Abstract
Background
Inflammatory breast carcinoma (IBC) appears to be a clinicopathologic entity distinct from noninflammatory locally advanced breast cancer (LABC). We examined incidence and survival trends for IBC in Surveillance, Epidemiology, and End Results (SEER) Program data with a case definition designed to capture many of its unique clinical and pathologic characteristics.
Methods
We analyzed breast cancer cases diagnosed in the SEER 9 Registries (n = 180 224), between 1988 and 2000. Breast cancer cases were categorized using SEER’s “ Extent of Disease” codes in combination with International Classification of Diseases for Oncology morphology code 8530/3 and classified as IBC (n = 3648), LABC (n = 3636), and non-T4 breast cancer (n = 172 940). We compared changes in incidence rates over 3-year intervals by breast cancer subtype and race using SEER*Stat. Survival differences by breast cancer subtype and race were assessed using Kaplan–Meier curves and log-rank statistics. All statistical tests were two-sided.
Results
Between 1988 and 1990 and 1997 and 1999, IBC incidence rates (per 100 000 woman-years) increased from 2.0 to 2.5 (P<.001), whereas those for LABC declined (2.5 to 2.0, P = .0025), as did those for non-T4 breast cancer (108 to 101, P = .0084). IBC incidence rates were statistically significantly higher in black women (3.1) than in white women (2.2) during the study period (P<.001). Women diagnosed with IBC had statistically significantly poorer survival than women with either LABC or non-T4 breast cancer (log-rank test, P<.001). Median survival of women with IBC (2.9 years) was statistically significantly shorter than that of women with LABC (6.4 years; P<.0001) or non-T4 breast cancer (>10 years, P<.0001). Black women with IBC or LABC had poorer survival than white women with IBC or LABC, respectively (log-rank test, P<.001).
Conclusions
Throughout the 1990s, IBC incidence rose, and survival improved modestly. Substantial racial differences were noted in age at diagnosis, age-specific incidence rates, and survival outcomes.
Inflammatory breast carcinoma (IBC) appears to be a relatively rare form of breast cancer with distinct clinicopathologic features (1-4). It is characterized by an early age at diagnosis, poor nuclear grade, negative hormone receptor status, and poor survival outcome. Although IBC is readily distinguishable from most forms of breast cancer, it can be confused with noninflammatory locally advanced breast cancer (LABC). Consistent with Haagensen’s original description of IBC (3), the American Joint Committee on Cancer (AJCC) provides the current gold standard definition for this form of breast cancer, describing it as a clinicopathologic entity that is characterized by diffuse erythema and edema (peau d’orange), often without an underlying palpable mass (5). The clinical presentation of IBC is due to tumor emboli within dermal lymphatic vessels, which may or may not be present on skin biopsy. Therefore, the AJCC relies on the clinical features of IBC and considers the pathologic features to be supportive of, but not necessary for, diagnosis. Some investigators (6-8) have classified IBC into three separate groups: 1) IBC cases with clinical features only, 2) IBC cases with clinical and pathologic features, and 3) IBC cases with pathologic features only. However, none of these definitions appears to capture all of the unique clinical and/or pathologic characteristics of the disease.
Possibly because of varying case definitions, population-based estimates for IBC incidence range widely, from <1% to 10%. For example, using codes from the Surveillance, Epidemiology, and End Results (SEER) Program, Levine et al. (6) noted IBC incidence rates of 6% and 10% among white and black women, respectively; however, the codes may have included LABC cases, inflating the IBC incidence estimates. Using a more conservative definition for IBC in SEER, the International Classification of Diseases for Oncology (ICD-O-2) morphology code 8530/3, Chang et al. (9) reported much lower incidence rates of 0.5% and 0.7% in white and black women, respectively. More recent population-based studies using clinical and pathologic (4) or pathologic only (10, 11) case definitions suggest that 1.0%–1.3% of all incident breast carcinomas in women are IBC.
In a previous study, we evaluated age-specific incidence rate patterns and survival outcome in both IBC and LABC patients; however, temporal trends were not examined and a less comprehensive SEER-based definition for IBC was employed (4). We recently reported comparisons of age-specific incidence rate patterns for seven different histopathologic types of breast carcinoma including IBC; however, survival outcomes and temporal trends were not reported, and a conservative case definition for IBC (ICD-O-2 8530/3) was used for the purposes of comparison (10). Thus, the primary purpose of this study was to determine the true population-based incidence of IBC. An additional goal was to evaluate changes in IBC incidence and survival over time, particularly by race. To examine these questions, we used a comprehensive definition designed to capture all of the clinically and/or pathologically defined IBC cases diagnosed in the National Cancer Institute’s SEER Program.
SUBJECTS AND METHODS
Data Source
We examined the publicly available records of the nine population-based registries in the SEER 9 Registries database (November 2002 submission) (12). The registries are located in San Francisco-Oakland, Metropolitan Detroit, Seattle (Puget Sound), Metropolitan Atlanta, Connecticut, Hawaii, Iowa, New Mexico, and Utah. Through these nine registries, SEER provides information on patient demographics and tumor characteristics of newly diagnosed malignancies for approximately 10% of the U.S. population.
SEER Case Definition and Selection
The SEER database provides modified AJCC staging information for breast cancer cases, which does not include tumor designations that distinguish between IBC and LABC cases. However, SEER’s “ Extent of Disease–Extent” (EOD-E) codes do provide tumor definitions similar to those of the AJCC (13) that can be used to uniquely define LABC and IBC cases (Table 1).
Table 1.
Classification of breast cancer subtypes by AJCC and SEER EOD staging*
| Breast cancer subtype | AJCC | EOD | Definition† |
|---|---|---|---|
| Non-T4 | T1-3 | EOD-E 10 | “Confined to breast tissue and fat including nipple and/or areola” |
| LABC | T4a | EOD-E 40 | “Invasion of (or fixation to) chest wall, ribs, intercostals, or serratus anterior muscles” |
| T4b | EOD-E 50 | “Extensive skin involvement: skin edema, peau d’orange, ‘pigskin,’ en cuirasse, lenticular nodule(s), inflammation of the skin, erythema, ulceration of skin of breast, satellite nodules(s) in skin of primary breast” |
|
| T4c | EOD-E 60 | EOD-E 60 includes EOD-E 40 and EOD-E 50. T4c includes AJCC categories T4a and T4b | |
| IBC | T4d | EOD-E 70 | “Inflammatory carcinoma, including diffuse (beyond that directly overlying the tumor) dermal lymphatic permeation or infiltration” |
| N/A | EOD-S 998 | “Diffuse; widespread: ¾’s or more of breast; inflammatory carcinoma” |
AJCC = American Joint Committee on Cancer; SEER, Surveillance, Epidemiology, and End Results; EOD = extent of disease; EOD-E = extent of disease-extension; EOD-S = extent of disease-size; LABC = locally advanced breast cancer; IBC = inflammatory breast cancer; N/A = not applicable.
Quotations are from Green et al. (5).
For this study, we used the EOD codes for tumor extension (EOD-E 70) and tumor size (EOD-S 998) in combination with ICD-O-2 morphology code 8530/3 (i.e., embolization of the dermal lymphatic vessels) (14) to define total IBC cases and clinical and/or pathologic subgroups of IBC. We defined total IBC as all cases with either clinical or pathologic features of IBC (EOD-E 70 or EOD-S 998 or ICD-O-2 8530/3), ClinPath IBC as cases with both clinical and pathologic features of IBC ([EOD-E 70 and/or EOD-S 998] + ICD-O-2 8530/3), PathOnly IBC as cases with pathologic features of IBC (ICD-O-2 8530/3), and ClinOnly IBC as cases with clinical features of IBC (EOD-E 70 and/or EOD-S 998).
Study Variables
The primary endpoints of this study were incidence rates and survival stratified by patient demographic characteristics and incident tumor characteristics. Patient demographics included age at diagnosis (<50, 50–59, 60–69, 70–79, or 80+ years), race (whites, blacks, or other), and a surrogate endpoint for menopausal status of 50 years of age. Morabia and Flandre (15) reported that 50 years of age is an appropriate surrogate for menopausal status when information about menstrual history is not available. Moreover, recent population-based studies of age-specific incidence rate patterns for IBC and other histopathologic subtypes of breast cancer used 50 years of age as a surrogate for menopausal status (4, 10).
Tumor characteristics included tumor size (≤2 cm versus >2 cm), axillary lymph node status (lymph node positive versus negative), estrogen receptor (ER) and progesterone receptor (PgR) expression (positive versus negative), and tumor grade. The variable for tumor size was dichotomized to approximate the AJCC criteria for T1 tumors (≤2cm) (5). Grade was defined according to ICD-O-2 coding conventions (14); histologic nuclear grades 1 (well-differentiated) and 2 (moderately differentiated) were defined as low-grade tumors, and histologic nuclear grades 3 (poorly differentiated) and 4 (undifferentiated) were defined as high-grade tumors. Data for patient demographics and/or tumor characteristics that were missing or coded as “ other or unknown” were not included in this analysis.
Statistical Analysis
The SEER 9 Registries database was accessed in ASCII format and analyzed with the SAS statistical software package (SAS for Windows Version 8.2, SAS Institute Inc., Cary, NC). The National Cancer Institute’s SEER*Stat software package version 5.0.20 was used to calculate incidence rates, 95% confidence intervals (CIs) estimated by the gamma method, cumulative relative survival, and median survival time. Incidence rates were expressed per 100 000 woman-years and were age-adjusted by the direct method to the 2000 U.S. standard population (16). Rate ratios (RRs) were calculated by divid ing the age-adjusted breast cancer incidence rate in women with a high-risk prognostic factor by the incidence rate in women with the corresponding low-risk prognostic factor. Low-risk prognostic factors were assigned a rate ratio of 1.0 and served as the reference group. Differences in the rate ratios for patient demographics and tumor characteristics were evaluated using 95% confidence limits that were calculated as previously described (17, 18).
To characterize the age distribution of cases in each breast cancer subtype, continuous 1-year age frequency distributions were constructed using density plots as previously described (19, 20). Briefly, age frequency distributions were constructed using a smoothing function with a filter width of 20; the vertical axis of each density plot represented the smoothed estimates of the proportion or density (where density × 100 = percent) of patients who developed breast cancer at the corresponding age at diagnosis on the horizontal axis. The area under the curve of each age density histogram included 100% of all breast cancer cases. Kolmogorov–Smirnov (KS) statistics define the maximum difference in the cumulative proportions of two nonparametric distributions (19). In this study, KS statistics were used to test for statistically significant differences in the age frequency distributions of IBC patientsstratified by race and ER receptor status (21).
Differences in the mean age at diagnosis by breast cancer subtype were evaluated using one-way analysis of variance and the Student’s t test (22). The Mantel–Haenszel chi-square test of trend statistic (16) was used to evaluate temporal trends in the age-adjusted incidence rates of each breast cancer subtype across 3-year intervals from 1988 through 1999. Age density histograms, age-specific incidence rate curves, and cumulative survival data were plotted using the S-Plus 6.0 software package (Insightful Corporation, Seattle, WA).
Cumulative relative survival was defined as breast cancer–specific survival corrected for corresponding national mortality rates (23). Kaplan–Meier product-limit curves (24) and log-rank tests (25) were used to evaluate differences in overall and breast cancer–specific survival by breast cancer subtype. In all statistical tests, P<.05 was considered to be statistically significant. All statistical tests were two-sided.
RESULTS
Of all microscopically confirmed malignancies of the breast included in the SEER 9 Registries database between 1988 and 2000 (N = 180 224), total IBC comprised approximately 2.0% (N = 3648). The mean age at diagnosis (years ± standard deviation) was statistically significantly different for IBC patients (58.8 ± 14.8) as compared with non-T4 (61.7 ± 14.4; P<.0001) and LABC (66.2 ± 15.7; P<.0001) patients. As shown in Table 2, the median age at diagnosis was older for patients with non-T4 breast cancer (63 years, interquartile range [IQR] = 50–73) or LABC (68, IQR = 54–79) than for patients with IBC (58, IQR = 47–70). Black women represented 7.6% (13 198 of 172 940), 14.7% (535 of 3636), and 12.7% (463 of 3648) of all patients with non-T4 breast cancer, LABC, and IBC, respectively. White women represented 86.7% (149 985 of 172 940), 79.5% (2892 of 3636), and 80.7% (2943 of 3648) of all patients with non-T4 breast cancer, LABC, and IBC, respectively.
Table 2.
Breast cancer incidence rates by patient demographics, tumor characteristics, and hormone receptor status*
| Breast cancer subgroup |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-T4 (T1–3)† |
LABC‡ |
Total IBC§ |
|||||||
| Characteristic | N | Rate | RR (95% CI) | N | Rate | RR (95% CI) | N | Rate | RR (95% CI) |
| Age group, y | |||||||||
| <50 | 40 665 | 35.5 | 1.0 (referent) | 641 | 0.6 | 1.0 (referent) | 1139 | 1.0 | 1.0 (referent) |
| 50–59 | 34 825 | 221.2 | 6.2 (6.1 to 6.3) | 579 | 3.7 | 6.6 (5.9 to 7.4) | 820 | 5.2 | 5.3 (4.8 to 5.8) |
| 60–69 | 39 777 | 315.7 | 8.9 (8.8 to 9.0) | 747 | 5.9 | 10.7 (9.6 to 11.9) | 738 | 5.9 | 5.9 (5.4 to 6.5) |
| 70–79 | 38 290 | 379.3 | 10.7 (10.5 to 10.8) | 824 | 8.2 | 14.7 (13.3 to 16.3) | 613 | 6.1 | 6.1 (5.6 to 6.8) |
| 80+ | 19 383 | 312.8 | 8.8 (8.7 to 9.0) | 845 | 13.5 | 24.3 (21.9 to 26.9) | 338 | 5.4 | 5.5 (4.9 to 6.2) |
| Race | |||||||||
| White | 149 985 | 111.3 | 1.0 (referent) | 2892 | 2.1 | 1.0 (referent) | 2943 | 2.2 | 1.0 (referent) |
| Black | 13 198 | 89.4 | 0.80 (0.79 to 0.82) | 535 | 3.8 | 1.8 (1.7 to 2.0) | 463 | 3.1 | 1.4 (1.3 to 1.5) |
| Other | 9241 | 65.3 | 201 | 1.5 | 239 | 1.7 | |||
| Tumor size | |||||||||
| ≤ 2.0 cm | 112 805 | 69.1 | 1.0 (referent) | 467 | 0.3 | 1.0 (referent) | 112 | 0.1 | 1.0 (referent) |
| >2.0 cm | 50 849 | 31.2 | 0.45 (0.44 to 0.46) | 2550 | 1.5 | 5.4 (4.9 to 6.0) | 588 | 0.4 | 5.1 (4.2 to 6.4) |
| Unknown | 9286 | 5.7 | 619 | 0.4 | 2948 | 1.8 | |||
| Nuclear grade | |||||||||
| Low | 75 943 | 46.5 | 1.0 (referent) | 996 | 0.6 | 1.0 (referent) | 626 | 0.4 | 1.0 (referent) |
| High | 49 809 | 30.8 | 0.66 (0.65 to 0.67) | 1604 | 1.0 | 1.6 (1.5 to 1.8) | 1939 | 1.2 | 3.1 (2.8 to 3.4) |
| Unknown | 47 188 | 28.8 | 1036 | 0.6 | 1083 | 0.7 | |||
| Lymph node status | |||||||||
| Negative | 115 381 | 70.7 | 1.0 (referent) | 822 | 0.5 | 1.0 (referent) | 400 | 0.3 | 1.0 (referent) |
| Positive | 45 485 | 28.3 | 0.399 (0.396 to 0.404) | 1991 | 1.2 | 2.5 (2.3 to 2.7) | 1917 | 1.2 | 4.7 (4.2 to 5.3) |
| Unknown | 12 074 | 7.1 | 823 | 0.5 | 1331 | 0.8 | |||
| ER status | |||||||||
| Positive | 93 408 | 57.2 | 1.0 (referent) | 1581 | 0.9 | 1.0 (referent) | 1264 | 0.8 | 1.0 (referent) |
| Negative | 27 404 | 17.1 | 0.299 (0.295 to 0.303) | 713 | 0.4 | 0.46 (0.43 to 0.51) | 1065 | 0.7 | 0.86 (0.79 to 0.93) |
| Unknown | 52 128 | 31.8 | 1342 | 0.8 | 1319 | 0.8 | |||
| PgR status | |||||||||
| Positive | 79 549 | 48.8 | 1.0 (referent) | 1292 | 0.8 | 1.0 (referent) | 1042 | 0.6 | 1.0 (referent) |
| Negative | 38 072 | 23.5 | 0.481 (0.475 to 0.487) | 965 | 0.6 | 0.76 (0.70 to 0.83) | 1239 | 0.8 | 1.2 (1.1 to 1.3) |
| Unknown | 55 319 | 33.7 | 1379 | 0.8 | 1367 | 0.8 | |||
RR = rate ratio; CI = confidence interval; LABC = non-inflammatory locally advanced breast cancer; IBC = inflammatory breast cancer; ER = estrogen receptor; PgR = progesterone receptor. Rates refer to age-adjusted incidence rates and are expressed per 100 000 woman-years.
A total of 172 940 women had non-T4 breast cancer, for a rate of 106.1; the median age was 63 (interquartile range [IQR] = 50–73).
A total of 3636 women had LABC, for a rate of 2.2; the median age was 68 (IQR = 54–79).
A total of 3648 women had IBC, for a rate of 2.3; the median age was 58 (IQR = 47–70).
Stratified by race, age-adjusted IBC incidence rates (per 100 000 woman-years) were higher in black (3.1) than in white (2.2) women (Table 2). Black women were at greater risk than white women of being diagnosed with either LABC (RR = 1.8, 95% CI = 1.7 to 2.0) or IBC (RR = 1.4, 95% CI = 1.3 to 1.5). However, their risk of non-T4 breast cancer (RR = 0.80, 95% CI = 0.79 to 0.82) was lower than that of white women.
Differences in tumor characteristics by breast cancer subtype were also evaluated (Table 2). Women diagnosed with LABC (RR = 5.4, 95% CI = 4.9 to 6.0) or IBC (RR = 5.1, 95% CI = 4.2 to 6.4) were more likely to present with larger tumors (>2.0 cm) at diagnosis than with smaller tumors (≤2.0 cm). In contrast, women diagnosed with non-T4 breast cancer were more likely to present with smaller tumors than with larger tumors (RR = 0.45, 95% CI = 0.44 to 0.46). High nuclear grade tumors were also more commonly observed than low nuclear grade tumors in women with LABC (RR = 1.6, 95% CI = 1.5 to 1.8) or IBC (RR = 3.1, 95% CI = 2.8 to 3.4), but not in women with non-T4 breast cancer (RR = 0.66, 95% CI = 0.65 to 0.67). Moreover, positive lymph node involvement was more common in LABC (RR = 2.5, 95% CI = 2.3 to 2.7) and IBC (RR = 4.7, 95% CI = 4.2 to 5.3) cases but was less common in non-T4 breast cancer cases (RR = 0.399, 95% CI = 0.396 to 0.404). In Table 3, the same patient and tumor characteristics were evaluated by IBC subtypes (ClinPath, PathOnly, and ClinOnly IBC). The patterns of association within each of these IBC subtypes were similar to those observed with total IBC.
Table 3.
Incidence rates of inflammatory breast cancer (IBC) subtypes by patient demographics, tumor characteristics, and hormone receptor status*
| IBC Subtype† |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| ClinPath IBC‡ |
PathOnly IBC§ |
ClinOnly IBC∥ |
|||||||
| Patient or tumor characteristic |
N | Rate | RR (95% CI) | N | Rate | RR (95% CI) | N | Rate | RR (95% CI) |
| Age group | |||||||||
| <50 | 555 | 0.5 | 1.0 (referent) | 54 | 0.0 | 1.0 (referent) | 530 | 0.5 | 1.0 (referent) |
| 50–59 | 383 | 2.4 | 5.1 (4.4 to 5.8) | 38 | 0.2 | 5.1 (3.4 to 7.6) | 399 | 2.5 | 5.5 (4.8 to 6.3) |
| 60–69 | 281 | 2.2 | 4.7 (4.0 to 5.4) | 24 | 0.2 | 4.0 (2.5 to 6.5) | 433 | 3.4 | 7.5 (6.6 to 8.5) |
| 70–79 | 237 | 2.3 | 4.9 (4.2 to 5.7) | 32 | 0.3 | 6.7 (4.4 to 10.3) | 344 | 3.4 | 7.4 (6.4 to 8.5) |
| 80+ | 122 | 2.0 | 4.1 (3.3 to 5.0) | 12 | 0.2 | 4.1 (2.2 to 7.5) | 204 | 3.3 | 7.1 (6.0 to 8.3) |
| Race | |||||||||
| White | 1299 | 1.0 | 1.0 (referent) | 125 | 0.1 | 1.0 (referent) | 1519 | 1.1 | 1.0 (referent) |
| Black | 196 | 1.3 | 1.3 (1.1 to 1.5) | 23 | 0.1 | 1.5 (1.0 to 2.4) | 244 | 1.7 | 1.5 (1.3 to 1.7) |
| Other | 82 | 0.6 | 11 | 0.1 | 146 | 1.0 | |||
| Tumor size | |||||||||
| ≤ 2.0 cm | 18 | 0.0 | 1.0 (referent) | 16 | 0.01 | 1.0 (referent) | 78 | 0.05 | 1.0 (referent) |
| >2.0 cm | 146 | 0.1 | 8.1 (4.6 to 14.1) | 66 | 0.04 | 4.1 (2.2 to 7.7) | 376 | 0.2 | 4.7 (3.6 to 6.1) |
| Unknown | 1414 | 0.9 | 78 | 0.05 | 1456 | 0.9 | |||
| Tumor grade | |||||||||
| Low | 254 | 0.2 | 1.0 (referent) | 20 | 0.01 | 1.0 (referent) | 352 | 0.2 | 1.0 (referent) |
| High | 836 | 0.5 | 3.3 (2.9 to 3.8) | 71 | 0.04 | 3.4 (2.0 to 5.6) | 1032 | 0.6 | 3.0 (2.6 to 3.4) |
| Unknown | 488 | 0.3 | 69 | 0.04 | 526 | 0.3 | |||
| Lymph node status | |||||||||
| Negative | 151 | 0.1 | 1.0 (referent) | 21 | 0.01 | 1.0 (referent) | 228 | 0.1 | 1.0 (referent) |
| Positive | 815 | 0.5 | 5.3 (4.5 to 6.4) | 64 | 0.04 | 3.1 (1.8 to 5.1) | 1038 | 0.6 | 4.5 (3.9 to 5.2) |
| Unknown | 612 | 0.4 | 75 | 0.05 | 644 | 0.4 | |||
| ER status | |||||||||
| Positive | 522 | 0.3 | 1.0 (referent) | 38 | 0.02 | 1.0 (referent) | 704 | 0.4 | 1.0 (referent) |
| Negative | 516 | 0.3 | 1.0 (0.9 to 1.1) | 35 | 0.02 | 1.0 (0.6 to 1.6) | 514 | 0.3 | 0.75 (0.67 to 0.83) |
| Unknown | 540 | 0.3 | 87 | 0.05 | 692 | 0.4 | |||
| PgR status | |||||||||
| Positive | 450 | 0.3 | 1.0 (referent) | 31 | 0.02 | 1.0 (referent) | 561 | 0.3 | 1.0 (referent) |
| Negative | 564 | 0.4 | 1.3 (1.1 to 1.4) | 41 | 0.03 | 1.4 (0.9 to 2.1) | 634 | 0.4 | 1.2 (1.0 to 1.3) |
| Unknown | 564 | 0.4 | 88 | 0.05 | 715 | 0.4 | |||
RR = rate ratio; CI = confidence interval; ER = estrogen receptor; PgR = progesterone receptor. Rates refer to age-adjusted incidence rates and are expressed per 100000 woman-years.
ClinPath IBC refers to cases with both clinical and pathologic features of IBC ([EOD-E 70 and/or EOD-S 998] and ICD-O-2 8530/3); PathOnly IBC refers to cases with pathologic features of IBC (ICD-O-2 8530/3); ClinOnly IBC refers to cases with clinical features of IBC (EOD-E 70 and/or EOD-S 998).
A total of 1578 women had ClinPath IBC, for a rate of 1.0. The median age was 55 (interquartile range [IQR] = 46–68).
A total of 160 women had PathOnly IBC, for a rate of 0.1. The median age was 58 (IQR = 47–71).
A total of 1910 women had ClinOnly IBC, for a rate of 1.2. The median age was 60 (IQR = 49–71).
The age-adjusted incidence rates of IBC increased from the 3-year interval 1988–1990 through the 3-year interval 1997–1999 (Table 4) (2.0 to 2.5, Ptrend<.001). This statistically significant temporal increase was limited to white patients (2.0 to 2.5, Ptrend<.001). In contrast to the increase in IBC incidence rates, overall incidence rates declined for LABC (2.5 to 2.0, Ptrend = .0025) and non-T4 breast cancer (107.7 to 100.5, Ptrend = .0084) over the same 3-year intervals.
Table 4.
Age-adjusted incidence rates and 95% confidence intervals of each breast cancer subtype (also stratified by race, in the case of IBC) by 3-year interval*
| 3-y interval | Non-T4 | LABC | IBC | IBC in whites | IBC in blacks |
|---|---|---|---|---|---|
| 1988–1990 | 107.7 (106.6 to 108.8) | 2.5 (2.4 to 2.7) | 2.0 (1.8 to 2.2) | 2.0 (1.8 to 2.2) | 2.6 (2.1 to 3.3) |
| 1991–1993 | 111.8 (110.7 to 112.9) | 2.1 (1.9 to 2.2) | 2.3 (2.1 to 2.4) | 2.2 (2.1 to 2.4) | 3.5 (2.8 to 4.2) |
| 1994–1996 | 112.9 (111.9 to 114.0) | 2.1 (1.9 to 2.2) | 2.4 (2.2 to 2.5) | 2.4 (2.2 to 2.5) | 3.0 (2.4 to 3.6) |
| 1997–1999 | 100.5 (99.5 to 101.4) | 2.0 (1.9 to 2.2) | 2.5 (2.3 to 2.6) | 2.5 (2.3 to 2.7) | 3.1 (2.5 to 3.7) |
| Chi-square test of trend | P = .0084 | P = .0025 | P<.0001 | P<.0001 | P = .3153 |
LABC = locally advanced breast cancer; IBC = inflammatory breast cancer. Age-adjusted incidence rates are expressed per 100 000 woman-years.
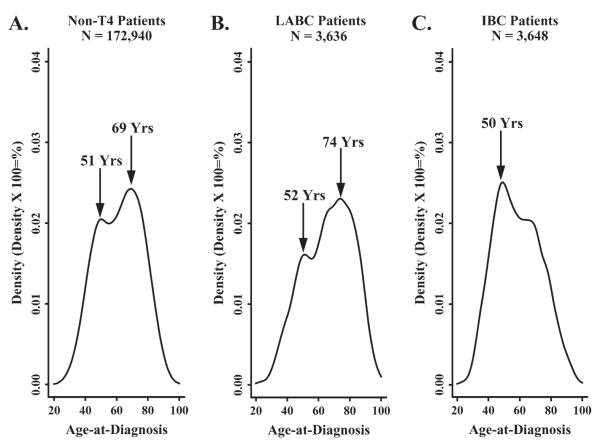
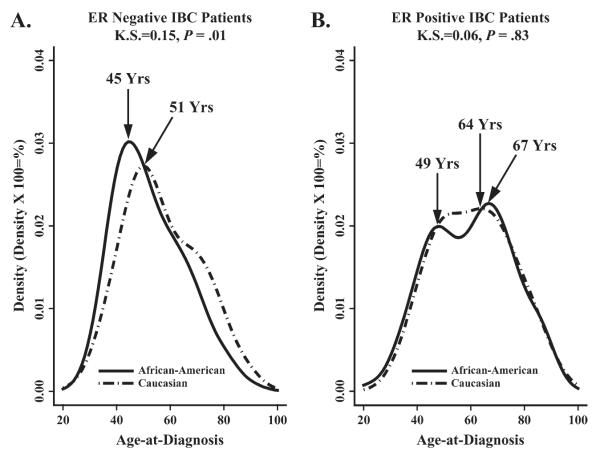
Age density histograms of non-T4 breast cancer (Fig. 1, A), LABC (Fig. 1, B), and IBC (Fig. 1, C) all revealed a bimodal pattern for age at diagnosis. The predominant age peaks for diagnosis of non-T4 breast cancer and LABC were at older ages, with prominent points of inflection at 69 and 74 years of age, respectively. In contrast, IBC exhibited an earlier prominent inflection point at 50 years of age (Fig. 1, C). When IBC patients were stratified by race and ER status, black IBC patients with ER-negative tumors (Fig. 2, A) exhibited a statistically significantly younger age at diagnosis than white patients diagnosed with ER-negative tumors (KS = 0.15, P = .01). However, there was no difference in age at diagnosis in IBC patients with ER-positive tumors by race (KS = 0.06, P = .83).
Fig. 1.
Age density histograms showing the age distributions of patients with different breast cancer subtypes. A) Non-T4 breast cancer; B) non-inflammatory locally advanced breast cancer (LABC); and C) inflammatory breast cancer (IBC). Data are from the Surveillance, Epidemiology, and End Results (SEER) 9 Registries, 1988–2000.
Fig. 2.
Age density histograms showing the age distributions of IBC patients with different estrogen receptor (ER) status. A) ER-negative status. B) ER-positive status. The solid line represents the age distribution in black women and the dotted line represents the age distribution in white women. Data are from the Surveillance, Epidemiology, and End Results (SEER) 9 Registries, 1988–2000. K.S. = Kolmogorov–Smirnov.
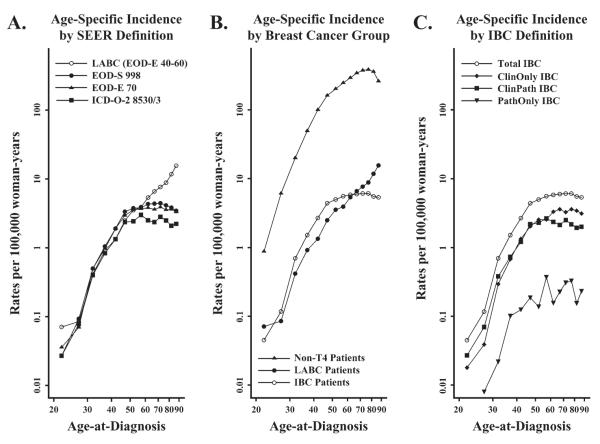
Age-specific incidence rate curves were created for each breast cancer subgroup. As Fig. 3, A shows, substantial differences in the age-specific rates were evident among patients older than 50 years of age, with the rates of IBC, as defined by codes EOD-E 70, EOD-S 998, and ICD-O-2 8530/3, leveling off whereas those for LABC continued to rise. When IBC was defined as a single entity, the age-specific incidence rates for total IBC rose until 50 years of age and then remained constant (Fig. 3, B). By contrast, the age-specific incidence rates of non-T4 breast cancer and LABC continued to rise in women older than 50 years of age (Fig. 3, B). This distinct age-specific incidence rate pattern for IBC was observed regardless of whether IBC was defined on the basis of clinical (ClinOnly IBC), pathologic (PathOnly IBC), or clinical and pathologic (ClinPath IBC) criteria (Fig. 3, C).
Fig.3.
Age-specific incidence rates of breast cancer per 100 000 woman-years. Breast cancer cases grouped: A) using the Surveillance, Epidemiology, and End Results (SEER) coding conventions for noninflammatory locally advanced breast cancer (LABC) and inflammatory breast cancer (IBC); B) by three malignant breast cancer subtypes; C) by IBC definition. Data are from SEER 9 Registries, 1988–2000.
Age-specific incidence rates of each breast cancer subtype were also stratified by ER status and race. The incidence rates of ER-positive and -negative tumors among patients with non-T4 breast cancer diverged in women older than 50 years of age, with the incidence rates of ER-positive tumors exceeding those of ER-negative tumors in both black and white women (Fig. 4, A). The age-specific incidence rates of LABC in black women were greater than those in white women diagnosed before 50 years of age, regardless of ER receptor status. Among women diagnosed at 50 years of age or older, the incidence rates of ER-positive LABC were higher than those of ER-negative LABC (Fig.4, B). Among IBC patients diagnosed before 50 years of age, the age-specific incidence rates of ER-negative tumors were slightly higher than those of ER-positive tumors (Fig. 4, C). In IBC patients who were 50 years of age or older, rates of ER-positive tumors were higher than those of ER-negative tumors in both black and white women (Fig. 4, C).
Fig.4.
Age-specific incidence rates of breast cancer per 100 000 woman-years stratified by estrogen receptor (ER) status and race. A) Non-T4 breast cancer. B) Noninflammatory locally advanced breast cancer (LABC). C) Inflammatory breast cancer (IBC). Data are from the Surveillance, Epidemiology, and End Results (SEER) 9 Registries, 1988–2000.
The age-specific incidence rates of ER-positive non-T4 breast cancer were consistently higher among white women than among black women of all ages (Fig. 4, A). In contrast, the age-specific incidence rates of ER-negative non-T4 breast cancer were consistently higher among black women at all ages (Fig. 4, A). For both LABC (Fig. 4, B) and IBC (Fig. 4, C) subtypes, the age-specific incidence rates of ER-positive and -negative tumors were consistently higher among black patients than among white patients diagnosed at 50 years of age or older.
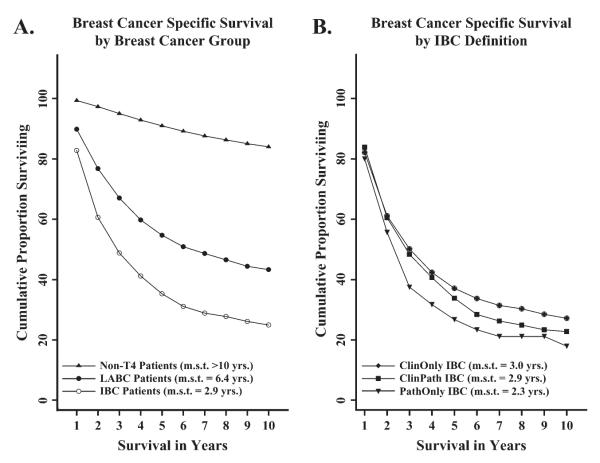
Of all microscopically confirmed malignancies of the breast diagnosed in the SEER 9 Registries database between 1988 and 2000, IBC comprised 7.0% (1936 of 27 747) of all breast cancer–specific mortality. Kaplan–Meier plots (Fig. 5) show that breast cancer–specific survival was statistically significantly poorer in patients with IBC than in patients with LABC or non-T4 breast cancer (log-rank test, P<.001). The median survival times of women with non-T4 breast cancer (greater than 10 years), LABC (6.4 years), and IBC (2.9 years) were also statistically significantly different (P<.0001) (Fig. 5, A). When actuarial breast cancer–specific survival among the IBC subtypes was compared (Fig. 5, B), patients with pathologically defined IBC (PathOnly IBC) had statistically significantly shorter survival than patients with IBC defined by clinical features only (ClinOnly IBC) or by both clinical and pathologic features (ClinPath IBC) (log-rank test, P<.001). However, the median survival time of patients with PathOnly IBC (2.3 years) was not statistically different from that of patients with ClinOnly IBC (3.0 years) or ClinPath IBC (2.9 years) (P = .058).
Fig. 5.
Kaplan–Meier curves showing relative breast cancer-specific survival curves. Median survival time (m.s.t.) is expressed in years for each group. A) Breast cancer subtype. B) Inflammatory breast cancer (IBC) definition. Data are from the Surveillance, Epidemiology, and End Results (SEER) 9 Registries, 1988–2000.
Breastcancer–specific survival among IBC patients improved throughout the 1990s; however, the median survival time in black women with IBC was only 2.0 years compared with 3.0 years among white women (log-rank test, P<.001). Breast cancer–specific survival was also statistically significantly poorer among black women with LABC (median of 3.1 years) than among white patients with LABC (median of 7.5 years) (log-rank test, P<.001).
When IBC patients were stratified by ER status for analysis of breast cancer–specific survival, IBC patients with ER-negative tumors had poorer survival than those with ER-positive tumors (log-rank test, P<.001). ER-negative patients had a median survival of 2.0 years, compared with 4.0 years for ER-positive patients (P<.0001).
DISCUSSION
IBC appears to be a relatively rare form of breast cancer; however, the varying case definitions used to classify IBC have limited our understanding of this disease. Here, we report the population-based incidence rate of IBC and describe patient demographics and tumor characteristics that are associated with IBC using data from the National Cancer Institute’s SEER 9 Registries. We used codes available in SEER to develop a comprehensive clinical and/or pathologic definition for IBC that was consistent with the AJCC definition (5). The extent of disease code for tumor extension (EOD-E 70) and a novel code for tumor size (EOD-S 998) were used to define the clinical features of IBC. The ICD-O-2 morphology code 8530/3 (i.e., embolization of the dermal lymphatic vessels) was used to define IBC based on pathologic criteria (14). The most recent population-based analyses of IBC have used either ICD-O-2 8530/3 (9-11) or EOD-E 70 (4) to define IBC, but none of these studies used the combination of clinical (EOD-E 70 or EOD-S 998) and pathologic (ICD-O-2 8530/3) coding conventions to construct the IBC case definition. Thus, our study adds additional information to the literature beyond that previously reported by 1) constructing a novel and comprehensive SEER-based case definition for IBC, 2) establishingtheage-adjusted incidence for total IBC in the U.S. population, and 3) examining temporal trends in IBC incidence and survival between 1988 and 2000.
The age distribution of IBC patients (rates and frequency) revealed a pattern of early age at diagnosis that was consistent with what has been seen in previous studies (4, 7, 9, 26, 27). In a recent study, the age-specific incidence rate pattern for IBC was similar to that for medullary breast carcinoma but different than that for other histopathologic subtypes (10). Further research is required to determine whether genetic predisposition and/or early life exposures account for this peculiar age distribution. Our findings also showed an earlier age at IBC diagnosis in black women, suggesting that substantial racial disparities exist in IBC patients relative to non-T4 breast cancer patients.
Total IBC comprised 2.0% of all incident breast cancer cases, similar to the lower end of earlier estimates (1-3, 6, 26, 28, 29) but higher than recent population-based studies of IBC (4, 9-11). A large number of cases were defined by clinical features of IBC without pathologic confirmation of dermal lymphatic invasion (ClinOnly IBC; n = 1910), suggesting that studies lacking a clinically defined IBC case definition may underestimate the true incidence of this disease.
In the 3-year intervals between 1988 and 1990 and 1997 and 1999, the age-adjusted incidence rate of IBC rose by 25%. This increase in IBC incidence was statistically significant only in white women, and the results suggest that IBC incidence rates in white women may be catching up to those in black women. By contrast, Chang et al. (9) reported that the incidence of IBC doubled in both blacks and whites between 1975 and 1977 and 1990 and 1992, with the rates of IBC in white patients lagging behind those in black patients.
Possible explanations for the increase in IBC incidence that we observed may be heightened clinical awareness and earlier detection with screening mammography. The period of our study indeed coincides with a rise in mammography use (30, 31). However, earlier detection over time with screening mammography is generally associated with a fall in the rate of late-stage disease (32), as seen for LABC but not IBC in this study. IBC may be difficult to detect by mammography for several reasons. First, studies examining the mammographic patterns of inflammatory changes associated with IBC commonly show skin thickening, stromal coarsening, and diffusely increased breast density, whereas an associated mass and/or malignant-type calcification, although common in patients with IBC, may be absent on mammography owing to diffusely increased breast density (33-36). Second, IBC by its clinicopathologic definition is a diffuse tumor (3, 5). Thus, the utility of mammographic screening in the early detection and diagnosis of IBC may be limited because of the combination of a diffuse tumor obscured by increased breast density brought on by inflammation.
Another possible explanation for the increased incidence of IBC is changing patterns of risk factor exposure. There is some evidence that reproductive hormone exposure plays a role in the etiology of IBC (37, 38). Although the differences did not reach statistical significance, Chang et al. (38) reported that IBC patients were younger at menarche and at the time of their first live birth and that a higher proportion of IBC patients were premenopausal than their non-IBC counterparts. In a subsequent study by Chang et al. (39), premenopausal IBC patients were shown to have statistically significantly worse survival than postmenopausal IBC patients. In our study, we found, as have others (3, 10), that the age-specific incidence rates of IBC rose rapidly until 50 years of age and then stabilized. We observed this pattern for IBC incidence irrespective of race or ER status. These data support the findings of Chang et al. (38, 39), suggesting that premenopausal events such as the premenopausal hormonal milieu may be important factors in the initiation and progression of IBC.
We observed a modest trend for improvement in IBC survival in this study; however, the median survival time of IBC patients improved by only 8.4 months between 1988 and 1990 and 1997 and 1999. A statistically significant improvement in IBC survival after the 1988–1990 interval coincided with the more common use of anthracycline-based neoadjuvant therapy and the introduction of paclitaxel-based regimens in the clinical management of IBC in the 1990s (40). Although we were unable to directly examine how changes in therapy for IBC affected survival, distinct tumor features, unique age distributions, and different survival outcomes for IBC and LABC suggest that future clinical trials should use a case definition for IBC that more effectively excludes LABC. Moreover, our survival analysis supports the conclusions of Somlo et al. (41), who argued for the need to develop standard staging criterion for treatment planning in which individuals at higher risk of developing more aggressive IBC subtypes receive more aggressive neoadjuvant and adjuvant chemotherapy.
Studies of breast cancer survival outcomes in equal-access health care systems demonstrate that African-American race/ethnicity is an independent predictor of elevated risk for breast cancer mortality (42, 43). These findings are consistent with our finding that among black women breast cancer mortality was higher in IBC patients than in patients with either LABC or non-T4 disease. It is interesting to note that the 5-year relative risk of death in black women diagnosed with breast cancer and treated in the U.S. Department of Defense health care system was 25%, whereas that for black women diagnosed in the SEER Registries database was 34% (42). These findings suggest that equal access to health care such as that provided in the U.S. Department of Defense health care system improves breast cancer mortality among black women but does not fully account for racial disparities in survival outcome between black and white women.
Race and socioeconomic status have been reported to be independent predictors of advanced stage at diagnosis in breast cancer (44, 45). Race is also a statistically significant predictor of tumor aggressiveness as measured by histologic grade within each stage of breast cancer (46). Although black and white women have equivalent response rates to local and systemic breast cancer therapy, higher rates of locoregional recurrence in black patients suggest the presence of a biologic determinant for more aggressive breast cancer (43). The early age at diagnosis, age-specific incidence rate patterns, and poor survival outcomes described for IBC patients in this study are consistent with the effects of both race/ethnicity and biologic determinants of aggressive breast cancer.
The strength of this study is its large-scale population-based design. The potential limitations of this study include 1) a lack of histopathologic slide review, 2) missing or incomplete data, 3) nonstandardized measurement of hormone receptor expression, and 4) lack of data for menstrual status, reproductive risk factors, method of detection, and treatment. Although SEER does not provide information about reproductive risk factors, it does provide information on the most important risk factor for female breast cancer, which is age at diagnosis. Therefore, we used 50 years of age as a surrogate for menopausal status (15). Without information about the method of detection, it is not possible for us to distinguish between the impact of heightened clinical awareness and screening mammography on the temporal trends in IBC incidence. Treatment records were unavailable; however, the standard tumor characteristics described in this study are beneficial in predicting prognosis irrespective of treatment (47).
Biomarkers for early detection and targeted therapies based on an understanding of the molecular determinants of IBC might improve the clinical management of this disease and improve survival among patients. The understanding of the molecular determinants of IBC is poised for advancement, with the efforts of several laboratories to develop microarray-based genomic profiles to define the molecular signature of IBC (48, 49). A gene expression profile that is characteristic of IBC may help resolve the debate over how to appropriately define IBC (7, 8, 50). Comparing the gene expression profiles of IBC patients stratified by menopausal status, race, and hormone receptor status should facilitate our understanding of the etiology of this disease and may help to identify IBC subtypes that may possess common therapeutic responses and clinical outcomes. With further characterization of the molecular profile and etiology of IBC, we may eventually determine why IBC incidence continues to rise and why the IBC phenotype is so aggressive and resistant to current treatment modalities.
REFERENCES
- (1).Lee BJ, Tannenbaum NE. Inflammatory carcinoma of the breast. Surg Gynecol Obstet. 1924;39:580–95. [Google Scholar]
- (2).Taylor GW, Meltzer A. “Inflammatory carcinoma” of the breast. Am J Cancer. 1938;33:33–49. [Google Scholar]
- (3).Haagensen CD. Diseases of the breast. 2nd ed Saunders; Philadelphia (PA): 1971. Inflammatory carcinoma; pp. 576–84. [Google Scholar]
- (4).Anderson WF, Chu KC, Chang S. Inflammatory breast carcinoma and non-inflammatory locally advanced breast carcinoma: distinct clinicopathologic entities? J Clin Oncol. 2003;21:2254–9. doi: 10.1200/JCO.2003.07.082. [DOI] [PubMed] [Google Scholar]
- (5).Green FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, et al., editors. AJCC cancer staging manual. 6th ed Springer-Verlag; New York (NY): 2002. Breast; pp. 255–81. [Google Scholar]
- (6).Levine PH, Steinhorn SC, Ries LG, Aron JL. Inflammatory breast cancer: the experience of the Surveillance, Epidemiology, and End Results (SEER) Program. J Natl Cancer Inst. 1985;74:291–7. [PubMed] [Google Scholar]
- (7).Lucas FV, Perez-Mesa C. Inflammatory carcinoma of the breast. Cancer. 1978;41:1595–605. doi: 10.1002/1097-0142(197804)41:4<1595::aid-cncr2820410450>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- (8).Bonnier P, Charpin C, Lejeune C, Romain S, Tubiana N, Beedassy B, et al. Inflammatory carcinomas of the breast: a clinical, pathological, or a clinical and pathological definition? Int J Cancer. 1995;62:382–5. doi: 10.1002/ijc.2910620404. [DOI] [PubMed] [Google Scholar]
- (9).Chang S, Parker SL, Pham T, Buzdar AU, Hursting SD. Inflammatory breast carcinoma incidence and survival: the Surveillance, Epidemiology, and End Results Program of the National Cancer Institute, 1975–1992. Cancer. 1998;82:2366–72. [PubMed] [Google Scholar]
- (10).Anderson WF, Chu KC, Chang S, Sherman ME. Comparison of age-specific incidence rate patterns for different histopathologic types of breast carcinoma. Cancer Epidemiol Biomarkers Prev. 2004;13:1128–35. [PubMed] [Google Scholar]
- (11).Wingo PA, Jamison PM, Young JL, Gargiullo P. Population-based statistics for women diagnosed with inflammatory breast cancer (United States) Cancer Causes and Control. 2004;15:321–8. doi: 10.1023/B:CACO.0000024222.61114.18. [DOI] [PubMed] [Google Scholar]
- (12).National Cancer Institute. DCCPS. Surveillance Research Program. Cancer Statistics Branch Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence—SEER 9 Regs Public-Use, Nov 2002 Sub (1973–2000) 2003 Available at: http://www.seer.cancer.gov.
- (13).SEER Extent of Disease: 1988 codes and coding instructions. (3rd ed) Available at: http://www.seer.cancer.gov/tools/codingmanuals.
- (14).World Health Organization . International Classification of Diseases for Oncology. 2nd ed The Organization; Geneva (Switzerland): 1990. [Google Scholar]
- (15).Morabia A, Flandre P. Misclassification bias related to definition of menopausal status in case-control studies of breast cancer. Int J Epidemiol. 1992;21:222–8. doi: 10.1093/ije/21.2.222. [DOI] [PubMed] [Google Scholar]
- (16).Methods for rates and proportions. 2nd ed John Wiley & Sons; New York (NY): 1981. [Google Scholar]
- (17).Groves FD, Linet MS, Travis LB, Devesa SS. Cancer surveillance series: Non-Hodgkin’s lymphoma incidence by histologic subtype in the United States from 1978 through 1995. J Natl Cancer Inst. 2000;92:1240–51. doi: 10.1093/jnci/92.15.1240. [DOI] [PubMed] [Google Scholar]
- (18).Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4:213–26. doi: 10.1002/sim.4780040211. [DOI] [PubMed] [Google Scholar]
- (19).Anderson WF, Chu KC, Chatterjee N, Brawley O, Brinton LA. Tumor variants by hormone receptor expression in white patients with node-negative breast cancer from the Surveillance, Epidemiology, and End Results database. J Clin Oncol. 2001;19:18–27. doi: 10.1200/JCO.2001.19.1.18. [DOI] [PubMed] [Google Scholar]
- (20).Anderson WF, Chatterjee N, Ershler WB, Brawley OW. Estrogen receptor breast cancer phenotypes in the Surveillance, Epidemiology, and End Results database. Breast Cancer Res Treat. 2002;76:27–36. doi: 10.1023/a:1020299707510. [DOI] [PubMed] [Google Scholar]
- (21).Introduction to the theory of non-parametric statistics. John Wiley & Sons; New York (NY): 1979. [Google Scholar]
- (22).The design of experiments. 8th ed Oliver and Boyd; Edinburgh (Scotland): 1966. [Google Scholar]
- (23).Surveillance Research Program. National Cancer Institute SEER*Stat Software. (version 5.0.20) 2003 Available at: http://www.seer.cancer.gov/seerstat.
- (24).Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- (25).Peto R, Peto J. Asymptomatically efficient rank variant test procedures. J R Stat Soc A. 1972;135:185–98. [Google Scholar]
- (26).Bonnier P, Piana L, Khouzami A, Romain S, Padaut J, Charpin C, et al. Inflammatory carcinoma of the breast. Eur J Gynaecol Oncol. 1992;13:7–11. [PubMed] [Google Scholar]
- (27).Anderson WF, Chu KC, Chang S. Inreply. J Clin Oncol. 2004;22:383. [Google Scholar]
- (28).Barber KW, Jr, Dockerty MB, Clagett OT. Inflammatory carcinoma of the breast. Suvr Med (Sofiia) 1961;112:406–10. [PubMed] [Google Scholar]
- (29).Grace WR, Cooperman AM. Inflammatory breast cancer. Surg Clin North Am. 1985;65:151–60. doi: 10.1016/s0039-6109(16)43539-5. [DOI] [PubMed] [Google Scholar]
- (30).Breen N, Wagener DK, Brown ML, Davis WW, Ballard-Barbash R. Progress in cancer screening over a decade: results of cancer screening from the 1987, 1992, and 1998 National Health Interview Surveys. J Natl Cancer Inst. 2001;93:1704–13. doi: 10.1093/jnci/93.22.1704. [DOI] [PubMed] [Google Scholar]
- (31).Swan J, Breen N, Coates RJ, Rimer BK, Lee NC. Progress in cancer screening practices in the United States: results from the 2000 National Health Interview Survey. Cancer. 2003;97:1528–40. doi: 10.1002/cncr.11208. [DOI] [PubMed] [Google Scholar]
- (32).Chu KC, Tarone RE, Kessler LG, Ries LA, Hankey BF, Miller BA, et al. Recent trends in U.S. breast cancer incidence, survival, and mortality rates. J Natl Cancer Inst. 1996;88:1571–9. doi: 10.1093/jnci/88.21.1571. [DOI] [PubMed] [Google Scholar]
- (33).Gunhan-Bilgen I, Ustun EE, Memis A. Inflammatory breast carcinoma: mammographic, ultrasonographic, clinical, and pathologic findings in 142 cases. Radiology. 2002;223:829–38. doi: 10.1148/radiol.2233010198. [DOI] [PubMed] [Google Scholar]
- (34).Kushwaha AC, Whitman GJ, Stelling CB, Cristofanilli M, Buzdar AU. Primary inflammatory carcinoma of the breast: retrospective review of mammographic findings. AJR Am J Roentgenol. 2000;174:535–8. doi: 10.2214/ajr.174.2.1740535. [DOI] [PubMed] [Google Scholar]
- (35).Tardivon AA, Viala J, Corvellec RA, Guinebretiere JM, Vanel D. Mammographic patterns of inflammatory breast carcinoma: a retrospective study of 92 cases. Eur J Radiol. 1997;24:124–30. doi: 10.1016/s0720-048x(96)01137-0. [DOI] [PubMed] [Google Scholar]
- (36).Dershaw DD, Moore MP, Liberman L, Deutch BM. Inflammatory breast carcinoma: mammographic findings. Radiology. 1994;190:831–4. doi: 10.1148/radiology.190.3.8115635. [DOI] [PubMed] [Google Scholar]
- (37).Mourali N, Muenz LR, Tabbane F, Belhassen S, Bahi J, Levine PH. Epidemiologic features of rapidly progressing breast cancer in Tunisia. Cancer. 1980;46:2741–6. doi: 10.1002/1097-0142(19801215)46:12<2741::aid-cncr2820461234>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- (38).Chang S, Buzdar AU, Hursting SD. Inflammatory breast cancer and body mass index. J Clin Oncol. 1998;16:3731–5. doi: 10.1200/JCO.1998.16.12.3731. [DOI] [PubMed] [Google Scholar]
- (39).Chang S, Alderfer JR, Asmar L, Buzdar AU. Inflammatory breast cancer survival: the role of obesity and menopausal status at diagnosis. Breast Cancer Res Treat. 2000;64:157–63. doi: 10.1023/a:1006489100283. [DOI] [PubMed] [Google Scholar]
- (40).Cristofanilli M, Buzdar AU, Hortobagyi GN. Update on the management of inflammatory breast cancer. Oncologist. 2003;8:141–8. doi: 10.1634/theoncologist.8-2-141. [DOI] [PubMed] [Google Scholar]
- (41).Somlo G, Frankel P, Chow W, Leong L, Margolin K, Morgan R, Jr, et al. Prognostic indicators and survival in patients with stage IIIB inflammatory breast carcinoma after dose-intense chemotherapy. J Clin Oncol. 2004;22:1839–48. doi: 10.1200/JCO.2004.10.147. [DOI] [PubMed] [Google Scholar]
- (42).Wojcik BE, Spinks MK, Optenberg SA. Breast carcinoma survival analysis for African American and white women in an equal-access health care system. Cancer. 1998;82:1310–8. doi: 10.1002/(sici)1097-0142(19980401)82:7<1310::aid-cncr14>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- (43).Newman LA, Mason J, Cote D, Vin Y, Carolin K, Bouwman D, et al. African-American ethnicity, socioeconomic status, and breast cancer survival: a meta-analysis of 14 studies involving over 10 000 African-American and 40 000 White American patients with carcinoma of the breast. Cancer. 2002;94:2844–54. doi: 10.1002/cncr.10575. [DOI] [PubMed] [Google Scholar]
- (44).Yood MU, Johnson CC, Blount A, Abrams J, Wolman E, McCarthy BD, et al. Race and differences in breast cancer survival in a managed care population. J Natl Cancer Inst. 1999;91:1487–91. doi: 10.1093/jnci/91.17.1487. [DOI] [PubMed] [Google Scholar]
- (45).Schwartz KL, Crossley-May H, Vigneau FD, Brown K, Banerjee M. Race, socioeconomic status and stage at diagnosis for five common malignancies. Cancer Causes Control. 2003;14:761–6. doi: 10.1023/a:1026321923883. [DOI] [PubMed] [Google Scholar]
- (46).Henson DE, Chu KC, Levine PH. Histologic grade, stage, and survival in breast carcinoma: comparison of African American and Caucasian women. Cancer. 2003;98:908–17. doi: 10.1002/cncr.11558. [DOI] [PubMed] [Google Scholar]
- (47).Henderson IC, Patek AJ. The relationship between prognostic and predictive factors in the management of breast cancer. Breast Cancer Res Treat. 1998;52:261–88. doi: 10.1023/a:1006141703224. [DOI] [PubMed] [Google Scholar]
- (48).Van den Eynden GG, Van der Auwera I, Van Laere S, Colpaert CG, van Dam P, Merajver S, et al. Validation of a tissue microarray to study differential protein expression in inflammatory and non-inflammatory breast cancer. Breast Cancer Res Treat. 2004;85:13–22. doi: 10.1023/B:BREA.0000021028.33926.a8. [DOI] [PubMed] [Google Scholar]
- (49).Wu M, Wu ZF, Kumar-Sinha C, Chinnaiyan A, Merajver SD. RhoC induces differential expression of genes involved in invasion and metastasis in MCF10A breast cells. Breast Cancer Res Treat. 2004;84:3–12. doi: 10.1023/B:BREA.0000018426.76893.21. [DOI] [PubMed] [Google Scholar]
- (50).Amparo RS, Angel CD, Ana LH, Antonio LC, Vicente MS, Carlos FM, et al. Inflammatory breast carcinoma: pathological or clinical entity? Breast Cancer Res Treat. 2000;64:269–73. doi: 10.1023/a:1026512722789. [DOI] [PubMed] [Google Scholar]