Abstract
Background: AZD5438 is an orally bioavailable inhibitor of cyclin E-cdk2, cyclin A-cdk2 and cyclin B-cdk1 complexes. Three phase I studies assessed the clinical safety, tolerability, pharmacokinetics and pharmacodynamics of AZD5438 when administered in different dosing schedules.
Patients and methods: AZD5438 was administered four times daily, once every 7 days (study 1), for 14 consecutive days followed by 7 days of rest (study 2), or continuously (study 3), to patients with advanced solid tumours. Dose escalation proceeded until the emergence of dose-limiting toxic effects.
Results: Sixty-four patients were included across the three studies (19, 17 and 28, respectively). Nausea and vomiting were the most common adverse events. When dosed continuously, 40 mg four times daily was considered intolerable, and due to safety issues, all studies were terminated prematurely. Consequently, no intolerable dose was identified during the weekly schedule. Pharmacokinetics demonstrated dose-proportional exposure, high interpatient variability and accumulation after multiple doses. Skin biopsies indicated reduced retinoblastoma protein phosphorylation at cdk2 phospho-sites; other pharmacodynamic assessments did not reveal consistent trends.
Conclusions: AZD5438 was generally well tolerated in a weekly dosing schedule, but not in continuous schedules. The clinical development programme for AZD5438 was discontinued owing to tolerability and exposure data from these studies.
Keywords: AZD5438, cyclin-dependent kinase inhibitor, hair follicle analysis, pharmacodynamics
introduction
Cyclins and cyclin-dependent kinases (CDKs) are core components of the cell cycle machinery and drive the transition between cell cycle phases. During the progression from G1 to S, cyclin D- and cyclin E-dependent kinases 4, 6 and 2 sequentially phosphorylate the Rb protein (Rb) [1], disrupting pRb-mediated E2F-1 repression and allowing transcription of genes required for S-phase transit [2, 3]. CDK2 also plays a role in S-phase and G2-phase progression, while CDK1 controls the G2/M transition [4]. Dysregulation of cell cycle CDK activity occurs universally in human cancer [5] so that CDKs have generated considerable interest as novel antineoplastic targets [6, 7].
AZD5438 is an orally bioavailable inhibitor of cyclin E-CDK2, cyclin A-CDK2 and cyclin B-CDK1 complexes (IC50 0.006, 0.045 and 0.016 μM, respectively), with 75-fold selectivity over cyclin D1-CDK4 (IC50 0.45 μM) [8]. AZD5438 inhibits phosphorylation of the CDK2 substrates pRb and p27Kip1, and phosphorylation of the CDK1 substrates nucleolin and protein phosphatase 1α, in a dose-dependent manner. In a panel of 23 cell lines (including lung, colorectal, breast, prostate and haematological tumour cells), IC50s varied from 0.17 μM (MCF-7 human breast cancer) to 1.7 μM (ARH-77 plasma cell leukaemia). In exponentially growing tumour cells, acute exposure to AZD5438 induces S- and G2-phase arrest. G1 arrest is evident in synchronised tumour cell populations. These observations are consistent with a CDK1/2 inhibitory phenotype [9,10,11].
Preclinical and healthy volunteer studies [12] demonstrated a promising safety and efficacy profile, prompting clinical dose-escalation and scheduling studies. In this report, we describe three clinical studies investigating the safety and tolerability of AZD5438 in patients with advanced solid tumours. The studies assessed weekly dosing and continuous daily dosing schemes with and without varying periods off-therapy.
In all three studies, AZD5438 was administered four times daily. Preclinical data demonstrated that the daily dose could be split to either twice daily or four times daily dosing, while maintaining tumour growth inhibition and avoiding peak plasma drug concentration (Cmax) effects. The most efficacious models were associated with evidence of a sustained reduction in the levels of phosphorylated pRb by ∼50% for up to 16 h after dose. Furthermore, in healthy volunteer studies, pharmacokinetics demonstrated a relatively short plasma half-life, with Cmax achieved 0.5–3 h after dose. Dose-limiting toxicity (DLT) at 160 mg was nausea and vomiting, with only one episode of grade 1 nausea among subjects receiving either 60 or 80 mg. The 160-mg dose level was also associated with a trend towards an increased time between the start of the Q wave and the end of the T wave in the heart's electrical cycle (QTc interval) [12]. Pharmacodynamic data demonstrated up to 30% reduction in phosphorylated Rb levels in buccal mucosa biopsies at 1.5 h after a 40- or 60-mg dose; this effect was not maintained at 6 h after dose [13]. Taken together, the preclinical and clinical data indicated that four times daily dosing could mitigate Cmax-related toxic effects, while sustaining target coverage despite the rapid elimination of AZD5438, and was therefore chosen for investigation.
patients and methods
patients
All three studies included patients aged ≥18 years of age with histologically or cytologically confirmed solid malignant tumours that were refractory to standard therapies or for whom no standard treatment exists. Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, adequate haematological, renal and hepatic function and should have received no more than three prior cytotoxic chemotherapy regimens. Patients were excluded if they had active intracerebral metastases, treatment with potent cytochrome P-450 inhibitors or inducers or if they had received radiation or chemotherapy within 3 weeks of the start of study treatment. All patients provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki, local institutional review board ethical approval, Good Clinical Practice and applicable regulatory requirements.
study design
Three open-label, dose-escalation multicentre phase I studies were carried out. AZD5438 was administered orally in all studies. In study 1, cohorts of three or four patients received four doses of AZD5438 orally on day 1 and subsequently every 7 days. The starting dose was 40 mg/day in four divided doses [10 mg q.i.d., 50% of the maximum tolerated dose (MTD) observed in a previously conducted single ascending dose study in healthy volunteers]. If a DLT was observed in one patient in a cohort within 21 days of commencing treatment, additional patients were to be recruited (to increase the cohort up to six) and treated at the same dose level. If a DLT was observed in two or more patients at a dose level, that dose was declared the non-tolerated dose (NTD) and no further dose escalation occurred. The MTD was defined as one dose level below the NTD. The cohort of patients at the MTD was expanded to six patients for confirmation. Using Common Terminology Criteria for Adverse Events (CTCAE), DLTs were defined as any of the following treatment-related events occurring during the first treatment cycle: any grade 4 haematologic event (excluding leucocytopenia and neutropenia if duration ≤8 days); grade 3 or 4 neutropenia with fever; thrombocytopenia associated with bleeding; grade ≥2 vomiting (2–5 episodes in 24 h) despite optimal anti-emetic therapy; any other grade 3 or 4 non-haematologic event (including biochemical findings) despite adequate supportive care; unscheduled interruption of dosing of >7 days; QTc interval >500 ms or increase >60 ms. The dose-escalation plans of studies 2 and 3 as well as the definitions for NTD, MTD and DLT were similar. In study 2, eligible patients received a single dose of AZD5438 on day 1 followed by four times daily dosing for 14 consecutive days, followed by a 7-day rest period. The starting dose was 20 mg/day (5 mg q.i.d.), which was 25% of the maximum well-tolerated dose observed in the previously mentioned single ascending dose study in healthy volunteers. In study 3, patients received a single dose of AZD5438 on the first day of the first cycle, followed by q.i.d. dosing thereafter in 28-day cycles, starting at 10 mg/day (2.5 mg q.i.d). With this lower dose, a dose intensity comparable with study 2 was obtained.
measurement of study variables
At enrolment, demographics were recorded, along with ECOG performance status, concomitant medications and radiological assessments from within the previous 28 days documented according to RECIST [14]. Each patient underwent a physical examination, measurement of vital signs; a pregnancy test if applicable and assessment of adequate contraception, and resting electrocardiogram (ECG), standard biochemistry, urinalysis and haematology tests. Assessments were carried out serially during study therapy. Electronic ECGs were collected and centrally analysed before each dose-escalation decision. Adverse events (AEs) were reported according to the National Cancer Institute CTCAE version 3.0. Tumour response was evaluated by RECIST every 8 weeks. No formal statistical analyses were carried out.
pharmacokinetics
Single-dose pharmacokinetic parameters were measured using venous blood samples taken before and at various time points following the first dose of AZD5438. In study 1, blood samples were taken on day 1 before dose and at 0.5, 1, 1.5, 2, 3, 4 and 5 h after dose. In studies 2 and 3, there was a single dose administered on day 1, enabling blood sampling up to 24 h after dose. In these studies, blood samples were drawn on day 1 before dose and at 0.5, 1, 1.5, 2, 3, 4, 5, 8 and 24 h after dose. In study 2, additional pharmacokinetic samples were taken on day 7 (before dose and 1.5 h after dose) and day 14 (before dose and up to 5 h after dose). In study 3, additional pharmacokinetic samples were taken at days 8, 15 and 22 (before dose and 1.5 h after dose) and on day 28 (before dose and up to 5 h after dose).
A validated high-performance liquid chromatography assay with tandem mass spectrometry detection was used to determine the total drug concentration in plasma. The following pharmacokinetic parameters were determined by non-compartmental analysis using WinNonLin version 3.1 (Pharsight Corporation, Mountain View, CA): maximum plasma concentration (Cmax), time to reach the Cmax (tmax), area under the plasma concentration–time curve from time 0 to 5 h (AUC(0–5)), area under the plasma concentration–time curve from time zero to the time of the last measurable concentration (AUC(0–t)), area under the plasma concentration–time curve from time zero to infinity (AUC), terminal half-life (t½), total plasma clearance following oral dosing (CL/F) and volume of distribution at steady-state following oral dosing (Vdss/F).
exploratory pharmacodynamic end points
To investigate the effects of AZD5438 on various biomarkers in surrogate proliferating tissues, studies were carried out in stimulated peripheral blood mononuclear cells (PBMCs) (in the presence of pre- and post-treatment plasma), as well as in hair follicles and keratinocytes. Peripheral blood samples were taken before dose on day 1 and 1.5 h after first dose on days 1 and 8 (studies 1 and 3), or before dose on day 1 and 1.5 h after first dose on days 1 and 7 (study 2). PBMCs were extracted and frozen together with autologous plasma. Alternatively, stimulated healthy volunteer PBMCs were mixed with pre- and post-treatment plasma. Proliferation of PBMCs stimulated by OKT3 ex vivo in the presence of pre- and post-treatment plasma was assessed after a 48-h incubation at 37°C by measuring the incorporation of 3H-thymidine into cellular DNA, administered with a 42-h incubation pulse. Cells were harvested using Tomtek 96 Harvester and counted with Wallac 1205 BettaPlate Liquid Scintillation Counter. Additionally, levels of phosphorylated p27Kip1, phosphorylated pRb and Ki67 were measured from scalp hair taken on days 1 and 8 of the first treatment cycle (study 1), on days 1 and 7 of the first treatment cycle (study 2) or on days 1 and 8 of every treatment cycle (study 3). Up to 40 hair follicles were plucked. Follicles in the first phase of the hair cycle were analysed using methodology previously described [13]. In a subset of participants treated at the Dana-Farber Cancer Institute (part of study 3), skin biopsies were carried out pre-treatment and within 2 h of the first dose on either day 15 or day 22 of treatment and analysed immunohistochemically for expression of phospho-Rb, total Rb, p27Kip1, cyclin D1, p53 and Ki67, as previously described [15, 16].
results
early study termination
A review of emerging safety data from studies 2 and 3, as well as the available clinical pharmacokinetic and pharmacodynamic data, led to a decision by AstraZeneca to discontinue the development of AZD5438 as a potential anticancer agent. Therefore, all three studies described in this report were terminated prematurely.
study population
A total of 64 patients with advanced solid malignancies, whose characteristics are summarised in Table 1, were entered into the studies. Fifteen of the 19 patients who entered study 1 completed the first cycle (21 days). Four patients were removed during the first cycle due to non-drug-related AEs (n = 2, 60 mg q.i.d. cohort) or disease progression (n = 1, 40 mg q.i.d. cohort; n = 1, 60 mg q.i.d. cohort) and were deemed not fully assessable for toxicity and replaced. The 15 patients who completed the first cycle were later withdrawn from the study due to disease progression (n = 13), AEs (n = 1) or withdrawal of consent (n = 1). Five patients died during the study: four due to disease progression (40 mg q.i.d., n = 1; 60 mg q.i.d., n = 3) and one due to pneumonia Common Terminology Criteria (CTC) grade 4 (60 mg q.i.d.).
Table 1.
Patient characteristics
| Patient characteristics | Study 1 (n = 19) | Study 2 (n = 17) | Study 3 (n = 28) |
| Median age, years (range) | 57.5 (41−71) | 61.3 (44–79) | 58.3(33–84) |
| Male/female, n | 12/7 | 12/5 | 18/10 |
| Race/ethnicity | |||
| Caucasian | 19 | 16 | 26 |
| Asian (non-Japanese) | 1 | 1 | |
| Black | 1 | ||
| Primary tumour location, n | |||
| Lung | 5 | 3 | |
| Pancreas | 5 | ||
| Colorectal | 4 | 4 | 7 |
| Renal | 4 | ||
| Skin/soft tissue | 3 | ||
| Adrenal | 2 | ||
| Head and neck | 2 | ||
| Oesophagus | 2 | ||
| Other | 5 | 9 | 9 |
Seventeen patients were enrolled in study 2, of whom three (all from the 40 mg q.i.d. cohort) did not complete the first cycle due to intestinal obstruction (n = 1), disease progression (n = 1) and fatal acute renal failure (n = 1). The 14 patients who completed the first cycle were later discontinued for disease progression (n = 11), intercurrent illness (n = 1), nausea and vomiting (n = 1) and premature study termination (n = 1).
Twenty-eight patients were enrolled in study 3. Thirteen patients did not complete the first treatment cycle: two in the 10 mg q.i.d. cohort due to disease progression; seven in the 20 mg q.i.d. cohort due to withdrawal of consent (n = 3), disease progression (n = 2) and AEs (n = 2) and four in the 40 mg q.i.d. cohort due to withdrawal of consent (n = 2) and AEs (n = 2). The 15 patients who completed the first cycle were later discontinued for disease progression (n = 13), withdrawal of consent (n = 1) and discontinuation at the investigator's discretion (n = 1). Three patients died during study 3; one due to disease progression and two due to AEs detailed below. Dose levels and numbers of enrolled are shown in Table 2.
Table 2.
Dose-escalation schemes
| Dose-escalation schemes |
||||
| Dose level, mg q.i.d. | Patients, n | Number of days on treatmenta, range | Number of patients with DLT in cycle 1 | |
| Study 1: weekly dosing | 10 | 3 | 36–106 | 0 |
| 20 | 3 | 51–106 | 0 | |
| 40 | 4 | 8–58 | 0 | |
| 60 | 6 | 1–141 | 0 | |
| 90 | 3 | 50–106 | 0 | |
| Study 2: 14 days continuous dosing, 1 week rest | 5 | 3 | 55–77 | 0 |
| 10 | 3 | 55–98 | 0 | |
| 20 | 3 | 55–119 | 0 | |
| 40 | 8 | 5–161 | 2 (G2 nausea and vomiting; G5 acute renal failure) | |
| Study 3: continuous dosing | 2.5 | 3 | 35–56 | 0 |
| 5 | 3 | 29–56 | 0 | |
| 10 | 5 | 19–87 | 0 | |
| 20 | 11 | 1–226 | 0 | |
| 40 | 6 | 3–56 | 2 (fatal pericarditis; G3 fatigue) | |
Period from first to last dose of AZD5438 treatment.
DLT, dose-limiting toxicity.
safety and tolerability
In all three studies, the most frequently reported treatment-emergent AEs were gastrointestinal in origin (Table 3), and most were CTC grades 1–2. The number of patients reporting gastrointestinal AEs increased in a dose-dependent manner. When administered four times daily once every week (study 1), AZD5438 was well tolerated up to doses of 90 mg q.i.d. At this dose, CTC grade 3 fatigue was reported, although none of the other observed safety findings over the dose range of 10–90 mg q.i.d. satisfied DLT criteria. Also, no treatment-related serious adverse events (SAEs) were reported. Due to early study termination, neither the NTD nor the MTD was established for this schedule.
Table 3.
Treatment-emergent AEs occurring in three or more patients irrespective of causality
| Number of patients experiencing AE | Study 1 AZD5438 dose (mg, q.i.d.) |
Study 2 AZD5438 dose (mg, q.i.d.) |
Study 3 AZD5438 dose (mg, q.i.d.) |
||||||||||||||
| 10 (3) | 20 (3) | 40 (4) | 60 (6) | 90 (3) | Total n (%) (19) | 5 (3) | 10 (3) | 20 (3) | 40 (8) | Total n (%) (17) | 2.5 (3) | 5(3) | 10 (5) | 20 (11) | 40 (6) | Total n (%) (28) | |
| Nausea | 1 | 3 | 3a | 4 | 2 | 13 (68) | 1 | 2 | 3 | 7a | 13 (76) | 1 | 1 | 3 | 5 | 4 | 14 (50) |
| Vomiting | 0 | 1 | 3a | 4 | 3 | 11 (58) | 1 | 0 | 2 | 4 | 7 (41) | 1 | 0 | 4 | 4a | 3 | 12 (43) |
| Diarrhoea | 1 | 1 | 0 | 4a | 1 | 7 (37) | 0 | 0 | 1 | 3 | 4 (24) | 1 | 0 | 0 | 1 | 2 | 4 (14) |
| Constipation | 2 | 1 | 2a | 1 | 0 | 6 (32) | 0 | 1 | 1 | 3 | 5 (29) | 1 | 0 | 2 | 3 | 0 | 6 (21) |
| Abdominal pain | 0 | 0 | 0 | 2 | 1 | 3 (16) | 0 | 0 | 0 | 3 | 3 (18) | ||||||
| Fatigue | 1 | 1 | 1 | 1 | 2a | 6 (32) | 1 | 1 | 1 | 5 | 8 (47) | 1 | 2 | 2 | 5 | 5a | 15 (54) |
| Pyrexia | 0 | 2 | 0 | 3 | 0 | 5 (26) | 0 | 0 | 0 | 3 | 3 (18) | 0 | 0 | 1 | 1 | 2 | 4 (14) |
| Nasopharyngitis | 1 | 1 | 1 | 0 | 1 | 4 (21) | |||||||||||
| Anorexia | 0 | 0 | 1 | 1 | 1 | 3 (16) | 0 | 2 | 2 | 3 | 7 (41) | 0 | 0 | 1 | 3a | 1 | 5 (18) |
| Back pain | 0 | 0 | 0 | 3a | 0 | 3 (16) | 1 | 0 | 1 | 1 | 3 (18) | ||||||
| Headache | 1 | 0 | 2 | 0 | 2 | 5 (26) | 1 | 0 | 1 | 1 | 3 (18) | 0 | 0 | 1 | 2 | 1 | 4 (14) |
| Urinary retention | 0 | 0 | 1 | 2 | 0 | 3 (16) | |||||||||||
| Dyspnoea | 1 | 0 | 0 | 1a | 1b | 3 (16) | 0 | 0 | 1a | 2 | 2 | 5 (18) | |||||
| Lethargy | 1 | 1 | 2 | 1 | 5 (29) | ||||||||||||
| Cough | 1 | 0 | 0 | 3 | 4 (24) | 1 | 0 | 1 | 1 | 0 | 3 (11) | ||||||
| Dyspepsia | 1 | 0 | 1 | 1 | 3 (18) | ||||||||||||
| Dehydration | 0 | 0 | 0 | 2 | 1 | 3 (11) | |||||||||||
| Dizziness | 0 | 1 | 0 | 2 | 0 | 3 (11) | |||||||||||
| Oedema | 0 | 1 | 0 | 1a | 1 | 3 (11) | |||||||||||
All AEs were CTC grade 1 or 2 except for CTC grade 3 AEs (n = 1).
All AEs were CTC grade 1 or 2 except for a single CTC grade 4 AE.
AE, adverse event.
Administration of AZD5438 four times daily in continuous schedules (studies 2 and 3) led to substantially increased toxic effects. All 17 patients who received AZD5438 in study 2 experienced at least one AE. Most AEs were mild to moderate (CTC grade 1 or 2, see Table 3). Eleven patients experienced AEs considered to be related to study drug, including nausea (n = 7), vomiting (n = 4), fatigue (n = 2) and lethargy (n = 2). DLTs were encountered at 40 mg q.i.d. in two patients (CTC grade 2 nausea and vomiting and CTC grade 5 acute renal failure). The first patient, a 48-year-old female diagnosed with advanced breast cancer with liver and bone metastasis, reported nausea, anorexia, fatigue and diarrhoea 4 days after initiating AZD5438 treatment. Despite anti-emetic treatment, her symptoms deteriorated, resulting in admission to hospital on day 5. Lactate dehydrogenase and C-reactive protein were elevated (337 UI/l and 95 mg/l, respectively), alkaline phosphatase, aspartate aminotransferase and alanine aminotransferase levels increased transiently and serum sodium levels decreased to 126 mmol/l. There were ECG features of pericarditis without evidence of myocardial infarction (normal creatinine kinase, troponin-T and echocardiogram). These symptoms resolved after study treatment was stopped.
The second patient, a 67-year-old woman with metastatic colorectal carcinoma and a history of pulmonary embolism, reported lethargy and nausea from the first day of therapy, with vomiting from day 3 despite anti-emetic treatment. On day 5 she complained of increased weakness, reduced fluid intake, diaphoresis and generalised abdominal pain. On admission to hospital she was dehydrated, hypotensive (blood pressure 84/60 mmHg) and tachycardic (heart rate 125 bpm). Chest X-ray was normal, ECG showed sinus tachycardia and chemistries showed serum creatinine of 252 mmol/l (baseline 102 mmol/l), normal serum electrolytes (K+ and Na+) and slightly elevated blood urea (12.5 mmol/l). Despite fluid therapy (central venous pressure +10 and recovery of blood pressure), the patient remained anuric. There was no response to high-dose furosemide, and serum creatinine elevated further to 404 mmol/l within 12 h of admission. Further aggressive management and dialysis were declined. The patient deteriorated and died on day 6. Blood culture was negative and C-reactive protein was elevated markedly (220 mg/l, baseline unknown). The cause of death was dehydration and acute renal failure, which was deemed to be study treatment related. Post-mortem examination was not carried out.
Of the six other patients treated with 40 mg q.i.d., two experienced elevations in C-reactive protein, white cell count and serum creatinine, which resolved after cessation of treatment. Treatment was resumed at 20 mg q.i.d. in one patient without recurrence of the elevations; the other patient was not re-treated with AZD5438. As DLTs were experienced by two of six assessable patients at 40 mg q.i.d., this dose was considered to be the NTD for the 14-days-on, 7-days-off schedule. The MTD could not be established due to study termination.
A total of 166 AEs were reported by 27 patients during study 3. Consistent with the other studies, the majority of AEs were gastrointestinal in origin, with the number of reports increasing in a dose-dependent manner. Of these, the AEs considered to be causally related to AZD5438 by the investigator were nausea (n = 8 patients), vomiting (n = 7 patients), diarrhoea (n = 3 patients) and abdominal pain (n = 1 patient). Six patients experienced a total of nine non-fatal SAEs, of which myocardial ischaemia, CTC grade 2 (20 mg q.i.d.), in a patient with hypertension was considered to be possibly related to the study drug.
Two patients died due to AEs. The first patient was a 53-year-old female in the 20 mg q.i.d. cohort with a primary tumour of the ampulla of Vater, who had a history of hypertension and rheumatic heart disease. On study day 4, she reported worsening bouts of vomiting that had started on day 1, following initiation of study treatment. AZD5438 was discontinued and she received i.v. fluids. Hours later, she became lethargic and confused, with respiratory distress, hypoxia, hypotension, hyponatraemia, marked leukocytosis, increased serum creatinine and markedly elevated aspartate aminotransferase, D-dimer and brain natriuretic peptide levels. ECG initially showed no acute changes, but there was evidence of previous myocardial infarction. Despite intubation, i.v. fluids and antibiotics, she decompensated and died on day 5. ECG showed changes consistent with acute myocardial infarction. No post-mortem examination was carried out. In the opinion of the investigator, a pulmonary embolism may have caused hypoxia and right heart failure (high B-type natriuretic peptide) with passive liver congestion (elevated aspartate aminotransferase). Additionally, with a history of rheumatic heart disease and strong family history for coronary artery disease, this patient may have suffered a myocardial infarction, leading to fatality. The event was not considered to be related to AZD5438.
The second patient, a 58-year-old female in the 40 mg cohort, died on study day 4. This patient, with previously untreated metastatic liposarcoma to the liver, had a medical history of hysterectomy, tubal ligation and three radical resections of retroperitoneal sarcoma (including right nephrectomy and adrenalectomy). Following initiation of AZD5438, she experienced a prodrome of fatigue, nausea and vomiting, which progressively worsened. On admission on day 3, study treatment was stopped. At presentation, she was hypotensive with white blood cell count 20 000 and elevated serum creatinine. ECG showed no acute changes, but low voltage, with unremarkable echocardiogram. Chest X-ray showed bilateral hilar infiltrates suggestive of aspiration pneumonia. Despite treatment of septic shock with antibiotics, pressors and a tumour necrosis factor α inhibitor, she became acidotic and anuric and died on day 4. Blood cultures were negative. A post-mortem examination concluded that the cause of death was pericarditis. The investigator considered this fatal event to be treatment related and therefore a DLT.
A second DLT was also observed in the 40 mg q.i.d. cohort presented as severe fatigue, CTC grade 3. This episode of fatigue, in a 60-year-old male with oesophageal carcinoma and liver metastases, emerged on treatment day 3, after nausea and vomiting the previous day. The condition improved significantly following discontinuation of study therapy. As a result of these DLTs, AZD5438 40 mg q.i.d. was delineated as the NTD for the continuous dosing schedule. Due to the premature termination of this study, the MTD was not established.
pharmacokinetics
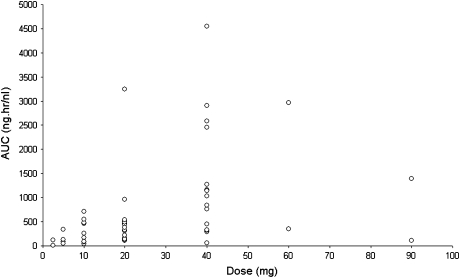
Pharmacokinetic data obtained following administration of single oral doses (all three studies) and multiple oral doses (studies 2 and 3) of AZD5438 are summarised in Table 4. Absorption of AZD5438 after single oral doses was rapid, with time to maximum plasma drug concentration generally ranging between 0.5 and 4 h after dose across the doses studied. Beyond the peak, plasma concentrations declined rapidly over the time period studied (up to 24 h after dose administration). The half-life ranged from 0.72 to 8.09 h. The variability in exposure between patients within a dose cohort was high (Figure 1), but exposure (AUC and maximum plasma drug concentration [Cmax]) generally increased with increasing dose.
Table 4.
Derived pharmacokinetic parameters following administration of a single dose and multiple doses of AZD5438
| AZD5438 dose (mg) |
||||||||
| Single dose (studies 1, 2 and 3) |
2.5 | 5 | 10 | 20 | 40 | 60 | 90 | |
| Study 3 only | Studies 2 + 3 | All studies | All studies | All studies | Study 1 only | Study 1 only | ||
| Cmax (ng/ml) | Median (range), n | 14.2 (2.9–24.9), 3 | 21.3 (12.5–37.5), 6 | 88 (10.7–182), 11 | 134 (30.9–301), 17 | 261 (25.1–754), 18 | 708 (239–1690), 6 | 438 (51.3–602), 3 |
| Tmax (h) | Median (range), n | 1.0 (1.0–2.0), 3 | 1.25 (1.0–3.0), 6 | 1.0 (0.5–2.0), 11 | 1.5 (0.5–3.25), 17 | 1.0 (0.5–4.0), 18 | 1.0 (0.5–1.5), 6 | 1.0 (0.5–2.0), 3 |
| AUC (ng h/ml) | Median (range), n | 62.9 (7.8–118), 2 | 89.3 (53.0–342), 5 | 256 (44.4–715), 9 | 411 (107–3246), 16 | 1028 (59.8–4550), 15 | 1655 (346–2964), 2 | 751 (109–1393), 2 |
| AUC(0–5) (ng h/ml) | Median (range), n | 38.8 (6.8–79.7), 3 | 44.9 (34.7–121), 6 | 169 (34.1–382), 10 | 303 (88.9–942), 17 | 724 (50.7–1607), 18 | 2220 (343–4510), 6 | 1179 (100–1272), 2 |
| Half-life (h) | Median (range), n | 2.32 (1.23–2.82), 3 | 2.54 (1.68–7.24), 6 | 2.09 (1.27–5.28), 9 | 2.0 (1.31–8.09), 17 | 2.60 (1.31–7.61), 15 | 1.27 (0.72–1.82), 2 | 1.18 (1.01–1.34), 2 |
| CL/F (l/h) | Median (range), n | 172 (21.2–322), 2 | 56 (14.6–94.4), 5 | 39.1 (14.0–225), 9 | 49 (6.2–188), 16 | 38.9 (8.8–669), 15 | 96.6 (20.2–173), 2 | 444 (64.6–824), 2 |
| Vdss/F (l) | Median (range), n | 511 (92.7–929), 2 | 321 (144–662), 5 | 113 (64.7–839), 9 | 168 (73.2–690), 16 | 136 (70.4–1851), 15 | 126 (61.8–190), 2 | 1203 (143–2262), 2 |
| Multiple doses (studies 2 and 3) |
2.5 | 5 | 5 | 10 | 10 | 20 | 20 | 40 | 40 | |
| Study 3 | Study 2 | Study 3 | Study 2 | Study 3 | Study 2 | Study3 | Study 2 | Study 3 | ||
| Cmax ss (ng/ml) | Median (range), n | 24.2 (3.6–49.8), 3 | 33.5 (33–41.2), 3 | 27.7 (22.1–36.8), 3 | 135 (22.3–284), 3 | 150 (97.3–202), 2 | 177 (86.9–197), 3 | 155 (72.4–171), 3 | 178.5 (173–184), 2 | 85.8, 1 |
| Tmax ss (h) | Median (range), n | 0.5 (0.5–1.5), 3 | 1.0 (0.5–1.5), 3 | 1.0 (0.5–1.0), 3 | 2.0 (0.5–2.0), 3 | 1.25 (1.0–1.5), 2 | 1.0 (1.0–3.0), 3 | 1.0 (1.0–2.0), 3 | 0.75 (0.5–1.0), 2 | 2, 1 |
| AUC(0–5) (ng h/ml) | Median (range), n | 58.7 (7.3–172), 3 | 94.9 (93.0–95.5), 3 | 62.2 (36.7–110), 3 | 422 (53.3–809), 3 | 411, 1 | 420 (309–531), 2 | 392 (115–426), 3 | 367, 1 | 245, 1 |
| Cmin ss (ng/ml)a | Median (range), n | <0.5–21.1 (5.39), 3 | 7.84 (7.32–9.96), 3 | 4.53 (2.19–15.1), 3 | 78.9 (7.09–92.9), 3 | 33.9, 1 | 71.3 (54.2–318), 3 | 32.8 (3.9–47.5), 3 | 16.3, 1 | 18, 1 |
| Predictabilityb | Median (range), n | 0.59 (0.25–0.94), 2 | 1.06 (0.72–1.72), 3 | 0.51 (0.32–0.69), 2 | 0.70 (0.59–1.46), 3 | 0.90, 1 | 1.68 (1.08–2.28), 2 | 0.89 (0.78–1.08), 3 | 0.82, 1 | 0.75, 1 |
| Accumulation ratio | Median (range), n | 1.51 (1.07–2.16), 3 | 2.65 (1.42–2.68), 3 | 0.91 (0.89–1.3), 3 | 1.1 (0.76–2.64), 3 | 1.11, 1 | 2.55 (1.61–3.48), 2 | 1.21 (1.08–1.40), 3 | 0.92, 1 | 0.86, 1 |
Concentration present at 5 h after dosing.
Assessment of how well predicted the multiple-dose exposure is from the single-dose data (ratio of day 14 AUC(0–5):day 1 AUC for study 2 and ratio of day 28 AUC(0-5):day 1 AUC for study 3).
AUC, area under the plasma concentration–time curve; AUC(0–5), area under the plasma concentration–time curve from time 0 to 5 h; CL/F, total apparent drug clearance; Cmax, maximum plasma drug concentration; Cmax ss, maximum plasma drug concentration at steady-state; Cmin ss, minimum plasma drug concentration at steady-state; tmax, time to reach maximum plasma drug concentration; tmax ss, time to reach maximum plasma drug concentration at steady-state; Vdss/F, apparent volume of distribution at steady-state.
Figure 1.
Exposure [area under the curve (AUC)] versus AZD5438 dose. This figure shows the AUC as a function of AZD5438 dose. The AUCs were calculated after a single dose of AZD5438, on day 1 of treatment. There is a dose-proportional increase in exposure, but a high interpatient variability in exposure to AZD5438.
Following multiple oral dosing of AZD5438, Cmax was again achieved rapidly after dosing with concentrations declining rapidly thereafter. Consistent with the single-dose data, exposure following multiple dosing of AZD5438 increased with increasing dose. Comparison of the plasma drug concentrations achieved on days 7 and 14 (study 2) and on days 8, 15 and 22 compared with drug levels obtained on day 29 (study 3) indicated that steady-state was achieved within the first 7 days of dosing with AZD5438.
An assessment of the predictability of multiple-dose exposure from the single-dose data [ratio of day 14 AUC (study 2) or day 28 AUC (study 3) from time 0 to 5 h to day 1 AUC for the same patient] showed that multiple-dose pharmacokinetics was not well predicted from single-dose pharmacokinetics. Accumulation occurred in some patients following multiple oral dosing of AZD5438 q.i.d. for 14 consecutive days (study 2) or continuously for 28 days (study 3). Among nine patients evaluated in study 2, the accumulation ratio ranged from 0.76 to 3.48; in 11 patients evaluated in study 3, the accumulation ratio ranged from 0.86 to 2.16.
tumour response
In all three studies, no objective responses were observed. Overall, 26 patients could not be evaluated for tumour response as they withdrew early. Of the 38 assessable patients, 24 had progressive disease and 14 had stable disease as best response (supplemental Table 1, available at Annals of Oncology online).
exploratory pharmacodynamic findings
Evaluation of stimulated patient PBMCs combined with autologous plasma was not possible across the three studies because the majority of PBMC samples had few or no viable cells. An alternative method involved using stimulated PBMCs from a healthy volunteer donor in combination with the patients’ pre- and post-treatment plasma to assess the drug effect. By this approach, plasma samples from 12 patients in study 1 generated informative data (stimulated proliferation counts >5000 cpm) at three time points. However, no consistent trends were observed across dose levels. In study 2, using the alternative approach, 24 of 37 pre-treatment plasma samples generated stimulated proliferation counts >5000 cpm. No meaningful effect on proliferation count was observed with post-treatment plasma obtained from patients receiving 5 or 10 mg (n = 3). Two of three samples in the 20 mg q.i.d. cohort and seven of eight samples in the 40 mg q.i.d. cohort had reduced proliferation counts, when plasma obtained 1.5 h after dose was compared with pre-treatment plasma on day 1. In study 3, with volunteer cells plus pre-treatment patient plasma, 11 samples demonstrated two or more values >5000 cpm. No consistent trends were observed in reduction of 3H incorporation using post-treatment plasma in the 10 mg q.i.d. cohort. At 20 and 40 mg q.i.d, there were reductions in proliferation counts at 1.5 h after dose compared with before dose on day 1 in five of six patients and in all patients, respectively. However, among small sample sets available, these trends were not confirmed with plasma obtained 1.5 h after dose on day 7 (study 2) or day 8 (study 3).
For hair follicle analysis, there were insufficient data to evaluate a treatment effect on biomarkers since only 17% of follicles collected in study 1, 38% collected in study 2 and 21% collected in study 3 had staining present.
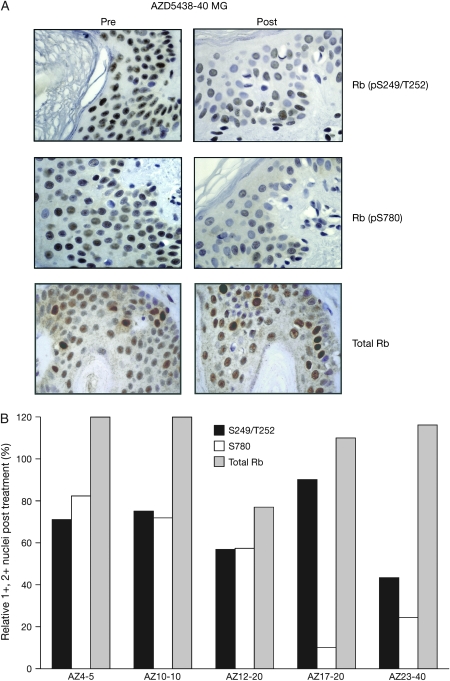
Seven paired skin biopsies were analysed over the course of study 3. Among samples obtained from patients receiving doses of 5 mg q.i.d. or higher (n = 5), the percent positive nuclei (scored as 1+ or 2+) staining for phospho-Rb at the S249/T252 and S780 epitopes declined after treatment, while total Rb staining was similar in pre- and post-treatment samples. Representative skin biopsies for one patient, treated at the 40 mg q.i.d. dose level, are shown in Figure 2, along with quantification of Rb staining. No significant change was seen in staining at S807/811 or T356 phospho-sites after treatment, with variable changes observed at the S795 epitope. Additionally, the reduction in Rb phosphorylation at the S249/T252 and S780 sites was incomplete and was not associated with a reduction in Ki67 staining after treatment. Among the seven paired samples, most demonstrated stable or increased p27Kip1 staining after treatment, although decreased staining was observed in two samples. No significant changes in cyclin D1 or p53 staining were observed after treatment.
Figure 2.
Total and phospho-Rb staining in skin biopsies. (A) Skin biopsies were obtained pre-treatment (pre) and 2 h after the first dose on day 22 of continuous ADZ5438 dosing. Five-micrometer sections from formalin-fixed, paraffin-embedded samples were subjected to immunohistochemistry with the indicated antibodies. Results from patient 23 are shown (treated at the 40-mg dose level), demonstrating reduced Rb staining at the S249/T252 and S780 phospho-sites in the keratinocyte layers, suggestive of reduced cyclin-dependent kinase activity after treatment. Total Rb staining is maintained in the post-treatment sample. (B) Immunohistochemical data were quantified by scoring the nuclei of 100–200 keratinocytes as 0, 1+ or 2+. The percentage of positive nuclei was considered as 100 for each pre-treatment sample. Bars indicate the percent positive staining (1+, 2+) in each post-treatment sample, relative to the pre-treatment sample. The x-axis designations indicate the patient number and the dose of AZD5438 administered. In these samples, reductions in phospho-Rb staining were noted, while total Rb staining was maintained after treatment.
post hoc analysis: exposure versus tolerability
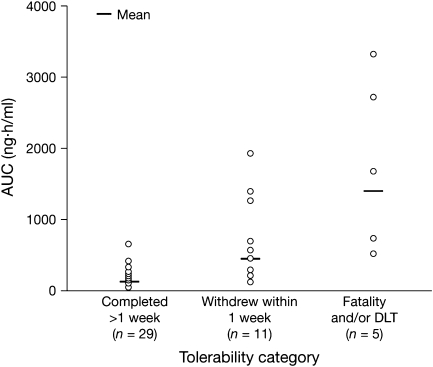
In light of the high variability in exposure, a post hoc analysis was carried out to investigate the relationship between drug exposure and tolerability. This analysis included the 45 patients treated with AZD5438 continuous dosing schedules in studies 2 and 3. Patients were divided into three categories: death and/or DLT, withdrawal within 1 week of starting treatment and completed >1 week of treatment. There was a trend associating increased exposure with decreased tolerability (Figure 3).
Figure 3.
Post hoc analysis: exposure to AZD5438 and tolerability outcome in continuous dosing studies. Owing to the lack of multiple-dose data for many of the patients, the total daily area under the curve was calculated from day 1 pharmacokinetic measurements. Tolerability categories were (1) patients who completed at least the initial safety assessment period (21 or 28 days) or withdrew after 1 week due to disease progression; (2) patients who withdrew within 1 week of starting treatment due to poor tolerability or for disease-related reasons and (3) patients who discontinued due to fatality and/or dose-limiting toxicity.
discussion
AZD5438 is one of a number of CDK inhibitors that have recently undergone clinical evaluation. The therapeutic benefit of these agents, including flavopiridol, seliciclib and BMS-387032 (SNS-032), has proved difficult to demonstrate in single-agent solid tumour studies [6], although recently it was shown that treatment with seliciclib can induce responses in patients with nasopharyngeal cancer [17]. AZD5438 had an advantage over some existing agents in being orally bioavailable, allowing sustained daily dosing, likely necessary for tumour growth inhibition in the absence of induction of apoptosis. This report covers three phase I studies designed to investigate different dosing schedules for AZD5438.
The majority of toxic effects observed in all three studies were gastrointestinal in origin, and increased in a dose-dependent manner, consistent with the toxicity profiles in preclinical and healthy volunteer studies with AZD5438 [12], and also in line with clinical experiences with other CDK inhibitors [6]. The weekly schedule was well tolerated up to 90 mg q.i.d., and no NTD was established due to the adverse safety findings from the continuous studies. Both studies with continuous AZD5438 administration progressed sufficiently to identify a NTD of 40 mg q.i.d., with both non-fatal gastrointestinal and constitutional DLTs as well as fatal DLTs.
Continuous dosing of AZD5438 was associated with profound anorexia and fatigue in some patients, accompanied by nausea and vomiting. This symptom complex occurred within the first few days of starting AZD5438 therapy and was associated with tachycardia, hypotension, hyponatraemia and increases in white blood cell counts, C-reactive peptide and serum creatinine. Given the complex nature of the events, and the presence of significant medical co-morbidities in this patient population, a clear aetiology for these events has not yet been established. Nonetheless, these cases share certain similarities, such as the complex of anorexia, lethargy/fatigue, nausea and vomiting leading to sudden decompensation that was seen in all three patients who experienced fatal events on the continuous dosing schedules. In light of the full profile of SAEs that subsequently emerged, the fatal myocardial infarction (suspected) in this study might retrospectively be considered to be possibly treatment related. It is notable that cardiovascular events, including myocardial infarction, have been reported in studies of flavopiridol [18, 19]. However, the severity of AEs observed in these studies was not expected and is not comparable with those observed in studies with other CDK inhibitors.
Single-dose pharmacokinetic evaluation in all three studies showed that AZD5438 was rapidly absorbed and eliminated. After q.i.d. dosing, multiple-dose pharmacokinetics were not well predicted from the single-dose data, with evidence of drug accumulation (studies 2 and 3). Exposure to AZD5438 showed high interpatient variability. The factors accounting for this variability have not been identified. Notwithstanding this variability, exposure generally increased with increasing dose.
In the studies described here, the analyses of pharmacodynamic markers in PBMCs and hair follicles led to disappointing results, with no observed treatment-related trends at all. The biological activity of flavopiridol, another CDK inhibitor, had been successfully investigated in PBMCs before [18,20], and PBMC and hair follicle analyses were employed successfully in a study of AZD5438 in healthy volunteers [13]. The results of the studies described here highlight the difficulties inherent in transferring surrogate tissue biomarker measurements from well-tolerated healthy volunteer studies to a setting of metastatic cancer in which patients have numerous confounding factors including previous and concurrent therapies, as well as their underlying disease.
The analyses of paired skin biopsies in study 3 revealed inhibition of CDK2, indicated by stable P27Kip1 expression [21] and reduced phosphorylation of Rb at the S249/T252 phospho-site [9, 10]. In buccal mucosae from healthy volunteers, no changes were induced by AZD5438 at this phospho-site [13], potentially reflecting differences in the tissues examined and the analysis after only a single dose, compared with a minimum of 15 days of dosing before post-treatment sampling here. Interestingly, reduced phosphorylation of Rb at S780 was also observed, a site reported to be phosphorylated by CDK4 [22]. AZD5438 is significantly less potent against CDK4, although phosphorylation at this site could be affected by cell cycle position, induced by combined CDK2/CDK1 inhibition [11]. Similarly, in the buccal mucosae of healthy volunteers, reduced phosphorylation at S807/811 was noted, which is another CDK4-specific site [23, 24]. In both data sets, changes in Rb phosphorylation did not translate to reduced Ki67 staining, indicating the necessity for more complete inhibition of CDK targets in order to achieve antiproliferative effects.
A post hoc analysis of observed exposure and tolerability outcome in the 45 patients treated with AZD5438 continuous dosing showed a clear trend to poorer outcome with increasing exposure. However, the high degree of variability in interindividual exposure and the inability to identify factors underlying this variability make it difficult to predict tolerable doses in individual patients. Together with the unpredictable nature of the serious toxic effects, this variability in exposure constituted a major clinical development challenge for the compound. The exposure-related toxicity might have resulted from accumulation of the drug in these patients, although the onset of severe toxic effects was frequently observed early in the course of treatment. In contrast to the exposure-related intolerability, dose-related efficacy was not observed. Clinical exposures did not reach the exposures associated with preclinical efficacy, even at NTDs. There appeared to be only minimal clinical efficacy, manifested by disease stabilisation for >4 months in three out of a total of 64 patients dosed across all three studies. Thus, the probability of obtaining a viable dose and schedule for further clinical evaluation of AZD5438 as a potential anticancer agent was considered to be minimal or non-existent.
In conclusion, the phase I AZD5438 clinical study programme has failed to establish an appropriate risk–benefit profile for this novel agent, and therefore its clinical development has been discontinued. The utility of CDK inhibitors as anticancer agents, as monotherapy or in combination regimens, remains to be established.
funding
AstraZeneca. The Dana-Farber substudy (part of study 3) was funded by AstraZeneca as well as the Dana-Farber/Harvard Cancer Center (DF/HCC) Specialized Program of Research Excellence in Lung Cancer and National Institutes of Health (P20 CA90578, R01 CA90687).
disclosure
Gary Schwartz served as a consultant to AstraZeneca. Mark Middleton received research funds and consultancy fees from AstraZeneca. Jan Schellens served on advisory board for AZD5438, sponsored by AstraZeneca. Payments went to The Netherlands Cancer Institute. Geoffrey Shapiro received research funding from AstraZeneca. Dereck Amakye, Helen Swaisland and Clive Morris are employees of AstraZeneca. All other authors have no potential conflict of interest to report.
Supplementary Material
Acknowledgments
We thank Cheryl Forder and Hazel Weir for technical assistance with PBMC assay development, Fiona Smith, Cheryl Forder and Syngenta for analysing PBMC and hair follicles, Kate Byth, Gareth D. Hughes, Robert Wilkinson and Alexandra McGregor for the conduct of supporting preclinical translational studies, Jim Growcott, Shampa Das, David Wilson, Andrew Hughes and Louise Garside for contribution to the clinical programme and analysis of clinical data and Matthew Lewis of Mudskipper Bioscience for editorial assistance. Susan Rigby, Anita Lindsay, John Freeman and Chris Bowen provided study logistics management. We thank Anat Stemmer-Rachamimov, Anna Kreshock, Donna Skinner, Janice Williams and Anna Levitz of the DF/HCC Pathology Core for assistance with skin biopsy processing and immunohistochemistry. We thank Drs Leonard J. Appleman and Andrew X. Zhu for assisting with recruitment at DF/HCC.
References
- 1.Reed SI. Control of the G1/S transition. Cancer Surv. 1997;29:7–23. [PubMed] [Google Scholar]
- 2.Lundberg AS, Weinberg RA. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol. 1998;18:753–761. doi: 10.1128/mcb.18.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harbour JW, Luo RX, Dei Santi A, et al. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98:859–869. doi: 10.1016/s0092-8674(00)81519-6. [DOI] [PubMed] [Google Scholar]
- 4.Pines J. The cell cycle kinases. Semin Cancer Biol. 1994;5:305–313. [PubMed] [Google Scholar]
- 5.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol. 2006;24:1770–1783. doi: 10.1200/JCO.2005.03.7689. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz GK, Shah MA. Targeting the cell cycle: a new approach to cancer therapy. J Clin Oncol. 2005;23:9408–9421. doi: 10.1200/JCO.2005.01.5594. [DOI] [PubMed] [Google Scholar]
- 8.Wheeler C, Stephens T, Byth K. Novel approaches in oncology at AstraZeneca. Eur J Cancer Suppl. 2003;1:3–8. [Google Scholar]
- 9.Cai D, Byth KF, Shapiro GI. AZ703, an imidazo[1,2-a]pyridine inhibitor of cyclin-dependent kinases 1 and 2, induces E2F-1-dependent apoptosis enhanced by depletion of cyclin-dependent kinase 9. Cancer Res. 2006;66:435–444. doi: 10.1158/0008-5472.CAN-05-1769. [DOI] [PubMed] [Google Scholar]
- 10.Byth KF, Geh C, Forder CL, et al. The cellular phenotype of AZ703, a novel selective imidazo[1,2-a]pyridine cyclin-dependent kinase inhibitor. Mol Cancer Ther. 2006;5:655–664. doi: 10.1158/1535-7163.MCT-05-0205. [DOI] [PubMed] [Google Scholar]
- 11.Cai D, Latham VM, Jr, Zhang X, Shapiro GI. Combined depletion of cell cycle and transcriptional cyclin-dependent kinase activities induces apoptosis in cancer cells. Cancer Res. 2006;66:9270–9280. doi: 10.1158/0008-5472.CAN-06-1758. [DOI] [PubMed] [Google Scholar]
- 12.Camidge DR, Smethurst D, Growcott J, et al. A first-in-man phase I tolerability and pharmacokinetic study of the cyclin-dependent kinase-inhibitor AZD5438 in healthy male volunteers. Cancer Chemother Pharmacol. 2007;60:391–398. doi: 10.1007/s00280-006-0371-x. [DOI] [PubMed] [Google Scholar]
- 13.Camidge DR, Pemberton M, Growcott J, et al. A phase I pharmacodynamic study of the effects of the cyclin-dependent kinase-inhibitor AZD5438 on cell cycle markers within the buccal mucosa, plucked scalp hairs and peripheral blood mononucleocytes of healthy male volunteers. Cancer Chemother Pharmacol. 2007;60:479–488. doi: 10.1007/s00280-006-0387-2. [DOI] [PubMed] [Google Scholar]
- 14.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 15.Haddad RI, Weinstein LJ, Wieczorek TJ, et al. A phase II clinical and pharmacodynamic study of E7070 in patients with metastatic, recurrent, or refractory squamous cell carcinoma of the head and neck: modulation of retinoblastoma protein phosphorylation by a novel chloroindolyl sulfonamide cell cycle inhibitor. Clin Cancer Res. 2004;10:4680–4687. doi: 10.1158/1078-0432.CCR-04-0229. [DOI] [PubMed] [Google Scholar]
- 16.Loda M, Cukor B, Tam SW, et al. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nature Med. 1997;3:231–234. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- 17.Hsieh WS, Soo R, Peh BK, et al. Pharmacodynamic effects of seliciclib, an orally administered cell cycle modulator, in undifferentiated nasopharyngeal cancer. Clin Cancer Res. 2009;15:1435–1442. doi: 10.1158/1078-0432.CCR-08-1748. [DOI] [PubMed] [Google Scholar]
- 18.Stadler WM, Vogelzang NJ, Amato R, et al. Flavopiridol, a novel cyclin-dependent kinase inhibitor, in metastatic renal cancer: a University of Chicago phase II consortium study. J Clin Oncol. 2000;18:371–375. doi: 10.1200/JCO.2000.18.2.371. [DOI] [PubMed] [Google Scholar]
- 19.George S, Kasimis BS, Cogswell J, et al. Phase I study of flavopiridol in combination with paclitaxel and carboplatin in patients with non-small-cell lung cancer. Clin Lung Cancer. 2008;9:160–165. doi: 10.3816/CLC.2008.n.024. [DOI] [PubMed] [Google Scholar]
- 20.Bible KC, Lensing JL, Nelson SA, et al. Phase 1 trial of flavopiridol combined with cisplatin or carboplatin in patients with advanced malignancies with the assessment of pharmacokinetic and pharmacodynamic end points. Clin Cancer Res. 2005;11:5935–5941. doi: 10.1158/1078-0432.CCR-04-2566. [DOI] [PubMed] [Google Scholar]
- 21.Zhang GJ, Safran M, Wei W, et al. Bioluminescent imaging of Cdk2 inhibition in vivo. Nat Med. 2004;10:643–648. doi: 10.1038/nm1047. [DOI] [PubMed] [Google Scholar]
- 22.Kitagawa M, Higashi H, Jung HK, et al. The consensus motif for phosphorylation by cyclin D1-cdk4 is different from that for phosphorylation by cyclin A/E-cdk2. EMBO J. 1996;15:7060–7069. [PMC free article] [PubMed] [Google Scholar]
- 23.Zarkowska T, Mittnacht S. Differential phosphorylation of the retinoblastoma protein by G1/S cyclin-dependent kinases. J Biol Chem. 1997;272:12738–12746. doi: 10.1074/jbc.272.19.12738. [DOI] [PubMed] [Google Scholar]
- 24.Brantley MA, Harbour JW. Inactivation of retinoblastoma protein in uveal melanoma by phosphorylation of sites in the COOH-terminal region. Cancer Res. 2000;60:4320–4323. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.