neurofilament (NF, red, inset). (B) CYP-2C11-IR (green) in rat cortical slices co-localizes with the astrocyte marker glial fibrillary acid protein (GFAP, red). (C) In rat trigeminal ganglia, CYP-2J-IR (green) is expressed in neuronal cell bodies throughout the ganglia. (D) In rat penetrating cortical arterioles, sEH (green) is expressed in vascular smooth muscle, as evidenced by the circumferential banding pattern of labeling. (E) sEH-IR is expressed in cerebral arterioles in mouse cortical slices. (F) In whole mount rat middle cerebral arteries, sEH-IR (green) dominantly localizes to extrinsic perivascular nerve fibers.
A second specific CYP epoxygenase isoform, CYP-4X1, was recently identified and characterized in the rat, mouse and human CNS. A so-called ‘orphan’ CYP enzyme, CYP-4X1 was initially cloned in the rat as a transcript expressed specifically in brain tissue [37]. In situ hybridization demonstrated widespread neuronal expression in the CNS, including neurons within the cerebral cortex, hippocampus, cerebellum and brain stem. Molecular cloning in the mouse later yielded the murine CYP-4X1 homologue [36], whose expression was localized to cortical, cerebellar, hippocampal and brain stem neurons by immunocytochemistry. The human CYP-4X1 homologue has likewise been cloned [23], and its mRNA has been identified in human brain tissue. In this most recent work, the metabolic profile of recombinant CYP-4X1 was characterized, including the identification of AA epoxygenase activity.
Recent work has additionally identified the expression of a specific family of CYP epoxygenases, the CYP-2Js, in extra-cranial parasympathetic and sensory ganglia [8]. While these structures do not technically fall within the CNS, one of the functions of the nerve fibers originating from these ganglia is the neurogenic regulation of the cerebral surface vasculature [38], a function intimately associated with CNS function and disease. Both RT-PCR and Western blot analysis demonstrated the expression of CYP-2J3 and CYP-2J4, homologues to the human CYP-2J2 isoform, in the rat parasympathetic sphenopalatine ganglia (SPG) and sensory trigeminal ganglia (TG). Immunocytochemistry demonstrated that CYP-2J localized primarily to neurons within these ganglia (Figure 2B), suggesting that these neurons, some of which project to the cerebral surface vasculature, possess the biochemical machinery for the synthesis of EETs [7; 8].
Soluble epoxide hydrolase (sEH) and EETs metabolism in the brain
The biological activity of EETs in vivo is terminated via their metabolism through multiple pathways, including acylation and incorporation into cellular phospholipids, glutathione conjugation, further oxidation by cyclooxygenase and CYP enzymes, β-oxidation, binding to intracellular fatty acid binding protein (FABP), and sEH-catalyzed hydration to vicinal diols termed dihydroxyeicosatrienoic acids (Figure 1) [2; 39].
Epoxide hydrolases (EHs) comprise a group of enzymes present in all living organisms that catalyze the addition of water molecules to epoxides to form corresponding diol species. Five EHs have been described in vertebrates: cholesterol epoxide hydrolase (chEH), hepoxilin epoxide hydrolase (hEH), leukotriene A4 (LTA4) hydrolase, sEH and microsomal epoxide hydrolase (mEH). The LTA4, cholesterol and hepoxilin EHs have strict substrate specificity, while sEH and mEH exhibit a wider profile of substrate preference [40]. Despite being active in the metabolism of several chemical epoxide species, sEH has recently received the most attention for its prominent role in the conversion of bioactive EETs to DHET products that are generally regarded as having reduced biological activity (Figure 1). Of the four EETs region-isomers, 14,15-EET possess the highest affinity for sEH [39], while increasing distance between the epoxide ring and the methyl-terminal carbon reduces the affinity for sEH, thus producing the following substrate preferences for sEH: 14,15-EET > 11,12-EET > 8,9-EET > 5,6-EET. 5,6-EET itself is metabolized poorly by sEH, and its primary route of metabolism may involve further oxidation by COX enzymes [41].
Soluble epoxide hydrolase is highly expressed in mouse, rat, and human brain, as demonstrated by Western blot of whole brain homogenate from a number of studies [42–46]. The cell type-specific expression of sEH in the brain, however, remains the subject of some uncertainty. Results from our group have identified dominant sEH expression within rat and mouse brain neurons. Immunohistochemical labeling of the rat and mouse cerebral cortex and striatum revealed sEH expression in neuronal soma and processes (Figure 2C) in addition to cerebral arterioles (Figures 2D and 2E)[43; 47]. Based upon the circumferential banding of sEH labeling in cortical arterioles (Figure 2D), this expression is likely specific to the vascular smooth muscle cell layer. In rat brain slices, sEH immunoreactivity did not co-localize with the astrocytic marker glial fibrillary acidic protein (GFAP) although sEH labeling was apparent in striatal white matter tracks, which is consistent with axonal expression of sEH. These findings were generally corroborated in a recent study by Sura et al. from human brain [48]. In that study, the authors document by immunohistochemistry the presence of sEH-immunoreactivity mainly in neurons and oligodendrocytes throughout the brain, including the cerebrum, basal ganglia, and brain stem, with only scattered astrocytes exhibiting sEH expression. The authors further noted the expression of sEH in choroid plexus ependymal cells. In contrast, one other group has recently reported sEH expression in rat astrocytes. In primary cultured rat cortical astrocytes, Rawal et al. detected sEH protein expression both by Western blot and immunofluorescence double labeling [46].
As was the case with CYP-2J epoxygenases, sEH expression has also been identified in peripheral neurons innervating the cerebral surface vasculature. In the rat, RT-PCR, Western blot and immunocytochemistry identified the specific localization of sEH within parasympathetic neurons of the SPG and sensory afferents in the TG [8]. Immunofluorescent labeling of whole-mount middle cerebral arteries revealed the presence of sEH-immunoreactivity in perivascular fibers surrounding these vessels (Figure 2F) [7]. Double labeling studies confirmed that sEH expression co-localized with neuronal nitric oxide synthase (nNOS), vasoactive intestinal peptide (VIP) and calcitonin gene-related peptide (CGRP), markers for parasympathetic and sensory perivascular vasodilator fibers in the cerebral circulation [7; 8].
One unique feature of P450 eicosanoids, the epoxide EETs and the hydroxyl HETEs, is their ability to be re-incorporated into membrane phospholipid pools. In the case of EETs, this pathway constitutes a novel route for the clearance and regulation of bioactive EETs levels in addition to providing a latent pool of releasable EETs not dependent upon de novo synthesis [4; 39]. Early experimental work in isolated mouse mast cells as well as endothelial and vascular smooth muscle cells from a variety of species demonstrated that all four EETs region-isomers were readily taken up into membrane phospholipids, including phosphatidylethanolamine (PE), phosphatidylcholine (PC) and phosphatidylinisitol (PI) [49–51]. Upon stimulation with calcium ionophore, 14,15-EET was released from the phospholipid pool at rates exceeding that of arachidonic acid, suggesting that this route of EETs mobilization may be of physiological importance [49]. EETs not incorporated into the phospholipid pool were observed to be rapidly hydrolyzed into DHET species (Figure 1) and released into the media [50; 51]. Subsequent work, however, demonstrated that DHET could likewise undergo phospholipid incorporation, albeit at a lower rate than the corresponding EET [52].
Based upon these findings, it has been hypothesized that sEH serves as a key regulator of EETs incorporation into membrane phospholipids through its hydrolysis of bioactive EETs to the more releasable DHET species [53; 54]. In porcine coronary endothelial cells, Weintraub et al. demonstrated that inhibition of sEH dramatically increased EETs incorporation into phospholipid pools. Interestingly, this effect was accompanied by an increase in overall EETs release evoked by stimulation with calcium ionophore, suggesting that through its effects on phospholipid EETs incorporation, sEH importantly regulates EETs’ release and biological activity [54].
Such membrane phospholipid incorporation of EETs has likewise been observed in the CNS. In a study in rat primary cortical astrocyte cultures, Shivachar et al. reported the incorporation of both 8,9-and 14,15-EET into astroglial phospholipids [55]. The authors observe that 8,9-EET was incorporated much more rapidly than 14,15-EET, a fact that they attribute the regio-selectivity of epoxide hydrolases. Indeed, pharmacological inhibition of EH activity markedly increased 14,15-EET phospholipid incorporation.
The above studies describing the cell type-specific localization of CYP epoxygenases and sEH throughout the CNS, including in neurons, astroglia, oligodendrocytes, and vascular cells suggest that the epoxyeicosanoid pathway may underlie many modes of cellular signaling throughout the CNS. As most of these cell types are unique to the CNS, it is likely that EETs regulate cellular function within this system in a manner not reflected in the periphery.
Mechanisms of EETs action in the CNS
EETs exert a myriad of autocrine and paracrine actions upon many distinct cell types, including those within the CNS. As detailed above, cellular EETs levels are determined on the supply side by de novo synthesis by CYP epoxygenase enzymes, phospholipase A2-dependent release from the membrane phospholipids and cellular uptake from the extracellular space [4; 39]. Regardless of their cellular source, EETs’ immediate molecular target remains unclear and the subject of vigorous research. Available evidence suggests that EETs directly interact with a number of signaling partners, including a putative membrane-bound G protein-coupled receptor (GPCR), ion channels including the transient receptor potential vanilloid-4 (TRPV4) cation channel and the peroxisome proliferator-activated receptor (PPAR) intracellular nuclear receptors [4] (Figure 3). The apparent promiscuity of epoxyeicosanoid signaling, in addition to their ability to act both via a putative GPCR and TRPV4 cation channels, is intriguing when compared to the signaling pathways employed by another lipid-based signaling pathway, the endocannabinoid system [56]. This system exerts its actions including those within the CNS via a family of GPCRs and through the direct activation of TRPV1 cation channels. Recent functional studies suggest the provocative notion that these pathways bear more than a mere resemblance to one another, but rather interact at multiple molecular levels to regulate cellular function.
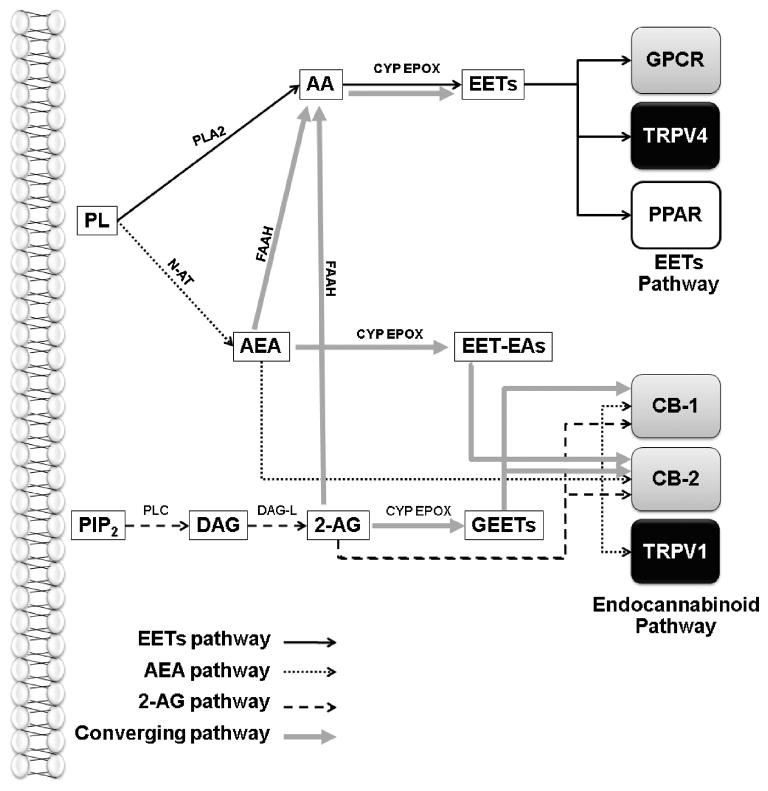
Figure 3.
Routes of interaction between epoxyeicosanoid and endocannabinoid signaling pathways. In the canonical EETs signaling pathway, EETs are produced from arachidonic acid (AA) by cytochrome P450 epoxygenase (CYP EPOX) enzymes. EETs exert their cellular actions indirectly through a putative G protein-coupled receptor (GPCR) or by directly stimulating ion channels such as the transient receptor potential vanilloid-4 (TRPV4) cation channel or the peroxisome proliferator activator receptor (PPAR) nuclear receptors. The canonical endocannabinoid signaling pathway includes the synthesis of the endocannabinoids anandamide (AEA) or 2-arachidonylglycerol (2-AG) which act as agonists at the CB-1 and CB-2 GPCRs. AEA also activates the TRPV1 cation channel. Both AEA and 2-AG can be hydrolyzed by fatty acid amido-hydroxylase or other enzymes to AA which feed directly into the CYP EPOX – EETs signaling cascade. Both AEA and 2-AG can be epoxidized by CYP EPOX to produce EET glycerol esters (GEETs) and EET ethanolamides (EET-EAs). GEETs are high affinity CB-1 and CB-2 agonists while EET-EA activates CB2 with high affinity. PL: phospholipids; N-AT: N-acetyl transferase; PIP2: phosphatidylinositol 4,5-bisphosphate; PLC: phospholipase C; DAG: diacylglycerol; DAG-L: DAG lipase;
A putative G protein-coupled receptor (GPCR) for EETs
Evidence for a specific membrane-bound, extracellular site of action for EETs has been steadily accumulating since the early 1990s. In a series of studies in guinea pig mononuclear cells and a human monocyte cell line, Wong and colleagues identified a specific, saturable and reversible binding site for radio-labeled 14(R),15(S)-EET [57–59]. This site is regio- and stereo-selective, having the highest affinity for this EET enantiomer [57; 58]. Ligand binding to this site was protease-sensitive, suggesting that the binding partner in question was a protein, and appeared to be under regulatory control of the PKA signaling pathway [59]. In cultured rat aortic endothelial cells, the cellular effect of a 14,15-EET analog upon aromatase activity persisted even when the molecule was chemically tethered to a silica bead [60], supporting the existence of an extracellular binding site for 14,15-EET. In a recent study, a radio-labeled EETs agonist, 20-iodo-14,15-epoxyeicosa-8(Z)-enoic acid (20-I-14,15-EE8ZE) was employed to further characterize in human U937 cells this putative EETs binding partner [61]. Based upon radio-ligand displacement, the authors describe a site exhibiting relative EETs affinities of 11,12-EET > 14,15-EET ≫ 14,15-DHET. Further, ligand binding decreased in the presence of GTPγS, suggesting that the binding site in question is a G protein-coupled receptor (GPCR). This last finding is in agreement with several previous studies that have suggested that the putative membrane-bound ‘EETs receptor’ is likely a GPCR [62–64].
Many of these early studies focusing on the role of EETs in the hyperpolarization and relaxation of vascular smooth muscle identified the large-conductance calcium-sensitive K+ channel (BK) as the primary effector pathway through which EETs exert their vasomotor actions. Treatment with EETs increased the open probability of BK channels in many cell types [63; 65], including cerebral vascular smooth muscle, while the membrane hyperpolarizing effects of EETs were sensitive to high extracellular K+ or K+ channel blockade with tetraethylammonium (TEA) [29]. Further experimental evidence has suggested that EETs do not interact directly with this ion channel, acting rather through a putative GPCR to exert their effects on BK channel function. EETs are unable to activate BK channel currents in inside-out detached patch recordings from VSM [62–64]. Treatment with an anti-Gsα antibody likewise blocks the effect of 11,12-EET on BK channel activity as does inhibition of ADP-ribosylation. The weight of this early data clearly suggests that EETs exert at least certain biological activities, including BK channel activation, through their action at a putative Gsα-coupled GPCR that involves the downstream activation of ADP ribosylation.
A recent study by Behm et al. has additionally identified a novel role for EETs in regulating signaling through the thromboxane prostaglandin (TP) receptor [66]. The authors report that 14,15-EET directly antagonizes this receptor with considerable specificity and affinity. The finding that EETs are an endogenous antagonist at this receptor is intriguing, and may have important implications regarding interactions between prostaglandin and EETs signaling pathways both in vasculature and extra-vascular tissues. However, these data have not yet been reproduced and the significance of this specific binding partner in mediating the cellular actions of EETs remains undetermined.
EETs activation of TRPV4 channel
While the classical understanding of EETs signaling involves the indirect activation of BK channels via a putative GPCR-mediated signaling pathway [3], further experimental evidence suggests that certain cellular actions of EETs may be mediated through their direct interaction with ion channels. In particular, the activation of the TRPV4 cation channel has received considerable recent attention. TRPV4 is a calcium-permeable member of the transient receptor potential vanilloid cation channel family, and closely related to the TRPV1 capsaicin receptor found on nociceptive primary afferent fibers [67]. In 2003, it was reported that 5,6- and 8,9-EET directly activate TRPV4 channels [10]. In this study, the authors report that the activation of TRPV4 by AA in transfected cells was sensitive to three distinct inhibitors of P450 enzymes, while direct application of 5,6- or 8,9-EET caused ruthenium red-sensitive elevations of intracellular Ca2+. 5,6-EET-evoked TRPV4 currents were observed both in whole-cell and detached inside-out patch recordings, demonstrating the direct activation of TRPV4 by these EETs regio-isomers. This effect was then confirmed in mouse aortic endothelial cells, in which TRPV4 activation by osmotic cell swelling and AA administration were sensitive to CYP epoxygenase inhibition [68]. Furthermore, 5,6-EET produced a ruthenium red-sensitive, Ca2+-sensitive current that appeared to reflect the activation of native endothelial TRPV4 by this EET regio-isomer. These and other studies have established that EETs-evoked TRPV4 activation is an important regulator of calcium-dependent endothelial function, including participation in flow-induced vasodilation [68; 69].
Two recent studies shed further light on the activation of TRPV4 by EETs and are more directly relevant to processes of CNS function and disease. Earley et al. describe TRPV4 expression in cerebral vascular smooth muscle cells which respond to 11,12-EET treatment with the activation of whole-cell TRPV4 currents [70]. 11,12-EET administration also increases Ca2+ spark and BK channel activity, effects that are sensitive to anti-sense oligonucleotide knockdown of TRPV4. Based upon these findings, the authors conclude that TRPV4 and the BK channel form a ‘novel Ca2+ signaling complex’ in cerebral vascular smooth muscle, in which EETs activate Ca2+ influx via TRPV4, resulting in sarcolemmal ryanodine receptor activation, Ca2+ spark formation, BK channel activation, membrane hyperpolarization and consequent vasorelaxation. Based upon these findings, the activation of TRPV4 may play a key role in the vasomotor action of EETs in the cerebral circulation. A second study by Sipe et al. reports that peripheral administration of 5,6-EET activates action potential spiking in mouse colonic primary afferents [71]. These fibers expressed TRPV4 and the activation of afferent spiking activity was sensitive to ruthenium red treatment and TRPV4 gene deletion. This study suggests that EETs, through their activation of TRPV4 may stimulate neuronal action potential firing, a finding that may be relevant to the activity of trigeminal afferents which also express TRPV4 in addition to EETs-synthetic CYP epoxygenases [7; 8; 72].
EETs activation of PPAR signaling
PPARs are a family of three intracellular nuclear receptors that upon ligand binding, recruit the retinoic X receptor binding partner, translocate to the nucleus, and bind to peroxisome proliferator response elements (PPREs) to regulate gene transcription. In particular, the PPARα receptor is known to serve as a lipid detector, being activated by fatty acids and eicosanoids and exerting regulatory control over genes involved in fatty acid metabolism and eicosanoid signaling pathways [73]. In 2002 it was initially reported that P450 ω-hydroxylase metabolites of 8,9-, 11,12- and 14,15-EET were potent trans-activators of PPARα signaling in a transfected cell line [74]. Subsequently, Ng et al. reported that in HepG2 cells, 11,12- and 14,15-EETs stimulated PPARα and PPARγ activity using a luciferase reporter assay [75]. Interestingly, the 8,9-EET regio-isomer demonstrated no activity in this assay, whereas the 14,15-DHET epoxide hydrolase metabolite exhibited robust activity. In primary rat hepatocytes, both 11,12- and 14,15-EETs were capable of inducing the expression of known PPARα-responsive gene products, suggesting that the activation of PPAR signaling by EETs and their metabolites may be physiologically relevant [76]. The specific participation of EETs-evoked PPAR signaling in the CNS function remains unexplored. Emerging evidence suggests that PPAR signaling is a potential therapeutic target in the treatment of disease states associated with oxidative stress such as hypoxic/ischemic injury and Alzheimer’s Disease [77]. The potential role for EETs signaling in these processes remains an obvious avenue for future research.
Interactions between epoxyeicosanoid and endocannabinoid signaling pathways
Reviewing the details of the EETs signaling system as they are currently understood, it is striking how many similarities exist between this pathway and the extensively studied endocannabinoid system [56]. Both pathways are based on lipophillic signaling molecules derived from membrane phospholipids. Endocannabinoids signal through distinct GPCRs (the CB1 and CB2 receptors) while experimental evidence supports existence of one or more putative EETs GPCRs [3; 4]. The endocannabinoid anandamide directly activates TRPV1 cation channels [56], while 5,6-, 8,9- and 11,12-EETs activate TRPV4 channels [10; 68–71]. Interestingly, recent experimental evidence suggests that these pathways do not simply resemble one another, but in fact converge in the regulation of cellular function, including in the vasculature and the brain (Figure 3). In 2003, Watanabe et al. reported that both 5,6-EET and the endocannabinoid anandamide (AEA) directly activate the TRPV4 channel in a transfected cell system [10]. The authors noted that the effects of anandamide were dependent first upon its hydrolysis to arachidonic acid by the enzyme fatty acid amido-hydrolase (FAAH) followed by its subsequent metabolism by P450 epoxygenases. Thus, at least in the case of AEA-evoked TRPV4 activation, the endocannabinoid signaling pathway converges with the P450 epoxygenase/EETs pathway. Similar findings have been reported in the bovine coronary endothelium, in which the endocannabinoid 2-arachidonylglycerol (2-AG) was produced in response to acetylcholine stimulation [11]. 2-AG was then hydrolyzed to arachidonic acid, contributing to EETs-mediated endothelium-dependent vasodilation. These studies demonstrate that the endocannabinoids AEA and 2-AG, following their hydrolysis to AA, can feed into pathways of the AA cascade, including the CYP epoxygenase pathway.
In a second mode of interaction between the endocannabinoid and P450 epoxygenase pathways, recent evidence also suggests the endocannabinoids AEA and 2-AG themselves may be substrates for P450 epoxygenase enzymes and that the resulting endocannabinoid epoxide metabolites may posess biological activity. In 2007, it was reported that human liver and kidney microsomes incubated with the AEA produced several P450 monoxygenase metabolites, including 20-HETE-ethanolamide (EA), 5,6-, 8,9-, 11,12- and 14,15-EET-EA [12]. In the liver, this AEA epoxygenase activity was attributed to the CYP-3A4 isoform, which is also expressed in activated mouse microglial cells [78]. The orphan CYP-4X1 isoform, which is expressed in human, mouse and rat brain, likewise converts AEA to 14,15-EET-EA [23; 36; 37]. Interestingly, 5,6-EET-EA appears to bind to and activate the human CB2 receptor with high affinity, suggesting a role for these endocannabinoid epoxygenase metabolites in immune function [78]. The endocannabinoid 2-AG can likewise serve as a substrate for CYP epoxygenases, resulting in the production of EET glycerol esters (GEETs) [13; 14]. These endocannabinoid epoxygenase metabolites, including 11,12-GEET and 14,15-GEET bind with high affinity to both CB1 and CB2 receptors in rat cerebellar and spleen membranes, respectively. In vitro, 11,12- and 14,15-GEET produce vasorelaxation in rat kidney arterioles and bovine coronary arteries. The former effect was sensitive to the CB1 antagonist AM251. In vivo, intravenous 11,12-GEET injection in mice produced hypomotility and hypothermia, effects that were also inhibited by CB1 receptor blockade [14].
These emerging studies thus demonstrate that the epoxyeicosanoid and endocannabinoid signaling pathways may interact at a number of levels. Endocannabinoids may be hydrolyzed to free AA which then feeds into the CYP epoxygenase pathway [10; 11], or these compounds may themselves be directly converted into bioactive epoxides [12–14] (Figure 3). Because these interactions have only recently become apparent, their physiological and pathophysiological significance remains unclear. The presence, however, of endocannabinoid epoxygenases and epoxide metabolites [23; 36; 37; 78] in the brain suggests that the interaction of these apparently similar signaling cascades may play an important role in CNS function and disease.
Physiological actions of EETs in the CNS
As has been described above, a substantial body of research identifies EETs as endogenous constituents of CNS tissue. CYP epoxygenase expression has been reported throughout the brain, including in neurons, astrocytes and vascular endothelium (Table 1), while cell type-specific EETs release has been described from cortical astrocytes and endothelium [19; 21]. Since the mid 1990s, investigations into the physiological role of EETs signaling in the CNS have centered predominantly upon their potent activity in regulating the function of the cerebral vasculature. This includes both their well-studied participation in the process of functional hyperemia as well as their regulation of cortical angiogenesis [3; 6; 21]. However, it is noteworthy that the initial characterization of EETs’ regulation of cellular function in the 1980s stemmed from their role as endogenous regulators of neurohormone release in the hypothalamus and anterior pituitary [15; 18]. These data, combined with recent evidence for the involvement of EETs in the neurogenic regulation of cerebral blood flow [7; 8] suggest that epoxyeicosanoids may under physiological conditions be key regulators of synaptic transmission, a cellular action that is distinctly CNS in nature.
Vasomotor effects of EETs in the cerebral circulation
Of their many cellular actions in tissues throughout the body, EETs are best known as potent vasodilators in various circulatory beds, including the heart, kidney and brain [3; 4]. In the coronary and renal circulation, EETs have been identified as EDHFs, being released from the vascular endothelium in response to stimulation and acting upon adjacent vascular smooth muscle to affect hyperpolarization and relaxation [3; 79]. In the cerebral circulation, several studies have documented first the direct vasoactive effects of EETs in this vascular bed and second their key role in mediating the process of functional hyperemia.
Beginning with the work of Ellis et al., EETs were initially identified as potent vasodilators in the cerebral vasculature. Using in vivo rabbit and cat cranial window preparations, the authors determined the effect of topically applied EETs on cerebrocortical surface arteriolar diameter [41; 80]. In this model, 5,6-EETs in micromolar concentrations produced a ~20% dilation of cerebral vessels while the 8,9-EET, 11,12-EET regio-isomers evoked less response. The dilator action of 5,6-EET was sensitive to COX inhibition with indomethacin, suggesting that a vasoactive COX metabolite of EETs was involved in this response. These findings were later extended by Gebremedhin et al. using isolated cat cerebral arteries [29]. In this study, the authors observed that 5,6-, 8,9- and 11,12-EET directly and concentration-dependently dilated cerebral arteries in vitro. More recent studies have confirmed EETs’ vasoactivity in the cerebral circulation in rats, including the effect of 11,12-EET in isolated rat cerebral artery and 14,15-EET in the rat cranial window model [8; 70]. These vasomotor actions of EETs in the cerebral vasculature are thought to be mediated by the activation of vascular smooth muscle K+ channels, as evidenced by the sensitivity of the EETs-evoked response to the K+ channel blocker TEA [3; 29].
More recently, the involvement of the TRPV4 cation channel in the vasomotor activity of EETs in rat cerebral arteries has been suggested. In dissociated rat cerebral vascular smooth muscle, Earley et al. reported that 11,12-EET administration produced TRPV4-like whole-cell currents that coincided with an increase in Ca2+ spark activity that was in turn linked to BK channel activity and membrane hyperpolarization [70]. Based upon these findings, the authors proposed the hypothesis that TRPV4 and the BK channel constitute a novel Ca2+ signaling complex in cerebral vascular smooth muscle cells that is the molecular target of vasoactive EETs. This model was supported by the attenuation of 11,12-EET-evoked vasodilation in isolated rat cerebral arteries by antisense oligonucleotide TRPV4 gene knockdown. These studies demonstrate that as in the peripheral circulatory beds such as the coronary, renal or mesenteric vasculature [3], all EETs regio-isomers act as potent vasodilators in the cerebral circulation.
EETs are mediators of cortical functional hyperemia
The physiological role of vasoactive EETs in the CNS has been explored most extensively in the context of the phenomenon termed functional hyperemia. Functional hyperemia is the process by which local changes in brain activity are coupled both spatially and temporally with corresponding increases in local cerebral blood flow [81]. Forming the physiological basis for the functional magnetic resonance imaging (fMRI) technique, this process of neurovascular coupling has been the subject of intense research that has identified numerous mediators and modulators of this process, including K+, adenosine, nitric oxide and EETs [6; 81]. In 1997, Alkayed et al. reported that stimulation of cultured rat astrocytes with the excitatory neurotransmitter glutamate increased cellular EETs production, an effect that was sensitive to P450 enzyme inhibition with miconazole [30]. The authors then demonstrated that miconazole blocked the hyperemic response to subdural glutamate infusion, supporting a role for EETs signaling in coupling excitatory neurotransmitter release with corresponding elevations in cerebral blood flow. A follow-up study produced similar results with the CYP epoxygenase inhibitor N-(methylsulfonyl)-2-(2-propynyloxy)-benzenehexanamide (MS-PPOH) in blocking the hyperemic response to subdural N-methyl-D-aspartate (NMDA) infusion [82]. In two further studies, MS-PPOH attenuated the cerebral blood flow response to functional activation of the rat cortex produced by whisker or electrical forepaw stimulation [33; 83]. These early studies thus established a role for astrocyte-derived EETs in mediating the process of functional hyperemia in the brain [5].
More recent studies have utilized sophisticated imaging and molecular techniques to define the cellular mechanisms governing the participation of EETs in neurovascular coupling. In isolated rat retina, Metea and Newman observed that arterioles dilated both in response to light stimulation and photolysis of caged Ca2+ molecules loaded into perivascular astrocytic endfeet [84]. In this study, the authors reported that the vasomotor response to both modes of stimulation were abolished by three distinct inhibitors of P450 epoxygenases. This elegant in vitro study extends functional activation studies carried out previously in the whole animal and confirms at the molecular level that astrocyte-derived EETs mediate neurovascular coupling in the CNS.
Within the field currently studying cerebral blood flow regulation, a puzzle has recently emerged concerning the occurrence of dilator versus constrictor coupling between astrocytes and their associated microvasculature. In contrast to the findings of Matea and Newman [84] and others [85; 86] utilizing both in vitro and in vivo two-photon imaging in rats and mice, Mulligan and MacVicar observed vasoconstriction of cerebral arterioles in cortical slices undergoing photolysis of astrocytic caged Ca2+ as well as with metabotropic glutamate receptor (mGlurR) agonist treatment [87]. These findings suggest that differing modes of neurovascular coupling can be produced, apparently in a context-dependent manner. A study by Blanco et al. sheds some light upon this puzzle by suggesting that the resting tone of cerebral arterioles is a key determinant of the polarity of neurovascular coupling, and that changes in the vasomotor responses to EETs may underpin these shifts in polarity [88]. In this study, the authors report that in acute rat cortical slices, the vasomotor responses to stimuli such as K+ or mGluR agonists shift in magnitude or polarity, depending on the resting tone of the vessel. Highly constricted vessels were observed to dilate avidly, while less constricted vessels responded either with less dilation (K+) or with constriction (mGluR agonists). Interestingly, the vasomotor response to exogenous 11,12-EET exhibited this same polarity. Under constricted conditions, 11,12-EET produced monophasic vasodilation that was TEA-sensitive. However, under conditions of reduced vascular tone, 11,12-EET produced a biphasic constriction followed by dilation. These findings, while still awaiting validation, present an intriguing mechanistic explanation for the regulation of dilator versus constrictor neurovascular coupling. Furthermore, they suggest that the vasomotor response to astrocyte-derived EETs may itself be one tunable element of this regulation.
The role of EETs in mediating cortical functional hyperemia within the so-called neurovascular unit is strikingly distinct from EETs-mediated regulation of the peripheral vasculature. Peripherally, EETs are commonly regarded as EDHFs, produced by the endothelium in response to stimulation; then acting on adjacent VSM to produce hyperpolarization and vasorelaxation [3; 4; 79]. In the cerebral circulation, while the end point of VSM hyperpolarization and relaxation remain the same, the cellular players involved are markedly different. Neuronal activity in sensed by astrocytic mGluR receptors, leading to the release of EETs from these intermediary cells, which transduce the neuronal signal to the adjacent microvasculature [6]. Thus it appears that even in the regulation of vascular tone, the role of epoxyeicosanoids in the CNS differs from the periphery in a manner reflective of the distinct structure-function relationships present in the CNS.
EETs regulate cortical angiogenesis
Angiogenesis, the process through which new microvasculature is produced, occurs both in the healthy and diseased brain. In addition to the initial development of the mature cerebral vasculature, normal physiological angiogenesis may represent the vascular projection of brain plasticity [89]. The stimulation and maintenance of cortical angiogenesis is importantly regulated through interactions between astrocytes that surround the cerebral microvasculature and endothelial cells which constitute the terminal branches of the circulatory bed and sites of angiogenic growth. The interplay between cortical astrocytes and brain microcirculation was examined in earlier studies demonstrating that astrocyte-derived EETs are key regulators of cortical angiogenesis [90; 91]. In the first study, the authors report that cultured cerebral microvascular endothelial cells (CMVECs) underwent avid proliferation when treated with conditioned media from primary cortical astrocyte cultures. When the two cell types were cultured together, CMVECs exhibited spontaneous tube formation, an in vitro model of angiogenesis. The CMVEC tube formation stimulated by co-culture with astrocytes was blocked with treatment with the P450 inhibitor 17-ODYA, suggesting that P450 metabolites mediated astrocyte-evoked angiogenic processes [90]. In the follow-up study by Zhang and Harder (2002), the authors report that inhibiting astrocytic P450 enzymes with 17-ODYA attenuated the mitogenic action of astrocyte-conditioned media on CMVECs, demonstrating that an astrocyte-derived P450 metabolite underlies this response [91]. The authors also observe that direct treatment of CMVECs with all four EETs regioisomers evokes cell proliferation, with 8,9-EET being the most potent in this process. Extending these findings in vivo, 8,9-EET-supplemented matrigel implanted subcutaneously exhibited more robust capillary infiltration than did control matrigel implants. These studies together provide strong evidence that cortical astrocyte-derived EETs exert pro-angiogenic effects on cerebral endothelial cells and that EETs signaling may mediate angiogenesis under normal physiological conditions.
EETs as regulators of neurohormone release
Despite the more recent focus upon the role of EETs in cerebrovascular regulation, the earliest studies of EETs’ function in the CNS detailed a non-vascular role for EETs signaling in stimulating neurohormone release from neuroendocrine regions of the brain. In one early study in rat hypothalamus, 5,6-EET stimulated the release of somatostatin and luteinizing hormone releasing hormone [15; 18]. These findings were later confirmed and extended to include the stimulation of somatostatin release from rat hypothalamus by the 8,9-EET regio-isomer. In the anterior pituitary, the four EETs regio-isomers were variously observed through the course of several studies to directly stimulate the release of luteinizing hormone, arginine vasopressin, oxytocin, prolactin and growth hormone [16; 92–95]. Interestingly, in addition to these direct effects upon neurohormone release, EETs appear to mediate the actions of other neuroendocrine secretagogues. In the hypothalamus, inhibition of CYP epoxygenase activity blocks dopamine-evoked somatostatin release [15]. In the anterior pituitary, inhibition of CYP epoxygenases blocks corticotropin-releasing factor-, β-adrenergic agonist-, and K+-evoked corticotropin release while thyrotropin-releasing hormone-evoked prolactin release was likewise sensitive to CYP epoxygenase inhibition [96; 97]. Importantly, it was observed that stimulation of these cells with 5,6-EET resulted in intracellular Ca2+ mobilization, suggesting a potential mechanism through which EETs might exert these pro-secretory actions. Although these findings have not been pursued in more than fifteen years, they identify a key role for EETs signaling in regulating the release of neurohormones that may be relevant to recent investigations regarding the role of neurogenic EETs in the regulation of neuropeptide release and cerebral blood flow [7; 8].
Role of EETs signaling in CNS disease
Since their identification as key mediators of cerebral blood flow regulation, increasing attention has been focused upon the role of EETs in CNS disease processes; particularly those related to cerebrovascular dysfunction. The role of the epoxyeicosanoid system and its therapeutic potential has undergone the most study in the field of cerebral ischemia [17]. Studies from our group have demonstrated the clear role for the EETs signaling pathway as an endogenous protective program activated in response to ischemic stress and protective against ischemic brain damage [98]. Interestingly, many of the protective actions of EETs have proven to be blood flow-independent and thus may reflect neuro-, glio-, or vasculoprotective actions of EETs independent of their vasomotor actions. In addition, further non-vascular actions of EETs have recently been elucidated, and examination of epoxyeicosanoid participation in CNS disease has expanded to include such processes as inflammation and nociception.
Genetic association of CYP epoxygenases and sEH to CNS disease risk
Experimental investigation of the involvement of the epoxyeicosanoid pathway in CNS disease has focused primarily upon cerebral ischemia in rodent stroke models [17]. These model systems have been useful in delineating the role of EETs in certain disease processes, but they do not necessarily indicate whether this system is important to the development of clinical CNS disease in humans. Recent genetic studies from human populations, however, have identified naturally-occurring variations in both CYP epoxygenase- and sEH-encoding genes [99]. Many of these variations are biochemically consequential, resulting in gene products with altered function [100; 101]. Intriguingly, a growing number of studies have identified associations between certain genetic variations in CYP epoxygenase and sEH genes and disease states relevant to CNS function. This includes cerebral and myocardial ischemia, hypertension, hypercholesterolemia, atherosclerosis and insulin resistance in type II diabetes. These studies, which are summarized on Table 2, highlight the potential importance of epoxyeicosanoid signaling pathways to CNS function and disease processes.
Table 2.
Association of CYP epoxygenase and EPHX2 gene sequence variation with cardiovascular and cerebrovascular disease risk.
| Enzyme | Variant | Atherosclerosis | Hyperlipidemia | Hypertension | Insulin Resistance | Myocardial Infarction | Cerebral Ischemia | References |
|---|---|---|---|---|---|---|---|---|
| CYP- | Intronic SNPs | + | N | [108] | ||||
| 2J2 | CYP-2J2*7 | N/+ | N/+ | N/+ | N | [100; 102–107] | ||
| CYP-2C8 | CYP-2C8*2 | N | N | N/+ | N | [103; 104; 108] | ||
| CYP-2C8*3 | N | N | N/+ | [103; 104; 108; 142] | ||||
| CYP-2C9 | CYP-2C9*2 | N | N | N/− | N | [103; 108; 142–146] | ||
| CYP-2C9*3 | N | N/− | N/− | N | [103; 108; 142–144; 146; 147] | |||
| Pharm. Inhibition | + | [146] | ||||||
| CYP-2C19 | CYP-2C19*3 | + | [144] | |||||
| sEH | K55R | + | [114] | |||||
| R287Q | + | + | N | + | − | [103; 109–113] | ||
| 403RIns | N | [103] | ||||||
| Intron 2 | + | [110] | ||||||
| Intron 11 | + | [109] | ||||||
| Intron 16 | + | [115] |
(+): Increased risk; N: no change in risk; (−): reduced risk. Pharm. Inhibition: Pharmacological CYP-2C9 inhibition.
Given the apparent role of endothelial EETs in cardiovascular regulation, numerous human genetics studies have been undertaken to investigate associations between polymorphisms in the EETs-synthetic CYP epoxygenase enzymes and cardiovascular risk (Table 2). Although many alternative alleles of the human CYP epoxygenases have been described [99], certain alleles have been repeatedly linked to altered cardiovascular risk. These include the CYP-2J2*7 variant, which is polymorphic in the gene promoter region and is transcription-deficient, as well as the CYP-2C8*2 and *3, and CYP-2C9*2 and *3 variants, which are loss-of-function polymorphisms [99; 100]. Of these, the most noteworthy is the CYP-2J2*7 variant which has been linked to increased risk of atherosclerosis, hypertension and myocardial infarction, although studies of varying sizes and within various populations report conflicting findings [100; 102–107]. While their impact upon these cardiovascular risk factors may modify the incidence and course of CNS disease, direct associations between polymorphisms in the CYP epoxygenase enzymes and CNS pathology such as cerebral ischemia [108] have not been reported.
Like CYP epoxygenases, naturally occurring genetic variations in the human EPHX2 gene have been described that result in enzymes with differing biochemical activity (Table 2). This includes the R287Q variant that has a glutamine substituted at the arginine 287th residue. When the resulting enzyme is reconstituted in vitro, it demonstrates both reduced hydrolase activity and protein stability, making this a ‘loss-of-function’ sEH variant [101]. In human studies, the R287Q polymorphism has been linked to a complex cardiovascular and cerebrovascular phenotype, including increased risk of atherosclerosis, hyperlipidemia, insulin resistance in type II diabetics, and a decreased risk of ischemic stroke [103; 109–113]. In a functional genomics study, transduction of this loss-of-function sEH variant into rat primary cortical neurons protected them from cell death resulting from simulated ischemia [47]. In addition to the R287Q variant, other EPXH2 polymorphisms have also been linked to cardiovascular and cerebrovascular risk [103; 109; 110; 114; 115]. These include non-synonymous amino acid substitutions such as the K55R variant that has been linked to elevated risk of atherosclerosis [114] as well as polymorphisms located in intronic regions that may alter splice efficiency and fidelity that have been linked to increased risk of atherosclerosis and cerebral ischemia [109; 110; 115].
Role of EETs signaling in cerebral ischemia
In the study of EETs’ influence upon CNS disease states, the role of EETs signaling in the development and treatment of ischemic stroke has been the subject of the most study. P450 enzymes are highly inducible in response to environmental toxins and oxidative stress [2; 3; 116]. Early studies on this subject investigated the impact of ischemic preconditioning (IPC) upon CYP epoxygenase expression in the brain and the participation of epoxygenase signaling in the protection afforded by IPC. In a rat model of transient focal cerebral ischemia, it was observed that brief periods of ischemia induce tolerance in brain against subsequent severe cerebral ischemia, concomitant with an increase in the expression levels of brain CYP-2C11 epoxygenase mRNA and protein [98]. To evaluate whether higher epoxygenase expression in the preconditioned brain corresponds with higher brain EETs levels during subsequent stroke, IPC- and sham-operated rats were implanted with microdialysis probes to collect and assay extracellular fluid for EETs during MCA occlusion. Probes were implanted in the ipsilateral MCA territory (striatum) 3 hours prior to 2-hour MCA occlusion and samples were collected during ischemia and analyzed for EETs content using a fluorescence-based high-performance liquid chromatography (HPLC) method in collaboration with Dr. Richard Roman at the Medical College of Wisconsin [117]. Figure 4 represents a reverse phase HPLC chromatogram from samples collected during cerebral ischemia and shows that at least one EETs regio-isomer, 14,15-EET, is increased during MCA occlusion in IPC versus sham brain.
Figure 4.
HPLC chromatograms demonstrating the presence of EETs in brain microdialysate collected during MCA occlusion from sham (left)- and ischemia-preconditioned (IPC) rats. Normalization to tridecanoic acid (TA) internal standard revealed that EETs are produced in brain at micromolar concentrations (pg/ml sample volume).
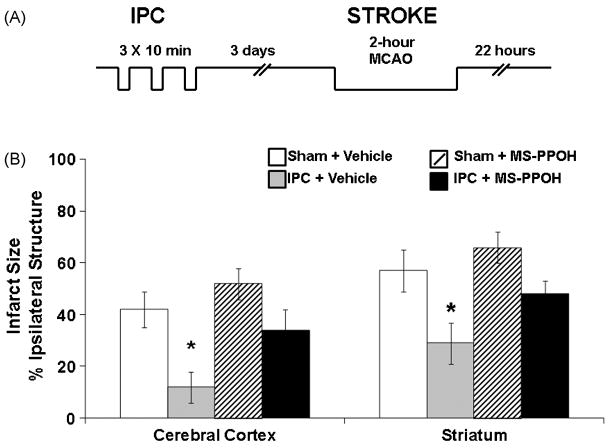
To evaluate whether the increase in brain EETs levels and epoxygenase expression contributes to the protection afforded by IPC, the effect of intra-cisternal injection of the P450 epoxygenase inhibitor MS-PPOH on ischemic tolerance was determined. Injecting the drug one hour prior to a 2-hour MCA occlusion in sham- and ischemia-preconditioned rats (Figure 5A depicts the experimental protocol) blocked the protective effect of IPC (Figure 5B) as determined by histological staining. Interestingly, MS-PPOH had no effect on infarct size in sham-preconditioned animals, suggesting that the CYP epoxygenase pathway is an inducible protective mechanism in brain that does not contribute to ischemic damage in unconditioned brain.
Figure 5.
Ischemic preconditioning (IPC) is protective against ischemic brain damage via P450 epoxygenase. (A) Schematic representative of IPC model. (B) Infarct size at 22 hrs after MCA occlusion (Stroke) in ischemia (IPC)- and sham-preconditioned (sham) rats pretreated with P450 epoxygenase inhibitor MS-PPOH or vehicle (n=8 per group). * Different from vehicle-treated groups.
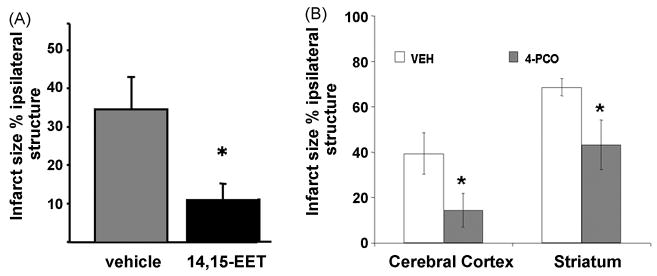
The data described in Figure 5 suggest that protection by IPC is in part mediated by EETs. However, measurement of CBF during MCA occlusion revealed no differences in blood flow between sham and IPC-preconditioned animals [98]. This observation suggests that EETs may exhibit other non-vascular protective properties in stroke. In agreement with this notion, EETs are directly protective against hypoxic-ischemic cell death in primary cultured astrocytes and neurons subjected to oxygen-glucose deprivation (OGD) [47; 118]. To ascertain whether this protective action of EETs can be translated in vivo, the effect of exogenously administered EETs on infarct size after MCA occlusion was determined. Figure 6A shows that exogenous administration of 14,15-EET is protective against experimental ischemic brain injury [44]. To achieve protection, however, it was necessary to administer EETs continuously for 24 hours via an osmotic minipump, suggesting that the in vivo effects of EETs are short-lived. The key explanation for the short-lived actions of EETs in the brain and circulation is their rapid metabolism and inactivation by soluble epoxide hydrolase.
Figure 6.
Exogenous EETs and sEH inhibition are protective against experimental cerebral ischemia. (A) Exogenous administration of 14,15-EET reduces infarct size after MCA occlusion in mice. Modified from Zhang et al. Stroke. 2008;39:2073–8. (B) Soluble epoxide hydrolase (sEH) as a regulator of EETs levels and a target for neuroprotection. EETs are metabolized and inactivated by sEH. Inhibition of sEH increases EETs and affords protection against cerebral ischemia.
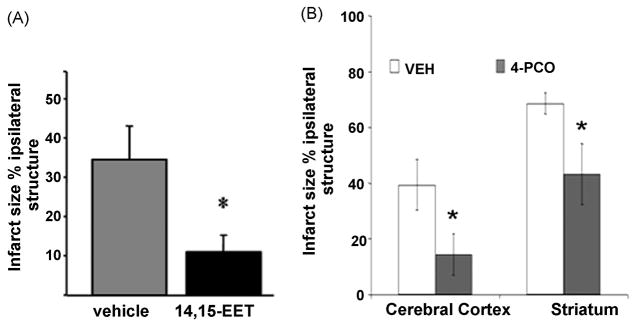
Because sEH is a key regulator of EETs’ biological actions [53], more recently our group has tested the hypothesis that sEH inhibition is protective against ischemic brain damage through a mechanism involving increased EETs availability. We initially utilized the transient epoxide hydrolase inhibitor 4-phenylchalcone oxide (4-PCO) to determine the effect of pharmacological sEH inhibition upon in vivo ischemic brain damage. Figure 6B shows that 4-PCO (administered 30 min prior to 2-hour MCA occlusion) reduced infarct size compared to vehicle-treated animals. A second more potent and selective sEH inhibitor N-adamantanyl-N′-dodecanoic acid urea butyl ester (AUDA-BE) reduced the infarct size in mice following MCA occlusion when the drug was administered 30 min prior to or at the time of reperfusion [43]. Most recently, our group examined the effect of EPHX2 gene deletion (sEH knockout mice, sEHKO) on infarct size in mice [44]. In this study, it was observed that sEHKO mice sustained smaller infarcts after MCA occlusion compared to wild type (WT) mice. Interestingly, ischemic protection in this case was associated with a significant increase in cortical blood flow during MCA occlusion in sEHKO compared to WT mice, suggesting that vasodilation likely contributes to the observed EETs-mediated protection. These findings support the hypothesis that inhibition of sEH is protective against experimental ischemic insult and that sEH may represent a promising therapeutic target in for the treatment of ischemic stroke [17].
The mechanism of protection by EETs in ischemic stroke is multi-factorial [17], which in addition to vasodilation likely involves the suppression of post-ischemic inflammatory cytokine expression [45]. EETs have additionally been shown to exert anti-thrombotic [119; 120], anti-pyretic [121], anti-oxidant [122], anti-apoptotic [123] and pro-angiogenic effects [90; 91], although these mechanisms have not yet been directly implicated in EETs-evoked ischemic protection. Similarly, EETs signaling has been linked to activation of multiple cell survival pathways, including opening of mitochondrial ATP-dependent potassium channels [124] and activation of the phosphatidylinositol 3-kinase (PI3-K)/AKT [125], mitogen-activated protein kinase (MAPK) [126] and cAMP/protein kinase A (PKA) pathways [127]. Whether activation of any of these pathways by EETs contributes to ischemic neuroprotection remains unknown.
Role of EETs in CNS tumor formation
The anti-apoptotic and pro-angiogenic effects of EETs in the cerebral circulation described above support their beneficial role in the context of cerebral ischemia. However, these cellular actions may exert a deleterious effect upon tumor formation, including tumors of CNS origin. In a study of human neoplastic tissue micro-arrays, Enayatallah et al. report the up-regulation of CYP-2C8, CYP-2C9, CYP-2J2 and sEH in several carcinoma samples [128]. In a second study of human carcinomas, CYP-2J2 was observed to be specifically up-regulated compared to surrounding normal tissue. In this study, forced CYP-2J2 over-expression or direct EETs administration to cultured carcinoma cells dramatically increased cell proliferation and resistance to tumor necrosis factor (TNF)-α-induced apoptosis. In contrast, anti-sense knockdown of CYP-2J2 expression or CYP epoxygenase inhibition with 17-ODYA blocked these responses [129]. Specifically in the CNS, a recent study of glioblastoma multiforme in the rat examined the effect of inhibition of EETs signaling upon tumor growth and progression [130]. Utilizing two distinct pharmacological agents, 17-ODYA and miconazole, the authors report that inhibition of CYP epoxygenase in rat glioblastoma tumors in vivo reduces tumor capillary density, retards tumor growth and improves animal survival. These findings appear in line with the reported pro-angiogenic and anti-apoptotic effects of EETs in the CNS [91; 118]. Interestingly, the authors of this article report that CYP epoxygenase inhibition did not result in a measurable decrease in tumor EETs levels. While the cause of this apparent inconsistency is unclear, the findings of this study suggest that epoxyeicosanoid signaling may underlie CNS tumor growth and progression and thus represent a novel therapeutic target for their treatment.
EETs as regulators of neuro-inflammation
Many disease states of the CNS involve an inflammatory component, either as a primary agent of injury, as in multiple sclerosis, or as a contributor to secondary injury, as in cerebral ischemia. As understanding of the mechanisms of CNS disease develops, so too does the recognition that satisfactory treatment regimes must take into account the complex interplay between CNS tissue, vasculature, injury and inflammatory processes. Beginning with the work of Node et al. in 1999, EETs have been recognized as possessing anti-inflammatory properties [131]. In this study, the authors reported that treatment of endothelial cells with the 5,6-, 8,9- and 11,12-EET, but not the 14,15-EET regio-isomer, prevented inflammatory cytokine-evoked up-regulation of adhesion molecule expression. This effect was attributed to a reduction in pro-inflammatory NF-κB signaling and resulted in a functional reduction of monocyte adhesion to the vascular endothelium. In a second line of investigation, Kozak and colleagues have reported that in isolated rat monocytes 11,12-EET inhibits LPS-evoked PGE2 production without altering the LPS-evoked elevation in COX-2 expression [120]. A similar effect of sEH inhibition upon LPS-evoked PGE2 production was observed by Schmelzer et al. [132]. These studies thus suggest that EETs negatively modulate inflammatory processes including pro-inflammatory PGE2 production.
It is important to note that the anti-inflammatory effects of epoxyeicosanoids have generally been assessed only peripherally and not in the context of CNS disease. However, one recent study begins to shed some light upon this subject in the context of cerebral ischemic injury [45]. This study reports that following MCA occlusion in mice, brain inflammatory cytokine levels are elevated. In the sEH knockout mouse, this inflammatory cytokine induction is attenuated compared to wild type controls. These findings suggest that CNS EETs signaling negatively regulates injury-evoked neuro-inflammatory processes. Thus far, this remains an isolated finding and the overall effects of epoxyeicosanoid signaling on inflammatory processes in the CNS continues to be a subject ripe for further investigation.
Anti-pyretic effect of central EETs
In work that parallels the peripheral anti-inflammatory actions of epoxyeicosanoids, including their modulation of PGE2 signaling [132], a group of studies demonstrated that endogenous EETs exert antipyretic effects in mice [133; 134]. In the initial studies, inhibition of CYP epoxygenase activity with three different pharmacological agents potentiated fever resulting from either i.p. LPS injection or central interleukin-1 (IL-1) beta injection. In a more recent study, Kozak et al. report that i.p. injection of P450 inducers attenuated the fever response to i.p. LPS, as did central administration of different EETs regio-isomers [121]. This latter study suggests that the CNS epoxyeicosanoid pathway contributes to endogenous anti-pyretic regulation. The efficacy of low-dose central EETs in these studies argues that this effect is centrally mediated and may be specific to their actions within the CNS.
Role of EETs in analgesia and anti-nociception
As a result of the observed anti-inflammatory effects of EETs, experimental work in animal models of inflammatory pain has begun to explore the potential role of EETs as endogenous analgesic factors or sEH inhibition as a potential therapeutic avenue for the amelioration of inflammatory pain. In the first study on this subject, Inceoglu et al. report that both exogenous EETs and sEH inhibitor administration reduce inflammatory pain associated with hindpaw LPS injection in the rat [135]. In this study, topical application of either of two distinct sEH inhibitors blocked LPS-evoked thermal sensitization and allodynia. Topically administered methyl esther EETs produced a similar reduction in thermal sensitization. These effects of sEH inhibitors were subsequently confirmed in a second model of inflammatory pain, subplantar carrageenan-evoked thermal hyperalgesia in the rat [136]. In the most recent study from this group, Inceoglu et al. report that intra-spinal injection of two distinct sEH inhibitors block LPS- and carrageenan-evoked thermal hyperalgesia [9]. These functional responses coincided with a reduction in LPS-evoked spinal COX-2 mRNA induction in addition to an up-regulation of Steroidogenic Acute Regulatory Protein (StARD1), an acute response neurosteroid-producing gene. The induction of spinal StARD1 expression could also be produced by intra-spinal injection of EETs plus cAMP, suggesting that the effects of the sEH inhibitors were the result of increased EETs signaling. Based upon these experimental findings, the authors conclude that EETs act through two distinct analgesic pathways, inhibiting COX-2 expression and inducing neurosteroid signaling to relieve inflammatory pain.
A recent study by Terashvili et al. added to these findings, demonstrating an anti-nociceptive effect of 14,15-EET, but not 5,6-, 8,9- or 11,12-EET, in the rat ventrolateral periaqueductal gray [137]. In this study, Terashvili et al. report that direct microinjection of 14,15-EET into this region dose-dependently reduced tail flick latency in rats. This anti-nociceptive effect of EETs involved activation of β-endorphin and Met-enkephalin signaling via the the μ and δ opioid receptors. Intriguingly, these recent studies demonstrate a potential role for EETs signaling both in peripheral and central pain processing pathways, suggesting that epoxyeicosanoids regulate physiological and pathophysiological CNS processes in ways not related to cerebrovascular function.
Conclusion
In summary, epoxyeicosanoids are important endogenous brain lipid signaling molecules involved in the regulation of many facets of CNS function. Under physiological conditions this includes processes such as neuovascular coupling and synaptic transmission. Because EETs’ are involved in cellular functions specific to the CNS, such as neural, neuroglial, neurovascular and gliovascular processes, the actions of EETs in the CNS are in many respects distinct from those observed in peripheral tissues. Under pathophysiological conditions such as ischemic stress, EETs protect neuronal, astroglial and vascular networks in a concerted manner, preserving CNS structural and functional integrity. The multiplicity of EETs’ cellular sources, targets and mechanisms of action make the epoxyeicosanoid pathway a unique and attractive target for ischemic neuroprotection. Finally, the emerging role for epoxyeicosanoids in processes specific to CNS function suggest that EETs may serve as a therapeutic target in other neurodegenerative disease such as Alzheimer’s and Parkinson’s Disease, or in cerebrovascular disorders beyond ischemic stroke such as migraine, traumatic brain injury or vasospasm after subarachnoid hemorrhage.
Acknowledgments
We would like to acknowledge Dr. Richard Roman, Medical College of Wisconsin, Milwaukee for EETs measurements in microdialysate from ischemic brain using fluorescent HPLC; LC-MS/MS analysis of EETs was performed by Dr. Dennis Koop at the OHSU Bioanalytical Shared Resource/Pharmacokinetics Core (BSR/PKC). MS-PPOH was kindly provided to us by Dr. J.R. Falck, University of Texas Southwestern Medical Center, Dallas; 4-PCO was purchased from Biomol International (Plymouth Meeting, PA).
References
- 1.Phillis JW, Horrocks LA, Farooqui AA. Cyclooxygenases, lipoxygenases, and epoxygenases in CNS: their role and involvement in neurological disorders. Brain Res Rev. 2006;52(2):201–43. doi: 10.1016/j.brainresrev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276(39):36059–62. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 3.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82(1):131–85. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 4.Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol. 2007;292(3):C996–1012. doi: 10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- 5.Harder DR, Alkayed NJ, Lange AR, Gebremedhin D, Roman RJ. Functional hyperemia in the brain: hypothesis for astrocyte-derived vasodilator metabolites. Stroke. 1998;29(1):229–34. doi: 10.1161/01.str.29.1.229. [DOI] [PubMed] [Google Scholar]
- 6.Koehler RC, Gebremedhin D, Harder DR. Role of astrocytes in cerebrovascular regulation. J Appl Physiol. 2006;100(1):307–17. doi: 10.1152/japplphysiol.00938.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iliff JJ, Close LN, Selden NR, Alkayed NJ. A novel role for P450 eicosanoids in the neurogenic control of cerebral blood flow in the rat. Exp Physiol. 2007;92(4):653–8. doi: 10.1113/expphysiol.2006/036889. [DOI] [PubMed] [Google Scholar]
- 8.Iliff JJ, Wang R, Zeldin DC, Alkayed NJ. Epoxyeicosanoids as mediators of neurogenic vasodilation in cerebral vessels. Am J Physiol Heart Circ Physiol. 2009;296(5):H1352–63. doi: 10.1152/ajpheart.00950.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inceoglu B, Jinks SL, Ulu A, Hegedus CM, Georgi K, Schmelzer KR, et al. Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesic pathways. Proc Natl Acad Sci U S A. 2008;105(48):18901–6. doi: 10.1073/pnas.0809765105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424(6947):434–8. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 11.Gauthier KM, Baewer DV, Hittner S, Hillard CJ, Nithipatikom K, Reddy DS, et al. Endothelium-derived 2-arachidonylglycerol: an intermediate in vasodilatory eicosanoid release in bovine coronary arteries. Am J Physiol Heart Circ Physiol. 2005;288(3):H1344–51. doi: 10.1152/ajpheart.00537.2004. [DOI] [PubMed] [Google Scholar]
- 12.Snider NT, Kornilov AM, Kent UM, Hollenberg PF. Anandamide metabolism by human liver and kidney microsomal cytochrome p450 enzymes to form hydroxyeicosatetraenoic and epoxyeicosatrienoic acid ethanolamides. J Pharmacol Exp Ther. 2007;321(2):590–7. doi: 10.1124/jpet.107.119321. [DOI] [PubMed] [Google Scholar]
- 13.Awumey EM, Hill SK, Diz DI, Bukoski RD. Cytochrome P-450 metabolites of 2-arachidonoylglycerol play a role in Ca2+-induced relaxation of rat mesenteric arteries. Am J Physiol Heart Circ Physiol. 2008;294(5):H2363–70. doi: 10.1152/ajpheart.01042.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Chen J, Imig JD, Wei S, Hachey DL, Guthi JS, et al. Identification of novel endogenous cytochrome p450 arachidonate metabolites with high affinity for cannabinoid receptors. J Biol Chem. 2008;283(36):24514–24. doi: 10.1074/jbc.M709873200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Capdevila J, Chacos N, Falck JR, Manna S, Negro-Vilar A, Ojeda SR. Novel hypothalamic arachidonate products stimulate somatostatin release from the median eminence. Endocrinology. 1983;113(1):421–3. doi: 10.1210/endo-113-1-421. [DOI] [PubMed] [Google Scholar]
- 16.Negro-Vilar A, Snyder GD, Falck JR, Manna S, Chacos N, Capdevila J. Involvement of eicosanoids in release of oxytocin and vasopressin from the neural lobe of the rat pituitary. Endocrinology. 1985;116(6):2663–8. doi: 10.1210/endo-116-6-2663. [DOI] [PubMed] [Google Scholar]
- 17.Iliff JJ, Alkayed NJ. Soluble epoxide hydrolase inhibition: targeting multiple mechanisms of ischemic brain injury with a single agent. Future Neurology. 2009;4(2):179–99. doi: 10.2217/14796708.4.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snyder GD, Capdevila J, Chacos N, Manna S, Falck JR. Action of luteinizing hormone-releasing hormone: involvement of novel arachidonic acid metabolites. Proc Natl Acad Sci U S A. 1983;80(11):3504–7. doi: 10.1073/pnas.80.11.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alkayed NJ, Narayanan J, Gebremedhin D, Medhora M, Roman RJ, Harder DR. Molecular characterization of an arachidonic acid epoxygenase in rat brain astrocytes. Stroke. 1996;27(5):971–9. doi: 10.1161/01.str.27.5.971. [DOI] [PubMed] [Google Scholar]
- 20.Amruthesh SC, Falck JR, Ellis EF. Brain synthesis and cerebrovascular action of epoxygenase metabolites of arachidonic acid. J Neurochem. 1992;58(2):503–10. doi: 10.1111/j.1471-4159.1992.tb09749.x. [DOI] [PubMed] [Google Scholar]
- 21.Medhora M, Narayanan J, Harder D. Dual regulation of the cerebral microvasculature by epoxyeicosatrienoic acids. Trends Cardiovasc Med. 2001;11(1):38–42. doi: 10.1016/s1050-1738(01)00082-2. [DOI] [PubMed] [Google Scholar]
- 22.Thompson CM, Capdevila JH, Strobel HW. Recombinant cytochrome P450 2D18 metabolism of dopamine and arachidonic acid. J Pharmacol Exp Ther. 2000;294(3):1120–30. [PubMed] [Google Scholar]
- 23.Stark K, Dostalek M, Guengerich FP. Expression and purification of orphan cytochrome P450 4X1 and oxidation of anandamide. FEBS J. 2008;275(14):3706–17. doi: 10.1111/j.1742-4658.2008.06518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daikh BE, Lasker JM, Raucy JL, Koop DR. Regio- and stereoselective epoxidation of arachidonic acid by human cytochromes P450 2C8 and 2C9. J Pharmacol Exp Ther. 1994;271(3):1427–33. [PubMed] [Google Scholar]
- 25.Liu M, Hurn PD, Alkayed NJ. Cytochrome P450 in neurological disease. Curr Drug Metab. 2004;5(3):225–34. doi: 10.2174/1389200043335540. [DOI] [PubMed] [Google Scholar]
- 26.Capdevila J, Snijder GD, Falck JR. Epoxygenation of arachidonic acid by rat anterior pituitary microsomal fractions. FEBS Lett. 1984;178(2):319–22. doi: 10.1016/0014-5793(84)80625-0. [DOI] [PubMed] [Google Scholar]
- 27.Junier MP, Dray F, Blair I, Capdevila J, Dishman E, Falck JR, et al. Epoxygenase products of arachidonic acid are endogenous constituents of the hypothalamus involved in D2 receptor-mediated, dopamine-induced release of somatostatin. Endocrinology. 1990;126(3):1534–40. doi: 10.1210/endo-126-3-1534. [DOI] [PubMed] [Google Scholar]
- 28.Amruthesh SC, Boerschel MF, McKinney JS, Willoughby KA, Ellis EF. Metabolism of arachidonic acid to epoxyeicosatrienoic acids, hydroxyeicosatetraenoic acids, and prostaglandins in cultured rat hippocampal astrocytes. J Neurochem. 1993;61(1):150–9. doi: 10.1111/j.1471-4159.1993.tb03550.x. [DOI] [PubMed] [Google Scholar]
- 29.Gebremedhin D, Ma YH, Falck JR, Roman RJ, VanRollins M, Harder DR. Mechanism of action of cerebral epoxyeicosatrienoic acids on cerebral arterial smooth muscle. Am J Physiol. 1992;263(2 Pt 2):H519–25. doi: 10.1152/ajpheart.1992.263.2.H519. [DOI] [PubMed] [Google Scholar]
- 30.Alkayed NJ, Birks EK, Narayanan J, Petrie KA, Kohler-Cabot AE, Harder DR. Role of P-450 arachidonic acid epoxygenase in the response of cerebral blood flow to glutamate in rats. Stroke. 1997;28(5):1066–72. doi: 10.1161/01.str.28.5.1066. [DOI] [PubMed] [Google Scholar]
- 31.Zaphiropoulos PG, Wood T. Identification of the major cytochrome P450s of subfamily 2C that are expressed in brain of female rats and in olfactory lobes of ethanol treated male rats. Biochem Biophys Res Commun. 1993;193(3):1006–13. doi: 10.1006/bbrc.1993.1725. [DOI] [PubMed] [Google Scholar]
- 32.Luo G, Zeldin DC, Blaisdell JA, Hodgson E, Goldstein JA. Cloning and expression of murine CYP2Cs and their ability to metabolize arachidonic acid. Arch Biochem Biophys. 1998;357(1):45–57. doi: 10.1006/abbi.1998.0806. [DOI] [PubMed] [Google Scholar]
- 33.Peng X, Zhang C, Alkayed NJ, Harder DR, Koehler RC. Dependency of cortical functional hyperemia to forepaw stimulation on epoxygenase and nitric oxide synthase activities in rats. J Cereb Blood Flow Metab. 2004;24(5):509–17. doi: 10.1097/00004647-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Kawashima H, Strobel HW. cDNA cloning of a novel rat brain cytochrome P450 belonging to the CYP2D subfamily. Biochem Biophys Res Commun. 1995;209(2):535–40. doi: 10.1006/bbrc.1995.1534. [DOI] [PubMed] [Google Scholar]
- 35.Warner M, Gustafsson JA. Effect of ethanol on cytochrome P450 in the rat brain. Proc Natl Acad Sci U S A. 1994;91(3):1019–23. doi: 10.1073/pnas.91.3.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Anizy M, Horley NJ, Kuo CS, Gillett LC, Laughton CA, Kendall D, et al. Cytochrome P450 Cyp4x1 is a major P450 protein in mouse brain. FEBS J. 2006;273(5):936–47. doi: 10.1111/j.1742-4658.2006.05119.x. [DOI] [PubMed] [Google Scholar]
- 37.Bylund J, Zhang C, Harder DR. Identification of a novel cytochrome P450, CYP4X1, with unique localization specific to the brain. Biochem Biophys Res Commun. 2002;296(3):677–84. doi: 10.1016/s0006-291x(02)00918-x. [DOI] [PubMed] [Google Scholar]
- 38.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol. 2006;100(3):1059–64. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- 39.Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res. 2004;43(1):55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 40.Newman JW, Morisseau C, Hammock BD. Epoxide hydrolases: their roles and interactions with lipid metabolism. Prog Lipid Res. 2005;44(1):1–51. doi: 10.1016/j.plipres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Ellis EF, Police RJ, Yancey L, McKinney JS, Amruthesh SC. Dilation of cerebral arterioles by cytochrome P-450 metabolites of arachidonic acid. Am J Physiol. 1990;259(4 Pt 2):H1171–7. doi: 10.1152/ajpheart.1990.259.4.H1171. [DOI] [PubMed] [Google Scholar]
- 42.Johansson C, Stark A, Sandberg M, Ek B, Rask L, Meijer J. Tissue specific basal expression of soluble murine epoxide hydrolase and effects of clofibrate on the mRNA levels in extrahepatic tissues and liver. Arch Toxicol. 1995;70(1):61–3. doi: 10.1007/s002040050250. [DOI] [PubMed] [Google Scholar]
- 43.Zhang W, Koerner IP, Noppens R, Grafe M, Tsai H, Morisseau C, et al. Soluble epoxide hydrolase: a novel therapeutic target in stroke. J Cereb Blood Flow Metab. 2007;27(12):1931–40. doi: 10.1038/sj.jcbfm.9600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, Iliff JJ, et al. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke. 2008;39(7):2073–8. doi: 10.1161/STROKEAHA.107.508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koerner IP, Zhang W, Cheng J, Parker S, Hurn PD, Alkayed NJ. Soluble epoxide hydrolase: regulation by estrogen and role in the inflammatory response to cerebral ischemia. Front Biosci. 2008;13:2833–41. doi: 10.2741/2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rawal S, Morisseau C, Hammock BD, Shivachar AC. Differential subcellular distribution and colocalization of the microsomal and soluble epoxide hydrolases in cultured neonatal rat brain cortical astrocytes. J Neurosci Res. 2009;87(1):218–27. doi: 10.1002/jnr.21827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koerner IP, Jacks R, DeBarber AE, Koop D, Mao P, Grant DF, et al. Polymorphisms in the human soluble epoxide hydrolase gene EPHX2 linked to neuronal survival after ischemic injury. J Neurosci. 2007;27(17):4642–9. doi: 10.1523/JNEUROSCI.0056-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sura P, Sura R, Enayetallah AE, Grant DF. Distribution and expression of soluble epoxide hydrolase in human brain. J Histochem Cytochem. 2008;56(6):551–9. doi: 10.1369/jhc.2008.950659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bernstrom K, Kayganich K, Murphy RC, Fitzpatrick FA. Incorporation and distribution of epoxyeicosatrienoic acids into cellular phospholipids. J Biol Chem. 1992;267(6):3686–90. [PubMed] [Google Scholar]
- 50.Fang X, VanRollins M, Kaduce TL, Spector AA. Epoxyeicosatrienoic acid metabolism in arterial smooth muscle cells. J Lipid Res. 1995;36(6):1236–46. [PubMed] [Google Scholar]
- 51.VanRollins M, Kaduce TL, Knapp HR, Spector AA. 14,15-Epoxyeicosatrienoic acid metabolism in endothelial cells. J Lipid Res. 1993;34(11):1931–42. [PubMed] [Google Scholar]
- 52.VanRollins M, Kaduce TL, Fang X, Knapp HR, Spector AA. Arachidonic acid diols produced by cytochrome P-450 monooxygenases are incorporated into phospholipids of vascular endothelial cells. J Biol Chem. 1996;271(24):14001–9. doi: 10.1074/jbc.271.24.14001. [DOI] [PubMed] [Google Scholar]
- 53.Fang X, Kaduce TL, Weintraub NL, Harmon S, Teesch LM, Morisseau C, et al. Pathways of epoxyeicosatrienoic acid metabolism in endothelial cells. Implications for the vascular effects of soluble epoxide hydrolase inhibition. J Biol Chem. 2001;276(18):14867–74. doi: 10.1074/jbc.M011761200. [DOI] [PubMed] [Google Scholar]
- 54.Weintraub NL, Fang X, Kaduce TL, VanRollins M, Chatterjee P, Spector AA. Epoxide hydrolases regulate epoxyeicosatrienoic acid incorporation into coronary endothelial phospholipids. Am J Physiol. 1999;277(5 Pt 2):H2098–108. doi: 10.1152/ajpheart.1999.277.5.H2098. [DOI] [PubMed] [Google Scholar]
- 55.Shivachar AC, Willoughby KA, Ellis EF. Effect of protein kinase C modulators on 14,15-epoxyeicosatrienoic acid incorporation into astroglial phospholipids. J Neurochem. 1995;65(1):338–46. doi: 10.1046/j.1471-4159.1995.65010338.x. [DOI] [PubMed] [Google Scholar]
- 56.Kim SR, Chung YC, Chung ES, Park KW, Won SY, Bok E, et al. Roles of transient receptor potential vanilloid subtype 1 and cannabinoid type 1 receptors in the brain: neuroprotection versus neurotoxicity. Mol Neurobiol. 2007;35(3):245–54. doi: 10.1007/s12035-007-0030-1. [DOI] [PubMed] [Google Scholar]
- 57.Wong PY, Lin KT, Yan YT, Ahern D, Iles J, Shen SY, et al. 14(R),15(S)-epoxyeicosatrienoic acid (14(R),15(S)-EET) receptor in guinea pig mononuclear cell membranes. J Lipid Mediat. 1993;6(1–3):199–208. [PubMed] [Google Scholar]
- 58.Wong PY, Lai PS, Shen SY, Belosludtsev YY, Falck JR. Post-receptor signal transduction and regulation of 14(R),15(S)-epoxyeicosatrienoic acid (14,15-EET) binding in U-937 cells. J Lipid Mediat Cell Signal. 1997;16(3):155–69. doi: 10.1016/s0929-7855(97)00005-9. [DOI] [PubMed] [Google Scholar]
- 59.Wong PY, Lai PS, Falck JR. Mechanism and signal transduction of 14 (R), 15 (S)-epoxyeicosatrienoic acid (14,15-EET) binding in guinea pig monocytes. Prostaglandins Other Lipid Mediat. 2000;62(4):321–33. doi: 10.1016/s0090-6980(00)00079-4. [DOI] [PubMed] [Google Scholar]
- 60.Snyder GD, Krishna UM, Falck JR, Spector AA. Evidence for a membrane site of action for 14,15-EET on expression of aromatase in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2002;283(5):H1936–42. doi: 10.1152/ajpheart.00321.2002. [DOI] [PubMed] [Google Scholar]
- 61.Yang W, Tuniki VR, Anjaiah S, Falck JR, Hillard CJ, Campbell WB. Characterization of epoxyeicosatrienoic acid binding site in U937 membranes using a novel radiolabeled agonist, 20-125i-14,15-epoxyeicosa-8(Z)-enoic acid. J Pharmacol Exp Ther. 2008;324(3):1019–27. doi: 10.1124/jpet.107.129577. [DOI] [PubMed] [Google Scholar]
- 62.Fukao M, Mason HS, Kenyon JL, Horowitz B, Keef KD. Regulation of BK(Ca) channels expressed in human embryonic kidney 293 cells by epoxyeicosatrienoic acid. Mol Pharmacol. 2001;59(1):16–23. doi: 10.1124/mol.59.1.16. [DOI] [PubMed] [Google Scholar]
- 63.Li PL, Campbell WB. Epoxyeicosatrienoic acids activate K+ channels in coronary smooth muscle through a guanine nucleotide binding protein. Circ Res. 1997;80(6):877–84. doi: 10.1161/01.res.80.6.877. [DOI] [PubMed] [Google Scholar]
- 64.Li PL, Chen CL, Bortell R, Campbell WB. 11,12-Epoxyeicosatrienoic acid stimulates endogenous mono-ADP-ribosylation in bovine coronary arterial smooth muscle. Circ Res. 1999;85(4):349–56. doi: 10.1161/01.res.85.4.349. [DOI] [PubMed] [Google Scholar]
- 65.Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, et al. Stereospecific effects of epoxyeicosatrienoic acids on renal vascular tone and K(+)-channel activity. Am J Physiol. 1996;270(5 Pt 2):F822–32. doi: 10.1152/ajprenal.1996.270.5.F822. [DOI] [PubMed] [Google Scholar]
- 66.Behm DJ, Ogbonna A, Wu C, Burns-Kurtis CL, Douglas SA. Epoxyeicosatrienoic acids function as selective, endogenous antagonists of native thromboxane receptors: identification of a novel mechanism of vasodilation. J Pharmacol Exp Ther. 2009;328(1):231–9. doi: 10.1124/jpet.108.145102. [DOI] [PubMed] [Google Scholar]
- 67.Plant TD, Strotmann R. TRPV4. Handb Exp Pharmacol. 2007;179:189–205. doi: 10.1007/978-3-540-34891-7_11. [DOI] [PubMed] [Google Scholar]
- 68.Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, et al. Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res. 2005;97(9):908–15. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- 69.Loot AE, Popp R, Fisslthaler B, Vriens J, Nilius B, Fleming I. Role of cytochrome P450-dependent transient receptor potential V4 activation in flow-induced vasodilatation. Cardiovasc Res. 2008;80(3):445–52. doi: 10.1093/cvr/cvn207. [DOI] [PubMed] [Google Scholar]
- 70.Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res. 2005;97(12):1270–9. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- 71.Sipe WEB, Brierley SM, Martin CM, Phillis BD, Cruz FB, Grady EF, et al. Transient receptor potential vanilloid 4 mediates protease activated receptor 2-induced sensitization of colonic afferent nerves and visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2008;294(5):G1288–98. doi: 10.1152/ajpgi.00002.2008. [DOI] [PubMed] [Google Scholar]
- 72.Hermanstyne TO, Markowitz K, Fan L, Gold MS. Mechanotransducers in rat pulpal afferents. J Dent Res. 2008;87(9):834–8. doi: 10.1177/154405910808700910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wray J, Bishop-Bailey D. Epoxygenases and peroxisome proliferator-activated receptors in mammalian vascular biology. Exp Physiol. 2008;93(1):148–54. doi: 10.1113/expphysiol.2007.038612. [DOI] [PubMed] [Google Scholar]
- 74.Cowart LA, Wei S, Hsu M, Johnson EF, Krishna MU, Falck JR, et al. The CYP4A isoforms hydroxylate epoxyeicosatrienoic acids to form high affinity peroxisome proliferator-activated receptor ligands. J Biol Chem. 2002;277(38):35105–12. doi: 10.1074/jbc.M201575200. [DOI] [PubMed] [Google Scholar]
- 75.Ng VY, Huang Y, Reddy LM, Falck JR, Lin ET, Kroetz DL. Cytochrome P450 eicosanoids are activators of peroxisome proliferator-activated receptor alpha. Drug Metab Dispos. 2007;35(7):1126–34. doi: 10.1124/dmd.106.013839. [DOI] [PubMed] [Google Scholar]
- 76.Liu Y, Zhang Y, Schmelzer K, Lee T, Fang X, Zhu Y, et al. The antiinflammatory effect of laminar flow: the role of PPARgamma, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc Natl Acad Sci U S A. 2005;102(46):16747–52. doi: 10.1073/pnas.0508081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sundararajan S, Jiang Q, Heneka M, Landreth G. PPARgamma as a therapeutic target in central nervous system diseases. Neurochem Int. 2006;49(2):136–44. doi: 10.1016/j.neuint.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 78.Snider NT, Nast JA, Tesmer LA, Hollenberg PF. A cytochrome P450-derived epoxygenated metabolite of anandamide is a potent cannabinoid receptor 2-selective agonist. Mol Pharmacol. 2009;75(4):965–72. doi: 10.1124/mol.108.053439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, et al. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401(6752):493–7. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- 80.Ellis EF, Amruthesh SC, Police RJ, Yancey LM. Brain synthesis and cerebrovascular action of cytochrome P-450/monooxygenase metabolites of arachidonic acid. Adv Prostaglandin Thromboxane Leukot Res. 1991;21A:201–4. [PubMed] [Google Scholar]
- 81.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006;100(1):328–35. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 82.Bhardwaj A, Northington FJ, Carhuapoma JR, Falck JR, Harder DR, Traystman RJ, et al. P-450 epoxygenase and NO synthase inhibitors reduce cerebral blood flow response to N-methyl-D-aspartate. Am J Physiol Heart Circ Physiol. 2000;279(4):H1616–24. doi: 10.1152/ajpheart.2000.279.4.H1616. [DOI] [PubMed] [Google Scholar]
- 83.Peng X, Carhuapoma JR, Bhardwaj A, Alkayed NJ, Falck JR, Harder DR, et al. Suppression of cortical functional hyperemia to vibrissal stimulation in the rat by epoxygenase inhibitors. Am J Physiol Heart Circ Physiol. 2002;283(5):H2029–37. doi: 10.1152/ajpheart.01130.2000. [DOI] [PubMed] [Google Scholar]
- 84.Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci. 2006;26(11):2862–70. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takano T, Tian G, Peng W, Lou N, Libionka W, Han X, et al. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9(2):260–7. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 86.Simard M, Arcuino G, Takano T, Liu QS, Nedergaard M. Signaling at the gliovascular interface. J Neurosci. 2003;23(27):9254–62. doi: 10.1523/JNEUROSCI.23-27-09254.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431(7005):195–9. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- 88.Blanco VM, Stern JE, Filosa JA. Tone-dependent vascular responses to astrocyte-derived signals. Am J Physiol Heart Circ Physiol. 2008;294(6):H2855–63. doi: 10.1152/ajpheart.91451.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Isaacs KR, Anderson BJ, Alcantara AA, Black JE, Greenough WT. Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J Cereb Blood Flow Metab. 1992;12(1):110–9. doi: 10.1038/jcbfm.1992.14. [DOI] [PubMed] [Google Scholar]
- 90.Munzenmaier DH, Harder DR. Cerebral microvascular endothelial cell tube formation: role of astrocytic epoxyeicosatrienoic acid release. Am J Physiol Heart Circ Physiol. 2000;278(4):H1163–7. doi: 10.1152/ajpheart.2000.278.4.H1163. [DOI] [PubMed] [Google Scholar]
- 91.Zhang C, Harder DR. Cerebral capillary endothelial cell mitogenesis and morphogenesis induced by astrocytic epoxyeicosatrienoic Acid. Stroke. 2002;33(12):2957–64. doi: 10.1161/01.str.0000037787.07479.9a. [DOI] [PubMed] [Google Scholar]
- 92.Snyder G, Lattanzio F, Yadagiri P, Falck JR, Capdevila J. 5,6-Epoxyeicosatrienoic acid mobilizes Ca2+ in anterior pituitary cells. Biochem Biophys Res Commun. 1986;139(3):1188–94. doi: 10.1016/s0006-291x(86)80303-5. [DOI] [PubMed] [Google Scholar]
- 93.Canonico PL, Judd AM, Koike K, Valdenegro CA, MacLeod RM. Arachidonate stimulates prolactin release in vitro: a role for the fatty acid and its metabolites as intracellular regulator(s) in mammotrophs. Endocrinology. 1985;116(1):218–25. doi: 10.1210/endo-116-1-218. [DOI] [PubMed] [Google Scholar]
- 94.Cashman JR, Hanks D, Weiner RI. Epoxy derivatives of arachidonic acid are potent stimulators of prolactin secretion. Neuroendocrinology. 1987;46(3):246–51. doi: 10.1159/000124827. [DOI] [PubMed] [Google Scholar]
- 95.Cashman JR. 5,6-Epoxyeicosatrienoic acid stimulates growth hormone release in rat anterior pituitary cells. Life Sci. 1989;44(19):1387–93. doi: 10.1016/0024-3205(89)90396-2. [DOI] [PubMed] [Google Scholar]
- 96.Cashman JR, Snowdowne KW. Prolactin secretion in anterior pituitary cells: effect of eicosanoids. Eicosanoids. 1992;5(3–4):153–61. [PubMed] [Google Scholar]
- 97.Luini AG, Axelrod J. Inhibitors of the cytochrome P-450 enzymes block the secretagogue-induced release of corticotropin in mouse pituitary tumor cells. Proc Natl Acad Sci U S A. 1985;82(4):1012–4. doi: 10.1073/pnas.82.4.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Alkayed NJ, Goyagi T, Joh H, Klaus J, Harder DR, Traystman RJ, et al. Neuroprotection and P450 2C11 upregulation after experimental transient ischemic attack. Stroke. 2002;33(6):1677–84. doi: 10.1161/01.str.0000016332.37292.59. [DOI] [PubMed] [Google Scholar]
- 99.Theken KN, Lee CR. Genetic variation in the cytochrome P450 epoxygenase pathway and cardiovascular disease risk. Pharmacogenomics. 2007;8(10):1369–83. doi: 10.2217/14622416.8.10.1369. [DOI] [PubMed] [Google Scholar]
- 100.Spiecker M, Darius H, Hankeln T, Soufi M, Sattler AM, Schaefer JR, et al. Risk of coronary artery disease associated with polymorphism of the cytochrome P450 epoxygenase CYP2J2. Circulation. 2004;110(15):2132–6. doi: 10.1161/01.CIR.0000143832.91812.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Przybyla-Zawislak BD, Srivastava PK, Vazquez-Matias J, Mohrenweiser HW, Maxwell JE, Hammock BD, et al. Polymorphisms in human soluble epoxide hydrolase. Mol Pharmacol. 2003;64(2):482–90. doi: 10.1124/mol.64.2.482. [DOI] [PubMed] [Google Scholar]
- 102.Liu P, Li Y, Chao T, Wu H, Lin L, Tsai L, et al. Synergistic effect of cytochrome P450 epoxygenase CYP2J2*7 polymorphism with smoking on the onset of premature myocardial infarction. Atherosclerosis. 2007;195(1):199–206. doi: 10.1016/j.atherosclerosis.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 103.Dreisbach AW, Japa S, Sigel A, Parenti MB, Hess AE, Srinouanprachanh SL, et al. The Prevalence of CYP2C8, 2C9, 2J2, and soluble epoxide hydrolase polymorphisms in African Americans with hypertension. Am J Hypertens. 2005;18(10):1276–81. doi: 10.1016/j.amjhyper.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 104.Lee CR, North KE, Bray MS, Couper DJ, Heiss G, Zeldin DC. CYP2J2 and CYP2C8 polymorphisms and coronary heart disease risk: the Atherosclerosis Risk in Communities (ARIC) study. Pharmacogenet Genomics. 2007;17(5):349–58. doi: 10.1097/FPC.0b013e32809913ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hoffmann MM, Bugert P, Seelhorst U, Wellnitz B, Winkelmann BR, Boehm BO, et al. The - 50G>T polymorphism in the promoter of the CYP2J2 gene in coronary heart disease: the Ludwigshafen Risk and Cardiovascular Health study. Clin Chem. 2007;53(3):539–40. doi: 10.1373/clinchem.2006.084756. [DOI] [PubMed] [Google Scholar]
- 106.Polonikov AV, Ivanov VP, Solodilova MA, Khoroshaya IV, Kozhuhov MA, Ivakin VE, et al. A common polymorphism G-50T in cytochrome P450 2J2 gene is associated with increased risk of essential hypertension in a Russian population. Dis Markers. 2008;24(2):119–26. doi: 10.1155/2008/626430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Borgel J, Bulut D, Hanefeld C, Neubauer H, Mugge A, Epplen JT, et al. The CYP2J2 G-50T polymorphism and myocardial infarction in patients with cardiovascular risk profile. BMC Cardiovasc Disord. 2008;8:41. doi: 10.1186/1471-2261-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marciante KD, Totah RA, Heckbert SR, Smith NL, Lemaitre RN, Lumley T, et al. Common variation in cytochrome P450 epoxygenase genes and the risk of incident nonfatal myocardial infarction and ischemic stroke. Pharmacogenet Genomics. 2008;18(6):535–43. doi: 10.1097/FPC.0b013e3282fd1287. [DOI] [PubMed] [Google Scholar]
- 109.Wei Q, Doris PA, Pollizotto MV, Boerwinkle E, Jacobs DRJ, Siscovick DS, et al. Sequence variation in the soluble epoxide hydrolase gene and subclinical coronary atherosclerosis: interaction with cigarette smoking. Atherosclerosis. 2007;190(1):26–34. doi: 10.1016/j.atherosclerosis.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 110.Burdon KP, Lehtinen AB, Langefeld CD, Carr JJ, Rich SS, Freedman BI, et al. Genetic analysis of the soluble epoxide hydrolase gene, EPHX2, in subclinical cardiovascular disease in the Diabetes Heart Study. Diab Vasc Dis Res. 2008;5(2):128–34. doi: 10.3132/dvdr.2008.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sato K, Emi M, Ezura Y, Fujita Y, Takada D, Ishigami T, et al. Soluble epoxide hydrolase variant (Glu287Arg) modifies plasma total cholesterol and triglyceride phenotype in familial hypercholesterolemia: intrafamilial association study in an eight-generation hyperlipidemic kindred. J Hum Genet. 2004;49(1):29–34. doi: 10.1007/s10038-003-0103-6. [DOI] [PubMed] [Google Scholar]
- 112.Zhang L, Ding H, Yan J, Hui R, Wang W, Kissling GE, et al. Genetic variation in cytochrome P450 2J2 and soluble epoxide hydrolase and risk of ischemic stroke in a Chinese population. Pharmacogenet Genomics. 2008;18(1):45–51. doi: 10.1097/FPC.0b013e3282f313e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ohtoshi K, Kaneto H, Node K, Nakamura Y, Shiraiwa T, Matsuhisa M, et al. Association of soluble epoxide hydrolase gene polymorphism with insulin resistance in type 2 diabetic patients. Biochem Biophys Res Commun. 2005;331(1):347–50. doi: 10.1016/j.bbrc.2005.03.171. [DOI] [PubMed] [Google Scholar]
- 114.Lee CR, North KE, Bray MS, Fornage M, Seubert JM, Newman JW, et al. Genetic variation in soluble epoxide hydrolase (EPHX2) and risk of coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) study. Hum Mol Genet. 2006;15(10):1640–9. doi: 10.1093/hmg/ddl085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gschwendtner A, Ripke S, Freilinger T, Lichtner P, Müller-Myhsok B, Wichmann H, et al. Genetic variation in soluble epoxide hydrolase (EPHX2) is associated with an increased risk of ischemic stroke in white Europeans. Stroke. 2008;39(5):1593–6. doi: 10.1161/STROKEAHA.107.502179. [DOI] [PubMed] [Google Scholar]
- 116.Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, Dalton TP. Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem Pharmacol. 2000;59(1):65–85. doi: 10.1016/s0006-2952(99)00310-x. [DOI] [PubMed] [Google Scholar]
- 117.Maier KG, Henderson L, Narayanan J, Alonso-Galicia M, Falck JR, Roman RJ. Fluorescent HPLC assay for 20-HETE and other P-450 metabolites of arachidonic acid. Am J Physiol Heart Circ Physiol. 2000;279(2):H863–71. doi: 10.1152/ajpheart.2000.279.2.H863. [DOI] [PubMed] [Google Scholar]
- 118.Liu M, Alkayed NJ. Hypoxic preconditioning and tolerance via hypoxia inducible factor (HIF) 1alpha-linked induction of P450 2C11 epoxygenase in astrocytes. J Cereb Blood Flow Metab. 2005;25(8):939–48. doi: 10.1038/sj.jcbfm.9600085. [DOI] [PubMed] [Google Scholar]
- 119.Heizer ML, McKinney JS, Ellis EF. 14,15-Epoxyeicosatrienoic acid inhibits platelet aggregation in mouse cerebral arterioles. Stroke. 1991;22(11):1389–93. doi: 10.1161/01.str.22.11.1389. [DOI] [PubMed] [Google Scholar]
- 120.Krötz F, Riexinger T, Buerkle MA, Nithipatikom K, Gloe T, Sohn H, et al. Membrane-potential-dependent inhibition of platelet adhesion to endothelial cells by epoxyeicosatrienoic acids. Arterioscler Thromb Vasc Biol. 2004;24(3):595–600. doi: 10.1161/01.ATV.0000116219.09040.8c. [DOI] [PubMed] [Google Scholar]
- 121.Kozak W, Kluger MJ, Kozak A, Wachulec M, Dokladny K. Role of cytochrome P-450 in endogenous antipyresis. Am J Physiol Regul Integr Comp Physiol. 2000;279(2):R455–60. doi: 10.1152/ajpregu.2000.279.2.R455. [DOI] [PubMed] [Google Scholar]
- 122.Spiecker M, Liao JK. Vascular protective effects of cytochrome p450 epoxygenase-derived eicosanoids. Arch Biochem Biophys. 2005;433(2):413–20. doi: 10.1016/j.abb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 123.Chen JK, Capdevila J, Harris RC. Cytochrome p450 epoxygenase metabolism of arachidonic acid inhibits apoptosis. Mol Cell Biol. 2001;21(18):6322–31. doi: 10.1128/MCB.21.18.6322-6331.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Seubert J, Yang B, Bradbury JA, Graves J, Degraff LM, Gabel S, et al. Enhanced postischemic functional recovery in CYP2J2 transgenic hearts involves mitochondrial ATP-sensitive K+ channels and p42/p44 MAPK pathway. Circ Res. 2004;95(5):506–14. doi: 10.1161/01.RES.0000139436.89654.c8. [DOI] [PubMed] [Google Scholar]
- 125.Potente M, Fisslthaler B, Busse R, Fleming I. 11,12-Epoxyeicosatrienoic acid-induced inhibition of FOXO factors promotes endothelial proliferation by down-regulating p27Kip1. J Biol Chem. 2003;278(32):29619–25. doi: 10.1074/jbc.M305385200. [DOI] [PubMed] [Google Scholar]
- 126.Fleming I, Fisslthaler B, Michaelis UR, Kiss L, Popp R, Busse R. The coronary endothelium-derived hyperpolarizing factor (EDHF) stimulates multiple signalling pathways and proliferation in vascular cells. Pflugers Arch. 2001;442(4):511–8. doi: 10.1007/s004240100565. [DOI] [PubMed] [Google Scholar]
- 127.Node K, Ruan XL, Dai J, Yang SX, Graham L, Zeldin DC, et al. Activation of Galpha s mediates induction of tissue-type plasminogen activator gene transcription by epoxyeicosatrienoic acids. J Biol Chem. 2001;276(19):15983–9. doi: 10.1074/jbc.M100439200. [DOI] [PubMed] [Google Scholar]
- 128.Enayetallah AE, French RA, Grant DF. Distribution of soluble epoxide hydrolase, cytochrome P450 2C8, 2C9 and 2J2 in human malignant neoplasms. J Mol Histol. 2006;37(3–4):133–41. doi: 10.1007/s10735-006-9050-9. [DOI] [PubMed] [Google Scholar]
- 129.Jiang J, Chen C, Card JW, Yang S, Chen J, Fu X, et al. Cytochrome P450 2J2 promotes the neoplastic phenotype of carcinoma cells and is up-regulated in human tumors. Cancer Res. 2005;65(11):4707–15. doi: 10.1158/0008-5472.CAN-04-4173. [DOI] [PubMed] [Google Scholar]
- 130.Zagorac D, Jakovcevic D, Gebremedhin D, Harder DR. Antiangiogenic effect of inhibitors of cytochrome P450 on rats with glioblastoma multiforme. J Cereb Blood Flow Metab. 2008;28(8):1431–9. doi: 10.1038/jcbfm.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285(5431):1276–9. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schmelzer KR, Inceoglu B, Kubala L, Kim I, Jinks SL, Eiserich JP, et al. Enhancement of antinociception by coadministration of nonsteroidal anti-inflammatory drugs and soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci U S A. 2006;103(37):13646–51. doi: 10.1073/pnas.0605908103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kozak W, Archuleta I, Mayfield KP, Kozak A, Rudolph K, Kluger MJ. Inhibitors of alternative pathways of arachidonate metabolism differentially affect fever in mice. Am J Physiol. 1998;275(4 Pt 2):R1031–40. doi: 10.1152/ajpregu.1998.275.4.R1031. [DOI] [PubMed] [Google Scholar]
- 134.Nakashima T, Harada Y, Miyata S, Kiyohara T. Inhibitors of cytochrome P-450 augment fever induced by interleukin-1 beta. Am J Physiol. 1996;271(5 Pt 2):R1274–9. doi: 10.1152/ajpregu.1996.271.5.R1274. [DOI] [PubMed] [Google Scholar]
- 135.Inceoglu B, Jinks SL, Schmelzer KR, Waite T, Kim IH, Hammock BD. Inhibition of soluble epoxide hydrolase reduces LPS-induced thermal hyperalgesia and mechanical allodynia in a rat model of inflammatory pain. Life Sci. 2006;79(24):2311–9. doi: 10.1016/j.lfs.2006.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Inceoglu B, Schmelzer KR, Morisseau C, Jinks SL, Hammock BD. Soluble epoxide hydrolase inhibition reveals novel biological functions of epoxyeicosatrienoic acids (EETs) Prostaglandins Other Lipid Mediat. 2007;82(1–4):42–9. doi: 10.1016/j.prostaglandins.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Terashvili M, Tseng LF, Wu H, Narayanan J, Hart LM, Falck JR, et al. Antinociception produced by 14,15-epoxyeicosatrienoic acid is mediated by the activation of beta-endorphin and met-enkephalin in the rat ventrolateral periaqueductal gray. J Pharmacol Exp Ther. 2008;326(2):614–22. doi: 10.1124/jpet.108.136739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Qu W, Rippe RA, Ma J, Scarborough P, Biagini C, Fiedorek FT, et al. Nutritional status modulates rat liver cytochrome P450 arachidonic acid metabolism. Mol Pharmacol. 1998;54(3):504–13. doi: 10.1124/mol.54.3.504. [DOI] [PubMed] [Google Scholar]
- 139.Imaoka S, Hashizume T, Funae Y. Localization of rat cytochrome P450 in various tissues and comparison of arachidonic acid metabolism by rat P450 with that by human P450 orthologs. Drug Metab Pharmacokinet. 2005;20(6):478–84. doi: 10.2133/dmpk.20.478. [DOI] [PubMed] [Google Scholar]
- 140.Wu S, Chen W, Murphy E, Gabel S, Tomer KB, Foley J, et al. Molecular cloning, expression, and functional significance of a cytochrome P450 highly expressed in rat heart myocytes. J Biol Chem. 1997;272(19):12551–9. doi: 10.1074/jbc.272.19.12551. [DOI] [PubMed] [Google Scholar]
- 141.Shin J, Engidawork E, Delabar J, Lubec G. Identification and characterisation of soluble epoxide hydrolase in mouse brain by a robust protein biochemical method. Amino Acids. 2005;28(1):63–9. doi: 10.1007/s00726-004-0145-x. [DOI] [PubMed] [Google Scholar]
- 142.Yasar U, Bennet AM, Eliasson E, Lundgren S, Wiman B, De Faire U, et al. Allelic variants of cytochromes P450 2C modify the risk for acute myocardial infarction. Pharmacogenetics. 2003;13(12):715–20. doi: 10.1097/00008571-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 143.Funk M, Freitag R, Endler G, Lalouschek W, Lang W, Mannhalter C, et al. Influence of cytochrome P450 2C9*2 and 2C9*3 variants on the risk of ischemic stroke: a cross-sectional case-control study. Clin Chem. 2005;51(9):1716–8. doi: 10.1373/clinchem.2005.050807. [DOI] [PubMed] [Google Scholar]
- 144.Ercan B, Ayaz L, Cicek D, Tamer L. Role of CYP2C9 and CYP2C19 polymorphisms in patients with atherosclerosis. Cell Biochem Funct. 2008;26(3):309–13. doi: 10.1002/cbf.1437. [DOI] [PubMed] [Google Scholar]
- 145.Funk M, Endler G, Freitag R, Wojta J, Huber K, Mannhalter C, et al. CYP2C9*2 and CYP2C9*3 alleles confer a lower risk for myocardial infarction. Clin Chem. 2004;50(12):2395–8. doi: 10.1373/clinchem.2004.038034. [DOI] [PubMed] [Google Scholar]
- 146.Visser LE, van Schaik RHN, Jan Danser AH, Hofman A, Witteman JCM, van Duijn CM, et al. The risk of myocardial infarction in patients with reduced activity of cytochrome P450 2C9. Pharmacogenet Genomics. 2007;17(7):473–9. doi: 10.1097/01.fpc.0000236335.57046.c8. [DOI] [PubMed] [Google Scholar]
- 147.Yu B, Luo C, Wang D, Wang A, Li Z, Zhang W, et al. CYP2C9 allele variants in Chinese hypertension patients and healthy controls. Clin Chim Acta. 2004;348(1–2):57–61. doi: 10.1016/j.cccn.2004.04.028. [DOI] [PubMed] [Google Scholar]