Abstract
The Smith–Lemli–Opitz syndrome (SLOS) is an autosomal recessive disease presenting with multiple congenital anomalies, caused by a defect in cholesterol biosynthesis that results in abnormally elevated levels of 7-dehydrocholesterol (7DHC). Progressive retinal degeneration has been demonstrated in a rat model of SLOS, which is markedly exacerbated by intense light, far more so than occurs in normal albino rats under the same conditions. Herein, we demonstrate that, by six postnatal weeks, retinas in the SLOS rat model contain levels of lipid hydroperoxides (LPOs) comparable to those found in light-damaged albino rats (twice the normal steady-state levels), and that intense light exposure results in a three-fold elevation of LPOs with concomitant severe retinal degeneration. These results suggest a correlation between retinal degeneration and LPO levels. We propose that the presence of 7DHC in the SLOS rat retina potentiates LPO formation, and promotes the observed hypersensitivity to light-induced retinal degeneration.
Keywords: AY9944, Smith–Lemli–Opitz syndrome, retina, degeneration, light damage, lipid hydroperoxide, rat
Retinal degenerations resulting either from hereditary or exogenous causes are thought to entail molecular events that, in part, involve oxidative damage to lipids, proteins, and/or nucleic acids, resulting in cellular dysfunction and, ultimately, cell death. Such events are known to be potentiated by exposure to intense light (Organisciak and Winkler, 1994; Boulton et al., 2001; Glickman, 2002; Wenzel et al., 2005). Certain metabolic conditions result in the formation of pro-oxidant molecules, the presence of which can promote cellular degeneration and death. The Smith–Lemli–Opitz syndrome (SLOS) likely represents such a condition. SLOS, the first described ‘multiple congenital anomalies syndrome’ (Smith et al., 1964; cf. Porter, 2000; Jira et al., 2003), is also the first member of a growing family of inborn errors of anabolic metabolism involving defective cholesterol biosynthesis (Kelley, 2000; Porter, 2003). SLOS specifically is due to a defect in 3β-hydroxysterol-Δ7-reductase (Waterham and Wanders, 2000; Correa-Cerro and Porter, 2005), resulting in abnormal and excessive accumulation of the cholesterol precursor, 7-dehydrocholesterol (7DHC) (Tint et al., 1994). 7DHC and other 7-dehydrosterols are photosensitive and extremely labile to oxidation (Girotti, 2002), generating cytotoxic oxysterols that have been implicated, indirectly, in SLOS (Gaoua et al., 1999; cf. Fliesler, 2002).
We have previously demonstrated a progressive retinal degeneration in a pharmacologically induced rat model of SLOS (Fliesler et al., 2004). More recently, we showed that intense light exposure markedly exacerbates that retinal degeneration, whereas treatment of rats with dimethylthiourea, a hydroxyl radical scavenger and anti-oxidant, prior to light exposure largely protects against the enhancement of degeneration (Vaughan et al., 2005), implicating a free radical mechanism in the degeneration. Herein, we provide evidence that correlates the severity of retinal degeneration in the SLOS rat model, relative to normal albino rats, with accumulated levels of lipid hydroxperoxides (LPOs) in the retina, particularly with intense light exposure.
The SLOS rat model was produced as previously described (Fliesler et al., 2004), by treatment of Sprague Dawley (albino) rats with AY9944, a selective inhibitor of 3β-hydroxysterol-Δ7-reductase (Dvornik et al., 1963; Givner and Dvornik, 1965). Age-matched Sprague Dawley rats, not treated with AY9944, served as controls. All procedures were pre-approved by Saint Louis University’s Animal Care Committee and conformed to the ARVO Statement on the Care and Use of Animals in Ophthalmic and Vision Research and with the NIH Guide for the Care and Use of Laboratory Animals. All animals were fed a cholesterol-free chow (Purina Test Mills, Inc.) upon weaning; rats were provided water, ad lib, and were maintained in dim cyclic light (20–40 lux, 12 hr light–12 hr dark) at 22–25 °C, unless otherwise stated. The retinal light damage paradigm was performed essentially as previously described (Organisciak et al., 1996, 1999; Vaughan et al., 2003, 2005). Rats (6-wk postnatal age, N=4 per group) were dark-adapted overnight, and then exposed for 24 hr to intense green light (1700 lux, 490–580 nm) in a temperature-controlled, Plexiglass chamber (kindly provided by D.T. Organisciak, Wright State University School of Medicine, Dayton, OH). In parallel, groups of control and AY9944-treated rats (hereafter referred to as ‘SLOS rats’) remained in darkness for the same 24 hr period. Thus, four groups of animals were employed, designated as follows: CU (controls, unexposed to intense light); CE (controls exposed to intense light); AU (SLOS rats unexposed to intense light); and AE (SLOS rats exposed to intense light). Within 30–45 min after light exposure, all rats were sacrificed by intracardiac pentobarbital overdose (i.e. there was no ‘recovery period’) and tissues were harvested for analysis. This timing was purposeful, since LPOs are extremely labile and short-lived (Girotti, 1998, Girotti, 2002). One eye of each animal was subjected to conventional histological analysis (Fliesler et al., 2004), while the contralateral retinas were harvested, placed into polypropylene microfuge tubes, and snap-frozen in liquid nitrogen. Individual retinas were subjected to LPO analysis, using a commercially available kit (Lipid Hydroperoxide Assay Kit; Caymen Chemical Co.), per the instructions of the manufacturer. This highly sensitive and simple assay is based on the reaction of any organic hydroperoxide (R-OOH) with Fe2+ to yield Fe3+, which then reacts with thiocyanate anion (SCN−), stoichiometrically, to yield a purple-colored Fe(SCN)52− product that can be quantified spectrophotometrically at 500 nm (ε 16 500 M−1 cm−1; linear range: 0 25–5 nmol) (Roomi and Hopkins, 1970; Mihaljevic et al., 1996). Each specimen was analyzed at least in triplicate. The data were then subjected to small-sample statistical analysis (Student’s t-test, two-tailed, assuming equal variances), with significance determined at p<0 001 (N=4).
As shown in Fig. 1, even the retinas of normal albino rats (CU group) contain measurable steady-state levels of LPOs (in this study, 1·5±0·3 nmoles/retina). Under the conditions used to induce retinal light damage in the control rats (CE group), retinal LPO levels increased approximately two-fold (2·7±1·2 nmol/retina; p<0·001, relative to CU). Notably, in the non-light-exposed SLOS rat retinas (AU group), the LPO levels (3·1±0·5 nmol/retina) were comparable to those observed in the light-exposed normal albino rats (CE vs. AU, p>0·5). Upon exposing the SLOS rats to intense green light for 24 hr (AE group), retinal LPO levels dramatically increased, more than three-fold (10·5±0·5 nmol/retina; p<0·001, relative to all other groups).
Fig. 1.
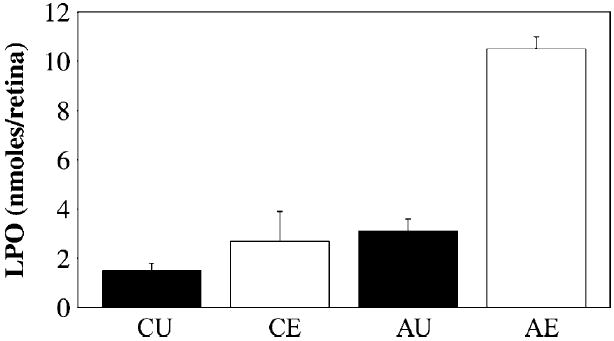
Lipid hydroperoxide (LPO) levels in retinas as a function of experimental treatment conditions. CU, non-light-exposed, normal albino rats; CE, albino rats exposed to intense green light for 24 hr; AU, non-light-exposed, AY9944-treated (SLOS) rats; AE, SLOS rats exposed to intense green light for 24 hr. Values (in nmoles/retina) are given as mean±S.D. (N=4).
Fig. 2 shows representative histological images of the superior central retina, approximately 1 5 mm from the optic nerve head, from eyes of groups CU (Fig. 2A), CE (Fig. 2B), and AE (Fig. 2C). In albino rats, this retinal region is well-known to be hypersensitive to intense light exposure, compared with other retinal areas (see Organisciak and Winkler, 1994; Boulton et al., 2001; Glickman, 2002; Wenzel et al., 2005). Under the conditions employed, considerable pyknosis of the outer nuclear layer (ONL) and cytological damage to the rod outer segment (ROS) layer is evident in this region of light-exposed control retinas (Fig. 2B), relative to non-light-exposed control retinas (Fig. 2A). However, no qualitative reductions in ONL thickness or ROS length were observed. Note that this represents a time point immediately after the period of intense light exposure (i.e. no ‘recovery period’), in contrast to prior studies, where a 2-week recovery period intervened between light exposure and histological analysis (see Organisciak et al., 1996; Vaughan et al., 2003, 2005). Remarkably, even at this very early post-exposure time point, this same region in light-exposed SLOS rat retinas (Fig. 2C) exhibits far more severe damage. This includes not only massive ONL pyknosis and vacuolization, but also marked photoreceptor loss and ONL thinning, and severe ROS shortening, damage, and loss. Furthermore, in at least two of the four eyes examined, gliosis was already apparent (data not shown). The retinal pigment epithelium (RPE) also contains numerous, densely stained inclusions. Considering the extent of ROS reduction and loss (indicative of gross derangement in the ROS turnover mechanism), these inclusions likely represent ROS-derived phagosomes and their degradation products, which are characteristic of the SLOS rat retina (Fliesler et al., 2004). These short-term cytological effects are predictive and consistent with the observed degeneration following a 2-week recovery period (Vaughan et al., 2005).
Fig. 2.
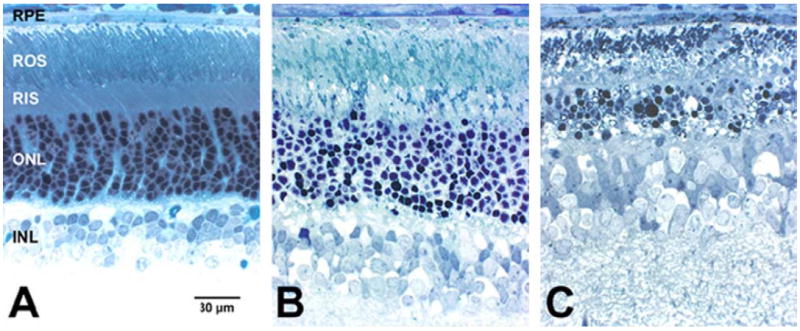
Histology of companion retinas (superior central zone, ca. 1·5 mm from the optic nerve head) as a function of experimental treatment conditions. (A) Non-light-exposed control (CU); (B) light-exposed control (CE); (C) AY9944-treated, light-exposed (AE). Spurr’s resin embedment; Toluidine blue stain. Abbreviations: RPE, retinal pigment epithelium; ROS, rod outer segment layer; RIS, rod inner segment layer; ONL, outer nuclear layer; INL, inner nuclear layer. Scale bar (panel A, for all panels), 30 μm.
Thus, the formation and steady-state levels of LPOs correlate, to some degree, with the observed severity of retinal degeneration. Indeed, LPOs have been correlated with a variety of pathologies and cellular dysfunction (Asano et al., 1989; Rao, 1990; Halliwell and Cross, 1994; Girotti, 1998; Spiteller, 2001). It should be noted that, whereas histological analysis only samples a very small portion of the total retina, the LPO assay provides a global measure of the entire retinal LPO content. Also, the relationship between LPO content and histological damage is not linear: for one thing, there is far more than a three-fold difference in the extent of histological damage comparing AE vs. AU retinas, whereas the LPO levels differ by a factor of about three. In addition, while there is obvious histological damage in the CE retinas, the AU retinas (which have essentially the same LPO levels) do not exhibit frank pathological changes at this stage of postnatal development (see Vaughan et al., 2005). However, AU retinas exhibit scotopic (rod) ERG amplitude changes comparable to those observed in light-damaged (CE) albino rats (Vaughan et al., 2005). Hence, we speculate that low levels of LPOs, above those typically found at steady-state in normal controls, can lead to functional deficits in advance of obvious histological signs of degeneration, whereas above some threshold LPO level, both cytological and functional defects ensue.
One cannot ascertain the biochemical identities of the constituent LPOs from this type of assay; such would require extensive lipidomic analysis and the appropriate authentic lipid standards. While far beyond the scope of the present study, such analyses are in progress in our lab currently. However, one known major difference between normal control rats and SLOS rats is the presence of 7DHC in the retina and other bodily tissues in the latter (Fliesler et al., 1999, 2004; Vaughan et al., 2005). Hence, the possible oxysterol products generated in normal rats (where cholesterol is the only appreciable sterol) vs. SLOS rats are quite different, as are the biological properties of those molecules (e.g. cytotoxicity potential). As mentioned above, 7DHC is highly prone to oxidation (far more so than is cholesterol), and its oxysterol products are highly cytotoxic. Also, it is possible that 7DHC or its by-products may potentiate the oxidation of other lipids, such as polyunsaturated fatty acids (PUFAs, e.g. docosahexaenoic acid), or visa versa. PUFA concentrations are exceptionally high in ROS membranes (Fliesler and Anderson, 1983). It is interesting to note that cells in the inner nuclear layer (INL) appear relatively unaffected in all cases. Since the degeneration is predominantly restricted to the outer retina (photoreceptors and RPE), this would suggest that LPO generation also is largely restricted to the outer retina under these conditions, consistent with the relative enrichment of PUFAs in photoreceptors. These factors may explain, in part, the progressive retinal degeneration observed in SLOS rats and their profound sensitivity to intense light exposure, relative to normal albino rats.
Acknowledgments
Supported by U.S.P.H.S. grant EY07361, by the March of Dimes, by the Norman J. Stupp Foundation, and by an unrestricted departmental grant from Research to Prevent Blindness. Authorship of this manuscript conforms to the ICMJE’s Uniform Requirements for Manuscripts Submitted to Biomedical Journals. The authors gratefully acknowledge Dr Daniel T. Organisciak for graciously providing the light damage chamber used in this study.
References
- Asano T, Koide T, Gotoh O, Joshita H, Hanamura T, Shigeno T, Takakura K. The role of free radicals and eicosanoids in the pathogenetic mechanism underlying ischemic brain edema. Mol Chem Neuropathol. 1989;10:101–133. doi: 10.1007/BF03159717. [DOI] [PubMed] [Google Scholar]
- Boulton M, Rozanowska M, Rozanowski B. Retinal photo-damage. J Photochem Photobiol B. 2001;64:144–161. doi: 10.1016/s1011-1344(01)00227-5. [DOI] [PubMed] [Google Scholar]
- Correa-Cerro LS, Porter FD. 3β-Hydroxysterol Δ7-reductase and the Smith–Lemli–Opitz syndrome. Mol Genet Metab. 2005;84:112–126. doi: 10.1016/j.ymgme.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Dvornik D, Kraml M, Dubuc J, Givner M, Gaudry R. A novel mode of inhibition of cholesterol biosynthesis. J Am Chem Soc. 1963;85:3309. [Google Scholar]
- Fliesler SJ. Effects of cholesterol biosynthesis inhibitors on retinal development, structure, and function. In: Fliesler SJ, editor. Sterols and Oxysterols: Chemistry, Biology, and Pathobiology. Research Signpost; Trivandrum, India: 2002. pp. 77–109. [Google Scholar]
- Fliesler SJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Prog Lipid Res. 1983;22:79–131. doi: 10.1016/0163-7827(83)90004-8. [DOI] [PubMed] [Google Scholar]
- Fliesler SJ, Richards MJ, Miller C-Y, Peachey NS. Marked alteration of sterol metabolism and composition without compromising retinal development or function. Invest Ophthalmol Vis Sci. 1999;40:1792–1801. [PubMed] [Google Scholar]
- Fliesler SJ, Peachey NS, Richards MJ, Nagel BA, Vaughan DK. Retinal degeneration in a rodent model of Smith–Lemli–Opitz syndrome: electrophysiologic, biochemical, and morphologic features. Arch Ophthalmol. 2004;122:1190–1200. doi: 10.1001/archopht.122.8.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaoua W, Chevy F, Roux C, Wolf C. Oxidized derivatives of 7-dehydrocholesterol induce growth retardation in cultured rat embryos: a model for antenatal growth retardation in the Smith–Lemli–Opitz syndrome. J Lipid Res. 1999;40:456–463. [PubMed] [Google Scholar]
- Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res. 1998;39:1529–1542. [PubMed] [Google Scholar]
- Girotti AW. Cholesterol-derived hydroperoxides: generation and reactivity in biological systems. In: Fliesler SJ, editor. Sterols and Oxysterols: Chemistry, Biology, and Pathobiology. Research Signpost; Trivandrum, India: 2002. pp. 121–139. [Google Scholar]
- Givner ML, Dvornik D. Agents affecting lipid metabolism-XV. Biochemical studies with the cholesterol biosynthesis inhibitor AY-9944 in young and mature rats. Biochem Pharmacol. 1965;14:611–619. doi: 10.1016/0006-2952(65)90233-9. [DOI] [PubMed] [Google Scholar]
- Glickman RD. Phototoxicity to the retina: mechanisms of damage. Int J Toxicol. 2002;21:473–490. doi: 10.1080/10915810290169909. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Cross CE. Oxygen-derived species: their relation to human disease and environmental stress. Environ Health Perspect. 1994;102(Suppl 10):5–12. doi: 10.1289/ehp.94102s105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jira PE, Waterham HR, Wanders RJ, Smeitink JA, Sengers RC, Wevers RA. Smith-Lemli-opitz syndrome and the DHCR7 gene. Ann Hum Genet. 2003;67(Pt 3):269–280. doi: 10.1046/j.1469-1809.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- Kelley RI. Inborn errors of cholesterol biosynthesis. Adv Pediatr. 2000;47:1–53. [PubMed] [Google Scholar]
- Mihaljevic B, Barusin-Razem B, Razem D. The reevaluation of the ferric thiocyanate assay for lipid hydroperoxides with special considerations of the mechanism aspects of the response. Free Radic Biol Med. 1996;21:53–63. doi: 10.1016/0891-5849(95)02224-4. [DOI] [PubMed] [Google Scholar]
- Organisciak DT, Winkler BS. Retinal light damage: practical and theoretical considerations. Prog Retin Eye Res. 1994;13:1–29. [Google Scholar]
- Organisciak DT, Darrow RA, Jiang Y-L, Blanks JC. Retinal light damage in rats with altered levels of rod outer segment docosahexaenoate. Invest Ophthalmol Vis Sci. 1996;37:2243–2257. [PubMed] [Google Scholar]
- Organisciak DT, Darrow RA, Barsalou L, Darrow RM, Lininger LA. Light-induced damage in the retina: differential effects of dimethylthiourea on photoreceptor survival, apoptosis and DNA oxidation. Photochem Photobiol. 1999;70:261–268. [PubMed] [Google Scholar]
- Porter FD. RSH/Smith–Lemli–Opitz syndrome: a multiple congenital anomaly/mental retardation syndrome due to an inborn error of cholesterol biosynthesis. Mol Genet Metab. 2000;71:163–174. doi: 10.1006/mgme.2000.3069. [DOI] [PubMed] [Google Scholar]
- Porter FD. Human malformation syndromes due to inborn errors of cholesterol synthesis. Curr Opin Pediatr. 2003;15:607–613. doi: 10.1097/00008480-200312000-00011. [DOI] [PubMed] [Google Scholar]
- Rao N. Role of oxygen free radicals in retinal damage associated with experimental uveitis. Trans Am Ophthalmol Soc. 1990;88:797–850. [PMC free article] [PubMed] [Google Scholar]
- Roomi MW, Hopkins CY. Some reactions of sterculic and malvalic acids. A new source of malvalic acid. Can J Biochem. 1970;48:759–762. doi: 10.1139/o70-119. [DOI] [PubMed] [Google Scholar]
- Smith JD, Lemli L, Opitz JM. A newly recognized syndrome of multiple congenital anomalies. J Pediatr. 1964;64:210–217. doi: 10.1016/s0022-3476(64)80264-x. [DOI] [PubMed] [Google Scholar]
- Spiteller G. Lipid peroxidation in aging and age-dependent diseases. Exp Gerontol. 2001;36:1425–1457. doi: 10.1016/s0531-5565(01)00131-0. [DOI] [PubMed] [Google Scholar]
- Tint GS, Irons M, Elias ER, et al. Defective cholesterol biosynthesis associated with the Smith–Lemli–Opitz syndrome. N Engl J Med. 1994;330:107–113. doi: 10.1056/NEJM199401133300205. [DOI] [PubMed] [Google Scholar]
- Vaughan DK, Coulibaly SF, Darrow RM, Organisciak DT. A morphometric study of light-induced damage in transgenic rat models of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2003;44:848–855. doi: 10.1167/iovs.02-0709. [DOI] [PubMed] [Google Scholar]
- Vaughan DK, Peachey NS, Richards MJ, Buchan B, Fliesler SJ. Light-induced exacerbation of retinal degeneration in a rat model of Smith–Lemli–Opitz syndrome. Exp Eye Res. doi: 10.1016/j.exer.2005.08.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterham HR, Wanders RJ. Biochemical and genetic aspects of 7-dehydrocholesterol reductase and Smith–Lemli–Opitz syndrome. Biochim Biophys Acta. 2000;1529:340–356. doi: 10.1016/s1388-1981(00)00159-1. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Grimm C, Samardzija M, Reme CE. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res. 2005;24:275–306. doi: 10.1016/j.preteyeres.2004.08.002. [DOI] [PubMed] [Google Scholar]


