Abstract
Importance of the field
Vascular delivery of several classes of therapeutic agents may benefit from carriage by red blood cells (RBC), for example, drugs that require delivery into phagocytic cells and those that must act within the vascular lumen. The fact that several protocols of infusion of RBC-encapsulated drugs are been currently explored in patients illustrates a high biomedical importance for the field.
Areas covered by this review
Two strategies for RBC drug delivery are discussed: encapsulation into isolated RBC ex vivo followed by infusion in compatible recipients and coupling therapeutics to surface of RBC. Studies of pharmacokinetics and effects in animal models and in human studies of diverse therapeutic enzymes, antibiotics and other drugs encapsulated in RBC are described and critically analyzed. Coupling to RBC surface of compounds regulating immune response and complement, affinity ligands, polyethylene glycol alleviating immune response to donor RBC and fibrinolytic plasminogen activators is d escribed. Also described is a novel, translation-prone approach for RBC drug delivery by injecting of therapeutics conjugated with fragments of antibodies providing safe anchoring of cargoes to circulating RBC, without need for ex vivo modification and infusion of RBC.
What the reader will gain
The readers will gain historical perspective, current status, challenges and perspectives of medical applications of RBC for drug delivery.
Take home message
RBC represent naturally designed carriers for intravascular drug delivery, characterized by unique longevity in the bloodstream, biocompatibility and safe physiological mechanisms for metabolism. Novel approaches for encapsulating drugs into RBC and coupling to RBC surface provide promising avenues for safe and widely useful improvement of drug delivery in the vascular system.
Keywords: carrier, drug delivery, intravascular, red blood cells
1. Introduction: red blood cells as carriers for drug delivery
The optimization of vascular delivery of drugs is an important biomedical problem. This problem is especially acute in case of delivery of potent and specific, yet labile and complex biotherapeutic agents including enzymes, which in most cases require precise localization in the target site. One means to achieve this goal is coupling drugs to carriers, such as synthetic or natural polymer structures of diverse geometries, phospholipid liposomes, albumin, antibodies or other biological molecules1. Use of drug carriers promises to enhance the specificity, effectiveness and safety of therapeutic, diagnostic or prophylactic interventions. Functions of drug carriers include: i) providing the optimal drug half-life in circulation and clearance mechanism; ii) restriction of unintended drug uptake by and effects in non-target tissues; iii) targeting to the intended therapeutic site; and, iv) providing optimal timing of the action, i.e., its timely initiation and termination.
Among other carriers, erythrocytes (red blood cells, RBC, non-nuclear biconcave discs with a diameter of ~7 µm, thickness ~2 µm and plasma membrane surface area ~160 µm2) represent a potentially attractive and, in some aspects, unique carrier for drug delivery (Table 1). One microliter of human blood contains about 5 million RBC and total number of RBC, the most abundant cellular constituent of the blood (>99%), in the human body approaches 30 trillion. Human erythrocytes normally have a life span of 100–120 days (of note, mouse RBC life-time is about a third of human’s counterpart), travel ~250 km through the cardiovascular system and function as natural carriers for oxygen. RBC life-span and size markedly exceed those of other drug delivery systems (e.g., <10 h and <100 nm for PEG-modified “stealth” liposomes). Thus, RBC are attractive carriers for intravascular delivery, e.g., for prolonging drug circulation and restricting unintended extravasation2–4.
Table 1. Comparison of erythrocytes with other drug delivery systems.
Note that half-life in circulation is shown for mice for the sake of consistency of the comparison (data for humans are not available for many drug delivery systems), whereas in humans RBC half-life is close to 60 days. Highly asymmetrical filomicelles have diameter of 40 nm and length ranging 1–30 micron. Size, shape and half-life in circulation vary immensely for proteins and protein conjugates. For example, protein molecules such as fibrinolytic plasminogen activators are small (<5 nm) and have very short half-life in the bloodstream (5–20 minutes), whereas immunoglobulins are slightly larger (10 nm) and circulate for a long time (half-life few hours in mice and up to few days in humans). There is no direct relationships between protein size and circulation time, e.g., albumin, a molecule of about the same size as plasminogen activators, circulates for much longer time (half-life of few hours). Protein conjugates can vary in size from 2–3 nm (fusion proteins) to microns (multi-molecular protein complexes and polyplexes). Proteins and protein conjugates are the least restricted to the bloodstream, which they can leave (i.e., extravasate) via diverse pathways including diffusion between endothelial cells and endocytosis via endothelium. Submicron liposomes and other carriers can extravasate in pathological tissues due to Enhanced Permeation and Retention (EPR) effect, and undergo endocytosis in endothelial cells. RBC carriers are the most restricted to the bloodstream; normally they can extravasate only in opening of reticuloendothelial system (e.g., hepatic sinuses and spleenic follicles).
| Size, nm | Shape | Half-life in blood | Diffusion in tissues | |
|---|---|---|---|---|
| RBC | 5,000–7,000 | Biconcave disc | 10–15 days | RES openings only |
| PEG-Liposomes | 50–500 | Spheres | 3–6 hours | Tumors (EPR), endocytosis |
| Polymersomes | 50–500 | Spheres | 10–20 hours | Tumors (EPR), endocytosis |
| Filomicelles | 40×20,000 | Filaments | 1–3 days | Unknown, possibly EPR |
| Polymer micelles | 20–300 | Spheres | 0.1–6 hours | Tumors (EPR), endocytosis |
| Proteins and conjugates | 5–5,000 | Irregular spheres | 10 min – 6 hours | Diffusion & endocytosis |
Injected substances interact with RBC, which partition and metabolize more than 50 known drugs (e.g., captopril, sulfanilamide, testosterone, insulin, catecholamines, tacrolimus, antibiotics and other agents)5. Therefore, RBC may unintentionally serve as a natural blood compartment participating in biodistribution, pharmacokinetics, slow release, metabolism and action of diverse drugs including anti-tumor agents6 and genetic materials7. However, the intentional use of RBC as drug carriers is arguably of even higher interest. This area of research has been incepted in early seventies, when it has been proposed that effects of certain drugs might benefit from encapsulation into autologous or immunologically compatible donor RBC that can be safely injected in the host, thereby improving circulation of a protected drug and its delivery to certain targets in the body8. A prolonged life-time in the circulation, availability, considerable surface and volume (mean corpuscular volume of human and mouse RBC is ~90 and ~50 µm3, respectively), high biocompatibility and natural mechanisms for safe elimination of RBC represent attractive features of this tentative drug carrier. Erythrocytes are champions among other drug delivery systems from the standpoint of longevity of circulation, biocompatibility and restriction of unintended permeation of drugs into extravascular compartments. Hydrodynamic forces driving RBC to the blood mainstream in the circulation and endothelial glycocalyx minimize RBC interaction with vascular walls9. Normally, RBC do not undergo extravasation from the circulation into tissues except hepatic sinuses and interstitium in the spleenic follicles, i.e., open to circulation sites of RBC genesis and elimination, part of reticuloendothelial system (RES). RES macrophages in the spleen and liver rapidly and effectively take up senescent, damaged and modified RBC via phagocytosis leading to their lysosomal degradation.
RBC transfer many substances in the bloodstream. Oxygen-carrying hemoglobin is the most important cargo among other molecules naturally encapsulated in the interior volume provided by erythrocyte plasma membrane. RBC membrane is supported from within by a complex cytoskeleton comprising of hexagonal lattice of actin-spectrin network interconnected with anchoring integral plasmalemmal proteins via numerous structural and connector proteins. Dynamic regulation and remodeling of this cytoskeleton maintained via activity of small GTP-ases asserts morphological stability, plasticity and deformability of RBC necessary for millions repetitive travels through tight capillaries10. As in other cell types, RBC plasma membrane represents an asymmetrical phospholipid-cholesterol composed bilayer, i.e., phosphatidylserine is enriched in the inner leaflet, whereas the outer surface is slightly negatively charged mostly due to anionic components of the glycocalyx extended from integral and surface glycoproteins associated with RBC plasmalemma, playing role of transporting (i.e., ion exchangers and channels), structural and protective elements. Glycophorin A and Band 3 represent two major integral glycoproteins among more than 300 proteins found in RBC plasma membrane11. Some of these proteins are expressed at relatively low and variable levels throughout human population, yet exert key functions of protecting RBC from damage and elimination from the bloodstream by immune system (see below).
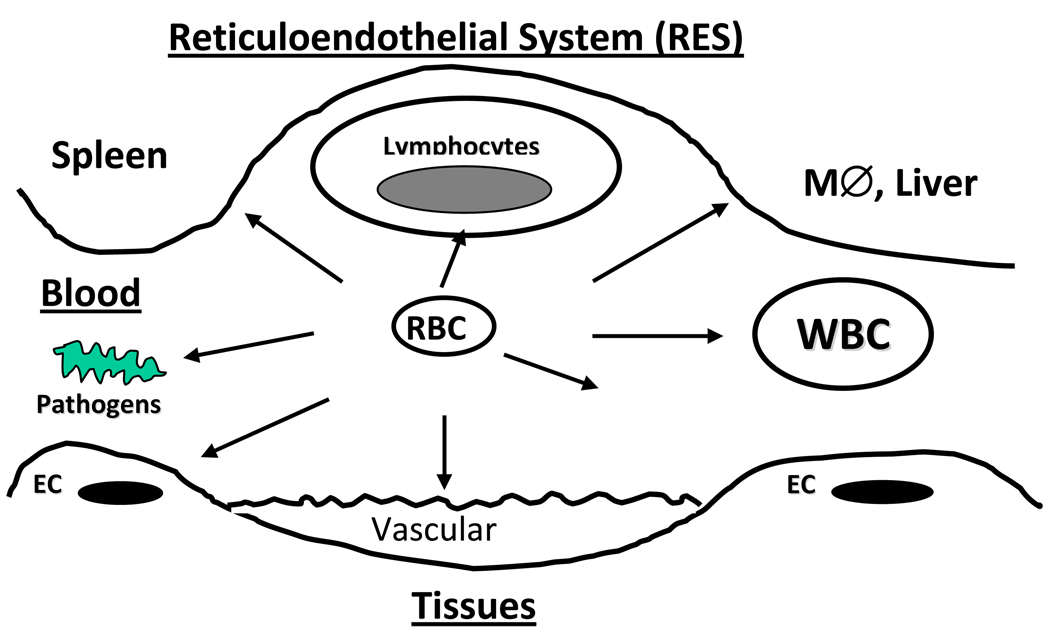
Due to these unique natural transporting features, RBC may provide optimal type of a carrier for drugs that are either needed to be delivered into the RBC-eliminating cells such as RES macrophages, or intended to work in the bloodstream (Figure 1). In the last three decades, significant efforts have been invested in order to prove the validity of this paradigm and establish clinically applicable strategies for RBC carriage of drugs. RBC are been tested for drug delivery in numerous animal4,12–14 and human studies14. Certain challenges and limitations of using RBC as drug carriers have been identified in these studies. Thus, use of RBC modified ex vivo (e.g., using donor or autologous blood) limits treatment options to hemotransfusion settings, technically challenging for a widespread use. Further, unintentional reduction of biocompatibility of modified RBC represents a serious potential problem. However, results of recent animal studies devised approaches to solve or circumvent these problems and boost confidence that RBC-based drug delivery systems can find medical utility relatively soon. Indeed, several RBC-drug delivery approaches are currently undergoing clinical testing and industrial development. This article will review two strategies for drug delivery by carrier RBC: loading into RBC and coupling to RBC surface.
Figure 1. Examples of therapeutic sites and targets accessible for intravascular drug delivery using RBC carriers.
Large, long-circulating RBC have an access and can deliver their cargo to targets in blood, including other blood cells (lymphocytes, white blood cells WBC), and pathogenic agents such as bacteria and toxins, to sites of vascular injury and endothelial cells lining vascular lumen (EC), and to diverse components of reticuloendothelial system (RES), such as macrophages in liver and spleen.
2. Vascular delivery of drugs encapsulated into carrier RBC
From a grossly oversimplified practical viewpoint, RBC resembles a durable sac-voyage made of elastic material. This might help explain why exploration of RBC transporting capacities in drug delivery field started with drug encapsulation inside RBC ghosts. Since early seventies, this area of research has received a substantial attention and produced first clinically tested RBC-based drug delivery systems. More than two hundred papers, monograph and proceeding chapters have been published on diverse aspects of drug encapsulation into carrier RBC; readers are addressed to the previous inclusive reviews for more complete literature references as well as more specific description of methods for encapsulation of drugs into carrier RBC15–17. Several excellent reviews outlining the ideology, methodology and outcomes of animal and human studies have been published on this subject including a recent article in this journal by one of the leading expert, Mauro Magnani16–20. It would be impossible to cover in sufficient depth all aspects of this strategy in a reasonably concise article; hence we provide rather a cursory overview of the field with focus on the most recent results and clinical studies.
2.1. Drug loading into carrier RBC and elimination of modified RBC
Originally, RBC carriage has been proposed to improve delivery of cargoes into target cells8,21. Methods of electrical insertion and hypotonic RBC loading followed by resealing provided encapsulation of diverse agents including antibiotics, steroids, anti-microbial agents, proteins and genetic materials into RBC with loading efficacy ranging from reasonable to fair and to excellent (10% to 30% and to 70%, respectively) of drugs retaining their functional activity22–24. In vitro studies showed that encapsulated agents can be released from RBC either slowly (e.g., via diffusion through RBC plasma membrane25 and/or its eventual degradation)13,26, or rapidly (e.g., via lysis of carrier RBC by plasma complement)27,28. Early studies also showed that RBC carriers facilitated intracellular delivery of encapsulated agents including proteins and DNA into cells in culture21.
Examples of pharmacological and imaging agents encapsulated into carrier RBC include diverse enzymes29,30, fluorescent labels30, erythropoietin for sustained stimulation of hemopoietic potential31, hemoglobin cofactor inositol hexaphosphate for enhancing RBC oxygen carrying capacity32,33, anti-thrombotic drugs heparin34 and thrombolytics35, amikacin for anti-parasitic therapy36,37, insulin38, fluoro-AMP25 and methotrexate for cancer chemotherapy35, isotopes for imaging of blood pool using gamma-scintigraphy and MRI39–42, antisense oligonucleotides for gene knock-outs43 and anti-viral interventions44, plasmid DNA for gene therapy45, and, more recently, magnetic nanoparticles46.
Organ distribution, pharmacokinetics and effects of some of these drug delivery systems have been tested in studies in lab animal species32,43 and, in a more fragmentary fashion, in human patients42,47–49. Some of these studies provided rather mixed results, perhaps, due to unintended damage inflicted to carrier RBC during drug loading that compromised its biocompatibility, or variability and heterogeneity of RBC loading (noted even within the same batch in the early studies)29,30. For example, RBC-encapsulated and free erythropoietin showed similar pharmacological properties in mice and its activity in plasma was essentially gone within less than a day after injection despite the fact that tracing of 51Cr-label showed relatively prolonged circulation of drug-loaded RBC, with half-life in blood ~6 days vs 7 days for intact RBC50. However, intravenous injection in mice of antisense oligonucleotides encapsulated into opsonized RBC enhanced hepatic delivery of the cargo43, whereas injection of RBC-encapsulated plasmid DNA resulted in a relatively prolonged transfection of the transgene in phagocytic cells in the RES and blood45. A relatively stable MRI signal has been detected in the blood samples taken from mice during a week post intraperitoneal injection of RBC-encapsulated magnetic nanoparticles46.
RES macrophages and other professional phagocytes eliminating senescent and damaged RBC represent natural target for drugs encapsulated in RBC. In fact, it is rather difficult to avoid uptake of carrier RBC by phagocytes and divert delivery to other targets of interest. Drug loading into RBC inevitably causes some extent of damage to its membrane and, in some cases, internal content (e.g., depletion of RBC systems for storage and utilization of energy51 and nitric oxide transport and metabolism52). In order to avoid these complications, major efforts have been dedicated to studies of mechanisms of RBC biocompatibility, its damage upon RBC modification and means for its preservation for drug delivery applications53–58.
Conformational changes and abnormal clustering of membrane glycoproteins including major components glycophorin A and band 3 caused by cross-linking agents and RBC membrane damage by osmotic stress during drug loading53 lead to cytoskeletal dysfunctions (loss of RBC plasticity and mechanical stability55), sensitize RBC to lysis caused by oxidants59 and provoke fixation of immunoglobulins that naturally present in plasma60. Exposure of phosphatidylserine from the inner leaflet of the RBC plasma membrane61 and other components normally absent on the RBC surface augments these processes and predisposes RBC to adhesion to endothelium62. Unintentional inactivation of specific RBC surface glycoproteins inhibiting complement (for example, Decay Acceleration Factor, DAF, and CD59)56 and masking RBC from recognition by phagocytes (for example, CD4763 and SHPS-164), further compromises RBC biocompatibility.
These adverse effects reduce RBC plasticity and resistance to osmotic and mechanical damage, lead to RBC opsonization by immunoglobulins and complement promoting phagocytosis (opsonization means “coating by compounds appetizing phagocytes”) and directly destroying RBC, which collectively may lead to RBC lysis, aggregation, immune reactions, cellular uptake, adhesion to vascular endothelium and rapid elimination via phagocytosis and entrapment in the microvasculature30,65–67. These adverse effects compromise drug delivery and may cause serious damage typical of intravascular hemolysis, including acute vascular, renal and immune reactions to free hemoglobin, hypoxia and toxic effects of RBC stroma towards RES macrophages68. On the other hand, rapid phagocytosis of altered RBC offers a direct and effective way for intracellular delivery, on a condition that RBC has not been destroyed by complement en route in the circulation.
Major technological advances have been made about a decade ago, i.e., design of devices allowing semi-automatic high-throughput blood filtration and hypotonic dialysis-based encapsulation into human RBC of drugs including steroids dexamethasone (DEX) and prednisolone, as well as oligonucleotides and peptides, producing amounts of loaded RBC sufficient for use in human patients69,70. Based on these developments, several companies embarked on industrial development of scaled-up production of drug-loaded human RBC and currently pursue clinical studies. Below we briefly overview few examples of delivery of specific types of drugs encapsulated in carrier RBC in animal and human studies. Most of these strategies pursue drug delivery to phagocytic cells in blood and RES (first of all hepatic and spleenic macrophages) or prolonged circulation of RBC-encapsulated enzymes detoxifying various toxic substances in the bloodstream (Figure 1).
2.2. RBC-mediated drug delivery to macrophages and other cell types
As expected, modifications that altered RBC surface antigens and membrane plasticity (e.g., treatment with cross-linking agents) resulted in their rapid phagocytosis by macrophages in the RES71,72, providing a mechanism to deliver encapsulated drugs into lysosomes in these and other cell types with active internalization processes including tumor cells26,73 (see section below). Pilot studies showed that microparticles made from RBC ghosts facilitate delivery of cytotoxic agents to malignant cells74.
2.2.1. Enzyme replacement therapies for lysosomal storage diseases
Genetic deficiency of lysosomal acidic hydrolytic enzymes results in pathological accumulation of their substrates in the cellular vesicles, leading to lysosomal storage disorders, LSD, manifested by inflammation, neurological, vascular, hepatic and pulmonary disorders75. Macrophages and other cells with active lysosomal metabolism represent the prime therapeutic target in LSD management. Pending development of effective and safe gene therapy, the only viable and clinically used option for therapeutic management of LSD is periodical infusion of deficient recombinant enzymes76, i.e., enzyme replacement therapy, ERT77. However, inactivation en route, poor pharmacokinetics and uptake by the target cells compromise this approach: only a minute fraction of these protein drugs get into the lysosomes; hence an acute need in improving delivery using drug carriers78.
Effective uptake and lysosomal addressing of drug-loaded RBC destined for fast phagocytosis due to membrane modification offers a natural mechanism for ERT delivery4,22. In fact, initial studies of RBC as carriers for drug delivery were focused on RBC loading with enzymes for ERT. Hypotonic exchange (i.e., reversible mild poring of RBC membrane in an enzyme suspension) was the most widely accepted and effective among methods for encapsulation in RBC enzymes used as ERT for treatment of Gaucher’s disease including galactosidase8, glucuronidase79 and glucocerebrosidase4,22. Efficacy of enzyme loading was reversely proportional to the molecular weight, yet even 180kD proteins have been encapsulated in RBC using hypotonic exchange8. RBC-encapsulated enzymes showed stable activity22 and delivered enzyme cargoes into cells in vitro4. Animal studies revealed relatively more effective lysosomal delivery of RBC-encapsulated vs. free enzymes, despite (or, perhaps, rather in agreement with) very limited circulating capacity of the former formulation79. Limited clinical studies in late seventies showed no overt toxicity of RBC-encapsulated glucocerebosidase in a patient with advanced adult type of Gaucher’s disease, yet therapeutic effect was rather modest and transient80. More recently, RBC-loaded enzyme replacement therapy using thymidine phosphorylase has been tested in a patient with a genetic mitochondrial deficiency of this enzyme: treatment improved biochemical readouts, but, unfortunately, the patient’s clinical condition did not improve81.
2.2.2. Anti-infectious drugs, antigens and toxic agents
Phagocytosis of RBC intentionally modified in the drug loading process to be taken up by macrophages in the RES serves as a natural delivery path for agents that help to eliminate invaders residing in these cells73,82. For example, anti-viral drug azidothymidine (AZT) was loaded in carrier RBC83 that delivered cargo to macrophages and provided enhanced anti-viral potency vs free drug in cell culture26,84 and in a mouse model of HIV infection85. Encouraging results have been obtained in cells with RBC-mediated delivery of other anti-viral agents86–90 including antisense agents inhibiting HIV-1 replication in infected cells91. These studies provide a basis for a wide-range antiviral strategy87,92,93 posed for systematic testing in animal models and human studies. It has to be noted, however, that loading of some anti-microbial drugs into RBC (i.e., anti-malarial agent clotrimazole) predisposes RBC to oxidative damage59, and oxidized RBC are rapidly taken up by hepatic RES macrophages (i.e., Kupffer cells) via scavenger receptor-mediated phagocytosis94.
Antigen-presenting function of macrophages and other immune cells eliminating altered RBC also offers an attractive natural mechanism for delivery of antigens to boost immune response95. In addition to optimized delivery of antigens, carrier RBC plays a role of the adjuvant, by presenting multiple copies of antigens and stimulating non-specific immune response96. Further, encapsulation of cytokines including interleukins and interferon into RBC facilitates delivery of these agents to macrophages97, resulting in stimulation of immune response in animal models98.
Alternatively, RBC encapsulated with toxic agents, such as ricin toxin kill target cells, first of all, phagocytes99. Injection of a toxin-loaded RBC leads to macrophage depletion100 providing anti-inflammatory effect and alleviation of acute graft injury after transplantation101. In theory, delivery of toxic agents by RBC to immune cells may be used for anti-inflammatory interventions, as well as for induction of antigen tolerance.
2.2.3. Anti-inflammatory agents
Delivery of RBC-loaded anti-inflammatory drugs such as glucocorticoids including dexamethasone (DEX) to pro-inflammatory cells such as macrophages represents further extension of the previous approach. It promises to optimize bioavailability of these poorly soluble drugs and selectivity of their delivery to active phagocytes in the RES and other components of immune system. Initial studies in vitro developed loading of DEX derivatives (e.g., more soluble DEX-phosphate) into RBC; since this procedure predisposes RBC to opsonization by complement facilitating phagocytosis, RBC-loaded DEX was taken up by cultured macrophages and inhibited their pro-inflammatory response to agonists70.
In a recent series of studies by Magnani’s team, this approach is currently being tested in human patients with several disease conditions associated with and aggravated by inflammation. For example, re-infusion of RBC-loaded DEX in ten patients suffering of chronic obstructive pulmonary disease, COPD, has been apparently well tolerated and resulted in a sustained elevation of blood DEX level after a single injection14. In other lung disease with a major inflammatory component, cystic fibrosis, re-infusions in the patients of autologous RBC-loaded DEX at monthly intervals provided sustained low-level of DEX for 28 days in the bloodstream, were well tolerated and provided significant reduction of inflammatory reactions102. Similar approach has been tested in the patients with inflammatory bowel disease (IBD, such as Crohn disease) and in this study three repetitive re-infusions of autologous RBC loaded with DEX (every four weeks) also provided a prolonged elevation of DEX blood level, permitting the patients to withdraw previously prescribed poorly tolerated steroids and still achieve remission that has been maintained for several months103. Similar encouraging results have been observed in a study involving 18 pediatric patients treated with monthly infusions of autologous RBC-loaded DEX for two years104. Finally, a very recent randomized controlled study of this treatment in forty IBD patients refractory to conventional steroids showed that by 8 weeks of treatment 75% of twenty patients that received RBC-loaded DEX were in clinical remission (vs 80% in the group that received prednisolone and 10% in the sham group, respectively), but no adverse effects have been detected in RBC-DEX treated group, in contrast with 80% patients showing adverse effects of prednisolone105. Certainly, number of the patients enrolled in these studies (10–15) was very small on the scale of regular clinical trails. However, these encouraging pilot results imply that RBC-mediated delivery of anti-inflammatory agents may find utility in treatment of acute and chronic inflammation.
2.2.4. Anti-cancer agents
Loading anti-cancer drugs into carriers restricts their toxicity to the body and improves their delivery to tumors relies via several mechanisms, both specific (e.g., antibody targeting) and less specific (e.g., Enhanced Permeation and Retention effect, EPR, typical of solid tumors). Liposomes, linear polymers and polymer micelles represent the most popular carriers for anti-cancer drugs. However, RBC carriers may find a niche in tumor treatment, for example, by providing formulations with prolonged circulation. In support of this notion, loading a hydrophobic anti-tumor agent dequalinium into mouse RBC provided much longer half-life in circulation than PEG-liposomal formulation (5–6 days vs 4 hours)106.
Anti-cancer drugs such as antibiotic doxorubicin have been encapsulated into carrier RBC using diverse loading schemas including glutaraldehyde cross-linking of RBC membrane13,107. This modification greatly enhances RBC uptake by macrophages and other cells exerting active phagocytosis71. Accordingly, doxorubicin-loaded RBC delivered the cargo into macrophages in culture108 and accumulated in the liver (predominantly, in macrophages) after intravenous injection in animals including dog109.
This intervention provided encouraging efficacy in treatment of lymphoid tumor in dogs without marked cardiac toxicity (a hallmark adverse effect of doxorubicin), yet inflicted unexpected substantial chronic suppression of myeloid cells110. Nevertheless, a formulation of human autologous RBC encapsulated with a related anthracycline antibiotic daunorubicin prepared using methodology avoiding RBC membrane cross-linking has been injected in patients with acute leukemia and showed a more prolonged drug level in plasma and lesser side effects than after injection of free drug111. Similarly, doxorubicin-loaded autologous RBC re-infused in patients with lymphomas, provided reduction of peak level and extension of drug level in plasma of patients resulting in significant elevation of the area under the curve and reduction of side effects comparing with free drug112.
Delivery of drug carriers to solid tumors relies in major part on the EPR effect mediated by abnormally high permeability of tumor vasculature and lack of effective lymphatic drainage. In the context of vascular permeability and tumor extravasation via EPR effect, large RBC represent less effective delivery platform than sub-micron carriers such as liposomes. In order to address this challenge, anti-tumor drugs (daunorubicin) have been conjugated with small vesicles (average diameter 100 nm) formed from RBC plasma membranes74. Soon after intravenous administration (<30 min) these RBC-based nano-vesicles are eliminated from the circulation mainly by the RES in liver and spleen113. However, injection of this formulation produced more potent anti-tumor effects than free drug in mouse models114, most likely due to slower drug release in the vicinity of tumor cells115, since these vesicles apparently do not enter target cells116.
2.2.5. Genetic materials
Finally, RBC ghosts have long been studied as means for intracellular delivery of genetic materials. Most efforts in this area have been focused on cytoplasmic delivery of small oligonucleotides for interference in protein synthesis. Early works demonstrated proof of principle in cell cultures and provided initial comparison of this and liposomal antisense delivery systems44. More recent studies confirmed intracellular delivery of oligonucleotides70 and other means of genetic interference, such as peptide nucleic acid inactivating viral RNA replication in culture of human macrophages infected with HIV91. Several recent studies took this direction into animal studies. Thus, injection of RBC-encapsulated DNA plasmid led to a prolonged (up to 3 days post injection) expression of a transgene in blood mononuclear leukocytes45. Of note, no transgene expression has been reported at this time in organs including hepatic Kupffer cells, which in most animal studies take the lion share of injected materials. The nature of targeting to blood leukocytes vs. RES macrophages indirectly inferred by this outcome remains enigmatic. In contrast, very recent study showed that antisense-loaded RBC getting opsonized in plasma deliver their cargo to the liver, consistently with known destination of RBC to this organ43.
2.3. Loading of detoxifying enzymes in RBC
Delivery of RBC-encapsulated drugs discussed above is based on uptake of modified RBC by macrophages, immune and tumor cells. In contrast, in theory, minimally altered RBC loaded with drugs degrading diffusible toxic compounds could circulate for a prolonged time avoiding rapid uptake by RES, thereby providing sustained antidotes to toxins.
Design of non-traumatic loading schemas and use of additional precautions (e.g., elimination of sub-population of senescent RBC from the mixture) yielded formulations of drug-loaded resealed RBC showing biocompatibility and pharmacokinetics similar to those of unloaded RBC in animal studies50,66. This provided marked prolongation of half-life in the bloodstream of RBC-encapsulated agents vs. free counterpart injected via the same route, including alcohol oxidase117, erythropoietin50, carbonic anhydrase30 and other agents30,46. Isotope-loaded RBC have been used to visualize blood pool imaging by gamma-scintigraphy and MRI41.
Loading capacity of RBC is limited; hence, highly potent and specific enzymes represent more effective cargo for this approach than non-enzymatic antidotes such as glutathione118. More than ten different detoxifying enzymes have been encapsulated into carrier RBC to test this hypothesis, including urikase to eliminate uric acid119, thiosulfate-cyanide sulfutransferase (AKA rhodanese) converting cyanides into less toxic thiocyanates120–122, phosphothioesterase to antagonize organo-phosphorus compounds including toxin paraoxon123–125, alcohol oxidase and alcohol dehydrogenase for elimination of methanol and other toxic alcohols117,126,127, L-asparaginase48,49,128, adenosine aminase and other enzymes (Table 2). Derivatives of some of these enzymes modified with polyethylene glycol (PEG-enzymes, partially masked from immune system) have also been encapsulated in mouse, human and sheep RBC129–131.
Table 2. Examples of therapeutic enzymes encapsulated in carrier RBC.
| Enzyme and ref | Size | Function | Condition to treat | Stage of development |
|---|---|---|---|---|
| Galactosidase8 | 464 kDa (tetramer) |
Degrades galactosides (sugars, lipids, proteins) |
Lysosomal storage diseases (LSD) |
Studies in primates |
| Glucuronidase79 | 80 kDa | Degrades heparin & mucopolysaccharides |
LSD (Gaucher’s disease) | Studies in rodents |
| Glucocerebrosidase4,22 | 60 kDa | Degrades beta- glycosides |
LSD (Gaucher’s disease) | Testing in humans |
| Thymidine phosphorylase81 |
100 kDa | Converts thymidine and phosphate into thymine and 2-deoxy-alpha-D- ribose 1-phosphate |
Genetic mitochondrial deficiency |
Testing in humans |
| Carbonic anhydrase30 | 30 kDa | Converts CO2 into bicarbonate |
Detoxification of CO2 | Studies in vitro |
| Uricase119 | 33 kDa | Oxidizes uric acid | Uric acid detoxification | Studies in mice |
| Thiosulfate-cyanide sulfurtransferase120–122 |
37 kDa | Converts cyanides into thiocyanate |
Cyanide detoxification | Studies in animals |
| Phosphothioesterase123–125 | 15–35 kDa | Thioester hydrolysis | Detoxification of organo- phosphorus compounds |
Studies in mice |
| Alcohol oxidase117,126,127 | 140–600 kDa |
Converts alcohols into aldehydes |
Detoxification of alcohols | Studies in mice |
| Alcohol dehydrogenase | 80 kD | Converts alcohols into ketones and aldehydes |
Detoxification of alcohols | Studies in mice |
| L-asparaginase48,49,128 | 80kD | Hydrolyses asparagine into aspartic acid |
Eradication of asparagine- dependent tumors |
Studies in mice137, monkeys128 and humans48 |
| Glutamine synthase140 | Variable | Forms glutamine from glutamate and ammonia |
Ammonia detoxification | Animal studies |
| Adenosine deaminase143 | 47 kD | Degrades adenosine | Elimination deoxiadenosine that inhibits immune cells in patients with reduced ADA |
Human studies144 |
In most of these studies, encapsulated enzymes retained activity and degraded their substrates in vitro. Studies of circulation, biodistribution and in vivo stability of enzyme-loaded RBC were rather fragmentary and, in many cases, revealed reduction of circulation time comparing with normal RBC132. Nevertheless, even this suboptimal approach afforded more prolonged blood level, as well as more potent and sustained detoxifying effects comparing with a free enzymes including rhodanese132 and alcohol oxidase117. Infusion of RBC-encapsulated phosphotriesterase protected mice against lethal dose of a toxin paraoxon125. In some cases, encapsulation of enzymes with cofactor molecules facilitated substrate influx and enzymatic conversion, e.g., kinetics of RBC-encapsulated rhodanese has been improved by co-loading of organic thiosulfonates122,133. Such binary detoxification system provided significant reduction of cyanide level132 and protected against lethal dose of cyanide in mice134, showing promising efficacy in a mouse model of cyanide poisoning135. Injection of homologous RBC loaded with PEG-urease/PEG-Aldehyde dehydrogenase in sheep provided more prolonged elevation of the enzyme activities in blood than injection of free PEG-modified enzymes (6 vs 2 days, respectively)136.
Several formulations of RBC-encapsulated detoxifying enzymes have been tested in primates and human patients. In an early study, RBC-encapsulated asparaginase showed more prolonged circulation (in the range of several days) and more prolonged reduction of asparagine plasma level than free enzyme injected in monkeys128. In mice, 51Cr-labeled RBC-encapsulated asparaginase circulated as a complex showing the same half-life for RBC carrier and the drug (although it was shorter that half-life of control RBC)137. In humans, pharmacokinetics of RBC-loaded asparaginase showed considerable variability between 13 tested patients, yet provided a significant prolongation of half-life in the plasma vs. free enzyme (27–29 days vs. 8–24 hours, respectively) and a more profound and prolonged reduction of asparagine level in plasma48. Based on this encouraging outcome, Kravtzoff and co-workers have studied tolerance of RBC-asparaginase in human patients and found no overt harmful effects, nor antibodies in plasma after a single injection49. Separation of marginal sub-populations of drug-loaded RBC (e.g., senescent or unintentionally damaged RBC) using gradient centrifugation helped to minimize dosing variability between and within the same batches138. New methods for asparaginase loading into RBC based on enzyme modification by low molecular weight protamine has been recently proposed, yielding formulation with longer half-life in mice than formulation than RBC-loaded asparaginase produced by hypotonic dialysis method and showing promising therapeutic effect in mouse model of leukemia dependent on exogenous asparagine139.
Other detoxifying enzymes encapsulated in carrier RBC include glutamine synthase for ammonia detoxification, providing circulation of detectable enzymatic activity for 48 hours and reduction of ammonium level by 50% in mouse blood140. Alcohol, glutamate and aldehyde dehydrogenases have been encapsulated into mouse RBC with variable yield and high resultant enzymatic activities (e.g., RBC/GDH effectively metabolized high quantities of ammonia) using electroporation127 and hypotonic dialysis141. These loading schemas reduced RBC stability, yet injection of RBC-encapsulated GDH alleviated ammonia toxicity in mice141. Isotope tracing of 51Cr-RBC showed that in contrast to control RBC, a major fraction (>50%) of electroporated RBC with encapsulated dehydrogenases was eliminated from the bloodstream within several hours, yet the kinetics of second phase of RBC/ADH removal was only slightly faster than control RBC (T1/2β approached 4.5 vs 5.3 days, respectively)142.
Native and PEG-modified adenosine deaminase (ADA) has been encapsulated in human RBC to achieve sustainable elimination of non-metabolized deoxiadenosine that accumulates in and inhibit immune cells in the patients with reduced adenosine deaminase level in blood143. Human studies showed that PEG-ADA loaded RBC circulate better than native ADA-loaded RBC, although both formulations have a fairly short half-life (20 vs. 12 days, respectively, which still was longer than that of PEG-ADA itself, 3–6 days)47. Clinical studies performed by this group involve very limited numbers of patients (1–2 per study), yet in a recent publication they have reported a patient that has been treated by RBC-loaded PEG-ADA for correction of a genetic deficiency for nine years with total 225 infusions every 2–3 weeks without overt adverse effects and with significant, although transient, improvement in lymphocyte numbers, sustained level of blood ADA activity and immunoglobulin level, and clinical improvements144.
3. Coupling therapeutics to the RBC surface
Coupling therapeutics to the surface of carrier RBC represents an alternative to encapsulation strategies that have been considered above. RBC membrane provides an extended surface area that may be used for anchoring multiple copies of protein or other therapeutic molecules. Lack of isolation of a drug from blood en route to the therapeutic site represents an obvious downside of surface coupling vs encapsulation. This concern, however, does not seem acute for drugs that are supposed to work in the bloodstream. Furthermore, using pro-drug formulations resistant to plasma inhibitors holds a promise to resolve issues associated with premature inactivation and side effects en route. On the other hand, surface coupling strategies avoid damaging encapsulation procedures and therefore offer theoretical advantages of drug loading without compromising RBC biocompatibility. In addition, coupling of drugs to RBC surface circumvents issues related to drug release (approaches to trigger drug release by using controlled lysis by complement have been suggested, yet practically useful controlled release from carrier RBC remains an elusive goal)28. Of note, coupling to RBC surface resolves diffusional limitations: even enzymes that react with small, membrane permeable substrate are more active when bound to the RBC surface than when incorporated within the cell145. Further, surface coupling offers a unique option to load drugs on circulating RBC without technically and logistically cumbersome need for their extraction necessary for drug encapsulation and re-infusion.
Generally speaking, techniques for coupling diverse molecules to RBC have been designed in the fifties, in the process of development of reagents for immunological reactions of agglutination. Numerous cross-linking agents and procedures have been applied to conjugate proteins and other antigens and biological molecules to RBC of different animal species including humans. Subsequent studies revealed that these conjugation methods grossly damage RBC membrane, reducing RBC plasticity, resistance to lytic agents and biocompatibility. Nevertheless, reliable methods of biocompatible conjugation molecules to RBC have been designed recently (see below).
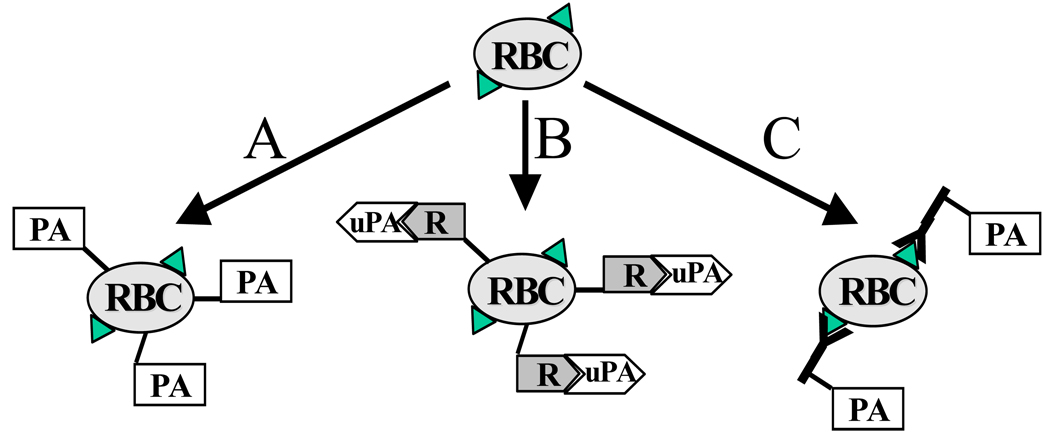
A theoretical paper of mid-nineties provided a speculative yet stimulating analysis of hypothetical RBC membrane anchors and methods of coupling of therapeutics using chemical conjugation to tentative affinity ligands and gave few examples of experimental studies in this area, available at the time20. Several practical strategies for coupling therapeutics to carrier RBC surface have evolved and have been tested in vitro and in vivo in last two decades. These strategies can be divided into three wide categories, described in further details in the following sections: i) chemical coupling of agents to RBC surface (either covalent, or non-covalent); ii) coupling to RBC membrane of a receptor that binds a therapeutic agent (and, in some cases, augments its functions); and, iii) conjugation of therapeutics or their receptors with affinity ligands (e.g., antibodies or their fragments) that bind to RBC thereby anchoring cargoes on RBC (Figure 2). This is the latter strategy that permits loading drugs on RBC surface by injecting these conjugates into the bloodstream. Both these strategies provide coupling of antibodies, antigens, enzymes, cytokines and other biologically active cargoes to RBC and are been explored for vascular delivery of several classes of therapeutics including, more recently, model polymer nanocarriers anchored to RBC either non-specifically146,147 or via affinity peptides148. Below we consider surface coupling to RBC for delivery of the following classes of biotherapeutics: i) antibodies and antigens; ii) affinity ligands for coupling and elimination circulating pathogens; iii) proteins regulating complement system; and, iv) proteins controlling formation and dissolution of blood clots.
Figure 2. Strategies for coupling therapeutic agents to RBC surface.
This schema uses plasminogen activators (PA, see section 3.5) to illustrate methods for coupling drugs to RBC, yet these principles are applicable to a wide variety of therapeutics. A. Direct coupling of drugs (PA) to RBC using covalent or non-covalent cross-linking agents is a prototype paradigm that involves modification of isolated or donor RBC followed by infusion in a patient. This paradigm is especially attractive for pursuing PEG-stealth RBC approach (Section 3.2). B. Non-covalent binding of a drug to its receptor conjugated to RBC may provide a more physiological anchorage some biotherapeutics and provide an additional modality for functional regulation of their activity (see an example of coupling urokinase uPA to its receptor suPAR coupled to RBC in Section 3.5.4). C. Conjugation of a drug with fragments of antibodies binding to RBC avoids ex vivo manipulations with RBC, eases administration and dosing, enhancing clinical feasibility (Sections 3.4 and 3.5.4).
3.1. Coupling of antigens and antibodies to surface of carrier RBC
In theory, RBC carrying antibodies or antigens on their surface can be employed to deliver diverse cargoes to intravascular targets149 or agents modulating immune response95, respectively. Since early eighties, many methods have been tested for coupling protein molecules to RBC for in vitro applications including use of non-specific chemical cross-linkers such as tannic acid150 and chromium chloride151,152. These methods have been surpassed by more specific cross-linkers, e.g., biotin-avidin pair offering modular anchoring of diverse biotinylated chemicals including proteins and nucleic acids to defined reactive groups on RBC membrane, i.e., amino acids153–155, sulfhydryl groups156, sugars157 and lipids158,159. In particular, controlled biotinylation of RBC lysine residues using NHS esters of biotin become arguably one of the most popular means for conjugation cargoes to RBC surface for a wide variety of applications in vitro and in vivo69,160–164.
One of such applications, namely, isolation and tracing of subpopulations of RBC in circulation165,166 as well as monitoring their survival167 and volume168, had found use in animal169,170 and human studies involving injection of normal, senescent and sickle-cell biotinylated RBC171,172. Furthermore, methods for direct biotinylation of RBC in the bloodstream using intravascular injection of biotin esters have been explored170,173 (this method likely yielded promiscuous modification of diverse blood cells and endothelium). On a cautionary note, an excessive biotinylation may reduce RBC biocompatibility, alter RBC antigens164 and repetitive injections of biotinylated RBC elicit immune response174. It has also been found in late eighties that excessive cross-linking of biotinylated RBC membrane by avidin, streptavidin or neutravidin, all of which have four high-affinity binding sites for biotin and thereby serve for coupling biotinylated molecules to RBC, causes to RBC lysis by complement activated via the alternative pathway27,175, leading to intravascular hemolysis and rapid elimination of modified RBC176. Studies of the molecular mechanisms of this phenomenon revealed that modification of RBC membrane glycoproteins Decay Acceleration Factor (DAF) and CD59 (that suppress complement activation and assembly of membrane-attacking complex in RBC membrane, respectively), is responsible for this unwanted side effect56,177,178. This allowed design of non-damaging means for coupling of molecules to RBC via biotin-avidin cross-linker without loss of RBC biocompatibility159,179; thus RBC carrying up to 105 molecules of protein cargo conjugated via streptavidin in a biocompatible manner circulate in animals similarly to naïve RBC, without enhanced clearance, lysis or organ uptake180,181.
Several labs explored use of RBC coated with antigens and cytokines for stimulation of immune response in vitro182,183 and, at more limited scale, in vivo184. Antibody-guided RBC formulations have also been tested for delivery to populations of lymphocytes to achieve selective modulation of immune response152,185. Further, model studies demonstrating proof of principle for targeting antibody-carrying RBC to selected sites in the vasculature have also been published3,149,151. However, use of antibody-coated RBC for targeted drug delivery in the vascular system has not been actively pursued in the last decade. The need for ex vivo modification of RBC for antibody conjugation and drug loading, as well as lack of practically adequate means for controlled release of drugs encapsulated into the carrier RBC represented a daunting technical challenge. In addition, safety issues associated with hemotransfusion inevitably involved in this strategy were viewed unfavorably by industrial sector in the age of burst of HIV and other blood transmitted infections. As a result, synthetic carriers including liposomes and polymer particles surpassed drug targeting strategies using antibody-carrying RBC.
3.2. Polyethylene glycol conjugation to RBC: an approach to universal donor blood
Conjugation of highly hydrophilic polyethylene glycol (PEG) with the chain length in the range MW 3–10kD has evolved since seventies as a universal “stealth” technology, prolonging circulation and masking from defense systems in the body of liposomes, polymer nanocarriers, proteins and other drug carriers and drugs themselves. The goal of producing PEG-ylated “stealth” RBC, proposed by Scott and co-authors a decade ago, is to obtain a ‘universal” donor blood for hemotransfusion by chemically camouflaging RBC antigens186–188. Indeed, PEG-coated RBC are less effectively opsonized, taken up by phagocytes and recognized by antibodies to RBC antigens189–191. Masking against immune reaction to mismatched ABO antigens is the most challenging goal; in this setting, PEG-coating offered no protection and rather aggravated hemolysis in the initial studies191. Additional studies using alternative PEG-coupling techniques and combinations of PEG with different chain length produced encouraging results in masking ABO and Rh(D) blood group antigens192–194. More recently, even masking of xeno-antigens on RBC has been attempted, yet the outcome was not too encouraging195.
Covalent coupling of cyanuric chloride activated PEG derivatives to amino acids is the most popular method for PEG conjugation to RBC191,196. PEG coupling using sulfo-group chemistry192,194, insertion of lipid derivatives of PEG197 and other methods have also been developed186,198,199. Extent of “stealth” feature or masking increases proportionally to PEG length in the range 5–35 kD, branching and surface density200. Studies of RBC electrophoresis showed that PEG conjugation increases apparent length and density of RBC glycocalyx201 and that long and especially branched PEG polymers work more effectively in this respect202. Similar results have been obtained when RBC was modified by Pluronic, a tri-block copolymer combining two PEG chains at the ends of a less hydrophilic moiety203. Of note, RBC modification using PEG length and surface density that does not mask complement receptor CR1 against antibodies, profoundly decreased binding of immune complexes to RBC, indicating that even relatively small steric hindrance may obstruct multivalent interactions of immune complexes with CR1204. This observation is important in the context of strategies for CR1-mediated clearance of pathogens discussed below.
PEG-modification at the extent that effectively masks RBC from recognition by immune system affects neither functions of RBC membrane glycoproteins (e.g., ion exchange function of Band 3), nor RBC stability, plasticity and biocompatibility190. Thus, PEG-RBC circulate with half-life similar to that of non-modified RBC in syngenic animals186,190. Additional fractionation of PEG-coated RBC provides the PEG-RBC sub-population that is most effectively masked, less damaged and circulates equally to native RBC205. Furthermore, PEG-coated RBC are less susceptible to parasite infection including malaria Plasmodium,206 due to masking their receptors196. In addition, PEG-modified RBC demonstrate reduced tendency to aggregation203,207 and improved rheological properties: reduced low shear flow viscosity189 and enhanced thickness of membrane protective layer and plasticity necessary to endure hydrodynamic stress in circulation196,208,209. In the last decade, PEG-RBC is an active area of research promising to alleviate acute problems of shortage of matched donor blood (see reviews by Garratti on this subject)210,211.
3.3. Elimination of circulating pathogens using RBC-coupled antibody heteropolymers
It has been found several decades ago that one of RBC transmembrane glycoproteins, Complement Receptor Type 1 (CR1), binds C3b component of activated complement and immune complexes containing this protein and that macrophages in the RES safely detach such immune complexes including antibody-pathogen complexes from circulating RBC without damaging the cell212. Two decades ago, Ron Taylor postulated that this natural function, i.e., immune clearance of pathogens from the bloodstream, can be performed as a controlled medical procedure that would not require complement activation, by injecting artificial immune complexes called heteropolymers that consist of a monoclonal antibody to CR1 conjugated with an antibody to a pathogen or toxin213. This strategy has been emulated later by a strategy for a decoy-type clearance of HIV-1 by RBC carrying electro-inserted CD4 in their plasma membrane that showed initial promise in vitro studies employing cell cultures of blood mononuclear cells from HIV-1 patients214.
In primates and humans, >90% of CR1 is expressed on RBC at levels of ~500–1,500 copies per cell, providing an anchorage site for CR1 antibodies and compounds conjugated with anti-CR1 (i.e., heteropolymers) injected in blood215–218. Loading of such heteropolymers on RBC provided effective binding of model antigens and bacteria in vitro213,219,220 and in non-human primates221 (of note, rodents do not express CR1, hence all in vivo studies of CR1-mediated blood clearance have been performed in monkeys until recent production of a transgenic mouse expressing human CR1222). CR1 represents an immunologically privileged site for antibody binding to RBC: isotope tracing and fluorescent imaging studies revealed that binding of neither anti-CR1 nor anti-CR1 conjugates targets carrier RBC for phagocytosis223, yet phagocytes recognize pathogens and immune complexes bound to CR1 either via C3b or anti-CR1224, cleave off extracellular domain of CR1225 and take up the pathological “cargo” from RBC surface226–228. Studies in primates demonstrate that diverse ligands can be coupled to circulating RBC using anti-CR1 conjugates and eliminated by phagocytes without damage or shortening RBC survival213,215,229,230.
This concept has been tested in primates and showed promising effectiveness of elimination of diverse bacterial231 and viral232–235 pathogens using CR1 heteropolymers targeted to these pathogens, thereby alleviating the infection235,236. Further, coupling to RBC of anti-CR1 conjugated with DNA, a common antigen for auto-antibodies in lupus afforded clearance of such auto-antibodies in monkeys230,237,238; the same principle of CR1-targeted heteropolymers has also been explored for elimination of cytokines as anti-inflammatory intervention239, indicating potentially wide biomedical application of this concept215.
3.4. Coupling inhibitors of complement to RBC and transfer of RBC-anchored proteins to endothelium
Complement system, consisting in more than 20 regulatory and executing proteins in blood plasma and cellular membranes, exerts key functions of innate immunity including opsonization and destruction of pathogens, tumor and foreign cells, and activation of pro-inflammatory cascades in the sites of invasion. However, in some pathological conditions, host cells suffer injury inflicted by overzealous or misguided activation of complement. Vascular endothelium and RBC represent the most common and vulnerable target for adverse effects of autologous complement activation, especially in situations of functional deficiency of membrane glycoproteins inhibiting complement: Decay Acceleration Factor (CD55 or DAF), CD59 and CR1 (CD 35, represented in mice by an analogue called Crry). DAF and CR1 inhibit early stages of complement activation, thereby protecting cells from lysis and reducing generation of pro-inflammatory mediators, whereas CD59 blocks formation of hemolytic pores in the plasmalemma. Complement-mediated hemolysis of RBC deficient in DAF and CD59 is involved in mechanism of paroxysmal nocturnal hemoglobinuria (PNH), whereas endothelial damage by complement is involved in acute vascular injury in ischemia-reperfusion syndrome, such as happens in organ transplantation.
Several groups pursued targeting complement inhibitors to RBC, to protect them from hemolysis and inhibit complement activation. Of note, natural DAF and CD59 are anchored on the luminal surface of RBC membrane via a lipid anchor, glycosylphosphatidylinositol (GPI). Accordingly, experimental methods for insertion of DAF, CD59 and other GPI-linked proteins into RBC plasmalemma using the lipid anchor have been designed240,241. Insertion of lipid-anchored CD59 in RBC plasmalemma protects against complement-mediated hemolysis242, providing a proof of principle for new approach for PNH therapy.
The plasma membrane domains that harbor artificially inserted GPI-linked proteins are not fully elucidated243, yet they differ from cholesterol-rich domains that tightly retain natural GPI-anchored proteins244. As a result, GPI-anchored proteins artificially inserted in membrane (“painted”) more easily release from RBC241. Perhaps, this is one of the reasons why RBC transfer artificially “painted” GPI-linked proteins to other cells in vitro245 and in vivo, where endothelium represents the preferential acceptor of GPI-inserted proteins from RBC246. As a result, injection of RBC carrying GPI-linked DAF and CD59 artificially inserted using gene therapy means alleviates acute ischemia-reperfusion injury in swine-to-primate model of xenograft transplantation247.
Of note, chemical conjugation to RBC plasmalemma is undesirable for coupling of complement inhibitors, because this approach would make coupling irreversible, which is not desirable for protein transfer from RBC to endothelium. In addition, it would require RBC isolation, modification and re-infusion, which inevitably reduce practical utility of the approach and RBC resistance to complement. Conjugation with antibodies also represents suboptimal mean for binding complement-regulating proteins to RBC, since cross-linking RBC membrane by multivalent conjugates can damage RBC. From the standpoint of safety, as well as industrial and clinical translation, small monovalent antigen-binding fragments of antibodies, i.e., recombinant single chain variable fragments (scFv) represent the preferable targeting moiety. Using modular gene engineering biotechnology methods, these recombinant proteins can be fused via short connecting peptides with variety of executing proteins. Thus recombinant scFv-fusions targeted to RBC hold promise to provide a series of clinically useful for loading biotherapeutics onto circulating RBC without need for RBC isolation, modification and hemotransfusion.
Expression of scFv in diverse vectors enables large-scale, GMP-quality production of homogeneous monovalent scFv/PA fusion proteins248,249. Advantages of scFv include: i) Lack of Fc-mediated side effects; ii) Lack of cross-linking of anchoring sites on RBC and RBC aggregation; iii) Due to its smaller size (~50 kDa), scFv/PA can be injected intramuscularly, as other recombinant protein therapies (e.g., insulin); iv) Established techniques for humanization and reduction of immunogenicity of scFv further help to minimize the likelihood of eliciting immune reactions; v) Modular recombinant format of scFv fusion supports synthesis of diverse fusions250.
For example, a recombinant fusion protein combining scFv directed to Rh(D) blood group antigen with human CR1 binds to CR1-deficient RBC and restores RBC ability to bind immune complexes251. This in vitro study implies that this approach may eventually be used to either replenish CR1 functions (i.e., immune clearance and complement inhibition) in CR1-deficient patients (~10% of humans are CR1-negative), or employed to boost CR1 immune clearance in a manner performed by heteropolymers described in the previous section. Recently, Spitzer, Atkinson and co-workers produced a scFv of a monoclonal antibody TER-119 recognizing a mouse analogue of human glycophorin A252 (GPA), fused it with DAF253 and Crry254 and demonstrated that this monovalent fusion constructs bind to RBC after intravascular injection in mice without damaging RBC, and, furthermore, enhance RBC resistance to lysis by complement in vivo253,254. Furthermore, recently this team performed a neonatal in vivo gene transfer of TER-119 scFv/Crry in mice using retroviral gene transfer vector and demonstrated that prolonged synthesis of this fusion protein in mice leading to its sustained coupling to circulating RBC, restores protection against excessive complement lysis in mice genetically deficient mice255. These studies, showing proof of principle for in vivo delivery to RBC of therapeutic fusion proteins (injected or encoded by gene therapy means) opens exciting applications, some of which will be discussed in the next section.
3.5. RBC carriage of agents regulating formation and dissolution of blood clots
An idea of intravascular delivery of agents controlling blood clotting and clot dissolution using carrier RBC has been proposed a quarter of century ago, as illustrated by an early work reporting conjugation of fibrinolytic drug streptokinase to RBC targeted to collagen, a thrombogenic component of extracellular matrix that gets exposed to blood in sites of vascular injury3. In theory, coupling drugs to RBC surface may favorably alter their pharmacokinetics (i.e., prolong life-time in circulation) and optimize interaction with components of blood coagulation and fibrinolytic systems accessible from RBC surface. Attempts in this direction included conjugation of heparin to RBC to enhance potency of anticoagulant thromboprophylaxis in patients predisposed to thrombosis256 and conjugation of pro-thrombotic RGD-containing peptide to RBC to design a substitute for platelet infusion in patients predisposed to hemorrhagic disorders.27
RBC carrying conjugated drugs reported in these studies exerted good functional activities in vitro2,3,256. However, until recently, no attempts to test these drug delivery systems in animal models have been reported, presumably because conjugation of drugs grossly compromised RBC biocompatibility. In particular, inactivation of complement inhibitors DAF and CD59 in RBC membrane257 led to hemolysis, phagocytosis and rapid elimination of RBC27,175–178,258. Eventually, methods to couple up to ~105 copies of therapeutic proteins per RBC without RBC damage have been devised.150,159,163,179,180 In vivo studies using radioisotope tracing showed that 51Cr-RBC/125I-drug complexes circulate similarly to control RBC for several days after injection in rats259 and mice180,181. This success brought back on the agenda attempts to couple fibrinolytic agents to RBC and test benefit/risk ratio of this approach in animal models of thrombosis.
3.5.2. Need for a safer and more effective management of thrombosis
Sealing of damaged blood vessels by mural clots prevents bleeding, while pathological vessel occlusion by intravascular clots (thrombosis) causes tissue ischemia and damage, leading to acute myocardial infarction (AMI), ischemic stroke, pulmonary embolism and peripheral vascular disease, among other complications. Thrombosis is the leading cause of mortality and disability in the US260,261. Of note, thrombi are prone to recur within hours to days after an AMI or stroke and the risk is great after transient ischemic attack or pulmonary embolism and in immobilized patients262–265. Thrombosis and embolism are also a common and dangerous complication of surgery that is especially difficult to manage due to the risk of acute bleeding at the operative site. Invasive interventions (e.g., angioplasty, carotid endarterectomy) may be complicated by formation of clots that embolize to the brain and cause neurological disorders. Thus, situations in which patients are at highest short-term risk for occurrence or recurrence of thrombosis are known. However, anti-platelet and anticoagulant agents provide limited protection and pose considerable risk of bleeding266–269, especially soon after the surgery; in addition some anticoagulants (e.g., Warfarin) require many hours to develop an effect, which is inadequate for acute short-term thromboprophylaxis.
Emergency therapy of thrombosis employs injection of plasminogen activators including tissue type (tPA) and urokinase (uPA), middle-size proteases (MW 30–60 kD) that generate plasmin, which cleave fibrin clots and thus restore perfusion249,270. However, inadequate delivery (blood clearance within <15 min271), inactivation by plasma inhibitors such as PAI-1272 and impermeability of occlusive clots273 restrict the effectiveness of therapeutic fibrinolysis by PA. Within minutes after infusion of mega-doses of fibrinolytics (e.g., ~100 mg of tPA) needed to overcome its inefficiency and achieve fibrinolysis locally, excess drug diffuses into pre-existing hemostatic mural clots predisposing to bleeding and into tissues such as the CNS274, where it may cause cerebral hemorrhage, damage to the blood-brain barrier (BBB) and toxicity within the brain275. Due to the danger of bleeding and the collateral CNS damage, fibrinolytics cannot be used in over 95% of stroke patients and in the post-operative period other than as an emergent potentially life-saving intervention. A more ideal thromboprophylactic agent would prevent occlusive thrombi from forming without lysing hemostatic mural (e.g., post-surgical) clots and causing extravascular toxicity.
Attempts to improve tPA delivery and its benefit/risk ratio have not yielded decisively better outcomes276–279. Ironically, the higher affinity of newly developed PA variants for clots promotes drug retention on the clot surface, impeding permeation into the occluding thrombi. The only currently employed use of tPA, i.e., post-thrombosis, is also marred by inevitable delays (time needed for diagnosis, injection and the lysis proper, slowed by clot impermeability), increasing the risk of ischemia-reperfusion (I/R) injury that worsens outcome. Prophylactic delivery of a fibrinolytic into the interior of growing, early nascent thrombi will arrest clot propagation and cause rapid clot lysis. Lysis of clots from within will be more homogeneous and complete than “therapeutic” external fibrinolysis from the clot surface. This would minimize the incidence of the secondary embolism and re-thrombosis. Prophylactic use of tPA in patients at high risk of imminent primary or recurrent thrombosis will lessen the formation of impermeable occlusive clots due to delays in fibrinolysis. However, fibrinolytics are not used for prophylaxis due to their rapid clearance and side effects. Newly designed mutant plasminogen activators with enhanced potency and fewer side effects may improve therapy279–284, but their diffusion into occluding clots has yet to be tested and no fibrinolytic has been designed for prophylactic use. All existing plasminogen activators are short-lived (<30 min, insufficient for prophylaxis) and small (<10 nm) agents, capable of diffusing into hemostatic clots (bleeding) and into the CNS (collateral damage). None is suited for use as prophylaxis in post-surgical or other patients at imminent risk of thrombosis.
3.5.3. Coupling plasminogen activators to RBC: a novel strategy for short-term thromboprophylaxis
Recent animal studies showed that biocompatible coupling to RBC converts plasminogen activators from problematic therapeutic agents into effective and safe agents for intravascular thromboprophylaxis. Hemodynamic factors are favorable, propelling RBC towards the blood mainstream285–287, restricting drug contact with vascular walls and mural clots. Infusion of RBC/tPA complex in rats and mice:
Does not cause complement activation, hemolysis, phagocytosis or accelerated clearance of RBC carrying up to 105 tPA molecules stably coupled per RBC288;
Blocks tPA diffusion into hemostatic clots288. In fact, RBC/tPA does not affect normal hemostasis or cause bleeding from fresh post-surgical hemostatic clots susceptible to free tPA289;
RBC glycocalyx protects tPA from plasma inhibitors290, while preserving its fibrin affinity291 and activation by fibrin291, which facilitates dissolution of clots;
Prolongs tPA circulation by many orders of magnitude291, permitting its use as prophylaxis288. In rats and mice <20% of RBC/tPA is cleared within 3 hours, which projects to a half-life of ~2–3 days. The half-life of RBC is ~3–4 weeks in mice vs 3–4 months in humans. Thus, the expected prophylactic window of RBC/PA in humans may vary from hours to many days and weeks depending on dose;
Delivers tPA into the interior of intravascular venous and arterial nascent clots, which are then lysed from within during clot extension in settings where even a 10-fold higher dose of soluble tPA (also indicated throughout the grant as “tPA” or “free tPA”) was ineffective288, i.e. Trojan Horse lysis291,292;
Furthermore, most recent animal studies showed that RBC/tPA provides effective and safe thromboprophylaxis in the most challenging and dangerous vascular bed, i.e., cerebral circulation, thereby preventing deleterious consequences of brain ischemia and stroke in mice293. This study showed that prophylactic RBC/tPA injection in mice provides dissolution of subsequently formed occlusive cerebrovascular thrombi, leading to rapid and stable reperfusion, marked alleviation of ischemic brain injury and improved survival, not attainable by even 10-fold higher doses of soluble tPA (which in fact greatly enhanced mortality). Of note, in contrast with free tPA, RBC/tPA did not aggravate brain edema, injury and hemorrhage in mice with cerebral ischemia and a rat models of post-thrombotic intracranial hemorrhage. Moreover, either prophylactic or post-insult injection of RBC/tPA attenuated disorders of vascular regulation and CNS tissue injury in the brain of pigs subjected to global ischemia/hypoxia, whereas free tPA grossly aggravated these pathological changes294.
3.5.4. Advanced approaches for coupling plasminogen activators with RBC
Encouraging outcomes of animal studies using injection of RBC/tPA complexes preformed ex vivo using chemical cross-linking pawed the way for most recent attempts to further advance coupling techniques. Thus, in addition to coupling to RBC via streptavidin cross-linker employed as a prototype method in all previous studies of RBC/tPA, urokinase has been coupled to RBC via urokinase receptor chemically conjugated to RBC without loss of biocompatibility295. This approach reduced undesirable interactions of urokinase with its vascular receptors and allowed trigger fibrinolysis, since interaction with RBC-coupled receptor activated urokinase295.
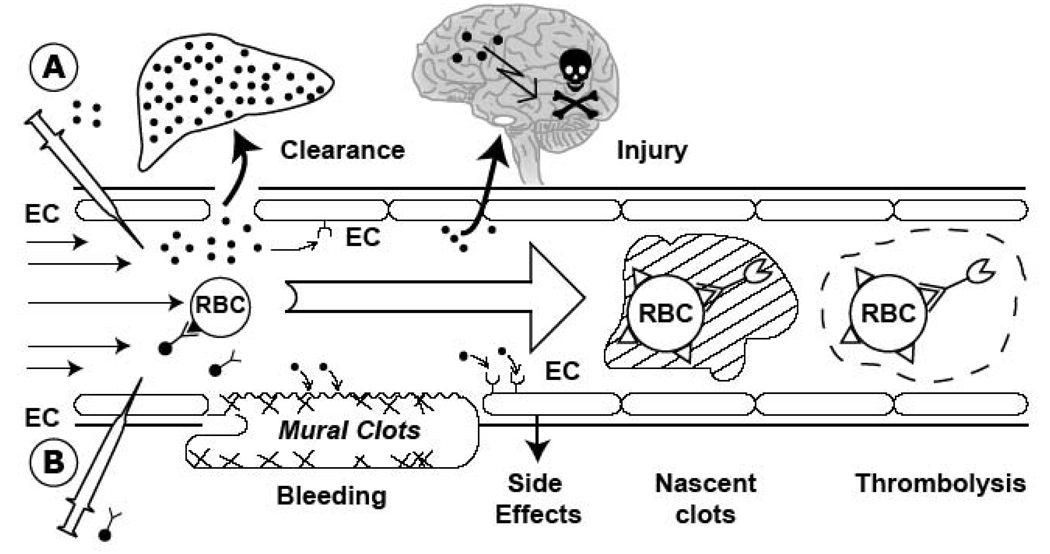
Preformed RBC/tPA conjugates have properties suitable for prophylactic use in settings where transfusion is common, but would be less practical in other settings and challenging to develop commercially. To avoid the need to couple tPA to isolated RBC ex vivo followed by transfusion, designed ways to target tPA to circulating RBC directly have also been designed. Figure 3 illustrates this strategy for coupling plasminogen activators to carrier RBC.
Figure 3. A concept of prophylactic fibrinolysis by scFv/tPA targeting to RBC.
(A). Plasminogen activators (dots) are relatively ineffective, in part due to rapid uptake by liver, and unsafe due to bleeding (indiscriminate lysis of hemostatic mural clots), vascular side effects (e.g. activation of receptors on endothelial cells, EC) and injurious effects of tPA diffusing into the CNS. (B) Coupling to RBC will dramatically prolong the longevity of scFv/tPA variant. RBC will restrain scFv/tPA binding to cellular receptors, and restrict its access into mural hemostatic clots and the CNS. Propulsion of RBC towards the mainstream will further offset interactions of the pro-drug with hemostatic clots and vascular walls. RBC-bound scFv/tPA will have virtually unlimited access to the interior of nascent pathological thrombi and thereby will dissolve pathological intravascular clots and prevent vascular occlusion.
The first approach capitalized on CR1 anchoring strategy, since agents conjugated with monoclonal antibodies to complement receptor-1 (CR1)296 bind to circulating RBC after iv injection in animals including primates, circulate with RBC and bind ligands in vivo without RBC damage213,221,297. Animal studies showed that tPA conjugated to a CR1 monoclonal antibody binds without harm to circulating RBC in mice and affords safe and effective prophylactic thrombolysis289 comparable to that provided by infusion of RBC/tPA288. To alleviate challenges associated with translation and application of chemically produced antibody/tPA conjugates and achieve predictable loading of RBC over a wide range of drug concentrations, a novel recombinant tPA mutant fused to an antigen-binding single chain variable fragment (scFv) of TER-119 monoclonal antibody to glycophorin A (anti-GPA scFv)298,299 has been very recently designed; pilot animal studies showed that this approach allows to load up to 20,000 copies of anti-GPA scFv/tPA fusion per RBC after a single intravenous injection of the fusion without detectable changes in behavior of circulating RBC. On an encouraging note, injection of scFv/tPA in mice provided swift dissolution of subsequently formed intravascular clots, which were impervious to injections of equimolar doses of soluble tPA (Zaitsev et al, JPET 2009, under revision).
4. Expert opinion
Erythrocytes represent a unique and promising, yet somewhat underdeveloped platform for drug delivery. After initial burst of interest and research efforts three decades age, drug delivery by RBC was overshadowed by artificial carriers offering wider range of applications, as well as better control over formulation, storage and utilization of drug delivery system than original RBC-based concepts. Thus, RBC is a good carrier for drug delivery to intravascular and RES targets, but many other important therapeutic targets (e.g., solid tumors, extravascular tissue components, CNS) are normally inaccessible to RBC. Since drug delivery field has been originated and developed predominantly in the context of targeted delivery of toxic anti-cancer drugs, such limitation of the spectrum of suitable targets RBC negatively impacted development of this drug delivery platform.
Studies of RBC drug delivery logically started with in vitro experimentation using isolated RBC and within two decades progressed to extensive animal studies, a necessary step for validation of the viability, efficacy and safety of this drug delivery system, including immune response to RBC-coupled proteins in settings similar to immunization procedures. Of course, certain specific aspects of the RBC drug delivery (e.g., immune response, subtle effects in the CNS and tolerability of RBC/drug complexes by specific patient populations) can be addressed only in clinical studies. The fact that several clinical trials of RBC drug delivery systems have recently been initiated instills a hope that these translational barriers will be eventually overcome.
Among potentially useful biomedical applications, RBC-mediated intracellular delivery of drugs including replacement therapies, imaging probes and toxic agents into RES macrophages and other cells exerting robust endocytic uptake seems as a reasonably promising goal. Delivery of anti-microbial, immunogenic or toxic agents into defense cells can be used for eradication of intracellular parasites, stimulation or suppression of immune reactions, respectively. It remains to be better understood, however, which particular advantages are offered by RBC in this context vs other drug delivery systems, all of which are effectively taken up by RES macrophages and other defense cells (it is more difficult to avoid this addressing). Further, in order to translate this concept into the clinical domain, we must more fully understand and optimize mechanisms of action of RBC-loaded drugs. The pharmacokinetics, stability and rate of release of drugs in plasma have to be systematically characterized and effects on diverse populations of cells in the body defined. This drug delivery system is also a bit promiscuous in terms of selectivity of targeting: many cell types in the body can take up RBC and their remnants. For example, phagocytes, immune and tumor cells all may be targets of antibiotic-loaded RBC. It is conceivable that more precise targeting towards some of these populations may greatly optimize therapeutic effect. It would be undesirable, however, if efforts in this direction would provide very complex, difficult to produce and utilize in medical practice formulations (to be fair, this concern is generally applicable to most of previous and current drug delivery carriers).
Strategies using RBC carriage to augment systemic effects of drugs in circulation such as sustained effects of detoxifying enzymes require no targeting and represent one of the unique areas of RBC drug delivery that offers clear potential advantages over other carriers, due to unique longevity of RBC. Maintenance of maximal biocompatibility of loaded RBC, yield of enzymes loading and kinetics of trans-membrane transport of their substrates and products, as well controlled release and metabolism of encapsulated cargoes represent challenging, yet theoretically surmountable aspects of this approach.
The need for ex-vivo manipulations with RBC, a relatively limited shelf-life and concerns related to the safety of donors matching and blood-born infections are recognized potential downsides of carriage drugs attained by ex vivo RBC loading and re-infusion. These concerns are shared by all types of hemotransfusion, cell replacement and stem cell therapies. Of note, transfusion of blood and blood products is a very widely used and generally safe therapeutic intervention worldwide, the safest and most effective type of cell transplantation strategy. Use of autologous blood (re-infusion) minimizes the safety concerns.
Drug delivery systems targeted to anchor molecules on RBC surface (e.g., scFv-fusion proteins) and therefore allowing safe and technically simple loading of circulating RBC hold a promise to completely solve this issue and greatly expand general applicability of RBC carriers in medicine. In particular, design of intravascular drug delivery platforms using coupling to circulating RBC agents that control immune system, eliminate pathogens and toxins from the bloodstream and correct pathological aspects of thrombosis and hemostasis seem most exciting and unique utilities of RBC drug delivery, promising timely translation into the industrial development and clinical practice.
Article highlights box 1
1. Introduction
RBC is naturally designed as a biocompatible carrier for vascular drug delivery, with prolonged life-time in circulation and safe mechanisms for eventual elimination including fixation of complement and uptake by macrophages. Drug encapsulation into RBC and coupling to RBC surface represent two approaches for RBC drug delivery.
2. Vascular delivery of drugs encapsulated into carrier RBC
Drugs can be encapsulated into isolated RBC using methods including hypotonic dialysis and resealing. RBC-encapsulated drugs are separated from blood, but can exert their activities in circulation if they or their substrates diffuse through RBC membrane. Unintentional damage to RBC in course of encapsulation compromises their biocompatibility and negatively impacts drug delivery except cases when macrophages are the target. Undamaging methods for RBC encapsulation provide clinically testable drug delivery systems that markedly prolong circulation time, bioavailability and effect of cargoes. Delivery RBC-encapsulated anti-inflammatory drugs into phagocytic cells and enhanced bioavailability of detoxifying enzymes in the bloodstream represent attractive examples of this strategy.
3. Coupling of therapeutics to the RBC surface
Drugs can be coupled to RBC surface using variety of covalent and non-covalent cross-linkers, as well as anchored onto circulating naïve RBC using recombinant fusion proteins with specific affinity to RBC. Animal studies show that surface coupling to RBC can be used for improvement of antigen delivery, masking of RBC antigens, clearance of pathogens from blood and intravascular delivery of therapeutics that suppose to act within the vascular lumen. In particular, coupling of fibrinolytic plasminogen activators to RBC provides the basis for a paradigm-shifting prophylactic thrombolysis, an approach that showed very promising results in animal models of peripheral, pulmonary and cerebral thromboses.
4. Expert opinion
RBC represents a versatile, safe and widely useful carrier for optimization of drug delivery in the vascular system. Several RBC-drug delivery strategies are posed for the translation into industrial development and clinical testing and use.
Acknowledgements
This work has been partially supported by NIH RO1 HL090697. Author thanks Dr. Juan-Carlos Murciano for help in preparation of the figures.
Footnotes
Declaration of interest:
The author states no conflicts of interest and has received no payment in the preparation of this manuscript.
REFERENCES
- 1.Simone EA, Dziubla TD, Muzykantov VR. Polymeric carriers: role of geometry in drug delivery. Expert Opin Drug Deliv. 2008;5:1283–1300. doi: 10.1517/17425240802567846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coller BS, Springer KT, Beer JH, et al. Thromboerythrocytes. In vitro studies of a potential autologous, semi-artificial alternative to platelet transfusions. J Clin Invest. 1992;89:546–555. doi: 10.1172/JCI115619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muzykantov VR, Sakharov DV, Smirnov MD, Samokhin GP, Smirnov VN. Immunotargeting of erythrocyte-bound streptokinase provides local lysis of a fibrin clot. Biochim Biophys Acta. 1986;884:355–362. doi: 10.1016/0304-4165(86)90184-4. [DOI] [PubMed] [Google Scholar]
- 4. Dale GL, Kuhl W, Beutler E. Incorporation of glucocerebrosidase into Gaucher's disease monocytes in vitro. Proc Natl Acad Sci U S A. 1979;76:473–475. doi: 10.1073/pnas.76.1.473. *This is one of the first papers describing RBC as a potential drug delivery system.
- 5.Hinderling PH. Red blood cells: a neglected compartment in pharmacokinetics and pharmacodynamics. Pharmacol Rev. 1997;49:279–295. [PubMed] [Google Scholar]
- 6.Dumez H, Highley M, Guetens G, et al. Erythrocytes and the transfer of anticancer drugs and metabolites: a possible relationship with therapeutic outcome. Semin Oncol. 2001;28:24–28. doi: 10.1016/s0093-7754(01)90209-x. [DOI] [PubMed] [Google Scholar]
- 7.Sakurai F, Nishioka T, Saito H, et al. Interaction between DNA-cationic liposome complexes and erythrocytes is an important factor in systemic gene transfer via the intravenous route in mice: the role of the neutral helper lipid. Gene Ther. 2001;8:677–686. doi: 10.1038/sj.gt.3301460. [DOI] [PubMed] [Google Scholar]
- 8.Ihler GM, Glew RH, Schnure FW. Enzyme loading of erythrocytes. Proc Natl Acad Sci U S A. 1973;70:2663–2666. doi: 10.1073/pnas.70.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ Res. 1996;79:581–589. doi: 10.1161/01.res.79.3.581. [DOI] [PubMed] [Google Scholar]
- 10.Kalfa TA, Pushkaran S, Mohandas N, et al. Rac GTPases regulate the morphology and deformability of the erythrocyte cytoskeleton. Blood. 2006;108:3637–3645. doi: 10.1182/blood-2006-03-005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasini EM, Kirkegaard M, Mortensen P, Lutz HU, Thomas AW, Mann M. Indepth analysis of the membrane and cytosolic proteome of red blood cells. Blood. 2006;108:791–801. doi: 10.1182/blood-2005-11-007799. [DOI] [PubMed] [Google Scholar]
- 12.Ktavtzoff R, Desbois I, Doinel C, et al. Immunological response to Lasparaginase loaded into red blood cells. Adv Exp Med Biol. 1992;326:175–182. doi: 10.1007/978-1-4615-3030-5_21. [DOI] [PubMed] [Google Scholar]
- 13.Tonetti M, Astroff B, Satterfield W, De Flora A, Benatti U, DeLoach JR. Construction and characterization of adriamycin-loaded canine red blood cells as a potential slow delivery system. Biotechnol Appl Biochem. 1990;12:621–629. [PubMed] [Google Scholar]
- 14.Rossi L, Serafini S, Cenerini L, et al. Erythrocyte-mediated delivery of dexamethasone in patients with chronic obstructive pulmonary disease. Biotechnol Appl Biochem. 2001;33:85–89. doi: 10.1042/ba20000087. [DOI] [PubMed] [Google Scholar]
- 15.Hamidi M, Tajerzadeh H. Carrier erythrocytes: an overview. Drug Deliv. 2003;10:9–20. doi: 10.1080/713840329. [DOI] [PubMed] [Google Scholar]
- 16. Hamidi M, Zarrin A, Foroozesh M, Mohammadi-Samani S. Applications of carrier erythrocytes in delivery of biopharmaceuticals. J Control Release. 2007;118:145–160. doi: 10.1016/j.jconrel.2006.06.032. **This and previous reviwes provide a comprehensive list of drugs encapsulated into RBC and briefly outline pharmacokinetic features of selected RBC-drug complexes in animal studies.
- 17.Pierige F, Serafini S, Rossi L, Magnani M. Cell-based drug delivery. Adv Drug Deliv Rev. 2008;60:286–295. doi: 10.1016/j.addr.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 18.Patel PD, Dand N, Hirlekar RS, Kadam VJ. Drug loaded erythrocytes: as novel drug delivery system. Curr Pharm Des. 2008;14:63–70. doi: 10.2174/138161208783330772. [DOI] [PubMed] [Google Scholar]
- 19. Rossi L, Serafini S, Pierige F, et al. Erythrocyte-based drug delivery. Expert Opin Drug Deliv. 2005;2:311–322. doi: 10.1517/17425247.2.2.311. **This authoritary review by one of the leaders of RBC-encapsulation describes methods for encapsulation and translational aspects of this drug delivery strategy, from animal to human studies.
- 20.Krantz A. Red cell-mediated therapy: opportunities and challenges. Blood Cells Mol Dis. 1997;23:58–68. doi: 10.1006/bcmd.1997.0119. [DOI] [PubMed] [Google Scholar]
- 21.Schlegel RA, Rechsteiner MC. Microinjection of thymidine kinase and bovine serum albumin into mammalian cells by fusion with red blood cells. Cell. 1975;5:371–379. doi: 10.1016/0092-8674(75)90056-2. [DOI] [PubMed] [Google Scholar]
- 22.Humphreys JD, Ihler G. Enhanced stability of erythrocyte-entrapped glucocerebrosidase activity. J Lab Clin Med. 1980;96:682–692. [PubMed] [Google Scholar]
- 23.Garin M, Rossi L, Luque J, Magnani M. Lactate catabolism by enzyme-loaded red blood cells. Biotechnol Appl Biochem. 1995;22(Pt 3):295–303. [PubMed] [Google Scholar]
- 24.Tajerzadeh H, Hamidi M. Evaluation of hypotonic preswelling method for encapsulation of enalaprilat in intact human erythrocytes. Drug Dev Ind Pharm. 2000;26:1247–1257. doi: 10.1081/ddc-100102306. [DOI] [PubMed] [Google Scholar]
- 25.Fraternale A, Rossi L, Magnani M. Encapsulation, metabolism and release of 2-fluoro-ara-AMP from human erythrocytes. Biochim Biophys Acta. 1996;1291:149–154. doi: 10.1016/0304-4165(96)00059-1. [DOI] [PubMed] [Google Scholar]
- 26. Magnani M, Casabianca A, Fraternale A, et al. Synthesis and targeted delivery of an azidothymidine homodinucleotide conferring protection to macrophages against retroviral infection. Proc Natl Acad Sci U S A. 1996;93:4403–4408. doi: 10.1073/pnas.93.9.4403. *This is one of the first experimental reports from Magnani's lab on use of RBC for drug delivery to macrophages.
- 27.Muzykantov VR, Smirnov MD, Samokhin GP. Avidin attachment to biotinylated erythrocytes induces homologous lysis via the alternative pathway of complement. Blood. 1991;78:2611–2618. [PubMed] [Google Scholar]
- 28.Muzykantov VR, Zaltsman AB, Smirnov MD, Samokhin GP, Morgan BP. Target-sensitive immunoerythrocytes: interaction of biotinylated red blood cells with immobilized avidin induces their lysis by complement. Biochim Biophys Acta. 1996;1279:137–143. doi: 10.1016/0005-2736(95)00260-x. [DOI] [PubMed] [Google Scholar]
- 29.Perez MT, Alvarez FJ, Garcia-Perez AI, Lucas L, Tejedor MC, Sancho P. Heterogeneity of hypotonically loaded rat erythrocyte populations as detected by countercurrent distribution in aqueous polymer two-phase systems. J Chromatogr B Biomed Appl. 1996;677:45–51. doi: 10.1016/0378-4347(95)00433-5. [DOI] [PubMed] [Google Scholar]
- 30.Alvarez FJ, Herraez A, Murciano JC, Jordan JA, Diez JC, Tejedor MC. In vivo survival and organ uptake of loaded carrier rat erythrocytes. J Biochem (Tokyo) 1996;120:286–291. doi: 10.1093/oxfordjournals.jbchem.a021411. [DOI] [PubMed] [Google Scholar]
- 31.Garin MI, Lopez RM, Sanz S, Pinilla M, Luque J. Erythrocytes as carriers for recombinant human erythropoietin. Pharm Res. 1996;13:869–874. doi: 10.1023/a:1016049027661. [DOI] [PubMed] [Google Scholar]
- 32.Teisseire B, Ropars C, Villereal MC, Nicolau C. Long-term physiological effects of enhanced O2 release by inositol hexaphosphate-loaded erythrocytes. Proc Natl Acad Sci U S A. 1987;84:6894–6898. doi: 10.1073/pnas.84.19.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boucher L, Chassaigne M, Ropars C. Internalization and distribution of inositol hexakisphosphate in red blood cells. Biotechnol Appl Biochem. 1996;24(Pt 1):73–78. [PubMed] [Google Scholar]
- 34.Eichler HG, Schneider W, Raberger G, Bacher S, Pabinger I. Erythrocytes as carriers for heparin. Preliminary in vitro and animal studies. Res Exp Med (Berl) 1986;186:407–412. doi: 10.1007/BF01852193. [DOI] [PubMed] [Google Scholar]
- 35.Flynn G, McHale L, McHale AP. Methotrexate-loaded, photosensitized erythrocytes: a photo-activatable carrier/delivery system for use in cancer therapy. Cancer Lett. 1994;82:225–229. doi: 10.1016/0304-3835(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 36.Millan CG, Castaneda AZ, Lopez FG, Marinero ML, Lanao JM, Arevalo M. Encapsulation and in vitro evaluation of amikacin-loaded erythrocytes. Drug Deliv. 2005;12:409–416. doi: 10.1080/10717540590968909. [DOI] [PubMed] [Google Scholar]
- 37.Gutierrez Millan C, Bax BE, Castaneda AZ, Marinero ML, Lanao JM. In vitro studies of amikacin-loaded human carrier erythrocytes. Transl Res. 2008;152:59–66. doi: 10.1016/j.trsl.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 38.Al-Achi A, Greenwood R. Erythrocytes as oral delivery systems for human insulin. Drug Dev Ind Pharm. 1998;24:67–72. doi: 10.3109/03639049809082354. [DOI] [PubMed] [Google Scholar]
- 39.Kravtzoff R, Urvoase E, Chambon C, Ropars C. Gd-DOTA loaded into red blood cells, a new magnetic resonance imaging contrast agents for vascular system. Adv Exp Med Biol. 1992;326:347–354. doi: 10.1007/978-1-4615-3030-5_42. [DOI] [PubMed] [Google Scholar]
- 40.Doucet D, Urvoas E, Kravtzoff R, et al. Blood-pool magnetic resonance imaging contrast agents. New developments. Invest Radiol. 1991;26 Suppl 1:S46–S47. doi: 10.1097/00004424-199111001-00014. discussion S60-44. [DOI] [PubMed] [Google Scholar]
- 41.Johnson KM, Tao JZ, Kennan RP, Gore JC. Gadolinium-bearing red cells as blood pool MRI contrast agents. Magn Reson Med. 1998;40:133–142. doi: 10.1002/mrm.1910400118. [DOI] [PubMed] [Google Scholar]
- 42.Hambye AS, Verbeke KA, Vandermeiren RP, Joosens EJ, Verbruggen AM, De Roo MJ. Comparison of modified technetium-99m albumin and technetium-99m red blood cells for equilibrium ventriculography. J Nucl Med. 1997;38:1521–1528. [PubMed] [Google Scholar]
- 43.Kim SH, Kim EJ, Hou JH, et al. Opsonized erythrocyte ghosts for liver-targeted delivery of antisense oligodeoxynucleotides. Biomaterials. 2009;30:959–967. doi: 10.1016/j.biomaterials.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 44.Grimaldi S, Lisi A, Pozzi D, Santoro N. Attempts to use liposomes and RBC ghosts as vectors in drug and antisense therapy of virus infection. Res Virol. 1997;148:177–180. doi: 10.1016/s0923-2516(97)89906-2. [DOI] [PubMed] [Google Scholar]
- 45.Byun HM, Suh D, Yoon H, et al. Erythrocyte ghost-mediated gene delivery for prolonged and blood-targeted expression. Gene Ther. 2004;11:492–496. doi: 10.1038/sj.gt.3302180. [DOI] [PubMed] [Google Scholar]
- 46.Antonelli A, Sfara C, Mosca L, Manuali E, Magnani M. New biomimetic constructs for improved in vivo circulation of superparamagnetic nanoparticles. J Nanosci Nanotechnol. 2008;8:2270–2278. doi: 10.1166/jnn.2008.190. [DOI] [PubMed] [Google Scholar]
- 47.Bax BE, Bain MD, Fairbanks LD, Webster AD, Chalmers RA. In vitro and in vivo studies with human carrier erythrocytes loaded with polyethylene glycol-conjugated and native adenosine deaminase. Br J Haematol. 2000;109:549–554. doi: 10.1046/j.1365-2141.2000.02059.x. [DOI] [PubMed] [Google Scholar]
- 48.Kravtzoff R, Desbois I, Lamagnere JP, et al. Improved pharmacodynamics of Lasparaginase-loaded in human red blood cells. Eur J Clin Pharmacol. 1996;49:465–470. doi: 10.1007/BF00195932. [DOI] [PubMed] [Google Scholar]
- 49.Kravtzoff R, Colombat PH, Desbois I, et al. Tolerance evaluation of Lasparaginase loaded in red blood cells. Eur J Clin Pharmacol. 1996;51:221–225. doi: 10.1007/s002280050187. [DOI] [PubMed] [Google Scholar]
- 50.Garin MI, Lopez RM, Luque J. Pharmacokinetic properties and in-vivo biological activity of recombinant human erythropoietin encapsulated in red blood cells. Cytokine. 1997;9:66–71. doi: 10.1006/cyto.1996.0137. [DOI] [PubMed] [Google Scholar]
- 51.van Wijk R, van Solinge WW. The energy-less red blood cell is lost: erythrocyte enzyme abnormalities of glycolysis. Blood. 2005;106:4034–4042. doi: 10.1182/blood-2005-04-1622. [DOI] [PubMed] [Google Scholar]
- 52.Dejam A, Hunter CJ, Pelletier MM, et al. Erythrocytes are the major intravascular storage sites of nitrite in human blood. Blood. 2005;106:734–739. doi: 10.1182/blood-2005-02-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Turrini F, Arese P, Yuan J, Low PS. Clustering of integral membrane proteins of the human erythrocyte membrane stimulates autologous IgG binding, complement deposition, and phagocytosis. J Biol Chem. 1991;266:23611–23617. [PubMed] [Google Scholar]
- 54.Chiarantini L, Rossi L, Fraternale A, Magnani M. Modulated red blood cell survival by membrane protein clustering. Mol Cell Biochem. 1995;144:53–59. doi: 10.1007/BF00926740. [DOI] [PubMed] [Google Scholar]
- 55.Paulitschke M, Nash GB, Anstee DJ, Tanner MJ, Gratzer WB. Perturbation of red blood cell membrane rigidity by extracellular ligands. Blood. 1995;86:342–348. [PubMed] [Google Scholar]
- 56.Zaltzman AB, Van den Berg CW, Muzykantov VR, Morgan BP. Enhanced complement susceptibility of avidin-biotin-treated human erythrocytes is a consequence of neutralization of the complement regulators CD59 and decay accelerating factor. Biochem J. 1995;307(Pt 3):651–656. doi: 10.1042/bj3070651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rancourt C, Robertson MW, 3rd, Wang M, et al. Endothelial cell vehicles for delivery of cytotoxic genes as a gene therapy approach for carcinoma of the ovary. Clin Cancer Res. 1998;4:265–270. [PubMed] [Google Scholar]
- 58.Jordan JA, Alvarez FJ, Lotero LA, et al. Differential induction of macrophage recognition of carrier erythrocytes by treatment with band 3 cross-linkers. Biotechnol Appl Biochem. 1998;27(Pt 2):133–137. [PubMed] [Google Scholar]
- 59.Lisovskaya IL, Shcherbachenko IM, Volkova RI, Ataullakhanov FI. Clotrimazole enhances lysis of human erythrocytes induced by t-BHP. Chem Biol Interact. 2009;180:433–439. doi: 10.1016/j.cbi.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 60.Turrini F, Mannu F, Arese P, Yuan J, Low PS. Characterization of the autologous antibodies that opsonize erythrocytes with clustered integral membrane proteins. Blood. 1993;81:3146–3152. [PubMed] [Google Scholar]
- 61.Geldwerth D, Helley D, de Jong K, et al. Detection of phosphatidylserine surface exposure on human erythrocytes using annexin V-ferrofluid. Biochem Biophys Res Commun. 1999;258:199–203. doi: 10.1006/bbrc.1999.0620. [DOI] [PubMed] [Google Scholar]
- 62.Closse C, Dachary-Prigent J, Boisseau MR. Phosphatidylserine-related adhesion of human erythrocytes to vascular endothelium. Br J Haematol. 1999;107:300–302. doi: 10.1046/j.1365-2141.1999.01718.x. [DOI] [PubMed] [Google Scholar]
- 63. Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288:2051–2054. doi: 10.1126/science.288.5473.2051. *This paper describes a recently discovered mechanism preventing uptake of RBC by host cells.
- 64.Ishikawa-Sekigami T, Kaneko Y, Okazawa H, et al. SHPS-1 promotes the survival of circulating erythrocytes through inhibition of phagocytosis by splenic macrophages. Blood. 2006;107:341–348. doi: 10.1182/blood-2005-05-1896. [DOI] [PubMed] [Google Scholar]
- 65.Jordan JA, Murciano JC, Lotero A, Herraez A, Diez JC. In vitro properties and organ uptake of rat band 3 cross-linked erythrocytes. Biochimie. 1997;79:53–61. doi: 10.1016/s0300-9084(97)87625-0. [DOI] [PubMed] [Google Scholar]
- 66.Chiarantini L, Johnson J, Deloach JR. Optimized recirculation survival of mouse carrier erythrocytes. Blood Cells. 1991;17:607–617. discussion 618–622. [PubMed] [Google Scholar]
- 67.Eichler HG, Gasic S, Bauer K, Korn A, Bacher S. In vivo clearance of antibody-sensitized human drug carrier erythrocytes. Clin Pharmacol Ther. 1986;40:300–303. doi: 10.1038/clpt.1986.180. [DOI] [PubMed] [Google Scholar]
- 68.Grover GJ, Loegering DJ. Effect of red blood cell stroma on the reticuloendothelial system clearance and killing of Streptococcus pneumoniae. Circ Shock. 1984;14:39–47. [PubMed] [Google Scholar]
- 69.Magnani M, Rossi L, D'Ascenzo M, Panzani I, Bigi L, Zanella A. Erythrocyte engineering for drug delivery and targeting. Biotechnol Appl Biochem. 1998;28(Pt 1):1–6. [PubMed] [Google Scholar]
- 70.Magnani M, Rossi L, Fraternale A, et al. Erythrocyte-mediated delivery of drugs, peptides and modified oligonucleotides. Gene Ther. 2002;9:749–751. doi: 10.1038/sj.gt.3301758. [DOI] [PubMed] [Google Scholar]
- 71.Alvarez FJ, Jordan JA, Calleja P, et al. Cross-linking treatment of loaded erythrocytes increases delivery of encapsulated substance to macrophages. Biotechnol Appl Biochem. 1998;27(Pt 2):139–143. [PubMed] [Google Scholar]
- 72.Alvarez FJ, Jordan JA, Herraez A, Diez JC, Tejedor MC. Hypotonically loaded rat erythrocytes deliver encapsulated substances into peritoneal macrophages. J Biochem. 1998;123:233–239. doi: 10.1093/oxfordjournals.jbchem.a021927. [DOI] [PubMed] [Google Scholar]
- 73.Magnani M, Rossi L, Brandi G, Schiavano GF, Montroni M, Piedimonte G. Targeting antiretroviral nucleoside analogues in phosphorylated form to macrophages: in vitro and in vivo studies. Proc Natl Acad Sci U S A. 1992;89:6477–6481. doi: 10.1073/pnas.89.14.6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gaudreault RC, Bellemare B, Lacroix J. Erythrocyte membrane-bound daunorubicin as a delivery system in anticancer treatment. Anticancer Res. 1989;9:1201–1205. [PubMed] [Google Scholar]
- 75.Schuchman E, Muro S. The development of enzyme replacement therapy for lysosomal diseases: Gaucher disease and beyond. In: Futerman T, Zimran A, editors. Gaucher Disease: Lessons Learned About Therapy of Lysosomal Diseases. CRC Press; 2006. pp. 125–140. [Google Scholar]
- 76.Schuchman EH, Suchi M, Takahashi T, Sandhoff K, Desnick RJ. Human acid sphingomyelinase. Isolation, nucleotide sequence and expression of the full-length and alternatively spliced cDNAs. J Biol Chem. 1991;266:8531–8539. [PubMed] [Google Scholar]
- 77.Schuchman EH. Hematopoietic stem cell gene therapy for Niemann-Pick disease and other lysosomal storage diseases. Chem Phys Lipids. 1999;102:179–188. doi: 10.1016/s0009-3084(99)00086-9. [DOI] [PubMed] [Google Scholar]
- 78.Muro S, Schuchman EH, Muzykantov VR. Lysosomal enzyme delivery by ICAM-1-targeted nanocarriers bypassing glycosylation- and clathrin-dependent endocytosis. Mol Ther. 2006;13:135–141. doi: 10.1016/j.ymthe.2005.07.687. [DOI] [PubMed] [Google Scholar]
- 79.Thorpe SR, Fiddler MB, Desnick RJ. Enzyme therapy. V. In vivo fate of erythrocyte-entrapped beta-glucuronidase in beta-glucuronidase-deficient mice. Pediatr Res. 1975;9:918–923. doi: 10.1203/00006450-197512000-00011. [DOI] [PubMed] [Google Scholar]
- 80.Beutler E, Dale GL, Guinto DE, Kuhl W. Enzyme replacement therapy in Gaucher's disease: preliminary clinical trial of a new enzyme preparation. Proc Natl Acad Sci U S A. 1977;74:4620–4623. doi: 10.1073/pnas.74.10.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Moran NF, Bain MD, Muqit MM, Bax BE. Carrier erythrocyte entrapped thymidine phosphorylase therapy for MNGIE. Neurology. 2008;71:686–688. doi: 10.1212/01.wnl.0000324602.97205.ab. *This paper reports outcome of RBC drug delivery study in human patients.
- 82.Magnani M, Rossi L, Casabianca A, et al. Red blood cells as advanced drug delivery systems for antiviral nucleoside analogues. Adv Exp Med Biol. 1992;326:239–245. doi: 10.1007/978-1-4615-3030-5_30. [DOI] [PubMed] [Google Scholar]
- 83.Benatti U, Giovine M, Damonte G, et al. Azidothymidine homodinucleotide-loaded erythrocytes and bioreactors for slow delivery of the antiretroviral drug azidothymidine. Biochem Biophys Res Commun. 1996;220:20–25. doi: 10.1006/bbrc.1996.0349. [DOI] [PubMed] [Google Scholar]
- 84.Rossi L, Brandi G, Schiavano GF, et al. Macrophage protection against human immunodeficiency virus or herpes simplex virus by red blood cell-mediated delivery of a heterodinucleotide of azidothymidine and acyclovir. AIDS Res Hum Retroviruses. 1998;14:435–444. doi: 10.1089/aid.1998.14.435. [DOI] [PubMed] [Google Scholar]
- 85.Fraternale A, Casabianca A, Orlandi C, et al. Macrophage protection by addition of glutathione (GSH)-loaded erythrocytes to AZT and DDI in a murine AIDS model. Antiviral Res. 2002;56:263–272. doi: 10.1016/s0166-3542(02)00128-6. [DOI] [PubMed] [Google Scholar]
- 86.Chiarantini L, Antonelli A, Rossi L, Fraternale A, Magnani M. Red blood cell phagocytosis following hexokinase inactivation. Cell Biochem Funct. 1994;12:217–220. doi: 10.1002/cbf.290120310. [DOI] [PubMed] [Google Scholar]
- 87.Magnani M, Casabianca A, Fraternale A, et al. Inhibition of murine AIDS by a new azidothymidine homodinucleotide. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17:189–195. doi: 10.1097/00042560-199803010-00001. [DOI] [PubMed] [Google Scholar]
- 88.Franchetti P, Cappellacci L, Petrelli R, et al. Inhibition of HIV-1 replication in macrophages by red blood cell-mediated delivery of a heterodinucleotide of lamivudine and tenofovir. Nucleosides Nucleotides Nucleic Acids. 2007;26:953–957. doi: 10.1080/15257770701508067. [DOI] [PubMed] [Google Scholar]
- 89.Rossi L, Brandi G, Schiavano GF, et al. Heterodimer-loaded erythrocytes as bioreactors for slow delivery of the antiviral drug azidothymidine and the antimycobacterial drug ethambutol. AIDS Res Hum Retroviruses. 1999;15:345–353. doi: 10.1089/088922299311312. [DOI] [PubMed] [Google Scholar]
- 90.Rossi L, Serafini S, Cappellacci L, et al. Erythrocyte-mediated delivery of a new homodinucleotide active against human immunodeficiency virus and herpes simplex virus. J Antimicrob Chemother. 2001;47:819–827. doi: 10.1093/jac/47.6.819. [DOI] [PubMed] [Google Scholar]
- 91.Fraternale A, Paoletti MF, Casabianca A, et al. Erythrocytes as carriers of antisense PNA addressed against HIV-1 gag-pol transframe domain. J Drug Target. 2009:1–8. doi: 10.1080/10611860902737474. [DOI] [PubMed] [Google Scholar]
- 92.Magnani M, Balestra E, Fraternale A, et al. Drug-loaded red blood cell-mediated clearance of HIV-1 macrophage reservoir by selective inhibition of STAT1 expression. J Leukoc Biol. 2003;74:764–771. doi: 10.1189/jlb.0403156. [DOI] [PubMed] [Google Scholar]
- 93.Magnani M, Rossi L, Fraternale A, et al. Targeting antiviral nucleotide analogues to macrophages. J Leukoc Biol. 1997;62:133–137. doi: 10.1002/jlb.62.1.133. [DOI] [PubMed] [Google Scholar]
- 94.Terpstra V, van Berkel TJ. Scavenger receptors on liver Kupffer cells mediate the in vivo uptake of oxidatively damaged red blood cells in mice. Blood. 2000;95:2157–2163. [PubMed] [Google Scholar]
- 95. Magnani M, Chiarantini L, Vittoria E, Mancini U, Rossi L, Fazi A. Red blood cells as an antigen-delivery system. Biotechnol Appl Biochem. 1992;16:188–194. *This paper describes a strategy of immune response modulation by RBC-antigen complexes.
- 96.Murray AM, Pearson IF, Fairbanks LD, Chalmers RA, Bain MD, Bax BE. The mouse immune response to carrier erythrocyte entrapped antigens. Vaccine. 2006;24:6129–6139. doi: 10.1016/j.vaccine.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 97.Olmos G, Lotero LA, Tejedor MC, Diez JC. Delivery to macrophages of interleukin 3 loaded in mouse erythrocytes. Biosci Rep. 2000;20:399–410. doi: 10.1023/a:1010334118492. [DOI] [PubMed] [Google Scholar]
- 98.Moyes RB, Kirch H, DeLoach JR. Enhanced biological activity of human recombinant interleukin 2 coupled to mouse red blood cells as evaluated using the mouse Meth A sarcoma model. Biotechnol Appl Biochem. 1996;23(Pt 1):29–36. [PubMed] [Google Scholar]
- 99.Chestier N, Kravtzoff R, Canepa S, Chassaigne M, Ropars C. Erythrocytes as carriers of ricin A chain: effects on the erythrophagocytic cells. Adv Exp Med Biol. 1992;326:279–289. doi: 10.1007/978-1-4615-3030-5_34. [DOI] [PubMed] [Google Scholar]
- 100.Rossi L, Serafini S, Antonelli A, et al. Macrophage depletion induced by clodronate-loaded erythrocytes. J Drug Target. 2005;13:99–111. doi: 10.1080/10611860500064123. [DOI] [PubMed] [Google Scholar]
- 101.Rossi L, Migliavacca B, Pierige F, et al. Prolonged islet allograft survival in diabetic mice upon macrophage depletion by clodronate-loaded erythrocytes. Transplantation. 2008;85:648–650. doi: 10.1097/TP.0b013e31816360f3. [DOI] [PubMed] [Google Scholar]
- 102.Rossi L, Castro M, D'Orio F, et al. Low doses of dexamethasone constantly delivered by autologous erythrocytes slow the progression of lung disease in cystic fibrosis patients. Blood Cells Mol Dis. 2004;33:57–63. doi: 10.1016/j.bcmd.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 103.Annese V, Latiano A, Rossi L, et al. Erythrocytes-mediated delivery of dexamethasone in steroid-dependent IBD patients-a pilot uncontrolled study. Am J Gastroenterol. 2005;100:1370–1375. doi: 10.1111/j.1572-0241.2005.41412.x. [DOI] [PubMed] [Google Scholar]
- 104.Castro M, Rossi L, Papadatou B, et al. Long-term treatment with autologous red blood cells loaded with dexamethasone 21-phosphate in pediatric patients affected by steroid-dependent Crohn disease. J Pediatr Gastroenterol Nutr. 2007;44:423–426. doi: 10.1097/MPG.0b013e3180320667. [DOI] [PubMed] [Google Scholar]
- 105.Bossa F, Latiano A, Rossi L, et al. Erythrocyte-mediated delivery of dexamethasone in patients with mild-to-moderate ulcerative colitis, refractory to mesalamine: a randomized, controlled study. Am J Gastroenterol. 2008;103:2509–2516. doi: 10.1111/j.1572-0241.2008.02103.x. [DOI] [PubMed] [Google Scholar]
- 106.Lizano C, Weissig V, Torchilin VP, Sancho P, Garcia-Perez AI, Pinilla M. In vivo biodistribution of erythrocytes and polyethyleneglycol-phosphatidylethanolamine micelles carrying the antitumour agent dequalinium. Eur J Pharm Biopharm. 2003;56:153–157. doi: 10.1016/s0939-6411(03)00089-4. [DOI] [PubMed] [Google Scholar]
- 107.Ataullakhanov FI, Kulikova EV, Vitvitsky VM. Reversible binding of anthracycline antibiotics to erythrocytes treated with glutaraldehyde. Biotechnol Appl Biochem. 1996;24(Pt 3):241–244. [PubMed] [Google Scholar]
- 108.Tonetti M, Zocchi E, Guida L, et al. Use of glutaraldehyde treated autologous human erythrocytes for hepatic targeting of doxorubicin. Adv Exp Med Biol. 1992;326:307–317. doi: 10.1007/978-1-4615-3030-5_37. [DOI] [PubMed] [Google Scholar]
- 109.Tonetti M, Astroff AB, Satterfield W, De Flora A, Benatti U, DeLoach JR. Pharmacokinetic properties of doxorubicin encapsulated in glutaraldehyde-treated canine erythrocytes. Am J Vet Res. 1991;52:1630–1635. [PubMed] [Google Scholar]
- 110.Matherne CM, Satterfield WC, Gasparini A, et al. Clinical efficacy and toxicity of doxorubicin encapsulated in glutaraldehyde-treated erythrocytes administered to dogs with lymphosarcoma. Am J Vet Res. 1994;55:847–853. [PubMed] [Google Scholar]
- 111. Skorokhod OA, Garmaeva T, Vitvitsky VM, et al. Pharmacokinetics of erythrocyte-bound daunorubicin in patients with acute leukemia. Med Sci Monit. 2004;10:PI55–PI64. *This and subsequent paper reports clinical studies of RBC drug delivery systems.
- 112.Skorokhod O, Kulikova EV, Galkina NM, et al. Doxorubicin pharmacokinetics in lymphoma patients treated with doxorubicin-loaded eythrocytes. Haematologica. 2007;92:570–571. doi: 10.3324/haematol.10770. [DOI] [PubMed] [Google Scholar]
- 113.Desilets J, Lejeune A, Mercer J, Gicquaud C. Nanoerythrosomes, a new derivative of erythrocyte ghost: IV. Fate of reinjected nanoerythrosomes. Anticancer Res. 2001;21:1741–1747. [PubMed] [Google Scholar]
- 114.Lejeune A, Moorjani M, Gicquaud C, Lacroix J, Poyet P, Gaudreault R. Nanoerythrosome, a new derivative of erythrocyte ghost: preparation and antineoplastic potential as drug carrier for daunorubicin. Anticancer Res. 1994;14:915–919. [PubMed] [Google Scholar]
- 115.Moorjani M, Lejeune A, Gicquaud C, Lacroix J, Poyet P, Gaudreault RC. Nanoerythrosomes, a new derivative of erythrocyte ghost II: identification of the mechanism of action. Anticancer Res. 1996;16:2831–2836. [PubMed] [Google Scholar]
- 116.Lejeune A, Poyet P, Gaudreault RC, Gicquaud C. Nanoerythrosomes, a new derivative of erythrocyte ghost: III. Is phagocytosis involved in the mechanism of action? Anticancer Res. 1997;17:3599–3603. [PubMed] [Google Scholar]
- 117.Magnani M, Fazi A, Mangani F, Rossi L, Mancini U. Methanol detoxification by enzyme-loaded erythrocytes. Biotechnol Appl Biochem. 1993;18(Pt 3):217–226. [PubMed] [Google Scholar]
- 118.Fazi A, Mancini U, Piatti E, Accorsi A, Magnani M. Human red blood cells as bioreactors for the inactivation of harmful xenobiotics. Biotechnol Appl Biochem. 1991;14:60–68. [PubMed] [Google Scholar]
- 119.Ihler G, Lantzy A, Purpura J, Glew RH. Enzymatic degradation of uric acid by uricase-loaded human erythrocytes. J Clin Invest. 1975;56:595–602. doi: 10.1172/JCI108129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Way JL, Leung P, Ray L, Sander C. Erythrocyte encapsulated thiosulfate sulfurtransferase. Bibl Haematol. 1985:75–81. doi: 10.1159/000410230. [DOI] [PubMed] [Google Scholar]
- 121.Leung P, Ray LE, Sander C, Way JL, Sylvester DM. Encapsulation of thiosulfate: cyanide sulfurtransferase by mouse erythrocytes. Toxicol Appl Pharmacol. 1986;83:101–107. doi: 10.1016/0041-008x(86)90327-3. [DOI] [PubMed] [Google Scholar]
- 122.Petrikovics I, Pei L, McGuinn WD, Cannon EP, Way JL. Encapsulation of rhodanese and organic thiosulfonates by mouse erythrocytes. Fundam Appl Toxicol. 1994;23:70–75. doi: 10.1006/faat.1994.1080. [DOI] [PubMed] [Google Scholar]
- 123.McGuinn WD, Cannon EP, Chui CT, Pei L, Petrikovics I, Way JL. The encapsulation of squid diisopropylphosphorofluoridate-hydrolyzing enzyme within mouse erythrocytes. Fundam Appl Toxicol. 1993;21:38–43. doi: 10.1006/faat.1993.1069. [DOI] [PubMed] [Google Scholar]
- 124.Pei L, Omburo G, McGuinn WD, et al. Encapsulation of phosphotriesterase within murine erythrocytes. Toxicol Appl Pharmacol. 1994;124:296–301. doi: 10.1006/taap.1994.1035. [DOI] [PubMed] [Google Scholar]
- 125.Pei L, Petrikovics I, Way JL. Antagonism of the lethal effects of paraoxon by carrier erythrocytes containing phosphotriesterase. Fundam Appl Toxicol. 1995;28:209–214. doi: 10.1006/faat.1995.1161. [DOI] [PubMed] [Google Scholar]
- 126.Sanz S, Pinilla M, Garin M, Tipton KF, Luque J. The influence of enzyme concentration on the encapsulation of glutamate dehydrogenase and alcohol dehydrogenase in red blood cells. Biotechnol Appl Biochem. 1995;22(Pt 2):223–231. [PubMed] [Google Scholar]
- 127.Lizano C, Sanz S, Luque J, Pinilla M. In vitro study of alcohol dehydrogenase and acetaldehyde dehydrogenase encapsulated into human erythrocytes by an electroporation procedure. Biochim Biophys Acta. 1998;1425:328–336. doi: 10.1016/s0304-4165(98)00085-3. [DOI] [PubMed] [Google Scholar]
- 128.Updike SJ, Wakamiya RT, Lightfoot EN., Jr Asparaginase entrapped in red blood cells: action and survival. Science. 1976;193:681–683. doi: 10.1126/science.821145. [DOI] [PubMed] [Google Scholar]
- 129.Baysal SH, Uslan AH. Encapsulation of Urease and PEG-Urease in erythrocyte. Artif Cells Blood Substit Immobil Biotechnol. 2000;28:263–271. doi: 10.3109/10731190009119357. [DOI] [PubMed] [Google Scholar]
- 130.Hamarat Baysal S, Uslan AH. Encapsulation of catalase and PEG-catalase in erythrocyte. Artif Cells Blood Substit Immobil Biotechnol. 2001;29:359–366. doi: 10.1081/bio-100106919. [DOI] [PubMed] [Google Scholar]
- 131.Hamarat Baysal S, Uslan AH. Encapsulation of PEG-urease/PEG-AlaDH enzyme system in erythrocyte. Artif Cells Blood Substit Immobil Biotechnol. 2001;29:405–412. doi: 10.1081/bio-100106924. [DOI] [PubMed] [Google Scholar]
- 132.Leung P, Cannon EP, Petrikovics I, Hawkins A, Way JL. In vivo studies on rhodanese encapsulation in mouse carrier erythrocytes. Toxicol Appl Pharmacol. 1991;110:268–274. doi: 10.1016/s0041-008x(05)80009-2. [DOI] [PubMed] [Google Scholar]
- 133.Way JL, Cannon EP, Leung P, Hawkins-Zitzer A, Pei L, Petrikovics I. Antagonism of the lethal effects of cyanide with resealed erythrocytes containing rhodanese and thiosulfate. Adv Exp Med Biol. 1992;326:159–163. doi: 10.1007/978-1-4615-3030-5_19. [DOI] [PubMed] [Google Scholar]
- 134.Cannon EP, Leung P, Hawkins A, Petrikovics I, DeLoach J, Way JL. Antagonism of cyanide intoxication with murine carrier erythrocytes containing bovine rhodanese and sodium thiosulfate. J Toxicol Environ Health. 1994;41:267–274. doi: 10.1080/15287399409531842. [DOI] [PubMed] [Google Scholar]
- 135.Petrikovics I, Cannon EP, McGuinn WD, et al. Cyanide antagonism with carrier erythrocytes and organic thiosulfonates. Fundam Appl Toxicol. 1995;24:86–93. doi: 10.1006/faat.1995.1010. [DOI] [PubMed] [Google Scholar]
- 136.Baysal SH, Uslan AH, Pala HH, Tuncoku O. Encapsulation of PEG-urease/PEG-AlaDH within sheep erythrocytes and determination of the system's activity in lowering blood levels of urea in animal models. Artif Cells Blood Substit Immobil Biotechnol. 2007;35:391–403. doi: 10.1080/10731190701460259. [DOI] [PubMed] [Google Scholar]
- 137.Kravtzoff R, Ropars C, Laguerre M, Muh JP, Chassaigne M. Erythrocytes as carriers for L-asparaginase. Methodological and mouse in-vivo studies. J Pharm Pharmacol. 1990;42:473–476. doi: 10.1111/j.2042-7158.1990.tb06598.x. [DOI] [PubMed] [Google Scholar]
- 138.Garin MI, Kravtzoff R, Chestier N, et al. Density gradient separation of L-asparaginase-loaded human erythrocytes. Biochem Mol Biol Int. 1994;33:807–814. [PubMed] [Google Scholar]
- 139.Kwon YM, Chung HS, Moon C, et al. L-Asparaginase encapsulated intact erythrocytes for treatment of acute lymphoblastic leukemia (ALL) J Control Release. 2009 doi: 10.1016/j.jconrel.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kosenko EA, Venediktova NI, Kudryavtsev AA, et al. Encapsulation of glutamine synthetase in mouse erythrocytes: a new procedure for ammonia detoxification. Biochem Cell Biol. 2008;86:469–476. doi: 10.1139/O08-134. [DOI] [PubMed] [Google Scholar]
- 141.Sanz S, Lizano C, Luque J, Pinilla M. In vitro and in vivo study of glutamate dehydrogenase encapsulated into mouse erythrocytes by a hypotonic dialysis procedure. Life Sci. 1999;65:2781–2789. doi: 10.1016/s0024-3205(99)00546-9. [DOI] [PubMed] [Google Scholar]
- 142.Lizano C, Perez MT, Pinilla M. Mouse erythrocytes as carriers for coencapsulated alcohol and aldehyde dehydrogenase obtained by electroporation in vivo survival rate in circulation, organ distribution and ethanol degradation. Life Sci. 2001;68:2001–2016. doi: 10.1016/s0024-3205(01)00991-2. [DOI] [PubMed] [Google Scholar]
- 143.Bax BE, Fairbanks LD, Bain MD, Simmonds HA, Chalmers RA. The entrapment of polyethylene glycol-bound adenosine deaminase (Pegademase) in human carrier erythrocytes. Biochem Soc Trans. 1996;24:442S. doi: 10.1042/bst024442s. [DOI] [PubMed] [Google Scholar]
- 144. Bax BE, Bain MD, Fairbanks LD, et al. A 9-yr evaluation of carrier erythrocyte encapsulated adenosine deaminase (ADA) therapy in a patient with adult-type ADA deficiency. Eur J Haematol. 2007;79:338–348. doi: 10.1111/j.1600-0609.2007.00927.x. *A longitudinal clinical study of a patient treatment with RBC-drug delivery approach.
- 145.Magnani M, Mancini U, Bianchi M, Fazi A. Comparison of uricase-bound and uricase-loaded erythrocytes as bioreactors for uric acid degradation. Adv Exp Med Biol. 1992;326:189–194. doi: 10.1007/978-1-4615-3030-5_23. [DOI] [PubMed] [Google Scholar]
- 146.Chambers E, Mitragotri S. Prolonged circulation of large polymeric nanoparticles by non-covalent adsorption on erythrocytes. J Control Release. 2004;100:111–119. doi: 10.1016/j.jconrel.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 147.Chambers E, Mitragotri S. Long circulating nanoparticles via adhesion on red blood cells: mechanism and extended circulation. Exp Biol Med (Maywood) 2007;232:958–966. [PubMed] [Google Scholar]
- 148.Hall SS, Mitragotri S, Daugherty PS. Identification of peptide ligands facilitating nanoparticle attachment to erythrocytes. Biotechnol Prog. 2007;23:749–754. doi: 10.1021/bp060333l. [DOI] [PubMed] [Google Scholar]
- 149.Smirnov VN, Domogatsky SP, Dolgov VV, et al. Carrier-directed targeting of liposomes and erythrocytes to denuded areas of vessel wall. Proc Natl Acad Sci U S A. 1986;83:6603–6607. doi: 10.1073/pnas.83.17.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Muzykantov VR, Smirnov MD, Zaltzman AB, Samokhin GP. Tannin-mediated attachment of avidin provides complement-resistant immunoerythrocytes that can be lysed in the presence of activator of complement. Anal Biochem. 1993;208:338–342. doi: 10.1006/abio.1993.1057. [DOI] [PubMed] [Google Scholar]
- 151.Muzykantov VR, Sakharov DV, Domogatsky SP, Goncharov NV, Danilov SM. Directed targeting of immunoerythrocytes provides local protection of endothelial cells from damage by hydrogen peroxide. Am J Pathol. 1987;128:276–285. [PMC free article] [PubMed] [Google Scholar]
- 152.Chiarantini L, Droleskey R, Magnani M, DeLoach JR. In vitro targeting of erythrocytes to cytotoxic T-cells by coupling of Thy-1.2 monoclonal antibody. Biotechnol Appl Biochem. 1992;15:171–184. [PubMed] [Google Scholar]
- 153.Orr GA. The use of the 2-iminobiotin-avidin interaction for the selective retrieval of labeled plasma membrane components. J Biol Chem. 1981;256:761–766. [PubMed] [Google Scholar]
- 154.Godfrey W, Doe B, Wallace EF, Bredt B, Wofsy L. Affinity targeting of membrane vesicles to cell surfaces. Exp Cell Res. 1981;135:137–145. doi: 10.1016/0014-4827(81)90306-2. [DOI] [PubMed] [Google Scholar]
- 155.Roffman E, Meromsky L, Ben-Hur H, Bayer EA, Wilchek M. Selective labeling of functional groups on membrane proteins or glycoproteins using reactive biotin derivatives and 125I-streptavidin. Biochem Biophys Res Commun. 1986;136:80–85. doi: 10.1016/0006-291x(86)90879-x. [DOI] [PubMed] [Google Scholar]
- 156.Bayer EA, Safars M, Wilchek M. Selective labeling of sulfhydryls and disulfides on blot transfers using avidin-biotin technology: studies on purified proteins and erythrocyte membranes. Anal Biochem. 1987;161:262–271. doi: 10.1016/0003-2697(87)90450-7. [DOI] [PubMed] [Google Scholar]
- 157.Wilchek M, Ben-Hur H, Bayer EA. p-Diazobenzoyl biocytin--a new biotinylating reagent for the labeling of tyrosines and histidines in proteins. Biochem Biophys Res Commun. 1986;138:872–879. doi: 10.1016/s0006-291x(86)80577-0. [DOI] [PubMed] [Google Scholar]
- 158.Samokhin GP, Smirnov MD, Muzykantov VR, Domogatsky SP, Smirnov VN. Red blood cell targeting to collagen-coated surfaces. FEBS Lett. 1983;154:257–261. doi: 10.1016/0014-5793(83)80160-4. [DOI] [PubMed] [Google Scholar]
- 159.Muzykantov VR, Smirnov MD, Klibanov AL. Avidin attachment to red blood cells via a phospholipid derivative of biotin provides complement-resistant immunoerythrocytes. J Immunol Methods. 1993;158:183–190. doi: 10.1016/0022-1759(93)90212-p. [DOI] [PubMed] [Google Scholar]
- 160.Smirnov MD, Samokhin GP, Muzykantov VR, Idelson GL, Domogatsky SP, Smirnov VN. Type I and III collagens as a possible target for drug delivery to the injured sites of vascular bed. Biochem Biophys Res Commun. 1983;116:99–105. doi: 10.1016/0006-291x(83)90386-8. [DOI] [PubMed] [Google Scholar]
- 161.Muzykantov VR, Sakharov DV, Smirnov MD, Domogatsky SP, Samokhin GP. Targeting of enzyme immobilized on erythrocyte membrane to collagen-coated surface. FEBS Lett. 1985;182:62–66. doi: 10.1016/0014-5793(85)81154-6. [DOI] [PubMed] [Google Scholar]
- 162.Magnani M, Chiarantini L, Mancini U. Preparation and characterization of biotinylated red blood cells. Biotechnol Appl Biochem. 1994;20(Pt 3):335–345. doi: 10.1111/j.1470-8744.1994.tb00321.x. [DOI] [PubMed] [Google Scholar]
- 163.Muzykantov VR, Taylor RP. Attachment of biotinylated antibody to red blood cells: antigen-binding capacity of immunoerythrocytes and their susceptibility to lysis by complement. Anal Biochem. 1994;223:142–148. doi: 10.1006/abio.1994.1559. [DOI] [PubMed] [Google Scholar]
- 164.Cowley H, Wojda U, Cipolone KM, Procter JL, Stroncek DF, Miller JL. Biotinylation modifies red cell antigens. Transfusion. 1999;39:163–168. doi: 10.1046/j.1537-2995.1999.39299154730.x. [DOI] [PubMed] [Google Scholar]
- 165.Suzuki T, Dale GL. Biotinylated erythrocytes: in vivo survival and in vitro recovery. Blood. 1987;70:791–795. [PubMed] [Google Scholar]
- 166.Suzuki T, Dale GL. Senescent erythrocytes: isolation of in vivo aged cells and their biochemical characteristics. Proc Natl Acad Sci U S A. 1988;85:1647–1651. doi: 10.1073/pnas.85.5.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mock DM, Lankford GL, Widness JA, Burmeister LF, Kahn D, Strauss RG. Measurement of red cell survival using biotin-labeled red cells: validation against 51Cr-labeled red cells. Transfusion. 1999;39:156–162. doi: 10.1046/j.1537-2995.1999.39299154729.x. [DOI] [PubMed] [Google Scholar]
- 168.Mock DM, Lankford GL, Widness JA, Burmeister LF, Kahn D, Strauss RG. Measurement of circulating red cell volume using biotin-labeled red cells: validation against 51Cr-labeled red cells. Transfusion. 1999;39:149–155. doi: 10.1046/j.1537-2995.1999.39299154728.x. [DOI] [PubMed] [Google Scholar]
- 169.Waugh RE, Narla M, Jackson CW, Mueller TJ, Suzuki T, Dale GL. Rheologic properties of senescent erythrocytes: loss of surface area and volume with red blood cell age. Blood. 1992;79:1351–1358. [PubMed] [Google Scholar]
- 170.Hoffmann-Fezer G, Mysliwietz J, Mortlbauer W, et al. Biotin labeling as an alternative nonradioactive approach to determination of red cell survival. Ann Hematol. 1993;67:81–87. doi: 10.1007/BF01788131. [DOI] [PubMed] [Google Scholar]
- 171.Franco RS, Lohmann J, Silberstein EB, et al. Time-dependent changes in the density and hemoglobin F content of biotin-labeled sickle cells. J Clin Invest. 1998;101:2730–2740. doi: 10.1172/JCI2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cavill I, Trevett D, Fisher J, Hoy T. The measurement of the total volume of red cells in man: a non-radioactive approach using biotin. Br J Haematol. 1988;70:491–493. doi: 10.1111/j.1365-2141.1988.tb02522.x. [DOI] [PubMed] [Google Scholar]
- 173.Hoffmann-Fezer G, Maschke H, Zeitler HJ, et al. Direct in vivo biotinylation of erythrocytes as an assay for red cell survival studies. Ann Hematol. 1991;63:214–217. doi: 10.1007/BF01703446. [DOI] [PubMed] [Google Scholar]
- 174.Cordle DG, Strauss RG, Lankford G, Mock DM. Antibodies provoked by the transfusion of biotin-labeled red cells. Transfusion. 1999;39:1065–1069. doi: 10.1046/j.1537-2995.1999.39101065.x. [DOI] [PubMed] [Google Scholar]
- 175.Muzykantov VR, Smirnov MD, Klibanov AL. Avidin attachment to biotinylated amino groups of the erythrocyte membrane eliminates homologous restriction of both classical and alternative pathways of the complement. FEBS Lett. 1993;318:108–112. doi: 10.1016/0014-5793(93)80002-c. [DOI] [PubMed] [Google Scholar]
- 176.Muzykantov VR, Seregina N, Smirnov MD. Fast lysis by complement and uptake by liver of avidin-carrying biotinylated erythrocytes. Int J Artif Organs. 1992;15:622–627. [PubMed] [Google Scholar]
- 177.Muzykantov VR, Smirnov MD, Samokhin GP. Streptavidin-induced lysis of homologous biotinylated erythrocytes. Evidence against the key role of the avidin charge in complement activation via the alternative pathway. FEBS Lett. 1991;280:112–114. doi: 10.1016/0014-5793(91)80216-p. [DOI] [PubMed] [Google Scholar]
- 178.Muzykantov VR, Smirnov MD, Samokhin GP. Avidin-induced lysis of biotinylated erythrocytes by homologous complement via the alternative pathway depends on avidin's ability of multipoint binding with biotinylated membrane. Biochim Biophys Acta. 1992;1107:119–125. doi: 10.1016/0005-2736(92)90336-k. [DOI] [PubMed] [Google Scholar]
- 179.Muzykantov VR, Smirnov MD, Samokhin GP. Avidin acylation prevents the complement-dependent lysis of avidin-carrying erythrocytes. Biochem J. 1991;273(Pt 2):393–397. doi: 10.1042/bj2730393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Muzykantov VR, Murciano JC. Attachment of antibody to biotinylated red blood cells: immuno-red blood cells display high affinity to immobilized antigen and normal biodistribution in rats. Biotechnol Appl Biochem. 1996;24(Pt 1):41–45. [PubMed] [Google Scholar]
- 181.Muzykantov VR, Murciano JC, Taylor RP, Atochina EN, Herraez A. Regulation of the complement-mediated elimination of red blood cells modified with biotin and streptavidin. Anal Biochem. 1996;241:109–119. doi: 10.1006/abio.1996.0384. [DOI] [PubMed] [Google Scholar]
- 182.Chiarantini L, Matteucci D, Pistello M, et al. AIDS vaccination studies using an ex vivo feline immunodeficiency virus model: homologous erythrocytes as a delivery system for preferential immunization with putative protective antigens. Clin Diagn Lab Immunol. 1998;5:235–241. doi: 10.1128/cdli.5.2.235-241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kirch HJ, Moyes RB, Chiarantini L, DeLoach JR. Effect of targeted erythrocytes coated with recombinant human interleukin 2 on T-lymphocyte proliferation in vitro. Biotechnol Appl Biochem. 1994;19(Pt 3):331–340. [PubMed] [Google Scholar]
- 184.Chiarantini L, Argnani R, Zucchini S, et al. Red blood cells as delivery system for recombinant HSV-1 glycoprotein B: immunogenicity and protection in mice. Vaccine. 1997;15:276–280. doi: 10.1016/s0264-410x(96)00181-8. [DOI] [PubMed] [Google Scholar]
- 185.Chiarantini L, Droleskey R, Magnani M, Kirch H, DeLoach JR. Targeting of erythrocytes to cytotoxic T-cells. Adv Exp Med Biol. 1992;326:257–267. doi: 10.1007/978-1-4615-3030-5_32. [DOI] [PubMed] [Google Scholar]
- 186. Scott MD, Murad KL, Koumpouras F, Talbot M, Eaton JW. Chemical camouflage of antigenic determinants: stealth erythrocytes. Proc Natl Acad Sci U S A. 1997;94:7566–7571. doi: 10.1073/pnas.94.14.7566. **This paper describes the concept of masking RBC antigens by PEG modification.
- 187.Scott MD, Bradley AJ, Murad KL. Camouflaged blood cells: low-technology bioengineering for transfusion medicine? Transfus Med Rev. 2000;14:53–63. doi: 10.1016/s0887-7963(00)80115-7. [DOI] [PubMed] [Google Scholar]
- 188.Garratty G. Stealth erythrocytes--a possible transfusion product for the new century? Vox Sang. 2000;78 Suppl 2:143–147. [PubMed] [Google Scholar]
- 189.Armstrong JK, Meiselman HJ, Fisher TC. Covalent binding of poly(ethylene glycol) (PEG) to the surface of red blood cells inhibits aggregation and reduces low shear blood viscosity. Am J Hematol. 1997;56:26–28. doi: 10.1002/(sici)1096-8652(199709)56:1<26::aid-ajh5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 190.Murad KL, Mahany KL, Brugnara C, Kuypers FA, Eaton JW, Scott MD. Structural and functional consequences of antigenic modulation of red blood cells with methoxypoly(ethylene glycol) Blood. 1999;93:2121–2127. [PubMed] [Google Scholar]
- 191.Bradley AJ, Test ST, Murad KL, Mitsuyoshi J, Scott MD. Interactions of IgM ABO antibodies and complement with methoxy-PEG-modified human RBCs. Transfusion. 2001;41:1225–1233. doi: 10.1046/j.1537-2995.2001.41101225.x. [DOI] [PubMed] [Google Scholar]
- 192.Nacharaju P, Boctor FN, Manjula BN, Acharya SA. Surface decoration of red blood cells with maleimidophenyl-polyethylene glycol facilitated by thiolation with iminothiolane: an approach to mask A, B, and D antigens to generate universal red blood cells. Transfusion. 2005;45:374–383. doi: 10.1111/j.1537-2995.2005.04290.x. [DOI] [PubMed] [Google Scholar]
- 193.Tan Y, Qiu Y, Xu H, et al. Decreased immunorejection in unmatched blood transfusions by attachment of methoxypolyethylene glycol on human red blood cells and the effect on D antigen. Transfusion. 2006;46:2122–2127. doi: 10.1111/j.1537-2995.2006.01038.x. [DOI] [PubMed] [Google Scholar]
- 194.Nacharaju P, Manjula BN, Acharya SA. Thiolation mediated pegylation platform to generate functional universal red blood cells. Artif Cells Blood Substit Immobil Biotechnol. 2007;35:107–118. doi: 10.1080/10731190600974657. [DOI] [PubMed] [Google Scholar]
- 195.Gundersen SI, Kennedy MS, Palmer AF. Immune recognition of exposed xenoantigens on the surface of PEGylated bovine red blood cells. Biotechnol Bioeng. 2008;101:337–344. doi: 10.1002/bit.21908. [DOI] [PubMed] [Google Scholar]
- 196.Sabolovic D, Sestier C, Perrotin P, Guillet R, Tefit M, Boynard M. Covalent binding of polyethylene glycol to the surface of red blood cells as detected and followed up by cell electrophoresis and rheological methods. Electrophoresis. 2000;21:301–306. doi: 10.1002/(SICI)1522-2683(20000101)21:2<301::AID-ELPS301>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 197.Chung HA, Kato K, Itoh C, Ohhashi S, Nagamune T. Casual cell surface remodeling using biocompatible lipid-poly(ethylene glycol)(n): development of stealth cells and monitoring of cell membrane behavior in serum-supplemented conditions. J Biomed Mater Res A. 2004;70:179–185. doi: 10.1002/jbm.a.20117. [DOI] [PubMed] [Google Scholar]
- 198.Leach JK, Hinman A, O'Rear EA. Investigation of deformability, viscosity, and aggregation of mPEG-modified erythrocytes. Biomed Sci Instrum. 2002;38:333–338. [PubMed] [Google Scholar]
- 199.Hashemi-Najafabadi S, Vasheghani-Farahani E, Shojaosadati SA, et al. A method to optimize PEG-coating of red blood cells. Bioconjug Chem. 2006;17:1288–1293. doi: 10.1021/bc060057w. [DOI] [PubMed] [Google Scholar]
- 200.Bradley AJ, Murad KL, Regan KL, Scott MD. Biophysical consequences of linker chemistry and polymer size on stealth erythrocytes: size does matter. Biochim Biophys Acta. 2002;1561:147–158. doi: 10.1016/s0005-2736(02)00339-5. [DOI] [PubMed] [Google Scholar]
- 201.Neu B, Armstrong JK, Fisher TC, Baumler H, Meiselman HJ. Electrophoretic mobility of human red blood cells coated with poly(ethylene glycol) Biorheology. 2001;38:389–403. [PubMed] [Google Scholar]
- 202.Neu B, Armstrong JK, Fisher TC, Meiselman HJ. Surface characterization of poly(ethylene glycol) coated human red blood cells by particle electrophoresis. Biorheology. 2003;40:477–487. [PubMed] [Google Scholar]
- 203.Armstrong JK, Meiselman HJ, Wenby RB, Fisher TC. Modulation of red blood cell aggregation and blood viscosity by the covalent attachment of Pluronic copolymers. Biorheology. 2001;38:239–247. [PubMed] [Google Scholar]
- 204.Bradley AJ, Scott MD. Immune complex binding by immunocamouflaged [poly(ethylene glycol)-grafted] erythrocytes. Am J Hematol. 2007;82:970–975. doi: 10.1002/ajh.20956. [DOI] [PubMed] [Google Scholar]
- 205.Bradley AJ, Scott MD. Separation and purification of methoxypoly(ethylene glycol) grafted red blood cells via two-phase partitioning. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;807:163–168. doi: 10.1016/j.jchromb.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 206.Blackall DP, Armstrong JK, Meiselman HJ, Fisher TC. Polyethylene glycolcoated red blood cells fail to bind glycophorin A-specific antibodies and are impervious to invasion by the Plasmodium falciparum malaria parasite. Blood. 2001;97:551–556. doi: 10.1182/blood.v97.2.551. [DOI] [PubMed] [Google Scholar]
- 207.Jeong ST, Byun SM. Decreased agglutinability of methoxy-polyethylene glycol attached red blood cells: significance as a blood substitute. Decreased agglutinability of methoxy-polyethylene glycol attached red blood cells: significance as a blood substitute. 1996;24:503–511. doi: 10.3109/10731199609117442. [DOI] [PubMed] [Google Scholar]
- 208.Chen PC, Huang W, Stassinopoulos A, Cheung AT. Effects of pegylated hamster red blood cells on microcirculation. Artif Cells Blood Substit Immobil Biotechnol. 2008;36:295–309. doi: 10.1080/10731190802239008. [DOI] [PubMed] [Google Scholar]
- 209.Jovtchev S, Stoeff S, Arnold K, Zschornig O. Studies on the aggregation behaviour of pegylated human red blood cells with the Zeta sedimentation technique. Clin Hemorheol Microcirc. 2008;39:229–233. [PubMed] [Google Scholar]
- 210.Garratty G. Modulating the red cell membrane to produce universal/stealth donor red cells suitable for transfusion. Vox Sang. 2008;94:87–95. doi: 10.1111/j.1423-0410.2007.01003.x. [DOI] [PubMed] [Google Scholar]
- 211.Garratty G. Progress in modulating the RBC membrane to produce transfusable universal/stealth donor RBCs. Transfus Med Rev. 2004;18:245–256. doi: 10.1016/j.tmrv.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 212.Hess C, Schifferli JA. Immune adherence revisited: novel players in an old game. News Physiol Sci. 2003;18:104–108. doi: 10.1152/nips.01425.2002. [DOI] [PubMed] [Google Scholar]
- 213.Taylor RP, Sutherland WM, Reist CJ, Webb DJ, Wright EL, Labuguen RH. Use of heteropolymeric monoclonal antibodies to attach antigens to the C3b receptor of human erythrocytes: a potential therapeutic treatment. Proc Natl Acad Sci U S A. 1991;88:3305–3309. doi: 10.1073/pnas.88.8.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tosi PF, Schwartz D, Sharma U, et al. Human erythrocytes bearing electroinserted CD4 neutralize infection in vitro by primary isolates of human immunodeficiency virus type 1. Blood. 1996;87:4839–4844. [PubMed] [Google Scholar]
- 215.Lindorfer MA, Hahn CS, Foley PL, Taylor RP. Heteropolymer-mediated clearance of immune complexes via erythrocyte CR1: mechanisms and applications. Immunol Rev. 2001;183:10–24. doi: 10.1034/j.1600-065x.2001.1830102.x. [DOI] [PubMed] [Google Scholar]
- 216.Fearon DT. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980;152:20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nickells M, Hauhart R, Krych M, et al. Mapping epitopes for 20 monoclonal antibodies to CR1. Clin Exp Immunol. 1998;112:27–33. doi: 10.1046/j.1365-2249.1998.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Krych-Goldberg M, Atkinson JP. Structure-function relationships of complement receptor type 1. Immunol Rev. 2001;180:112–122. doi: 10.1034/j.1600-065x.2001.1800110.x. [DOI] [PubMed] [Google Scholar]
- 219.Kuhn SE, Nardin A, Klebba PE, Taylor RP. Escherichia coli bound to the primate erythrocyte complement receptor via bispecific monoclonal antibodies are transferred to and phagocytosed by human monocytes in an in vitro model. J Immunol. 1998;160:5088–5097. [PubMed] [Google Scholar]
- 220.Nardin A, Sutherland WM, Hevey M, Schmaljohn A, Taylor RP. Quantitative studies of heteropolymer-mediated binding of inactivated Marburg virus to the complement receptor on primate erythrocytes. J Immunol Methods. 1998;211:21–31. doi: 10.1016/s0022-1759(97)00168-3. [DOI] [PubMed] [Google Scholar]
- 221. Taylor RP, Reist CJ, Sutherland WM, Otto A, Labuguen RH, Wright EL, et al. In vivo binding and clearance of circulating antigen by bispecific heteropolymer-mediated binding to primate erythrocyte complement receptor. J Immunol. 1992;148:2462–2468. **This paper introduces the strategy of pathogens clearance from blood using CR1-mediated anchoring of antibody conjugates.
- 222.Repik A, Pincus SE, Ghiran I. A transgenic mouse model for studying the clearance of blood-borne pathogens via human complement receptor 1 (CR1) Clin Exp Immunol. 2005;140:230–240. doi: 10.1111/j.1365-2249.2005.02764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Reinagel ML, Gezen M, Ferguson PJ, Kuhn S, Martin EN, Taylor RP. The primate erythrocyte complement receptor (CR1) as a privileged site: binding of immunoglobulin G to erythrocyte CR1 does not target erythrocytes for phagocytosis. Blood. 1997;89:1068–1077. [PubMed] [Google Scholar]
- 224.Gyimesi E, Bankovich AJ, Schuman TA, Goldberg JB, Lindorfer MA, Taylor RP. Staphylococcus aureus bound to complement receptor 1 on human erythrocytes by bispecific monoclonal antibodies is phagocytosed by acceptor macrophages. Immunol Lett. 2004;95:185–192. doi: 10.1016/j.imlet.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 225.Nardin A, Lindorfer MA, Taylor RP. How are immune complexes bound to the primate erythrocyte complement receptor transferred to acceptor phagocytic cells? Mol Immunol. 1999;36:827–835. doi: 10.1016/s0161-5890(99)00103-0. [DOI] [PubMed] [Google Scholar]
- 226.Taylor RP, Ferguson PJ, Martin EN, et al. Immune complexes bound to the primate erythrocyte complement receptor (CR1) via anti-CR1 mAbs are cleared simultaneously with loss of CR1 in a concerted reaction in a rhesus monkey model. Clin Immunol Immunopathol. 1997;82:49–59. doi: 10.1006/clin.1996.4286. [DOI] [PubMed] [Google Scholar]
- 227.Craig ML, Bankovich AJ, Taylor RP. Visualization of the transfer reaction: tracking immune complexes from erythrocyte complement receptor 1 to macrophages. Clin Immunol. 2002;105:36–47. doi: 10.1006/clim.2002.5266. [DOI] [PubMed] [Google Scholar]
- 228.Craig ML, Waitumbi JN, Taylor RP. Processing of C3b-opsonized immune complexes bound to non-complement receptor 1 (CR1) sites on red cells: phagocytosis, transfer, and associations with CR1. J Immunol. 2005;174:3059–3066. doi: 10.4049/jimmunol.174.5.3059. [DOI] [PubMed] [Google Scholar]
- 229.Reist CJ, Liang HY, Denny D, Martin EN, Scheld WM, Taylor RP. Cross-linked bispecific monoclonal antibody heteropolymers facilitate the clearance of human IgM from the circulation of squirrel monkeys. Eur J Immunol. 1994;24:2018–2025. doi: 10.1002/eji.1830240913. [DOI] [PubMed] [Google Scholar]
- 230.Ferguson PJ, Martin EN, Greene KL, et al. Antigen-based heteropolymers facilitate, via primate erythrocyte complement receptor type 1, rapid erythrocyte binding of an autoantibody and its clearance from the circulation in rhesus monkeys. J Immunol. 1995;155:339–347. [PubMed] [Google Scholar]
- 231.Lindorfer MA, Nardin A, Foley PL, et al. Targeting of Pseudomonas aeruginosa in the bloodstream with bispecific monoclonal antibodies. J Immunol. 2001;167:2240–2249. doi: 10.4049/jimmunol.167.4.2240. [DOI] [PubMed] [Google Scholar]
- 232.Taylor RP, Sutherland WM, Martin EN, et al. Bispecific monoclonal antibody complexes bound to primate erythrocyte complement receptor 1 facilitate virus clearance in a monkey model. J Immunol. 1997;158:842–850. [PubMed] [Google Scholar]
- 233.Taylor RP, Martin EN, Reinagel ML, et al. Bispecific monoclonal antibody complexes facilitate erythrocyte binding and liver clearance of a prototype particulate pathogen in a monkey model. J Immunol. 1997;159:4035–4044. [PubMed] [Google Scholar]
- 234.Hahn CS, French OG, Foley P, Martin EN, Taylor RP. Bispecific monoclonal antibodies mediate binding of dengue virus to erythrocytes in a monkey model of passive viremia. J Immunol. 2001;166:1057–1065. doi: 10.4049/jimmunol.166.2.1057. [DOI] [PubMed] [Google Scholar]
- 235.Asher DR, Cerny AM, Finberg RW. The erythrocyte viral trap: transgenic expression of viral receptor on erythrocytes attenuates coxsackievirus B infection. Proc Natl Acad Sci U S A. 2005;102:12897–12902. doi: 10.1073/pnas.0506211102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Craig ML, Reinagel ML, Martin EN, Schlimgen R, Nardin A, Taylor RP. Infusion of bispecific monoclonal antibody complexes into monkeys provides immunologic protection against later challenge with a model pathogen. Clin Immunol. 1999;92:170–180. doi: 10.1006/clim.1999.4739. [DOI] [PubMed] [Google Scholar]
- 237.Ferguson PJ, Reist CJ, Martin EN, et al. Antigen-based heteropolymers. A potential therapy for binding and clearing autoantibodies via erythrocyte CR1. Arthritis Rheum. 1995;38:190–200. doi: 10.1002/art.1780380207. [DOI] [PubMed] [Google Scholar]
- 238.Pincus SE, Lukacher N, Mohamed N, et al. Evaluation of antigen-based heteropolymer for treatment of systemic lupus erythematosus in a nonhuman primate model. Clin Immunol. 2002;105:141–154. doi: 10.1006/clim.2002.5274. [DOI] [PubMed] [Google Scholar]
- 239.Buster BL, Mattes KA, Scheld WM. Monoclonal antibody-mediated, complement-independent binding of human tumor necrosis factor-alpha to primate erythrocytes via complement receptor 1. J Infect Dis. 1997;176:1041–1046. doi: 10.1086/516536. [DOI] [PubMed] [Google Scholar]
- 240.Medof ME, Nagarajan S, Tykocinski ML. Cell-surface engineering with GPI-anchored proteins. FASEB J. 1996;10:574–586. doi: 10.1096/fasebj.10.5.8621057. [DOI] [PubMed] [Google Scholar]
- 241.Civenni G, Test ST, Brodbeck U, Butikofer P. In vitro incorporation of GPI-anchored proteins into human erythrocytes and their fate in the membrane. Blood. 1998;91:1784–1792. [PubMed] [Google Scholar]
- 242.Hill A, Ridley SH, Esser D, et al. Protection of erythrocytes from human complement-mediated lysis by membrane-targeted recombinant soluble CD59: a new approach to PNH therapy. Blood. 2006;107:2131–2137. doi: 10.1182/blood-2005-02-0782. [DOI] [PubMed] [Google Scholar]
- 243.Chen R, Walter EI, Parker G, et al. Mammalian glycophosphatidylinositol anchor transfer to proteins and posttransfer deacylation. Proc Natl Acad Sci U S A. 1998;95:9512–9517. doi: 10.1073/pnas.95.16.9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Suzuki K, Okumura Y. GPI-linked proteins do not transfer spontaneously from erythrocytes to liposomes. New aspects of reorganization of the cell membrane. Biochemistry. 2000;39:9477–9485. doi: 10.1021/bi000113v. [DOI] [PubMed] [Google Scholar]
- 245.Anderson SM, Yu G, Giattina M, Miller JL. Intercellular transfer of a glycosylphosphatidylinositol (GPI)-linked protein: release and uptake of CD4-GPI from recombinant adeno-associated virus-transduced HeLa cells. Proc Natl Acad Sci U S A. 1996;93:5894–5898. doi: 10.1073/pnas.93.12.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Kooyman DL, Byrne GW, McClellan S, et al. In vivo transfer of GPI-linked complement restriction factors from erythrocytes to the endothelium. Science. 1995;269:89–92. doi: 10.1126/science.7541557. *This paper introduces the conept of intercellular transfer of RBC-associated GPI-anchored proteins in the vasculature.
- 247.McCurry KR, Kooyman DL, Alvarado CG, et al. Human complement regulatory proteins protect swine-to-primate cardiac xenografts from humoral injury. Nat Med. 1995;1:423–427. doi: 10.1038/nm0595-423. [DOI] [PubMed] [Google Scholar]
- 248.Runge MS, Quertermous T, Zavodny PJ, et al. A recombinant chimeric plasminogen activator with high affinity for fibrin has increased thrombolytic potency in vitro and in vivo. Proc Natl Acad Sci U S A. 1991;88:10337–10341. doi: 10.1073/pnas.88.22.10337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Holvoet P, Laroche Y, Stassen JM, et al. Pharmacokinetic and thrombolytic properties of chimeric plasminogen activators consisting of a single-chain Fv fragment of a fibrin-specific antibody fused to single-chain urokinase. Blood. 1993;81:696–703. [PubMed] [Google Scholar]
- 250.Ding BS, Gottstein C, Grunow A, et al. Endothelial targeting of a recombinant construct fusing a PECAM-1 single-chain variable antibody fragment (scFv) with prourokinase facilitates prophylactic thrombolysis in the pulmonary vasculature. Blood. 2005;106:4191–4198. doi: 10.1182/blood-2005-05-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Oudin S, Libyh MT, Goossens D, et al. A soluble recombinant multimeric anti-Rh(D) single-chain Fv/CR1 molecule restores the immune complex binding ability of CR1-deficient erythrocytes. J Immunol. 2000;164:1505–1513. doi: 10.4049/jimmunol.164.3.1505. [DOI] [PubMed] [Google Scholar]
- 252.Kina T, Ikuta K, Takayama E, et al. The monoclonal antibody TER-119 recognizes a molecule associated with glycophorin A and specifically marks the late stages of murine erythroid lineage. Br J Haematol. 2000;109:280–287. doi: 10.1046/j.1365-2141.2000.02037.x. [DOI] [PubMed] [Google Scholar]
- 253. Spitzer D, Unsinger J, Bessler M, Atkinson JP. ScFv-mediated in vivo targeting of DAF to erythrocytes inhibits lysis by complement. Mol Immunol. 2004;40:911–919. doi: 10.1016/j.molimm.2003.10.017. *This paper introduced recombinant fusion proteins safely anchoring complement regulating proteins on RBC.
- 254.Spitzer D, Unsinger J, Mao D, Wu X, Molina H, Atkinson JP. In vivo correction of complement regulatory protein deficiency with an inhibitor targeting the red blood cell membrane. J Immunol. 2005;175:7763–7770. doi: 10.4049/jimmunol.175.11.7763. [DOI] [PubMed] [Google Scholar]
- 255.Spitzer D, Wu X, Ma X, Xu L, Ponder KP, Atkinson JP. Cutting edge: treatment of complement regulatory protein deficiency by retroviral in vivo gene therapy. J Immunol. 2006;177:4953–4956. doi: 10.4049/jimmunol.177.8.4953. [DOI] [PubMed] [Google Scholar]
- 256.Muller M, Buchi L, Woodtli K, Haeberli A, Beer JH. Preparation and characterization of 'heparinocytes': erythrocytes with covalently bound low molecular weight heparin. FEBS Lett. 2000;468:115–119. doi: 10.1016/s0014-5793(00)01204-7. [DOI] [PubMed] [Google Scholar]
- 257.Medof ME, Kinoshita T, Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984;160:1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Hoffman JF. On red blood cells, hemolysis and resealed ghosts. Adv Exp Med Biol. 1992;326:1–15. doi: 10.1007/978-1-4615-3030-5_1. [DOI] [PubMed] [Google Scholar]
- 259.Muzykantov VR, Murciano JC. Streptavidin-mediated coupling of therapeutic proteins to carrier erythrocytes. In: Magnani M, editor. Erythrocyte engineering for drug delivery and targeting. Boston-London: Eurekah.com; 2001. pp. 37–67. [Google Scholar]
- 260.Jackson MR, Clagett GP. Antithrombotic therapy in peripheral arterial occlusive disease. Chest. 1998;114:666S–682S. doi: 10.1378/chest.114.5_supplement.666s. [DOI] [PubMed] [Google Scholar]
- 261.Bradberry JC. Peripheral arterial disease: pathophysiology, risk factors, and role of antithrombotic therapy. J Am Pharm Assoc (Wash DC) 2004;44:S37–S44. doi: 10.1331/154434504322904596. quiz S44-35. [DOI] [PubMed] [Google Scholar]
- 262.Albers GW. Expanding the window for thrombolytic therapy in acute stroke. The potential role of acute MRI for patient selection. Stroke. 1999;30:2230–2237. doi: 10.1161/01.str.30.10.2230. [DOI] [PubMed] [Google Scholar]
- 263.Kang DW, Latour LL, Chalela JA, Dambrosia JA, Warach S. Early and late recurrence of ischemic lesion on MRI: evidence for a prolonged stroke-prone state? Neurology. 2004;63:2261–2265. doi: 10.1212/01.wnl.0000147295.50029.67. [DOI] [PubMed] [Google Scholar]
- 264.Wartenberg KE, Patsalides A, Yepes MS. Is magnetic resonance spectroscopy superior to conventional diagnostic tools in hypoxic-ischemic encephalopathy? J Neuroimaging. 2004;14:180–186. [PubMed] [Google Scholar]
- 265.Zivin JA. Thrombolytic stroke therapy: past, present, and future. Neurology. 1999;53:14–19. doi: 10.1212/wnl.53.1.14. [DOI] [PubMed] [Google Scholar]
- 266.Imperiale TF, Speroff T. A meta-analysis of methods to prevent venous thromboembolism following total hip replacement. Jama. 1994;271:1780–1785. [PubMed] [Google Scholar]
- 267.Zlokovic BV. Antithrombotic, procoagulant, and fibrinolytic mechanisms in cerebral circulation: implications for brain injury and protection. Neurosurg Focus. 1997;2:e5. [PubMed] [Google Scholar]
- 268.Topol EJ, Califf RM, Weisman HF, et al. Randomised trial of coronary intervention with antibody against platelet IIb/IIIa integrin for reduction of clinical restenosis: results at six months. The EPIC Investigators. Lancet. 1994;343:881–886. doi: 10.1016/s0140-6736(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 269.Konstantopoulos K, Mousa SA. Antiplatelet therapies: platelet GPIIb/IIIa antagonists and beyond. Curr Opin Investig Drugs. 2001;2:1086–1092. [PubMed] [Google Scholar]
- 270.Topol EJ, Morris DC, Smalling RW, et al. A multicenter, randomized, placebo-controlled trial of a new form of intravenous recombinant tissue-type plasminogen activator (activase) in acute myocardial infarction. J Am Coll Cardiol. 1987;9:1205–1213. doi: 10.1016/s0735-1097(87)80457-6. [DOI] [PubMed] [Google Scholar]
- 271.Narita M, Bu G, Herz J, Schwartz AL. Two receptor systems are involved in the plasma clearance of tissue-type plasminogen activator (t-PA) in vivo. J Clin Invest. 1995;96:1164–1168. doi: 10.1172/JCI118105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Reilly CF, Fujita T, Mayer EJ, Siegfried ME. Both circulating and clot-bound plasminogen activator inhibitor-1 inhibit endogenous fibrinolysis in the rat. Arterioscler Thromb. 1991;11:1276–1286. doi: 10.1161/01.atv.11.5.1276. [DOI] [PubMed] [Google Scholar]
- 273.Rijken DC, Barrett-Bergshoeff MM, Jie AF, Criscuoli M, Sakharov DV. Clot penetration and fibrin binding of amediplase, a chimeric plasminogen activator (K2 tu-PA) Thromb Haemost. 2004;91:52–60. doi: 10.1160/TH03-07-0435. [DOI] [PubMed] [Google Scholar]
- 274.Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med. 1998;4:228–231. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- 275.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 276.Collen D, Van Hoef B, Schlott B, Hartmann M, Guhrs KH, Lijnen HR. Mechanisms of activation of mammalian plasma fibrinolytic systems with streptokinase and with recombinant staphylokinase. Eur J Biochem. 1993;216:307–314. doi: 10.1111/j.1432-1033.1993.tb18147.x. [DOI] [PubMed] [Google Scholar]
- 277.Holvoet P, Dewerchin M, Stassen JM, et al. Thrombolytic profiles of clot-targeted plasminogen activators. Parameters determining potency and initial and maximal rates. Circulation. 1993;87:1007–1016. doi: 10.1161/01.cir.87.3.1007. [DOI] [PubMed] [Google Scholar]
- 278.Muzykantov VR, Barnathan ES, Atochina EN, Kuo A, Danilov SM, Fisher AB. Targeting of antibody-conjugated plasminogen activators to the pulmonary vasculature. J Pharmacol Exp Ther. 1996;279:1026–1034. [PubMed] [Google Scholar]
- 279.Runge MS, Harker LA, Bode C, et al. Enhanced thrombolytic and antithrombotic potency of afibrin-targeted plasminogen activator in baboons. Circulation. 1996;94:1412–1422. doi: 10.1161/01.cir.94.6.1412. [DOI] [PubMed] [Google Scholar]
- 280.Benedict CR, Refino CJ, Keyt BA, et al. New variant of human tissue plasminogen activator (TPA) with enhanced efficacy and lower incidence of bleeding compared with recombinant human TPA. Circulation. 1995;92:3032–3040. doi: 10.1161/01.cir.92.10.3032. [DOI] [PubMed] [Google Scholar]
- 281.Chapman DF, Lyden P, Lapchak PA, Nunez S, Thibodeaux H, Zivin J. Comparison of TNK with wild-type tissue plasminogen activator in a rabbit embolic stroke model. Stroke. 2001;32:748–752. doi: 10.1161/01.str.32.3.748. [DOI] [PubMed] [Google Scholar]
- 282.Marder VJ, Stewart D. Towards safer thrombolytic therapy. Semin Hematol. 2002;39:206–216. doi: 10.1053/shem.2002.34088. [DOI] [PubMed] [Google Scholar]
- 283.Thomas GR, Thibodeaux H, Errett CJ, et al. A long-half-life and fibrin-specific form of tissue plasminogen activator in rabbit models of embolic stroke and peripheral bleeding. Stroke. 1994;25:2072–2078. doi: 10.1161/01.str.25.10.2072. discussion 2078–2079. [DOI] [PubMed] [Google Scholar]
- 284.Zhang RL, Zhang L, Jiang Q, Zhang ZG, Goussev A, Chopp M. Postischemic intracarotid treatment with TNK-tPA reduces infarct volume and improves neurological deficits in embolic stroke in the unanesthetized rat. Brain Res. 2000;878:64–71. doi: 10.1016/s0006-8993(00)02693-7. [DOI] [PubMed] [Google Scholar]
- 285.Simon SI, Goldsmith HL. Leukocyte adhesion dynamics in shear flow. Ann Biomed Eng. 2002;30:315–332. doi: 10.1114/1.1467677. [DOI] [PubMed] [Google Scholar]
- 286.Graham DA, Huang TC, Keyt BA, Alevriadou BR. Real-time measurement of lysis of mural platelet deposits by fibrinolytic agents under arterial flow. Ann Biomed Eng. 1998;26:712–724. doi: 10.1114/1.46. [DOI] [PubMed] [Google Scholar]
- 287.Konstantopoulos K, Kukreti S, McIntire LV. Biomechanics of cell interactions in shear fields. Adv Drug Deliv Rev. 1998;33:141–164. doi: 10.1016/s0169-409x(98)00024-6. [DOI] [PubMed] [Google Scholar]
- 288. Murciano JC, Medinilla S, Eslin D, Atochina E, Cines DB, Muzykantov VR. Prophylactic fibrinolysis through selective dissolution of nascent clots by tPA-carrying erythrocytes. Nat Biotechnol. 2003;21:891–896. doi: 10.1038/nbt846. This paper describes safe coupling of **fibrinolytics to RBC and their use for paradigm-shifting prophylactic thrombolysis in animal models.
- 289.Zaitsev S, Danielyan K, Murciano JC, et al. Human complement receptor type 1-directed loading of tissue plasminogen activator on circulating erythrocytes for prophylactic fibrinolysis. Blood. 2006;108:1895–1902. doi: 10.1182/blood-2005-11-012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ganguly K, Murciano JC, Westrick R, Leferovich J, Cines DB, Muzykantov VR. The glycocalyx protects erythrocyte-bound tissue-type plasminogen activator from enzymatic inhibition. J Pharmacol Exp Ther. 2007;321:158–164. doi: 10.1124/jpet.106.114405. [DOI] [PubMed] [Google Scholar]
- 291.Ganguly K, Krasik T, Medinilla S, et al. Blood clearance and activity of erythrocyte-coupled fibrinolytics. J Pharmacol Exp Ther. 2005;312:1106–1113. doi: 10.1124/jpet.104.075770. [DOI] [PubMed] [Google Scholar]
- 292.Ganguly K, Goel MS, Krasik T, Bdeir K, Diamond SL, Cines D, Muzykantov VR, Murciano JC. Fibrin affinity of erythrocyte-coupled tPA resists hemodynamic forces and enhances fibrinolysis in vivo. J Pharmacol Exp Ther. 2006;216:1130–1136. doi: 10.1124/jpet.105.093450. [DOI] [PubMed] [Google Scholar]
- 293. Danielyan K, Ganguly K, Ding BS, et al. Cerebrovascular thromboprophylaxis in mice by erythrocyte-coupled tissue-type plasminogen activator. Circulation. 2008;118:1442–1449. doi: 10.1161/CIRCULATIONAHA.107.750257. **This paper describes advantages of safe prophylactic thrombolysis attained by RBC-tPA conjugate in animal models of stroke.
- 294.Armstead WM, Ganguly K, Kiessling JW, et al. Red blood cells-coupled tPA prevents impairment of cerebral vasodilatory responses and tissue injury in pediatric cerebral hypoxia/ischemia through inhibition of ERK MAPK activation. J Cereb Blood Flow Metab. 2009;29:1463–1474. doi: 10.1038/jcbfm.2009.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Murciano JC, Higazi AA, Cines DB, Muzykantov VR. Soluble urokinase receptor conjugated to carrier red blood cells binds latent pro-urokinase and alters its functional profile. J Control Release. 2009;139:190–196. doi: 10.1016/j.jconrel.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Cornacoff JB, Hebert LA, Smead WL, VanAman ME, Birmingham DJ, Waxman FJ. Primate erythrocyte-immune complex-clearing mechanism. J Clin Invest. 1983;71:236–247. doi: 10.1172/JCI110764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Whipple EC, Shanahan RS, Ditto AH, Taylor RP, Lindorfer MA. Analyses of the in vivo trafficking of stoichiometric doses of an anti-complement receptor 1/2 monoclonal antibody infused intravenously in mice. J Immunol. 2004;173:2297–2306. doi: 10.4049/jimmunol.173.4.2297. [DOI] [PubMed] [Google Scholar]
- 298.Reid ME. Some concepts relating to the molecular genetic basis of certain MNS blood group antigens. Transfus Med. 1994;4:99–111. doi: 10.1111/j.1365-3148.1994.tb00250.x. [DOI] [PubMed] [Google Scholar]
- 299.Chasis JA, Mohandas N. Red blood cell glycophorins. Blood. 1992;80:1869–1879. [PubMed] [Google Scholar]