Abstract
Cytokine-induced activation of indoleamine 2,3 dioxygenase (IDO) catabolizes L-tryptophan (TRP) into L-kynurenine (KYN), which is metabolized to quinolinic acid (QUIN) and kynurenic acid (KA). QUIN and KA are neuroactive and may contribute to the behavioral changes experienced by some patients during exposure to inflammatory stimuli such as interferon (IFN)-alpha. A relationship between depressive symptoms and peripheral blood TRP, KYN and KA during IFN-alpha treatment has been described. However, whether peripheral blood changes in these IDO catabolites are manifest in the brain and whether they are related to central nervous system cytokine responses and/or behavior is unknown. Accordingly, TRP, KYN, QUIN and KA were measured in cerebrospinal fluid (CSF) and blood along with CSF concentrations of relevant cytokines, chemokines and soluble cytokine receptors in 27 patients with hepatitis C after ~12 weeks of either treatment with IFN-alpha (n=16) or no treatment (n=11). Depressive symptoms were assessed using the Montgomery Asberg Depression Rating Scale. IFN-alpha significantly increased peripheral blood KYN, which was accompanied by marked increases in CSF KYN. Increased CSF KYN was in turn associated with significant increases in CSF QUIN and KA. Despite significant decreases in peripheral blood TRP, IFN-alpha had no effect on CSF TRP concentrations. Increases in CSF KYN and QUIN were correlated with increased CSF IFN-alpha, soluble tumor necrosis factor-alpha receptor 2 (sTNFR2) and monocyte chemoattractant protein (MCP)-1 as well as increased depressive symptoms. In conclusion, peripheral administration of IFN-alpha activated IDO in concert with central cytokine responses, resulting in increased brain KYN, QUIN, KA, and ultimately depressive symptoms.
Keywords: interferon-alpha, indoleamine 2, 3 dioxygenase, kynurenine, quinolinic acid, kynurenic acid, tryptophan, cytokines, chemokines, depression
Introduction
Converging evidence suggests that activation of inflammatory responses including the release of inflammatory cytokines may contribute to the pathogenesis of major depression.1-4 When compared to non-depressed individuals, some medically ill and medically healthy patients with major depression have been found to exhibit evidence of inflammation, including elevations in inflammatory cytokines and their soluble receptors in peripheral blood and cerebrospinal fluid (CSF), as well as elevations in peripheral blood concentrations of acute phase proteins, chemokines, adhesion molecules and inflammatory mediators such as prostaglandins.1,2,5 Complementing these cross-sectional data are studies showing that some individuals acutely or chronically exposed to inflammatory stimuli, including lipopolysaccharide (LPS), typhoid vaccination and the innate immune cytokine, interferon (IFN)-alpha, develop symptoms indistinguishable from those of major depression.6-8 Moreover, depressive symptoms can be reduced by cytokine antagonists in patients with inflammatory disorders,9 and administration of anti-inflammatory drugs can enhance responsiveness to conventional antidepressant medications.10 Finally, abundant data from laboratory animals and increasing evidence from human studies demonstrate that inflammatory cytokines and activated immune cells can access the brain and influence numerous pathways believed to be involved in depression including neurotransmitter metabolism, neuroendocrine function and neural plasticity.1,3,11-17
One pathway that may be relatively unique to the mechanisms by which inflammatory stimuli contribute to the symptoms of depression is activation of the enzyme, indoleamine 2,3-dioxygenase (IDO). IDO is expressed in cell types throughout the body including fibroblasts, dendritic cells, monocytes, macrophages and microglia and can be induced by a number of cytokines such as IFN-gamma, IFN-alpha and tumor necrosis factor (TNF)-alpha either alone or in combination.18-20 Cytokine-induced activation of IDO can occur through several relevant inflammatory signaling pathways including signal transducer and activator of transcription 1 (STAT1), interferon regulatory factor (IRF)-1, p38 mitogen activated protein kinase (MAPK), and nuclear factor kappa B (NF-kB).19,21 Once activated, IDO leads to the breakdown of L-tryptophan (TRP) into L-kynurenine (KYN), thereby reducing the availability of TRP, which not only plays an important role in the regulation of T cells,22 but also is the primary amino acid precursor of serotonin, a monoamine that is believed to play a prominent role in the neurobiology of mood disorders.23,24
Of relevance to regulation of behavior during immune activation, activation of IDO appears to play a critical role in the development of depressive-like symptoms in laboratory animals following exposure to inflammatory stimuli such as LPS and bacille Calmette-Guérin (BCG), an attenuated form of Mycobacterium bovis.25,26 For example, treatment of mice with LPS or BCG induces IDO mRNA in the brain, and administration of the specific IDO inhibitor, 1-methyltryptophan, blocks both LPS- and BCG-induced increases in immobility in the forced swim test and the tail suspension test.25,26 In addition, mice genetically deficient in IDO are resistant to the development of depressive-like behaviors following BCG inoculation, despite induction of inflammatory cytokines.26 In humans, a number of studies indicate that activation of IDO pathways also may contribute to depression in the context of immune stimulation. Indeed, administration of IFN-alpha decreases peripheral blood concentrations of TRP and increases peripheral blood KYN, which in turn, are associated with IFN-alpha-induced depressive symptoms, which develop in 20-50% of IFN-alpha-treated patients depending on the dose.27-31 Similar relationships between evidence of IDO activation and behavioral changes are observed in patients with cancer and patients infected with human immunodeficiency virus (HIV).32,33
Interestingly, however, peripheral administration of KYN alone can induce depressive-like behavior in laboratory animals,25,34 indicating that TRP depletion may not be required for the induction of behavioral changes following IDO activation as has been previously suggested.35 Moreover, these data are consistent with findings that during IFN-alpha treatment, no changes were found in the availability of TRP relative to other amino acids that compete with TRP for transport into the brain.30 Taken together, these results support the hypothesis that KYN and its neuroactive metabolites may be more relevant to cytokine-induced behavioral changes than TRP depletion.30,36
Blood borne KYN, which is a primary source for central nervous system (CNS) kynurenines, is taken up into the brain through the large amino acid transporter, where it can be further metabolized in glial cells (microglia and astrocytes) and macrophages to quinolinic acid (QUIN) and kynurenic acid (KA).37 QUIN, which is produced in the brain preferentially by microglia or infiltrating monocytes/macrophages, is an excitotoxic neuromodulator which promotes the release of glutamate through direct stimulation of N-methyl-D-aspartate (NMDA) receptors, while also inducing oxidative stress including lipid peroxidation.37-41 Of note, increased QUIN in the central nervous system (CNS) has been associated with a number of neurodegenerative disorders such as Alzheimer's Disease, amyotrophic lateral sclerosis, and Huntington's Disease, as well as inflammatory and infectious diseases including the dementia complex associated with infection with the human immunodeficiency virus (HIV) and Acquired Immune Deficiency Symdrome.42-46 In contradistinction to QUIN, KA, which is produced primarily in astrocytes by kynurenine aminotransferase (KAT) II (an enzyme that is expressed in very low amounts in microglia), has been shown to antagonize glutamate release and block excitatory neurotransmission.37 Based on these properties, it has been suggested that the relative balance between QUIN and KA may underlie the development of behavioral toxicity during exposure to inflammatory stimuli such as IFN-alpha.30,36,47 To date however, no study has measured CNS concentrations of QUIN and KA during IFN-alpha administration. Moreover, the relationship of these metabolites with CSF concentrations of KYN and TRP as well as measures of CNS immune responses and depressive symptoms has yet to be determined. To further examine the role of brain kynurenines in the development of behavioral changes during immune activation in humans, we sampled blood and CSF from patients with hepatitis C virus (HCV) infection undergoing treatment with IFN-alpha versus HCV patients awaiting IFN-alpha therapy. As noted above, IFN-alpha is associated with the development of depressive symptoms that have been correlated with changes in peripheral blood KYN and TRP concentrations.27-31,48,49
Methods
Sample
Twenty-seven HCV patients participated in the study. To qualify, subjects were required to be serum positive for anti-HCV antibodies or HCV-RNA positive by reverse transcription-polymerase chain reaction. Exclusion criteria included: unstable cardiovascular, endocrine, hematologic, renal or neurologic disease (as determined by physical examination and laboratory testing); HIV infection (as reported by the subjects’ treating physician); decompensated liver disease; liver disease from any cause other than HCV; history of schizophrenia, bipolar disorder or a diagnosis of major depression (MD) or substance abuse/dependence within six months of study entry [as determined by the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders—Fourth Edition (SCID)];50 and/or a score <24 on the Mini Mental State Exam, indicating more than mild cognitive impairment.51 Patients were required to be off all antidepressant, antipsychotic or mood stabilizer medications for at least 12 weeks prior to blood or CSF sampling. Subjects were also required to discontinue other agents that might affect study results (i.e. narcotic analgesics, benzodiazepines, and anti-inflammatory agents) at least two weeks prior to undergoing lumbar puncture (LP) or blood collection. The subjects in the current study represent a subsample of subjects included in previous studies on the effects of IFN-alpha on cognitive performance, neuroendocrine function and CSF concentrations of cytokines, chemokines and monoamine metabolites.13,52,53 All subjects provided written informed consent, and study procedures were approved a priori by the Emory University Institutional Review Board.
Study design
Participants were enrolled in a longitudinal study examining behavioral, neurobiological and immune variables at baseline and after ~12 weeks of either no treatment or treatment with IFN-alpha plus ribavirin. For the purposes of this report, cross-sectional assessments of TRP, KYN and KYN metabolites in CSF and peripheral blood as well as CSF immune variables and behavior were evaluated in HCV patients treated with IFN-alpha/ribavirin for ~12 weeks (treatment group, n=16) versus HCV patients awaiting IFN-alpha/ribavirin therapy (controls, n=11). Subjects in the treatment group received either pegylated IFN-alfa-2b (Pegintron®, Schering Plough) 1.5μg/kg weekly (n=10) or pegylated IFN-alfa-2a (Pegasys®, Roche) (n=6) 180μg weekly, both administered subcutaneously. IFN-alpha-treated subjects also received ribavirin (800-1400 mg/d). Participation in the treatment versus control group was determined by patients and their treating physicians based on scheduling constraints and personal preferences, and was not based on standardized criteria or controlled by study protocol.
For LP, subjects were admitted to the Emory University General Clinical Research Center (GCRC) at 1pm, and the LP was performed between 4-5 p.m. by a trained physician. For each subject, ~10cc of CSF was withdrawn, after discarding the initial 1cc to avoid blood contamination. Samples were collected into chilled tubes, aliquoted into 1cc vials, and immediately frozen at −80°C until assay. Subjects were then discharged after an overnight stay. In order to limit the impact of the stress of CSF sampling on peripheral blood immune parameters and assessments of depression, blood sampling and depression assessments were conducted 6-7 days after LP. For blood sampling, subjects were admitted to the Emory GCRC in the evening with lights out at 10pm. The following morning, subjects were awakened at 7:15am and served breakfast, and neuropsychiatric assessments were conducted followed by lunch. At 4pm (corresponding to the time of the LP), blood was withdrawn from an indwelling catheter into chilled EDTA-coated tubes. Subjects were asked to rest quietly for 30 minutes prior to blood withdrawal. Following sampling, blood was immediately centrifuged at 1000Xg for 10 minutes at 4°C. Plasma was then removed and frozen at −80°C until assay. Because behavioral effects of pegylated IFN-alpha tend to be most pronounced immediately following the weekly injection, both LP and blood sampling were scheduled 4-5 days following each subject's last injection. Urine drug screens were conducted at all visits to the Emory GCRC to rule out substance abuse. Control subjects participated in all study procedures in parallel with IFN-alpha/ribavirin-treated patients.
Behavioral Assessments
Depression was evaluated using the mood disorders module of the SCID and the Montgomery-Asberg Depression Rating Scale (MADRS).54 The MADRS is a 10-item, clinician-administered scale that assesses the severity of depressive symptoms.
Laboratory Assessments
All biological samples were analyzed by research staff blinded to the clinical status of study participants.
TRP, KYN and KYN Metabolites
TRP and KYN were measured by high-performance liquid chromatography (HPLC) with a spectrophotometric detector (TOSOH UV-8000) or fluorescence spectrometric detector (HITACHI, Tokyo, Japan) as described previously with minor modifications.38 Briefly, separation was obtained with a reverse-phase column (Brave ODS 3 μm 150 mm × 4.6 mm; Alltech, IL, U.S.A.) and a mobile phase (flow rate 0.75 ml/min) composed of 0.1 M sodium acetate, 0.1 M acetic acid and 1% acetonitrile. The fluorescence excitation and emission wavelengths were set at 270 and 360 nm, respectively. UV signals were monitored at 355 nm for KYN. QUIN was determined by HPLC or gas chromatography/mass spectrometry (GC/MS) method. The assay is based on the conversion of QUIN to nicotinic acid mononucleotide, using recombinant quinolinate-nucleotide phosphorylase, and subsequently nicotinic acid D-ribonucleotide, determined by using recombinant nicotinamide nucleotide and fluorescent adenosine 5'-triphosphate, BODIPY® FL 2'-(or-3')-O-(N-(2-aminoethyl)urethane), trisodium salt. The reaction product, fluorescent deamido-NAD, could be clearly and efficiently separated from the substrate using a reverse-phase column. The data are consistent with data previously published using GC/MS method.
Human CSF samples were analyzed for KA by HPLC using an ESA Model 530 Fluorescence detector. Mobile phase (pH = 6.2) consisted of 250 mM Zinc Acetate, 50 mM Sodium Acetate, in acetonitrile:water (5:95 v:v) was pumped at 1ml/minute through a Hypersil Gold Reverse Phase C18 column measuring 4.6 mm × 100 mm with a 3 μm particle size (Thermo Scientific, Waltham, MA). KA was detected with an excitation wavelength of 344 nm and an emission wavelength of 398 nm. The chromatograms were integrated and quantified using ESA EZ Chrom SI software (ESA Inc., Chelmsford, MA). Human CSF was diluted 1:4 with 0.02 N HClO4 and then samples were centrifuged at 12,000xg for 5 min at 4°C. The supernatant (80 μl) was extracted and loaded into a refrigerated auto-sampler and maintained at 4°C until injection into the HPLC system. A standard curve was generated on each day from concentrated (2 μM) KA standards made up in 0.02 N HClO4 and held at 4°C until a 20 μl volume was injected into the system. Standards were made using serial dilution to encompass expected levels in the CSF samples. The standard curve was created using the system software, and samples were not run unless a linear standard curve with r2 greater than 0.98 was achieved.
CSF Immune Factors
Interleukin (IL)-6 was measured in duplicate by high sensitivity quantitative enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN). Cytokine soluble receptors [soluble IL-6 receptor (sIL6R) and soluble tumor necrosis factor receptor 2 (sTNFR2)] and monocyte chemoattractant protein (MCP)-1 were determined by R&D Quantikine ELISA kits. CSF IFN-alpha was measured by a high sensitivity quantitative ELISA (Amersham Biosciences, Piscataway, NJ), which was found to identify both recombinant human IFN-alpha as well as both pegylated IFN-alpha preparations. Inter- and intra-assay variability were reliably <12% for IL-6 and <10% for sIL6R, sTNFR2, MCP-1 and IFN-alpha. Assay sensitivities were as follows: IL-6: 0.04 pg/ml; IL-6sR: 6.5 pg/ml; sTNFR2: 1.0 pg/ml; MCP-1: 5.0 pg/ml; IFN-alpha: 0.1 pg/ml.
Statistical Analysis
All statistical analyses were conducted using SPSS 16.0 for Windows. For continuous measures, differences between groups were assessed using t-tests or Wilcoxon Rank Sum tests for variables with a non-normal distribution (including TRP, KYN, QUIN and KA). In t-tests where the Levene's test indicated non-homogeneity of variances, Welch's t tests were employed. Chi-square or Fischer tests were used to evaluate group differences in categorical variables. Generalized linear models (GLM) were employed to complement statistical comparisons of CSF and blood TRP/KYN pathway variables between groups, while controlling for factors that may have influenced relevant CSF and plasma biomarkers including age, sex, body mass index (BMI), and history of MD. Due to the non-normal distribution of the biological variables, relationships among continuous behavioral and biological measures were examined using Spearman's rank correlation coefficient (Spearman's rho). Based on the sample size for these statistical analyses, the study had >80% power to detect large effect sizes (Spearman's rho=0.50) using two-tailed tests of significance and an alpha level of 0.05.55 Where indicated, the Holm-Bonferroni method was used to evaluate statistical significance in the context of multiple correlations. To explore the relative contribution of relevant CSF KYN pathway variables and CSF immune biomarkers to scores of depression, multivariate analyses (multiple linear regressions) were performed including IFN-alpha treatment preparation and other relevant covariates as predictors. In addition, an exploratory factor analysis was conducted to evaluate the convergence of clinical, immune and CSF KYN pathway variables. An a priori decision was made to require eigenvalues of 1 or greater in order to interpret a factor as meaningful. All tests of significance were two-tailed with a significance level set at p<0.05.
Results
Sample Characteristics
As shown in Table 1, no significant differences in age, race, gender, education, past history of substance abuse or BMI were found between IFN-alpha-treated subjects and controls. Nevertheless, 4 IFN-alpha-treated subjects endorsed a past history of major depression compared to 0 control subjects (p=0.12). At the time of study, scores of depression (MADRS) were significantly higher in IFN-alpha-treated subjects versus controls. No patients met symptom criteria for major depression at the time of CSF or blood sampling.
Table 1.
Demographic and Clinical Characteristics of Study Participants
| Characteristic | Control Subjects (n = 11) | Interferon-Alpha (n = 16) | p-value |
|---|---|---|---|
| Age (mean, SD) | 48.2 (3.7) | 47.7 (6.8) | 0.83 |
| Gender (n, %) Males | 6 (54.5) | 10 (62.5) | 0.71 |
| Race (n, %) | |||
| Caucasian | 5 (45.5) | 5 (31.3) | 0.75 |
| Black | 5 (45.5) | 9 (56.3) | |
| Other | 1 (9.1) | 2 (12.5) | |
| Education (n, %) | |||
| College (1 or more years) | 9 (81.8) | 9 (56.3) | 0.23 |
| Past History Major Depression (n, %) | 0 (0) | 4 (25.0) | 0.12 |
| Past Substance Abuse (n, %) | 6 (54.5) | 9 (60.0) | 1.00 |
| MADRS (mean, SD) | 3.4 (3.9) | 11.8 (9.6) | 0.01 |
| BMI (mean, SD) | 29.4 (5.8) | 30.7 (4.3) | 0.52 |
BMI: body mass index; MADRS: Montgomery-Asberg Depression Rating Scale
CSF Concentrations of TRP, KYN and KYN Metabolites
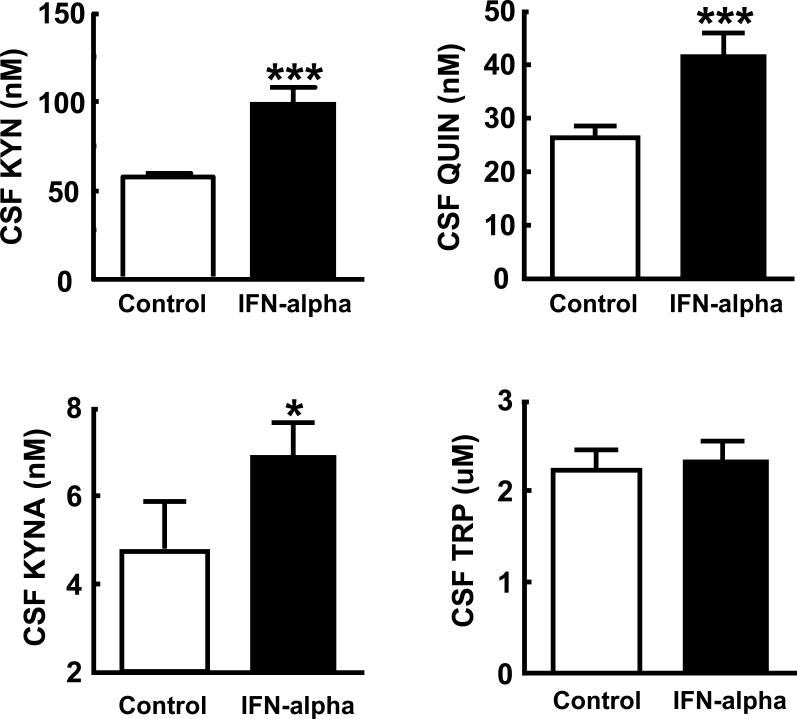
Evaluation of TRP, KYN and KYN metabolites in the CSF of study participants revealed that IFN-alpha-treated patients exhibited significantly higher concentrations of CSF KYN, QUIN and KA compared to controls (Figure 1). No differences in CSF TRP were found between groups. To confirm differences in CSF KYN and its metabolites as a function of IFN-alpha treatment, multivariate analysis of covariance was conducted by entering KYN, QUIN and KA into the model as dependent variables and controlling for relevant covariates including sex, age, BMI, and past history of major depression. A significant main effect of IFN-alpha treatment on KYN and the KYN metabolites was found (Wilks’ Lambda=0.624, p=0.03, Pillai's APPROX F[3,19]=3.81, p=0.03). Of note, no differences in CSF TRP, QUIN or KA were observed between IFN-alpha treatment groups (IFN-alpha-2a and IFN-alpha-2b) (data not shown). However, CSF KYN was significantly higher in patients treated with IFN-alpha-2a versus those treated with IFN-alpha-2b (111.5 SD 32.6 versus 76.6 SD 19.2, t=2.36, df=14, p=0.03). Nevertheless, CSF KYN concentrations in both IFN-alpha treatment groups were significantly higher than controls (p<0.01). Finally, no difference in the ratio of CSF QUIN to KA (QUIN/KA) in IFN-alpha-treated subjects versus controls was found (7.2 SD 3.5 versus 7.3 SD 3.0, respectively, p=0.94).
Figure 1. CSF Kynurenine, Quinolinic Acid, Kynurenic Acid and Tryptophan Concentrations in Control versus IFN-alpha/ribavirin-treated patients with HCV.
Cerebrospinal fluid (CSF) samples were obtained from control (n=11) and interferon (IFN)-alpha/ribavirin-treated (n=14-16) patients with hepatitis C virus (HCV) infection. IFN-alpha/ribavirin-treated subjects were studied after ~12 weeks on IFN-alpha/ribavirin therapy. CSF concentrations of kynurenine (KYN), quinolinic acid (QUIN), and kynurenic acid (KA) were significantly elevated in IFN-alpha-treated subjects compared to controls. No differences between groups in CSF tryptophan (TRP) concentrations were found. *p<0.05, **p<0.01, ***p<0.001 using Wilcoxon Rank Sum test.
Correlational analyses among KYN and KYN metabolites in the CSF revealed high correlations between KYN and both QUIN and KA (Spearman's rho=0.72, df=23, p<0.001, and Spearman's rh=0.48, df=23, p=0.001, respectively) as well as a significant correlation between KA and QUIN (r=0.44, df=21, p=0.025). No significant correlations were found between TRP and KYN or its metabolites (all p>0.40).
Relationship between CSF TRP, KYN and KYN Metabolites and CSF Immune Variables
To examine the relationship between TRP, KYN and KYN metabolites in the CSF and relevant CSF immune variables, CSF TRP, KYN, QUIN and KA were correlated with CSF cytokines and chemokines that were previously reported to be elevated in the CSF of IFN-alpha-treated patients (Table 2).13
Table 2.
Correlations between CSF Kynurenine and Kynurenine Metabolites and Immune Variables
| Immune Measure |
KYN (nM) |
QUIN (nM) |
KA (nM) |
|---|---|---|---|
| IFN-alpha (pg/ml) | 0.62***† | 0.42* | 0.28 |
| sTNFR2 (ng/ml) | 0.48*† | 0.56**† | 0.46* |
| IL-6 (pg/ml) | 0.26 | 0.16 | 0.01 |
| sIL-6R (ng/ml) | 0.36 | 0.37 | 0.36 |
| MCP-1 (ng/ml) | 0.59**† | 0.45* | 0.34 |
KYN: L-kynurenine; QUIN: quinolinic acid; KA: kynurenic acid; IFN: interferon; sTNFR2: soluble tumornecrosis factor-alpha receptor 2; IL: interleukin; sIL-6R: soluble IL-6 receptor; MCP-1: monocyte chemoattractant protein-1; n=25-27 Values reported as Spearman's rho
-p<0.05
-p<0.01
-p<0.001
-p<0.05 after controlling for multiple comparisons (Holm-Bonferroni method)
No significant correlations were found between TRP and any of the immune variables (all p>0.40). In contrast, high correlations were found between CSF KYN and CSF IFN-alpha, sTNFR2 and MCP-1, whereas no significant correlation was found between CSF KYN and IL-6 or sIL-6R (Table 2). Similar but smaller correlations were found between CSF QUIN and CSF IFN-alpha, sTNFR2 and MCP-1. CSF QUIN and IL-6 or sIL-6R were not significantly correlated. There was a significant correlation between CSF KA and sTNFR2 and a trend level correlation with MCP-1. No significant correlations were observed between KA and either CSF IFN-alpha, IL-6 of sIL-6R (all p>0.05).
Relationship of CSF TRP, KYN and KYN Metabolites and Immune Variables to Depression
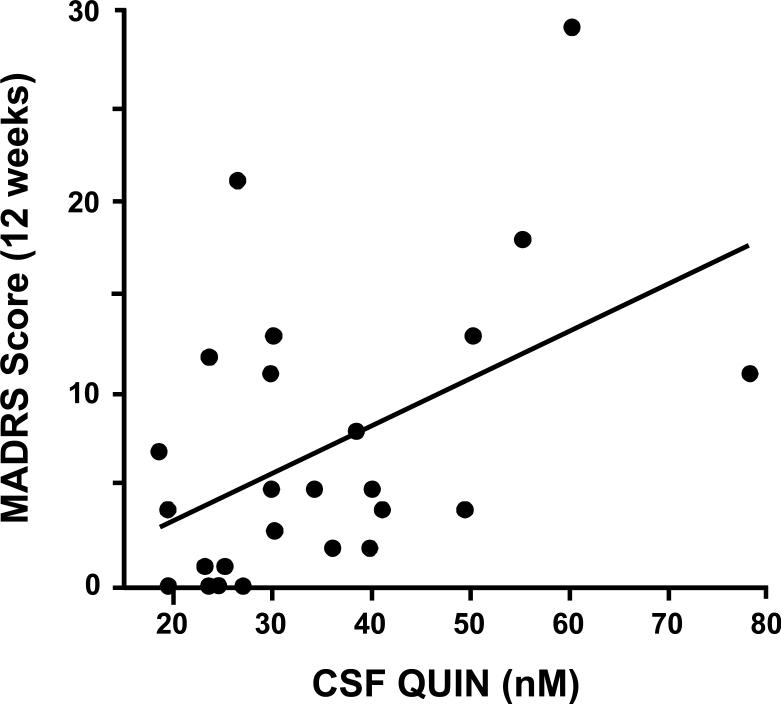
Among CSF KYN and its metabolites, CSF QUIN exhibited the highest correlation with depression scores (Figure 2) followed by CSF KYN (Spearman's rho=0.47, df=23, p=0.019 and Spearman's rho=0.39, df=25, p=0.043, respectively). KA did not significantly correlate with MADRS scores (Spearman's rho=0.25, df=23, p=0.22), nor did CSF TRP (Spearman's rho=0.002, df=25, p=.99).
Figure 2. Correlation between CSF Quinolinic Acid and Depression Scores in Control and IFN-alpha/ribavirin-treated patients with HCV.
Cerebrospinal fluid (CSF) samples were obtained from control (n=11) and interferon (IFN)-alpha/ribavirin-treated (n=14) patients with hepatitis C virus (HCV) infection. IFN-alpha/ribavirin-treated subjects were studied after ~12 weeks on IFN-alpha/ribavirin therapy. Symptoms of depression were assessed using the Montgomery Asberg Depression Rating Scale (MADRS). CSF quinolinic acid (QUIN) significantly correlated with MADRS scores (Spearman's rho=0.47, df=23, p=0.019).
To determine the relative contribution of CSF TRP, KYN and KYN metabolites and the immune variables to MADRS scores, multiple regression analyses were conducted. The regression model included the CSF TRP, KYN, QUIN and KA as well as the CSF immune variables IFN-alpha, IL-6, sIL-6R, sTNFR2, and MCP-1. Treatment group (control, IFN-alpha-2a, and IFN-alpha-2b) and history of depression were also entered into the model. Backward stepwise elimination of non-significant variables was employed using a stepping method criteria of 0.05 for entry into the model and a 0.10 probability for exclusion. Remaining in the model and accounting for 72% of the variance (R2) in MADRS scores was CSF QUIN, sTNFR2, MCP-1 and treatment group (control, IFN-alpha-2a, and IFN-alpha-2b). Forward stepwise elimination yielded the same results. Entering treatment group into the regression analysis first indicated that IFN-alpha treatment accounted for 37% of the variance (R2) in MADRS scores, whereas QUIN, sTNFR2 and MCP-1 accounted for an additional 35% of the variance. Finally, an exploratory factor analysis was performed rotating the principal components using Equamax rotation to examine the convergence of the clinical, immune and CSF KYN pathway variables noted above. A three-factor solution emerged, all with eigenvalues greater than 1.0, accounting for 66% of the variance. The first factor was loaded with the immune variables, CSF IFN-alpha, IL-6, and MCP1 (all with a loading of 0.80 or higher). CSF KYN and its metabolites as well as depression (MADRS scores) appeared in the second factor (all loading greater than 0.50). The third factor contained the cytokine soluble receptors for TNF-alpha and IL-6 in addition to CSF TRP which was negatively correlated.
Plasma Concentrations of TRP, KYN and KYN metabolites
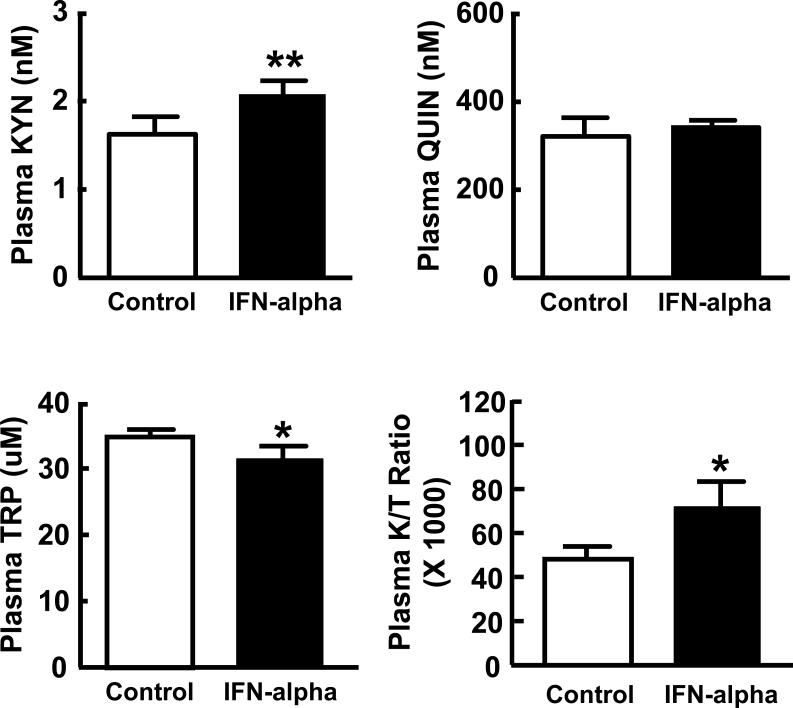
Evaluation of plasma concentrations of TRP, KYN and QUIN as well as the KYN/TRP ratio revealed significant differences between groups in TRP, KYN and the KYN/TRP ratio (Figure 3). Although plasma QUIN concentrations were higher in IFN-alpha-treated subjects than controls the difference did not reach significance. To confirm differences in plasma TRP and KYN as a function of IFN-alpha treatment, multivariate analysis of covariance was conducted entering TRP and KYN in the model and controlling for relevant covariates. No main effect of IFN-alpha treatment was found on plasma TRP and KYN after controlling for sex, age, BMI, and past history of major depression (p>.0.40). Of note, no differences were found between IFN-alpha treatment groups (IFN-alpha-2a and IFN-alpha-2b) in plasma TRP or KYN (data not shown). Moreover, plasma KYN in both IFN-alpha treatment groups was significantly higher than controls (p<0.05). Plasma TRP in patients treated with IFN-alpha 2a was significantly lower than controls, however, the difference in plasma TRP concentrations between patients treated with IFN-alpha 2b and controls did not reach statistical significance.
Figure 3. Plasma Kynurenine, Quinolinic Acid, and Tryptophan Concentrations and the Kynurenine to Tryptophan Ratio in Control versus IFN-alpha/ribavirin-treated patients with HCV.
Plasma samples were obtained from control (n=11) and interferon (IFN)-alpha/ribavirin-treated (n=16) patients with hepatitis C virus (HCV) infection. IFN-alpha/ribavirin-treated subjects were studied after ~12 weeks on IFN-alpha/ribavirin therapy. Plasma concentrations of kynurenine (KYN) and the kynurenine to tryptophan (K/T) ratio were significantly elevated in IFN-alpha-treated subjects compared to controls, whereas plasma tryptophan (TRP) concentrations were significantly decreased in IFN-alpha-treated patients. Values are depicted as the Mean ± Standard Error of the Mean. QUIN-quinolinic acid; *p<0.05, **p<0.01 using Wilcoxon Rank Sum test.
Plasma KYN concentrations highly correlated with plasma QUIN concentrations (Spearman's rho=0.73, df=25, p<0.001), whereas no correlation was found between plasma TRP and either plasma KYN or QUIN (Spearman's rho=−0.21, df=25, p=0.29 and Spearman's rho=-0.32, df=25, p<0.10, respectively).
To examine the relationship between plasma and CSF TRP, KYN, and QUIN, correlational analyses were performed (Table 3). High correlations were found between CSF and plasma KYN as well as CSF and plasma QUIN. However, no correlation was found between CSF and plasma TRP. Of note, peripheral blood TRP was negatively correlated with CSF KYN.
Table 3.
Correlations between CSF and Plasma Tryptophan, Kynurenine and Kynurenine Metabolites
| |
CSF TRP (μM) |
CSF KYN (nM) |
CSF QUIN (nM) |
|---|---|---|---|
| Plasma TRP (uM) | 0.14 | −0.46*† | −0.26 |
| Plasma KYN (nM) | 0.14 | 0.53**† | 0.71***† |
| Plasma QUIN (nM) | 0.32 | 0.46*† | 0.72***† |
TRP: L-tryptophan; KYN: L-kynurenine; QUIN: quinolinic acid; n=25-27
Values reported as Spearman's rho
−p<0.05
−p<0.01
−p<0.001
−p<0.05 after controlling for multiple comparisons (Holm-Bonferroni method)
Discussion
The data indicate that administration of IFN-alpha leads to increased KYN in peripheral blood as well as increased KYN, QUIN and KA in the CNS, likely secondary to activation of IDO. Interestingly, although significant decreases in TRP and increases in the KYN/TRP ratio were found in peripheral blood, no IFN-alpha-induced changes in TRP concentrations were found in the brain as reflected in the CSF. Correlations between CSF markers of inflammation and KYN and its metabolites provide evidence that changes in KYN metabolism are linked to central cytokine responses, which together were associated with IFN-alpha-induced depression.
Previous studies have emphasized the role of TRP depletion in the mechanism by which cytokine-induced activation of IDO leads to behavioral alterations.35 The lack of changes in CSF TRP following IFN-alpha administration, despite decreases in peripheral blood TRP, indicates that the access of TRP to the brain relative to other amino acids which compete with TRP for transport across the blood brain barrier is unaffected. These findings are consistent with previous research demonstrating that the ratio of TRP to competing amino acids does not change during IFN-alpha therapy,30 and may provide an explanation for the lack of a correlation between plasma and CSF TRP concentrations in the current study. Moreover, no relationships were found between TRP and CNS inflammatory markers or CSF KYN and its metabolites or IFN-alpha-induced mood changes. Taken together, these findings indicate that although decreased peripheral blood TRP may reflect inflammation-induced activation of peripheral IDO pathways and their impact on the brain (as indicated by the inverse relationship between peripheral blood TRP and CSF KYN), the data do not support the notion that changes in peripheral blood or CNS availability of TRP are a primary mediator of IFN-alpha-induced mood disturbances.
In contrast to TRP, KYN was found to be significantly elevated in both the periphery and the CSF with a high correlation in KYN concentrations between these two tissue compartments. These data complement previous findings that CNS KYN is derived largely from TRP metabolism in peripheral tissues and is taken up into the brain at a significant rate by the large amino acid transporter.56 In contrast, KYN metabolites including QUIN and KA cross the blood brain barrier at relatively low rates through passive diffusion, suggesting that peripheral stores of QUIN and KA contribute minimally to concentrations in the brain.37,56 Thus, peripheral blood KYN may provide an accessible “window” into the CNS regarding activation of IDO pathways relevant to the brain and behavior in humans.
Additionally compatible with the notion that TRP may not be a primary mediator of behavioral change during IFN-alpha therapy, is that peripheral administration of KYN has been shown to induce depressive-like symptoms in rodents.25,34 Upon entry into the brain, KYN is metabolized into QUIN and KA, both of which are neuromodulators that can influence neurotransmission and ultimately behavior.37 KA inhibits the release of glutamate, which, by extension, may inhibit the release of dopamine (DA), whose release is regulated in part by glutamatergic activity.37,57,58 Indeed, intrastriatal administration of KA dramatically reduces extracellular DA in the rat striatum.58 Of relevance in this regard, decreases in the DA metabolite, homovanillic acid, have been reported in IFN-alpha-treated rhesus monkeys in association with depressive-like huddling behavior,12 and mice treated with IFN-alpha exhibit decreased whole brain DA in association with reduced locomotor activity.59 In contrast to KA, QUIN promotes glutamate release through activation of NMDA receptors.37 QUIN also induces oxidative stress, which in combination with glutamate release may contribute to CNS excitotoxicity.36,37,41,47 As mentioned previously, excess QUIN is associated with several neurodegenerative disorders with behavioral alterations,42-45 and in the current study was found to be correlated with depression both alone and in concert with inflammatory mediators (sTNFR2 and MCP-1) previously found to be elevated in the CSF of IFN-alpha-treated subjects.13 Of note, in mice treated with BCG, the only brain KYN pathway enzyme whose mRNA exhibited a significant increase compared to saline was 3-hydroxyanthranilic acid oxygenase, which converts 3-hydroxyanthranillic acid to QUIN.26
Previous investigators have suggested that the balance between QUIN, which activates glutamate release, and KA which inhibits glutamate release (through inhibition of the alpha 7 subunit of the nicotinic acetylcholine receptor), may determine the relative impact of inflammatory stimuli.30,36,47 Nevertheless, no differences in the ratio between CSF QUIN and KA were found between IFN-alpha-treated and control subjects. These data indicate that both enzymatic pathways leading to the production of these relevant KYN metabolites may be equally activated and can contribute to behavioral changes in the context of inflammatory stimuli such as IFN-alpha.
A high correlation was found between QUIN in the plasma and QUIN in the CSF. However, as noted above, direct access of QUIN to the brain has been shown to occur through passive diffusion at a relatively low rate.56 These data indicate that QUIN may be produced directly in the brain by cells that are represented both in the periphery and the CNS. One cell type that has been shown to actively produce QUIN is macrophages.40,60 In the context of peripheral immune stimulation, activated macrophages can be recruited to the brain by MCP-1 secreted by microglia following activation by peripherally released TNF-alpha.15 Previous studies have shown that IFN-alpha administration is associated with increased MCP-1 both in the peripheral blood and CSF, and IFN-alpha-induced TNF-alpha has been associated with IFN-alpha-induced depression.13,53 Upon activation, macrophages produce QUIN in amounts 20-fold greater than microglia.40 Thus, consistent with previous studies using pokeweed mitogen as a peripheral immune stimulus in gerbils,38 correlations between QUIN in the periphery and the brain may be related to activated macrophages, which are apparent in both peripheral and central compartments and can convert KYN to QUIN.40,60 Interestingly, QUIN has been found to induce the production of MCP-1,61 potentially creating a feed forward cascade in which QUIN leads to increased MCP-1 which in turn recruits more peripheral blood macrophages to the brain that can produce high quantities of QUIN. Blockade of macrophage access to the brain through inhibition of MCP-1 or its receptor CCL2R may be one strategy to prevent this cascade. Indeed, CCLR2 knock out mice were shown to exhibit marked decreases in infiltrating macrophages in a model of peripherally-induced liver inflammation.15 Finally, increased CSF KA suggests activation of astrocytes, which express the appropriate enzymatic machinery to produce KA,37 and like microglia can be stimulated to produce MCP-1.62 Of note, cells at the blood brain barrier can also synthesize KA as well as KYN upon activation.63
Although activation of IDO pathways has been implicated in depression and depressive-like behavior in the context of medical illnesses as well as the administration of inflammatory stimuli including IFN-alpha, LPS and BCG in laboratory animals and humans, data supporting a role for IDO activation in depression in otherwise medically healthy individuals is somewhat limited. Nevertheless, several studies have implicated a role for activation of IDO pathways in pre- and postpartum mood alterations,64-66 where inflammation may also be involved.66 In addition, at least one study found a correlation between KYN and symptoms of anxiety in patients with a variety of affective disorders including major depression, bipolar disorder and schizoaffective disorders.67 However, there have been several reports where no differences in KYN were found between depressed and non-depressed subjects.68-70 These data indicate that activation of IDO pathways and the generation of KYN and its metabolites may be relatively unique to inflammation-induced depression, and further investigation into IDO pathway activation in patients with depression should focus on depressed individuals with increased inflammatory markers.
Several limitations of the current study warrant consideration. Although treatment with IFN-alpha has been widely used as a model system for examining mechanisms by which chronic innate immune activation produces behavioral disturbances in humans, it is important to recognize that exogenously administered IFN-alpha may not be equivalent to endogenously produced IFN-alpha (or cytokines induced by endogenous IFN-alpha) in terms of either physiological or behavioral effects. Similarly, although the analysis of CSF provides a unique “window” into the CNS effects of chronic IFN-alpha exposure, findings in CSF cannot be directly compared to results from animal studies of IFN-alpha or other cytokines and cytokine-inducers where specific brain regions can be examined directly. As a result of practical and ethical issues related to randomizing patients who qualify for IFN-alpha/ribavirin therapy to “no treatment”, assignment to treatment and control conditions was dictated by clinical decisions occurring between patients and treating physicians. Although treatment and control groups were well matched on key variables at baseline, it is possible that differences between the groups in variables, such as motivation or expectation bias, which we did not measure, may have influenced outcomes. Additional limitations include the fact that the small sample size in the current study provided sufficient statistical power to detect only relatively large effect sizes. Moreover, correlations between CSF, blood and behavioral variables may have been compromised by the separation in time (of one week) between blood and behavioral variables and measures in the CSF. However, this time separation allowed blood and behavioral assessments to be uncontaminated by the stress of knowing one was about to receive a LP. IFN-alpha-treated subjects were also on two different preparations of pegylated IFN-alpha, although statistical analyses revealed significant differences between IFN-alpha treatment preparations only for CSF KYN, and multivariate analyses examining the association of CSF TRP, KYN, QUIN, KA and the immune variables with depression were conducted using IFN-alpha preparation as a covariate. Finally, all subjects in the current study were infected with HCV, which may have amplified observed effects through the interaction of viral infection with IFN-alpha administration. Consistent with current standards of care for the treatment of HCV, all subjects who received IFN-alpha also received the antiviral agent ribavirin, which may have contributed to study findings, especially in light of evidence that ribavirin may contribute to the depressive burden of HCV treatment.49 Of note, however, is the consistency between behavioral findings from the current study and studies in patients receiving IFN-alpha monotherapy for other therapeutic indications.71 Current findings are also consistent with those seen across a range of inflammatory stimuli (including IFN-alpha, LPS and BCG), indicating the relevance of IDO and KYN metabolic pathways in contributing to behavioral change during immune activation in both humans and laboratory animals.
Acknowledgements
This study was supported by grants from the National Institutes of Health to CLR (K23 MH064619 and R01 MH070553), RD (R01 MH 71349 and R01 MH 079829), KWK (R01 AG 029573) and AHM (K05 MH069124 and R01 HL073921) as well as the Centers for Disease Control and Prevention. In addition, the study was supported by PHS Grant UL1 RR025008 from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources. The authors would also like to thank Philip Harvey, Ph.D. for his assistance with statistical analyses.
Source of Support:
This study was supported by grants from the National Institutes of Health to CLR (K23 MH064619, R01 MH070553), RD (R01 MH 71349, R01 MH 079829), KWK (R01 AG 029573) and AHM (K05 MH069124, R01 HL073921, T32 MH020018) as well as the Centers for Disease Control and Prevention. In addition, the study was supported by PHS Grant UL1 RR025008 from the Clinical and Translational Science Award program and PHS Grant M01 RR0039 from the General Clinical Research Center program, National Institutes of Health, National Center for Research Resources.
Footnotes
Financial Disclosure
Charles L. Raison has served as a speaker for Lilly and Wyeth and as a consultant or an advisory board member for Lilly and Wyeth; Robert Dantzer has served as a consultant for Astra-Zeneca, Lundbeck and Danone; Keith W. Kelley has served as a consultant for Astra-Zeneca; Andrew H. Miller has served as a consultant for Schering-Plough, AstraZeneca and Centocor, and has received research funding from Centocor, GlaxoSmithKline, and Schering-Plough; Marcus A. Lawson, Bobbi J Woolwine, Gerald Vogt, James R. Spivey, and Kuniaki Saito have nothing to declare.
References
- 1.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller GE, Rohleder N, Cole SW. Chronic interpersonal stress predicts activation of pro- and anti-inflammatory signaling pathways 6 months later. Psychosom Med. 2009;71:57–62. doi: 10.1097/PSY.0b013e318190d7de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anisman H, Merali Z, Hayley S. Neurotransmitter, peptide and cytokine processes in relation to depressive disorder: comorbidity between depression and neurodegenerative disorders. Prog Neurobiol. 2008;85:1–74. doi: 10.1016/j.pneurobio.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711:163–174. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- 5.Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]
- 6.Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 7.Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol Psychiatry. 2008;63:1022–1029. doi: 10.1016/j.biopsych.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capuron L, Fornwalt FB, Knight BT, Harvey PD, Ninan PT, Miller AH. Does Cytokine-induced depression differ from idiopathic major depression in medically healthy individuals? Journal of Affective Disorders. doi: 10.1016/j.jad.2009.02.017. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- 10.Muller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Muller B, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11:680–684. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- 11.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felger JC, Alagbe O, Hu F, Mook D, Freeman AA, Sanchez MM, et al. Effects of interferon-alpha on rhesus monkeys: a nonhuman primate model of cytokine-induced depression. Biol Psychiatry. 2007;62:1324–1333. doi: 10.1016/j.biopsych.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, et al. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry. 2009;65:296–303. doi: 10.1016/j.biopsych.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quan N, Banks WA. Brain-immune communication pathways. Brain Behav Immun. 2007;21:727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 15.D'Mello C, Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J Neurosci. 2009;29:2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weidenfeld J, Yirmiya R. Effects of bacterial endotoxin on the glucocorticoid feedback regulation of adrenocortical response to stress. Neuroimmunomodulation. 1996;3:352–357. doi: 10.1159/000097295. [DOI] [PubMed] [Google Scholar]
- 17.Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CB, Miller AH. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry. 2003;160:1342–1345. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- 18.Robinson CM, Shirey KA, Carlin JM. Synergistic transcriptional activation of indoleamine dioxygenase by IFN-gamma and tumor necrosis factor-alpha. J Interferon Cytokine Res. 2003;23:413–421. doi: 10.1089/107999003322277829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson CM, Hale PT, Carlin JM. NF-kappa B activation contributes to indoleamine dioxygenase transcriptional synergy induced by IFN-gamma and tumor necrosis factor-alpha. Cytokine. 2006;35:53–61. doi: 10.1016/j.cyto.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Pemberton LA, Kerr SJ, Smythe G, Brew BJ. Quinolinic acid production by macrophages stimulated with IFN-gamma, TNF-alpha, and IFN-alpha. J Interferon Cytokine Res. 1997;17:589–595. doi: 10.1089/jir.1997.17.589. [DOI] [PubMed] [Google Scholar]
- 21.Fujigaki H, Saito K, Fujigaki S, Takemura M, Sudo K, Ishiguro H, et al. The signal transducer and activator of transcription 1alpha and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways, and synergistic effect of several proinflammatory cytokines. J Biochem. 2006;139:655–662. doi: 10.1093/jb/mvj072. [DOI] [PubMed] [Google Scholar]
- 22.Mellor AL, Munn D, Chandler P, Keskin D, Johnson T, Marshall B, et al. Tryptophan catabolism and T cell responses. Adv Exp Med Biol. 2003;527:27–35. doi: 10.1007/978-1-4615-0135-0_3. [DOI] [PubMed] [Google Scholar]
- 23.Owens MJ, Nemeroff CB. Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin Chem. 1994;40:288–295. [PubMed] [Google Scholar]
- 24.Flores BH, Musselman DL, DeBattista C, Garlow SJ, Schatzberg AF, Nemeroff CB. Biology of Mood Disorders. In: Schatzberg AF, Nemeroff CB, editors. Textbook of Psychopharmacology. Third ed. America Psychiatric Publishing, Inc.; Washington DC: 2004. pp. 717–763. [Google Scholar]
- 25.O'Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2008 doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Connor JC, Lawson MA, Andre C, Briley EM, Szegedi SS, Lestage J, et al. Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. J Immunol. 2009;182:3202–3212. doi: 10.4049/jimmunol.0802722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maes M, Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, et al. Treatment with interferon-alpha (IFN alpha) of hepatitis C patients induces lower serum dipeptidyl peptidase IV activity, which is related to IFN alpha-induced depressive and anxiety symptoms and immune activation. Mol Psychiatry. 2001;6:475–480. doi: 10.1038/sj.mp.4000872. [DOI] [PubMed] [Google Scholar]
- 28.Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002;7:468–473. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- 29.Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, et al. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol Psychiatry. 2003;54:906–914. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 30.Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpe S, Maes M. IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol Psychiatry. 2005;10:538–544. doi: 10.1038/sj.mp.4001600. [DOI] [PubMed] [Google Scholar]
- 31.Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, et al. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J Clin Psychopharmacol. 2002;22:86–90. doi: 10.1097/00004714-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Schroecksnadel K, Fiegl M, Prassl K, Winkler C, Denz HA, Fuchs D. Diminished quality of life in patients with cancer correlates with tryptophan degradation. J Cancer Res Clin Oncol. 2007;133:477–485. doi: 10.1007/s00432-007-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fuchs D, Moller AA, Reibnegger G, Stockle E, Werner ER, Wachter H. Decreased serum tryptophan in patients with HIV-1 infection correlates with increased serum neopterin and with neurologic/psychiatric symptoms. J Acquir Immune Defic Syndr. 1990;3:873–876. [PubMed] [Google Scholar]
- 34.Vecsei L, Beal MF. Influence of kynurenine treatment on open-field activity, elevated plus-maze, avoidance behaviors and seizures in rats. Pharmacol Biochem Behav. 1990;37:71–76. doi: 10.1016/0091-3057(90)90043-h. [DOI] [PubMed] [Google Scholar]
- 35.Widner B, Laich A, Sperner-Unterweger B, Ledochowski M, Fuchs D. Neopterin production, tryptophan degradation, and mental depression--what is the link? Brain Behav Immun. 2002;16:590–595. doi: 10.1016/s0889-1591(02)00006-5. [DOI] [PubMed] [Google Scholar]
- 36.Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12:988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- 37.Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther. 2002;303:1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- 38.Saito K, Crowley JS, Markey SP, Heyes MP. A mechanism for increased quinolinic acid formation following acute systemic immune stimulation. J Biol Chem. 1993;268:15496–15503. [PubMed] [Google Scholar]
- 39.Guillemin GJ, Smythe G, Takikawa O, Brew BJ. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia. 2005;49:15–23. doi: 10.1002/glia.20090. [DOI] [PubMed] [Google Scholar]
- 40.Guillemin GJ, Smith DG, Smythe GA, Armati PJ, Brew BJ. Expression of the kynurenine pathway enzymes in human microglia and macrophages. Adv Exp Med Biol. 2003;527:105–112. doi: 10.1007/978-1-4615-0135-0_12. [DOI] [PubMed] [Google Scholar]
- 41.Rios C, Santamaria A. Quinolinic acid is a potent lipid peroxidant in rat brain homogenates. Neurochem Res. 1991;16:1139–1143. doi: 10.1007/BF00966592. [DOI] [PubMed] [Google Scholar]
- 42.Guillemin GJ, Wang L, Brew BJ. Quinolinic acid selectively induces apoptosis of human astrocytes: potential role in AIDS dementia complex. J Neuroinflammation. 2005;2:16. doi: 10.1186/1742-2094-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guillemin GJ, Brew BJ, Noonan CE, Takikawa O, Cullen KM. Indoleamine 2,3 dioxygenase and quinolinic acid Immunoreactivity in Alzheimer's disease hippocampus. Neuropathol Appl Neurobiol. 2005;31:395–404. doi: 10.1111/j.1365-2990.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- 44.Guillemin GJ, Meininger V, Brew BJ. Implications for the kynurenine pathway and quinolinic acid in amyotrophic lateral sclerosis. Neurodegener Dis. 2005;2:166–176. doi: 10.1159/000089622. [DOI] [PubMed] [Google Scholar]
- 45.Guidetti P, Schwarcz R. 3-Hydroxykynurenine and quinolinate: pathogenic synergism in early grade Huntington's disease? Adv Exp Med Biol. 2003;527:137–145. doi: 10.1007/978-1-4615-0135-0_16. [DOI] [PubMed] [Google Scholar]
- 46.Heyes MP, Saito K, Crowley JS, Davis LE, Demitrack MA, Der M, et al. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain. 1992;115(Pt 5):1249–1273. doi: 10.1093/brain/115.5.1249. [DOI] [PubMed] [Google Scholar]
- 47.McNally L, Bhagwagar Z, Hannestad J. Inflammation, glutamate, and glia in depression: a literature review. CNS Spectr. 2008;13:501–510. doi: 10.1017/s1092852900016734. [DOI] [PubMed] [Google Scholar]
- 48.Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry. 2004;56:819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 49.Raison CL, Borisov AS, Broadwell SD, Capuron L, Woolwine BJ, Jacobson IM, et al. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005;66:41–48. doi: 10.4088/jcp.v66n0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV. American Psychiatric Press; Washington DC: 1997. [Google Scholar]
- 51.Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Arch Gen Psychiatry. 1983;40:812. doi: 10.1001/archpsyc.1983.01790060110016. [DOI] [PubMed] [Google Scholar]
- 52.Majer M, Welberg LA, Capuron L, Pagnoni G, Raison CL, Miller AH. IFN-alpha-induced motor slowing is associated with increased depression and fatigue in patients with chronic hepatitis C. Brain Behav Immun. 2008;22:870–880. doi: 10.1016/j.bbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raison CL, Borisov AS, Woolwine BJ, Massung B, Vogt G, Miller AH. Interferon-alpha effects on diurnal hypothalamic-pituitary-adrenal axis activity: relationship with proinflammatory cytokines and behavior. Mol Psychiatry. 2009 doi: 10.1038/mp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 55.Cohen JD. Statistical power analysis for the behavioral sciences. 2nd ed. Lawrence Erlbaum Associates; Hillsdale, N.J.: 1988. [Google Scholar]
- 56.Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem. 1991;56:2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- 57.Borland LM, Michael AC. Voltammetric study of the control of striatal dopamine release by glutamate. J Neurochem. 2004;91:220–229. doi: 10.1111/j.1471-4159.2004.02708.x. [DOI] [PubMed] [Google Scholar]
- 58.Wu HQ, Rassoulpour A, Schwarcz R. Kynurenic acid leads, dopamine follows: a new case of volume transmission in the brain? J Neural Transm. 2007;114:33–41. doi: 10.1007/s00702-006-0562-y. [DOI] [PubMed] [Google Scholar]
- 59.Shuto H, Kataoka Y, Horikawa T, Fujihara N, Oishi R. Repeated interferon-alpha administration inhibits dopaminergic neural activity in the mouse brain. Brain Res. 1997;747:348–351. doi: 10.1016/s0006-8993(96)01371-6. [DOI] [PubMed] [Google Scholar]
- 60.Heyes MP, Saito K, Major EO, Milstien S, Markey SP, Vickers JH. A mechanism of quinolinic acid formation by brain in inflammatory neurological disease. Attenuation of synthesis from L-tryptophan by 6-chlorotryptophan and 4-chloro-3-hydroxyanthranilate. Brain. 1993;116(Pt 6):1425–1450. doi: 10.1093/brain/116.6.1425. [DOI] [PubMed] [Google Scholar]
- 61.Guillemin GJ, Croitoru-Lamoury J, Dormont D, Armati PJ, Brew BJ. Quinolinic acid upregulates chemokine production and chemokine receptor expression in astrocytes. Glia. 2003;41:371–381. doi: 10.1002/glia.10175. [DOI] [PubMed] [Google Scholar]
- 62.Weiss JM, Downie SA, Lyman WD, Berman JW. Astrocyte-derived monocytechemoattractant protein-1 directs the transmigration of leukocytes across a model of the human blood-brain barrier. J Immunol. 1998;161:6896–6903. [PubMed] [Google Scholar]
- 63.Owe-Young R, Webster NL, Mukhtar M, Pomerantz RJ, Smythe G, Walker D, et al. Kynurenine pathway metabolism in human blood-brain-barrier cells: implications for immune tolerance and neurotoxicity. J Neurochem. 2008;105:1346–1357. doi: 10.1111/j.1471-4159.2008.05241.x. [DOI] [PubMed] [Google Scholar]
- 64.Scrandis DA, Langenberg P, Tonelli LH, Sheikh TM, Manogura AC, Alberico LA, et al. Prepartum Depressive Symptoms Correlate Positively with C-Reactive Protein Levels and Negatively with Tryptophan Levels: A Preliminary Report. Int J Child Health Hum Dev. 2008;1:167–174. [PMC free article] [PubMed] [Google Scholar]
- 65.Kohl C, Walch T, Huber R, Kemmler G, Neurauter G, Fuchs D, et al. Measurement of tryptophan, kynurenine and neopterin in women with and without postpartum blues. J Affect Disord. 2005;86:135–142. doi: 10.1016/j.jad.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 66.Maes M, Verkerk R, Bonaccorso S, Ombelet W, Bosmans E, Scharpe S. Depressive and anxiety symptoms in the early puerperium are related to increased degradation of tryptophan into kynurenine, a phenomenon which is related to immune activation. Life Sci. 2002;71:1837–1848. doi: 10.1016/s0024-3205(02)01853-2. [DOI] [PubMed] [Google Scholar]
- 67.Orlikov AB, Prakhye IB, Ryzov IV. Kynurenine in blood plasma and DST in patients with endogenous anxiety and endogenous depression. Biol Psychiatry. 1994;36:97–102. doi: 10.1016/0006-3223(94)91189-4. [DOI] [PubMed] [Google Scholar]
- 68.Moller SE, Kirk L, Honore P. Tryptophan tolerance and metabolism in endogenous depression. Psychopharmacology (Berl) 1982;76:79–83. doi: 10.1007/BF00430761. [DOI] [PubMed] [Google Scholar]
- 69.Wood K, Harwood J, Coppen A. The effect of antidepressant drugs on plasma kynurenine in depressed patients. Psychopharmacology (Berl) 1978;59:263–266. doi: 10.1007/BF00426632. [DOI] [PubMed] [Google Scholar]
- 70.Myint AM, Kim YK, Verkerk R, Scharpe S, Steinbusch H, Leonard B. Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Disord. 2007;98:143–151. doi: 10.1016/j.jad.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 71.Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]