Abstract
Focusing on symptoms of attention deficit hyperactivity disorder (ADHD) in a sample obtained from the general population, we aimed to investigate the effects of incentives and event rate on reaction time (RT) performance and response inhibition. We assessed 1156 children, at a mean age of 8 years, on their performance on an inhibition task and a RT task under different experimental conditions that manipulated event rate and incentives. Children with high ADHD (ADHD-H) symptoms showed cognitive performance deficits only under some of the experimental conditions compared to a control group. The fast-incentive condition of the RT task succeeded in normalizing the RT variability, as well as the slow overall speed, in the ADHD-H group. Analyses of ADHD symptom scores as a quantitative trait in the total sample were overall consistent with these findings. The findings suggest that at least some cognitive performance deficits in children with high ADHD symptoms do notreflect stable cognitive deficits. The degree to which cognitive impairments in ADHD can be modulated by energetic or motivational factors has important implications for clinical and educational interventions.
Keywords: Attention deficit hyperactivity disorder, Reaction times, Response time, Inhibition, Attention, Incentives
INTRODUCTION
Studies of the cognitive profile in attention deficit hyperactivity disorder (ADHD) indicate poor performance across multiple executive, as well as nonexecutive, domains (Kuntsi et al., 2006a; Willcutt et al., 2005). Yet there is inconsistency in findings across studies. Rather than considering the inconsistency as “noise”, the factors underlying it may inform us on the underlying processes.
A recent detailed investigation confirmed earlier observations that the largest differences in comparisons between children with ADHD and control children across several commonly used cognitive tasks emerge for reaction time (RT) variability (Klein et al., 2006). In some studies, this emerged initially as a “side” finding when other cognitive variables failed to distinguish between the groups (Kuntsi & Stevenson, 2001; Kuntsi et al., 2001). The strength and consistency of the association have, however, placed RT variability firmly on the scientific agenda. The proposed underlying causes for RT variability in ADHD continue to be debated (Bellgrove et al., 2005; Castellanos & Tannock, 2002; Castellanos et al., 2005; Kuntsi et al., 2006a), but several of the models require further development to include explicit testable predictions that enable them to be properly proved or falsified.
Based on predictions emerging from theories of a deficit in the regulation of energetic state in ADHD (Sergeant, 2005; van der Meere, 2002), we recently investigated the extent to which RT variability can be “normalized” in ADHD using a simple RT task unaffected by more complex cognitive demands—referred to as the “fast task” (Andreou et al., 2007). We showed that under a condition with a fast event rate and incentives, the ADHD group improved significantly more than the control group in both speed and RT variability. Yet the performance of the ADHD group did not completely reach the level of the control group in the fast-incentive condition, suggesting that neither the manipulations of event rate or incentives were fully successful in optimizing the energetic state of the participants with ADHD or that additional processes may be involved.
ADHD is also linked to performance deficits on inhibitory control tasks (Willcutt et al., 2005). However, several authors have questioned the primacy of an inhibition deficit in ADHD as the underlying mechanism for the observed difference in performance. Recent meta-analyses indicate that poor performance in ADHD on the stop task, which involves the suppression of an ongoing response, is not due to real differences in the inhibition measure of stopping speed between ADHD and control groups but reflects differences in children's mean RT (MRT) to go stimuli (Alderson et al., 2007; Lijffijt et al., 2005). A carefully controlled cognitive experiment indicated poor overall performance in a combined continuous performance test—go/no-go task in young children at risk for ADHD, but no evidence of a deficit specific to either inhibitory control or sustained attention (Berwid et al., 2005). Electrophysiological data further indicate that inhibitory control difficulties in ADHD are accompanied by altered response preparation and execution processes in children (Banaschewski et al., 2004; Brandeis et al., 1998; Pliszka et al., 2000; van Leeuwen et al., 1998) and adults (McLoughlin et al., under review), which may indicate a more general state or response regulation problem (Banaschewski et al., 2004).
The primacy of an inhibition deficit in ADHD has been further studied using event rate and incentive manipulations. In two studies, an apparent response inhibition deficit in ADHD “disappeared” following the introduction of incentives (Konrad et al., 2000; Slusarek et al., 2001); yet this is not consistently found, and in general, study results appear highly sensitive to task parameters (Luman et al., 2005). Using event rate manipulations, some studies report no inhibition deficit in ADHD under any event rate, whereas other studies find ADHD–control differences only in a slow condition or, conversely, only in a fast condition (van der Meere, 2002; Wiersema et al., 2006). The effects of such manipulations on RT performance, when measured by the stop and go/no-go tasks, also vary across studies, emphasizing both the sensitivity of ADHD performance to task parameters and the differential sensitivity of different aspects of task performance to the same task manipulation. For example, a fast event rate may lead to improved speed but worse inhibition performance. Yet a key finding emerges: Children with ADHD do not show a stable inhibition deficit across varying task conditions.
The cognitive studies on ADHD commonly focus on clinically diagnosed ADHD. Given that selection biases may influence the composition of clinic-based samples, a complementary approach that focuses on ADHD symptoms in a sample obtained from the general population is required for a detailed understanding of the underlying processes. Such an approach is especially common in quantitative genetic research that assumes a liability threshold model, in which ADHD symptoms are continuously distributed throughout the population, with the “extreme” group defined on the basis of a predetermined cutoff point on a quantitative scale. The underlying assumption is that multiple susceptibility factors (genes and environment) contribute to an underlying distribution of liability for the disorder. The evidence from quantitative genetic (twin and sibling) studies, as well as from epidemiological studies of environmental risk factors and prediction of adverse outcomes, is consistent with the hypothesis that ADHD represents the extreme of a quantitative trait in the population (Chen et al., 2008).
This study aimed to investigate the effects of incentives and event rate on RT performance and response inhibition by comparing the top 5% of the highest scoring individuals on ADHD symptoms from a large general population sample to the remainder of the sample. A 5% threshold was chosen to be comparable to estimated prevalence rates of ADHD in the United Kingdom of 4% for males and 1% for females (Ford et al., 2003) while also ensuring an adequate sample size for the analyses. Specifically, we investigated the extent to which RT variability, MRT, and inhibition performance can be normalized under conditions that aim to optimize the energetic state in ADHD. We have previously carried out a small-scale pilot investigation of the association between ADHD symptom scores in the general population and performance on the fast task and a go/no-go task under different conditions (Kuntsi et al., 2005). Here, we extend this research to a separate large sample of 1156 children aged 7–9 years, and we compare the top 5% of the highest scoring individuals on the ADHD scale with the rest of the sample. We investigate the effects of event rate and incentives both separately (go/no-go task) and in combination (fast task). The latter approach specifically examines the extent to which a response style characterized by slow and variable speed of responding can be maximally reduced. In addition, we investigate associations with task performance for continuous ADHD symptom scores in the total sample.
METHODS
Sample and Procedure
Participants are members of the Study of Activity and Impulsivity Levels in children (SAIL) (Kuntsi et al., 2006b), a study of a general population sample of twins aged 7–10 years. The sample was recruited from a birth cohort study, the Twins' Early Development Study (TEDS; Trouton et al., 2002), which had invited parents of all twins born in England and Wales during 1994–1996 to enroll. Zygosity was determined using a standard zygosity questionnaire that has been shown to have 95% accuracy (Price et al., 2000).
Families on the TEDS register were invited to take part if they fulfilled the following SAIL project inclusion criteria: twins' birthdates between September 1, 1995, and December 31, 1996; lived within a feasible traveling distance from the research center; ethnic origin White European (to reduce population heterogeneity for molecular genetic studies); recent participation in TEDS, as indicated by return of questionnaires at either 4- or 7-year data collection point; no extreme pregnancy or perinatal difficulties or specific medical syndromes, chromosomal anomalies, or epilepsy; not participating in other current TEDS substudies; and not on stimulant or other neuropsychiatric medications.
Of the 1230 suitable families contacted, 672 families agreed to participate, reflecting a participation rate of 55%. Thirty individual children were subsequently excluded because of intelligence quotient (IQ) <70, epilepsy, autism, obsessive–compulsive disorder or neurodevelopmental disorder illness during testing, or placement on stimulant medication for ADHD. For the current analyses, we only included participants from whom we had the ADHD ratings from parents and teachers, reflecting 82% of the total available SAIL sample. The final sample for the current analyses consisted of 1156 children. The mean age was 8.79 years (SD = 0.66). The children's IQs ranged from 70 to 158 (mean = 109.70, SD = 14.72). The sample was 50% male. Parents of all participants have given informed consent, and the Institute of Psychiatry Ethical Committee approved the study.
The families visited the research center for the assessments. Two testers assessed the twins simultaneously in separate testing rooms. The tasks were administered in a fixed order as part of a more extensive test session, which in total lasted approximately 2.5 hr.
Measures
Wechsler Intelligence Scales for Children, Third Edition
The Vocabulary, Similarities, Picture Completion and Block Design subtests from the Wechsler Intelligence Scale for Children, Third Edition (WISC-III; Wechsler, 1991), were used to obtain an estimate of the child's IQ following procedures described by Sattler (Sattler, 1992).
The go/no-go task
On each trial of the go/no-go task (Borger & van der Meere, 2000; Kuntsi et al., 2005; van der Meere, et al., 1995), one of two possible stimuli appeared for 300 ms in the middle of the computer screen. The child was instructed to respond only to the “go” stimuli and to react as quickly as possible but to maintain a high level of accuracy. The proportion of “go” stimuli to “no-go” stimuli was 4:1. The response variables were commission errors (an index of inhibition), MRT to “go” stimuli, and SD of the RTs. Omission errors were rare—mean in each condition 2–12%—and were therefore not included in analyses.
The children performed the task under three different conditions matched for length of time on task. The fast condition consisted of 462 trials and had an interstimulus interval (ISI) of 1 s. The ISI dropped to 8 s in the slow presentation condition, which consisted of 72 trials. The order of presentation of the slow and fast conditions was varied randomly across children.
The incentive condition was administered last to ensure that the possibility of earning rewards would not adversely affect performance on the other conditions where rewards could not be earned. This condition is a modification of the incentive condition used in the study on the stop task by Slusarek et al. (2001). Each correct response to the letter X and each correct nonresponse to the letter O earned the child 1 point. The child lost 1 point for each omission error (failure to respond to X) and for each failure to respond within 2 s. Each commission error (incorrect response to O) led to the loss of 5 points. The points were shown in a box, immediately right of the screen center, and were updated continuously throughout. The child started with 40 points to avoid the possibility of a negative tally. The child was asked to try to win as many points as possible and was told that the points will be exchanged for a real prize when the game ends. This condition consisted of 72 trials and had an ISI of 8 s. A practice session preceded each experimental condition.
The fast task
The baseline condition of the fast task (Andreou et al., 2007; Kuntsi et al., 2005; Kuntsi et al., 2006b) followed a standard warned four-choice RT, as outlined in Leth-Steensen et al. (2000). A warning signal (four empty circles arranged side by side) first appeared on the screen. At the end of the fore period (presentation interval for the warning signal), the circle designated as the target signal for that trial was filled (colored) in. The child was asked to make a compatible choice by pressing the response key that directly corresponded in position to the location of the target stimulus. Following a response, the stimuli disappeared from the screen and a fixed intertrial interval of 2.5 s followed. Speed and accuracy were emphasized equally. If the child did not respond within 10 s, the trial terminated.
First, a practice session was administered, during which the child had to respond correctly to five consecutive trials. The baseline condition, with a fore period of 8 s and consisting of 72 trials, then followed.
To investigate the extent to which a response style characterized by slow and variable speed of responding can be maximally reduced, the task includes a comparison condition that uses a fast event rate (1 s) and incentives. This condition started immediately after the baseline condition and consisted of 80 trials using the faster event rate conditions employed by Leth-Steensen et al. (2000). The children were told to respond really quickly one after another to win smiley faces and earn real prizes in the end. The children won a smiley face for responding faster than their own MRT during the baseline (first) condition consecutively for three trials. The baseline MRT was calculated here based on the middle 94% of responses, therefore excluding extremely fast and extremely slow responses. The smiley faces appeared below the circles in the middle of the screen and were updated continuously.
The response variables were MRT and SD of the RTs, calculated for each condition based on correct responses only. For analyses that compared performance across the baseline and fast-incentive conditions, data from the second set of 30 trials of the baseline condition were used to provide a match on length of time on task with the fast-incentive condition (Andreou et al., 2007) as twin data suggest greater reliability and heritability for the second than for the first set of 30 trials (Kuntsi & Asherson, unpublished data). The fast-incentive condition is always administered after the baseline condition and, as such, does not involve a similar learning phase.
Rating scales
ADHD symptoms were measured using the Long Version of Conners' Parent Rating Scale (Conners et al., 1998b) and the Long Version of Conners' Teacher Rating Scale (Conners et al., 1998a). On both the parent and the teacher Conners' scales, adding up the scores on the nine-item hyperactive–impulsive and nine-item inattentive Diagnostic and Statistical Manual, 4th Edition (DSM-IV) symptoms subscales forms a total DSM-IV ADHD symptoms subscale. We created an ADHD composite score by adding up the scores on the parent and teacher DSM-IV ADHD symptoms subscales.
Analyses
We conducted analyses using STATA Statistical Software release 9.2 (Stata Corporation, College Station, TX). As the sample consisted of twins, we used the cluster command to remove any effects of familial clustering. Analysis of untrans-formed data was justified by the large sample size, with central limit theorem showing that the large sample size means that normality assumptions will not be violated for parametric tests (Lumley et al., 2002), and the use of logistic regression for group comparisons. The advantage of this analytic approach over analysis of variance or multivariate analysis of variance is that it corrected for the nonindependence of the data and maximized power. A further advantage of logistic regression is its insensitivity to unequal cell sizes (Tabachnick & Fidell, 2007). Age and sex were included as covariates in the partial correlation and regression analyses. Given the clear a priori hypotheses for the direction of group differences for each variable and the aim of replicating findings across several of the variables, exact p values are presented. A false discovery rate (FDR) correction for multiple testing was additionally used (Benjamini et al., 2001) based on an alpha of p <.05. Those p values that remained significant after this adjustment are indicated with an asterisk in Table 2.
Table 2.
Means (SDs) and between-group comparisons on the fast task and go/no-go task variables, controlling for age and sex
| Mean (SD)a |
|||||
|---|---|---|---|---|---|
| ADHD-H (n = 52–57) | Control (n = 1043–1092) | Zb | p | ORc | |
| Fast task | |||||
| Baseline (all trials) | |||||
| MRT | 1018. 25 (259.03) | 942.76 (232.55) | 3.07 | .002* | 1.49 |
| SD of RTs | 494.63 (272.32) | 403.73 (273.94) | 3.05 | .002* | 1.37 |
| Baseline (30 trials) | |||||
| MRT | 1091.76 (300.34) | 1007.12 (268.96) | 3.19 | .001* | 1.47 |
| SD of RTs | 482.68 (257.99) | 402.25 (316.12) | 2.97 | .003* | 1.32 |
| Fast-incentive | |||||
| MRT | 653.17 (143.64) | 648.13 (161.92) | 0.47 | .64 | 1.07 |
| SD of RTs | 216.99 (84.76) | 202.61 (133.59) | 0.78 | .44 | 1.06 |
| Difference score (baseline–fast-incentive) | |||||
| MRT | 438.59 (251.73) | 359.14 (202.98) | 3.54 | <.001* | 1.54 |
| SD of RTs | 265.69 (234.89) | 198.76 (293.55) | 2.82 | .01* | 1.30 |
| Go/no-go task | |||||
| Slow | |||||
| MRT | 616.13 (179.49) | 584.38 (129.02) | 2.42 | .02* | 1.36 |
| SD of RTs | 311.04 (232.44) | 219.26 (143.83) | 3.77 | <.001* | 1.51 |
| Commission errors | 64.36 (20.58) | 54.52 (23.12) | 1.54 | .12 | 1.26 |
| Fast | |||||
| MRT | 434.44 (71.95) | 420.86 (62.52) | 2.79 | .01* | 1.50 |
| SD of RTs | 202.23 (69.04) | 159.64 (56.89) | 4.61 | <.001* | 1.70 |
| Commission errors | 61.80 (14.48) | 50.86 (16.36) | 3.45 | .001* | 1.66 |
| Incentive | |||||
| MRT | 533.73 (94.72) | 555.16 (112.84) | −.31 | .76 | 0.96 |
| SD of RTs | 173.18 (87.37) | 144.08 (71.08) | 2.43 | .02* | 1.25 |
| Commission errors | 42.02 (19.07) | 31.36 (20.69) | 3.06 | .002* | 1.46 |
| Difference score (slow–fast) | |||||
| MRT | 183.13 (159.41) | 163.62 (106.76) | 1.43 | .15 | 1.21 |
| SD of RTs | 109.07 (224.18) | 59.88 (138.29) | 2.01 | .04 | 1.30 |
| Commission errors | 2.36 (19.78) | 3.67 (20.16) | −1.15 | .25 | 0.86 |
| Difference score (slow–incentive) | |||||
| MRT | 90.11 (157.46) | 29.18 (93.51) | 3.54 | <.001* | 1.57 |
| SD of RTs | 141.78 (230.19) | 75.19 (142.52) | 2.58 | .01* | 1.34 |
| Commission errors | 21.85 (24.24) | 23.17 (21.35) | −1.10 | .27 | 0.85 |
| Difference score (fast–incentive) | |||||
| MRT | −97.98 (87.09) | −134.02 (96.52) | 1.97 | .05 | 1.41 |
| SD of RTs | 30.77 (107.31) | 15.82 (79.29) | 0.94 | .35 | 1.18 |
| Commission errors | 19.74 (23.95) | 19.48 (19.18) | −0.13 | .90 | 0.98 |
| Combined data | |||||
| Fast task baseline + go/no-go slow | |||||
| MRT | 1620.19 (395.78) | 1521.81 (309.42) | 2.82 | .01* | 1.51 |
| SD of RTs | 781.01 (405.75) | 615.91 (342.91) | 3.78 | <.001* | 1.54 |
| Fast task baseline + go/no-go fast | |||||
| MRT | 1450.25 (296.86) | 1361.04 (268.27) | 3.35 | .001* | 1.59 |
| SD of RTs | 696.43 (294.57) | 560.55 (290.36) | 4.16 | <.001* | 1.55 |
Means and SDs calculated from raw data.
OR test statistic.
OR calculated from standardized cognitive score and represents the increase in risk associated with a 1 SD change in cognitive score.
Indicates p values that remain significant at p < .05 after FDR correction for multiple testing (Benjamini et al., 2001).
In the categorical analyses that compared the two groups, we first examined each task condition separately. Group status was entered into the logistic model as response variable and cognitive performance variables (MRT, SD of RTs, and commission errors) and age and sex as predictor variables. Next, we examined the extent of improvement across task conditions, both within each group and between the groups, to investigate whether children with high ADHD symptoms (ADHD-H) group improved more than the comparison group. For between-group improvement analyses, we entered group status as the response variable in the logistic regression model and difference score (performance on the variable in baseline condition – performance on the same variable in the comparison condition), along with age and sex, as the predictor variables. To ensure comparability across scores, odds ratios (ORs) are presented for standardized cognitive scores. Each OR can thus be interpreted as the increase in risk of being in the ADHD-H group associated with a change of 1 SD in cognitive score. Standardized scores are calculated by rescaling the group score distribution such that it has a mean of 0 and an SD of 1. For within-group improvement analyses, we used a linear regression with the xi: interaction expansion command within STATA.
RESULTS
Categorical Analyses
The ADHD-H group was created by selecting the highest scoring 5% on the ADHD composite score (n = 58). The rest of the sample formed the control group (n = 1098). The groups matched well on age and IQ, but there was an excess of boys in the ADHD-H group (Table 1). Examination of this sex difference revealed that the mean difference between males and females was significantly greater in the highest (fifth) quintile of the distribution of ADHD composite scores compared to the rest of the sample, t (601) = −2.51, p = .01, or compared to the fourth quintile, t (348) = −4.38, p <.001.
Table 1.
Sample characteristics: ADHD-H and control groups
| ADHD-H (n = 58) | Control (n = 1098) | Group comparison statistic | |
|---|---|---|---|
| Age | 8.79 (0.66) | 8.79 (0.66) | t(1154) = 0.00, p > .99 |
| % Male | 91.38 | 48.09 | χ2 = 41.30, p < .001 |
| Full scale IQ | 108.31 (14.72) | 109.77 (14.72) | t(1154) = 0.74, p = .46 |
| Parent Conners': total DSM-IV ADHD symptoms subscale | 33.05 (8.56) | 10.91 (8.04) | t(1154) = 20.37, p < .001 |
| Teacher Conners': total DSM-IV ADHD symptoms subscale | 30.33 (9.82) | 7.12 (7.38) | t(1154) = 22.90, p < .001 |
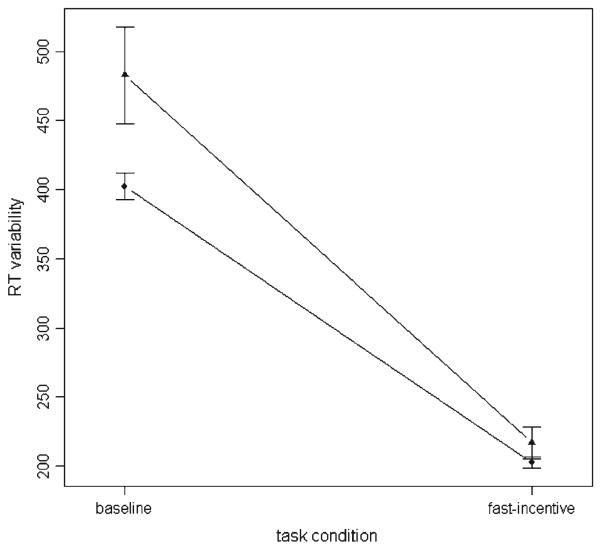
The fast task analyses indicated that the ADHD-H group, compared to the control group, had slower MRT and greater SD of RTs in the baseline condition (both for all trials and for 30 trials), whereas the groups did not differ in the fast-incentive condition (Figure 1; Table 2). The within-group analyses indicated that both groups improved significantly both for MRT [ADHD-H: t (606) = 10.73, p =.003; control: t (606) = 10.30, p <.001] and for SD of RTs [ADHD-H: t (606) = 5.07, p <.001; control: t (606) = 4.39, p <.001] from the baseline to the fast-incentive condition, with the between-group analyses showing that the improvement was significantly greater for the ADHD-H group (Table 2).
Fig. 1.
RT variability (SD of RTs) in baseline and fast-incentive conditions of the fast task (with standard errors): ADHD-H (▲) and control (●) groups. Data presented on raw data; age and sex not controlled for.
In the go/no-go task analyses, significant group differences emerged for MRT in fast and slow conditions, for SD of RTs in each of the conditions, and for commission errors in fast and incentive conditions (Table 2). The within-group analyses indicated that both groups improved significantly from slow to fast condition both for MRT [ADHD-H: t(609) = 5.92, p <.001; control: t(609) = 5.79, p <.001] and for SD of RTs [ADHD-H: t(609) = 3.32, p =.001; control: t(609) = 2.87, p =.004] but not for commission errors [ADHD-H: t(609) = 0.72, p =.47; control: t(609) = 1.09, p =.27]. Both groups similarly improved significantly from slow to incentive condition for MRT [ADHD-H: t(606) = 5.17, p <.001; control: t(606) = 4.17, p <.001] and SD of RTs [ADHD-H: t(606) = 3.71, p <.001; control: t(606) = 3.03, p =.003] but not for commission errors [ADHD-H: t(606) = 1.22, p =.22; control: t(606) = 1.69, p =.09]. Between fast and incentive conditions, only the control group improved significantly for MRT [ADHD-H: t(608) = −1.72, p =.09; control: t(608) = −2.42, p = .02] and neither group for SD of RTs [ADHD-H: t(608) = 0.95, p =.34; control: t(606) = 0.52, p =.60] or for commission errors [ADHD-H: t(608) = 0.65, p =.52; control: t(608) = 0.73, p = .46]. All significant p values remained significant at the alpha level of p < .05 after FDR correction for multiple testing (Benjamini et al., 2001), run separately for both sets of within-group analyses. The between-group comparisons for difference scores indicated greater improvement in the ADHD-H group than the control group for MRT from the slow to the incentive condition and from the fast to the incentive condition and for SD of RTs from slow to the incentive condition (Table 2). Greater improvement across conditions was not observed for commission errors.
As combining data across theoretically related measures improves reliability (Kuntsi et al., 2006b), we conducted additional analyses of RT data combined across the two tasks to investigate whether such composite scores best distinguish between the groups. For the go/no-go task, our a priori prediction for largest ADHD–control group differences on RT data was for the slow condition, but the data indicated good group discrimination on RT data also for the fast condition. We therefore created RT composite scores in two ways: (1) combining data from fast task baseline condition and go/no-go task slow condition and (2) combining data from fast task baseline condition and go/no-go task fast condition. The ORs for the composite scores were of similar magnitude as those for the individual variables that best discriminated between the groups (Table 2).
The pattern and significance of the results were similar when additional analyses were carried out on data from boys only (data not shown). Additional analyses were also conducted to examine speed–accuracy trade-off by obtaining correlations between MRT and commission errors. For both groups, the correlations were in the range of −.34 to −.50 (p < .01) for the slow and incentive conditions. This indicates an association between fast speed and more errors and hence speed–accuracy trade-off. For the fast condition, there was no association between MRT and commission errors for the ADHD-H group (r = .06, ns) and a correlation of −.16 (p < .01) for the control group.
Dimensional Analyses
To study the association between the continuous dimension of ADHD symptom scores and the cognitive performance, we obtained Pearson correlations between the ADHD composite score and each of the cognitive variables, controlling for age and sex (Table 3). The correlations were.2–.3 (effect sizes of 0.4–0.5) for the variables most strongly associated with the ADHD composite score, with the highest correlation obtained for SD of RTs when combined across fast task baseline condition and go/no-go task slow condition. We carried out additional analyses controlling for IQ, but this did not change the pattern of findings (data not shown).
Table 3.
Association between ADHD composite score and fast task and go/no-go task variables: Partial correlations controlling for age and sex, with associated effect sizes (d)
| ADHD composite score |
||
|---|---|---|
| Partial r | d | |
| Fast task | ||
| Baseline (all trials) | ||
| MRT | .21** | 0.4 |
| SD of RTs | .22** | 0.5 |
| Baseline (30 trials) | ||
| MRT | .20** | 0.4 |
| SD of RTs | .17** | 0.3 |
| Fast-incentive | ||
| MRT | .11** | 0.2 |
| SD of RTs | .11** | 0.2 |
| Difference score (baseline–fast-incentive) | ||
| MRT | .17** | 0.3 |
| SD of RTs | .14** | 0.3 |
| Go/no-go | ||
| Slow | ||
| MRT | .15** | 0.3 |
| SD of RTs | .23** | 0.5 |
| Commission errors | .08** | 0.2 |
| Fast | ||
| MRT | .13** | 0.3 |
| SD of RTs | .20** | 0.5 |
| Commission errors | .15** | 0.3 |
| Incentive | ||
| MRT | .04 | 0.1 |
| SD of RTs | .13** | 0.3 |
| Commission errors | .13** | 0.3 |
| Difference score (slow–fast) | ||
| MRT | .11** | 0.2 |
| SD of RTs | .16** | 0.3 |
| Commission errors | .03 | 0.1 |
| Difference score (slow–incentive) | ||
| MRT | .17** | 0.3 |
| SD of RTs | .17** | 0.3 |
| Commission errors | .04 | 0.1 |
| Difference score (fast–incentive) | ||
| MRT | .03 | 0.1 |
| SD of RTs | .03 | 0.1 |
| Commission errors | .00 | 0.0 |
| Combined data | ||
| Fast task baseline + go/no-go slow | ||
| MRT | .21** | 0.4 |
| SD of RTs | .26** | 0.5 |
| Fast task baseline + go/no-go fast | ||
| MRT | .21** | 0.4 |
| SD of RTs | .24** | 0.5 |
p ≤ .01.
DISCUSSION
Cognitive deficits in ADHD have sometimes been characterized as having a “now you see it, now you don't” quality to them. Studies that have included within-task manipulations, such as varied event rate and incentives, have investigated the stability of cognitive impairments in ADHD. In a study of clinic-referred participants with a research diagnosis of DSM-IV combined type ADHD, we previously demonstrated greater improvement in RT variability in a condition of the fast task with a fast event rate and incentives (Andreou et al., 2007). Here, using the same RT task in a large sample of children, we showed that RT variability normalized in the fast-incentive condition in children who represented the highest scoring 5% on an ADHD composite rating scale score. The finding applied also to speed of responding (MRT). The go/no-go data similarly suggested that slow speed is not a stable characteristic of children with high levels of ADHD symptoms since the groups differed in MRT in the slow and fast event rate conditions but were indistinguishable in the incentive condition. For both MRT and RT variability, incentives led to significantly greater improvements in the ADHD-H compared to the control group and hence were more effective than a fast event rate.
Overall, the fast task was more effective than the go/no-go task in normalizing the RT variability in the children with high levels of ADHD symptoms. This may reflect one or both of two key differences between the tasks: (1) the fast task focuses on the combined effects of incentives and fast event rate, whereas in the go/no-go task, the effects of each are studied individually and (2) in the fast task, the aspect of performance that is rewarded is reduced RT variability, whereas in the go/no-go task, accuracy is rewarded.
For commission errors in the go/no-go task, the groups differed significantly in the fast and incentive conditions but not in the slow condition. While this appears to suggest a lack of a stable inhibition deficit in the ADHD-H group, we also note that neither group improved significantly across the conditions, which calls for some caution in the interpretation of the results. Furthermore, the pattern of response inhibition performance (commission errors) across conditions did not confirm our expectations that group differences would be the most pronounced in the slow condition and that the ADHD-H group would show relatively greater improvement than the controls from the slow to the fast and incentive conditions. Our results failed to replicate previous findings using the stop task on clinically diagnosed samples in which ADHD–control differences in response inhibition disappeared in an incentive condition (Konrad et al., 2000; Slusarek et al., 2001). These findings add to the previous research that indicates highly mixed findings from studies of event rate using inhibition tasks (van der Meere, 2002; Wiersema et al., 2006). The go/no-go task is one of the most popular tasks in ADHD research, but findings obtained depend crucially on the exact task parameters, highlighting the complexity of tasks that involve multiple cognitive demands. The task requires the child to be both fast and accurate, but we showed that the speed–accuracy trade-off did not remain stable across task conditions and groups. For example, incentives led to faster speed in the ADHD-H group but not to a similar improvement in the percentage of commission errors, suggesting that the higher speed set a limit on any incentive-induced improvement in accuracy.
Findings from the analysis of the continuous ADHD symptom scores in the total sample were overall consistent with those obtained in the categorical (group) analyses. The correlations for key task variables were significant but somewhat lower than the highest correlations obtained with teacher-rated ADHD symptom scores in a previous small-scale preliminary investigation (Kuntsi et al., 2005). Among the highest correlations with the ADHD composite score in the current study was that obtained for the RT variability composite score (r =.3; effect size 0.5), calculated by summing up fast task baseline and go/no-go task slow condition data.
The composition of the ADHD-H group, defined as the top scoring 5% on the ADHD composite score, indicated a marked excess of boys (91%), replicating the well-documented finding of gender imbalance in clinical ADHD samples (Ford et al., 2003). Analyses of a boys-only sample indicated largely similar findings as for the whole sample, with theoretical conclusions remaining the same.
The 5% threshold for the ADHD-H group was based on both prevalence rate and statistical power considerations. It is beyond the scope of the present study to examine effects of different possible cutoffs, but we acknowledge this as a limitation of the current study and as a worthwhile future topic of investigation. The applicability of the findings across a wider age range—beyond ages 7–10 years as studied here—also needs to be established in future research.
Overall, two main findings emerged from this study. First, children representing the top scoring 5% on an ADHD composite score showed poor inhibition and RT performance under some of the experimental conditions, replicating previous findings with clinically diagnosed samples on aspects of cognitive processing affected in ADHD. Second, these performance deficits, particularly on MRT and RT variability, may not reflect stable cognitive deficits as there were no group differences in some of the comparison conditions. This is consistent with predictions based on theories of a deficit in the regulation of energetic state in ADHD (Sergeant, 2005; van der Meere, 2002). It is not clear how alternative theoretical accounts of RT variability, such as the temporal processing/time estimation deficit hypothesis (Castellanos & Tannock, 2002), would account for the overall pattern of findings, particularly the greater improvement in RT variability in the ADHD-H group when both incentives and faster event rate were combined (or with incentives rather than a fast event rate when studied separately in the go/no-go task). Yet we did not set out specifically to test predictions of competing hypotheses for RT variability here and this requires further research.
In recent years, the association between RT variability and ADHD has become one of the key topics in cognitive research on ADHD (Bellgrove et al., 2005; Castellanos et al., 2005; Klein et al., 2006). Without experimental manipulations or other theory-driven probing of the underlying mechanisms, RT variability itself is a nonspecific finding that may reflect multiple potential causes. The finding here that RT variability (as well as MRT) normalized in children with ADHD-H symptoms under a fast-incentive condition extends our previous finding with a clinically diagnosed ADHD group, where RT variability improved more among the ADHD than among the control group but did not completely normalize (Andreou et al., 2007). Clinic-referred individuals with ADHD diagnoses may either have impairments beyond those directly linked to their behavioral symptoms or their behavioral symptoms—and associated cognitive or energetic deficits—may be more severe than those observed in children representing the highest scoring 5% on an ADHD composite score. The specificity of the improvement in RT variability in ADHD also requires further study in relation to disorders that frequently co-occur with ADHD, as well as other disorders where RT variability may be observed.
A recent electrophysiological study reported an association between increased theta power, associated with cortical under-arousal, and RT variability (Loo & Smalley, 2008), which is also consistent with the hypothesis that RT variability may index lapses in attention due to an energetic state that is nonoptimal for task performance. The proposal that energetic processes are affected in ADHD links with findings that indicate how the mesolimbic dopamine system is involved in behavioral activation and effort-related processes (Salamone et al., 2007). Salamone et al. (2007) conclude in their review that “nucleus accumbens dopamine regulates response speed and the exertion of effort in reinforcer-seeking behaviour, and participates with other brain areas in the regulation of decisions based upon effort expenditure” (p. 475). Such neuroscience research will inform the further development of models of ADHD.
The degree to which cognitive impairments in ADHD can be modulated by energetic or motivational factors has important implications for clinical and educational interventions. In addition to highlighting the importance of using such energetic or motivational factors in helping children with ADHD stay focused, a further implication is the need to avoid labeling children with ADHD as “low ability” where tasks may not have revealed their true ability. Further investigation of the factors that help children with ADHD achieve their potential seems a key priority.
ACKNOWLEDGMENTS
The SAIL is funded by a project grant from the Wellcome Trust (GR070345MF). Thank-you to all who made this research possible: the TEDS-SAIL families, who gave their time and support so unstintingly; Rebecca Gibbs, Hannah Rogers, Eda Salih, Greer Swinard, Kate Lievesley, Kayley O'Flynn, Suzi Marquis, and Rebecca Whittemore; and everyone in the TEDS team.
REFERENCES
- Alderson RM, Rapport MD, Kofler MJ. Attention-deficit/hyperactivity disorder and behavioral inhibition: A meta-analytic review of the stop-signal paradigm. Journal of Abnormal Child Psychology. 2007;35:745–758. doi: 10.1007/s10802-007-9131-6. [DOI] [PubMed] [Google Scholar]
- Andreou P, Neale BM, Chen W, Christiansen H, Gabriels I, Heise A, Meidad S, Muller UC, Uebel H, Banaschewski T, Manor I, Oades R, Roeyers H, Rothenberger A, Sham P, Steinhausen HC, Asherson P, Kuntsi J. Reaction time performance in ADHD: Improvement under fast-incentive condition and familial effects. Psychological Medicine. 2007;37:1703–1715. doi: 10.1017/S0033291707000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaschewski T, Brandeis D, Heinrich H, Albrecht B, Brunner E, Rothenberger A. Questioning inhibitory control as the specific deficit of ADHD—Evidence from brain electrical activity. Journal of Neural Transmission. 2004;111:841–864. doi: 10.1007/s00702-003-0040-8. [DOI] [PubMed] [Google Scholar]
- Bellgrove MA, Hawi Z, Kirley A, Gill M, Robertson IH. Dissecting the attention deficit hyperactivity disorder (ADHD) phenotype: Sustained attention, response variability and spatial attentional asymmetries in relation to dopamine transporter (DAT1) genotype. Neuropsychologia. 2005;43:1847–1857. doi: 10.1016/j.neuropsychologia.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behavioural Brain Research. 2001;125:279–284. doi: 10.1016/s0166-4328(01)00297-2. [DOI] [PubMed] [Google Scholar]
- Berwid OG, Curko Kera EA, Marks DJ, Santra A, Bender HA, Halperin JM. Sustained attention and response inhibition in young children at risk for attention deficit/hyperactivity disorder. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2005;46:1219–1229. doi: 10.1111/j.1469-7610.2005.00417.x. [DOI] [PubMed] [Google Scholar]
- Borger N, van der Meere J. Motor control and state regulation in children with ADHD: A cardiac response study. Biological Psychology. 2000;51:247–267. doi: 10.1016/s0301-0511(99)00040-x. [DOI] [PubMed] [Google Scholar]
- Brandeis D, van Leeuwen TH, Rubia K, Vitacco D, Steger J, Pascual-Marqui RD, Steinhausen HC. Neuroelectric mapping reveals precursor of stop failures in children with attention deficits. Behavioural Brain Research. 1998;94:111–125. doi: 10.1016/s0166-4328(97)00174-5. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga-Barke EJ, Scheres A, Di Martino A, Hyde C, Walters JR. Varieties of attention-deficit/hyperactivity disorder-related intra-individual variability. Biological Psychiatry. 2005;57:1416–1423. doi: 10.1016/j.biopsych.2004.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: The search for endophenotypes. Nature Reviews. Neuroscience. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Chen W, Zhou K, Sham P, Franke B, Kuntsi J, Campbell D, Fleischman K, Knight J, Andreou P, Arnold R, Altink M, Boer F, Boholst MJ, Buschgens C, Butler L, Christiansen H, Fliers E, Howe-Forbes R, Gabriëls I, Heise A, Korn-Lubetzki I, Marco R, Medad S, Minderaa R, Müller UC, Mulligan A, Psychogiou L, Rommelse N, Sethna V, Uebel H, McGuffin P, Plomin R, Banaschewski T, Buitelaar J, Ebstein R, Eisenberg J, Gill M, Manor I, Miranda A, Mulas F, Oades RD, Roeyers H, Rothenberger A, Sergeant J, Sonuga-Barke E, Steinhausen HC, Taylor E, Thompson M, Faraone SV, Asherson P. DSM-IV combined type ADHD shows familial association with sibling trait scores: A sampling strategy for QTL linkage. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2008;147:1450–1460. doi: 10.1002/ajmg.b.30672. [DOI] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JD, Epstein JN. Revision and restandardization of the Conners Teacher Rating Scale (CTRS-R): Factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology. 1998a;26:279–291. doi: 10.1023/a:1022606501530. [DOI] [PubMed] [Google Scholar]
- Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners' Parent Rating Scale (CPRS-R): Factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology. 1998b;26:257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- Ford T, Goodman R, Meltzer H. The British Child and Adolescent Mental Health Survey 1999: The prevalence of DSM-IV disorders. Journal of American Academy of Child and Adolescent Psychiatry. 2003;42:1203–1211. doi: 10.1097/00004583-200310000-00011. [DOI] [PubMed] [Google Scholar]
- Klein C, Wendling K, Huettner P, Ruder H, Peper M. Intra-subject variability in attention-deficit hyperactivity disorder. Biological Psychiatry. 2006;60:1088–1097. doi: 10.1016/j.biopsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Konrad K, Gauggel S, Manz A, Scholl M. Lack of inhibition: A motivational deficit in children with attention deficit/hyperactivity disorder and children with traumatic brain injury. Child Neuropsycholology. 2000;6:286–296. doi: 10.1076/chin.6.4.286.3145. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Andreou P, Ma J, Borger NA, van der Meere JJ. Testing assumptions for endophenotype studies in ADHD: Reliability and validity of tasks in a general population sample. BMC Psychiatry. 2005;5:40. doi: 10.1186/1471-244X-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsi J, McLoughlin G, Asherson P. Attention deficit hyperactivity disorder. Neuromolecular Medicine. 2006a;8:461–484. doi: 10.1385/NMM:8:4:461. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Oosterlaan J, Stevenson J. Psychological mechanisms in hyperactivity: I. Response inhibition deficit, working memory impairment, delay aversion, or something else? Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2001;42:199–210. [PubMed] [Google Scholar]
- Kuntsi J, Rogers H, Swinard G, Borger N, van der MJ, Rijsdijk F, Asherson P. Reaction time, inhibition, working memory and ‘delay aversion’ performance: Genetic influences and their interpretation. Psychological Medicine. 2006b;36:1613–1624. doi: 10.1017/S0033291706008580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsi J, Stevenson J. Psychological mechanisms in hyperactivity: II. The role of genetic factors. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2001;42:211–219. [PubMed] [Google Scholar]
- Leth-Steensen C, Elbaz ZK, Douglas VI. Mean response times, variability, and skew in the responding of ADHD children: A response time distributional approach. Acta Psycho-logica. 2000;104:167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- Lijffijt M, Kenemans JL, Verbaten MN, van EH. A meta-analytic review of stopping performance in attention-deficit/ hyperactivity disorder: Deficient inhibitory motor control? Journal of Abnormal Psychology. 2005;114:216–222. doi: 10.1037/0021-843X.114.2.216. [DOI] [PubMed] [Google Scholar]
- Loo SK, Smalley SL. Preliminary report of familial clustering of EEG measures in ADHD. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2008;147:107–109. doi: 10.1002/ajmg.b.30575. [DOI] [PubMed] [Google Scholar]
- Luman M, Oosterlaan J, Sergeant JA. The impact of reinforcement contingencies on AD/HD: A review and theoretical appraisal. Clinical Psychology Review. 2005;25:183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Lumley T, Diehr P, Emerson S, Chen L. The importance of the normality assumption in large public health data sets. Annual Review of Public Health. 2002;23:151–169. doi: 10.1146/annurev.publhealth.23.100901.140546. [DOI] [PubMed] [Google Scholar]
- McLoughlin G, Albrecht B, Banaschewski T, Rothenberger A, Brandeis D, Asherson P, Kuntsi J. Developmental Stability of abnormal preparatory states and inhibitory processing in attention deficit hyperactivity disorder: Electro-physiological evidence from an adult sample. under review. [Google Scholar]
- Pliszka SR, Liotti M, Woldorff MG. Inhibitory control in children with attention-deficit/hyperactivity disorder: Event-related potentials identify the processing component and timing of an impaired right-frontal response-inhibition mechanism. Biological Psychiatry. 2000;48:238–246. doi: 10.1016/s0006-3223(00)00890-8. [DOI] [PubMed] [Google Scholar]
- Price TS, Freeman B, Craig I, Petrill SA, Ebersole L, Plomin R. Infant zygosity can be assigned by parental report questionnaire data. Twin Research. 2000;3:129–133. doi: 10.1375/136905200320565391. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Sattler JM. Assessment of children: WISC-III and WPPSI-R supplement. Jerome M. Sattler; San Diego, CA: 1992. [Google Scholar]
- Sergeant JA. Modeling attention-deficit/hyperactivity disorder: A critical appraisal of the cognitive-energetic model. Biological Psychiatry. 2005;57:1248–1255. doi: 10.1016/j.biopsych.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Slusarek M, Velling S, Bunk D, Eggers C. Motivational effects on inhibitory control in children with ADHD. Journal of American Academy of Child and Adolescent Psychiatry. 2001;40:355–363. doi: 10.1097/00004583-200103000-00016. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 5th ed. Allyn & Bacon; Boston, MA: 2007. [Google Scholar]
- Trouton A, Spinath FM, Plomin R. Twins early development study (TEDS): A multivariate, longitudinal genetic investigation of language, cognition and behavior problems in childhood. Twin Research. 2002;5:444–448. doi: 10.1375/136905202320906255. [DOI] [PubMed] [Google Scholar]
- van der Meere J, Stemerdink N, Gunning B. Effects of presentation rate of stimuli on response inhibition in ADHD children with and without tics. Perceptual and Motor Skills. 1995;81:259–262. doi: 10.2466/pms.1995.81.1.259. [DOI] [PubMed] [Google Scholar]
- van der Meere JJ. The role of attention. In: Sandberg S, editor. Hyperactivity disorders of childhood. Cambridge, UK. 2nd ed. Cambridge University Press; 2002. pp. 162–213. [Google Scholar]
- van Leeuwen TH, Steinhausen HC, Overtoom CC, Pascual-Marqui RD, van't KB, Rothenberger A, Sergeant JA, Brandeis D. The continuous performance test revisited with neuroelectric mapping: Impaired orienting in children with attention deficits. Behavioural Brain Research. 1998;94:97–110. doi: 10.1016/s0166-4328(97)00173-3. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3rd ed. The Psychological Corporation; London, UK: 1991. [Google Scholar]
- Wiersema R, van der MJ, Roeyers H, Van CR, Baeyens D. Event rate and event-related potentials in ADHD. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2006;47:560–567. doi: 10.1111/j.1469-7610.2005.01592.x. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]