Abstract
There is a growing consensus that the various forms of cell death (necrosis, apoptosis and autophagy) are not separated by strict boundaries, but rather share molecular effectors and signaling routes. Among the latter, a clear role is played by calcium (Ca2+), the ubiquitous second messenger involved in the control of a broad variety of physiological events. Fine tuning of intracellular Ca2+ homeostasis by anti- and proapoptotic proteins shapes the Ca2+ signal to which mitochondria and other cellular effectors are exposed, and hence the efficiency of various cell death inducers. Here, we will review: (i) the evidence linking calcium homeostasis to the regulation of apoptotic, and more recently autophagic cell death, (ii) the discussion of mitochondria as a critical, although not unique checkpoint and (iii) the molecular and functional elucidation of ER/mitochondria contacts, corresponding to the mitochondria-associated membrane (MAM) subfraction and proposed to be a specialized signaling microdomain.
Keywords: cell death, Bcl-2, endoplasmic reticulum, autophagy, mitochondria-associated membranes (MAM)
Introduction
Apoptosis is an essential, genetically regulated and finely tuned process of cell elimination essential for embryogenesis, development and tissue homeostasis of multicellular organisms (Kerr et al., 1972). Apoptosis takes part in the normal development and functions of organisms as diverse as nematodes, insects or humans (Twomey and McCarthy, 2005). Dysregulation or impairment of apoptosis has deleterious consequences. In humans, pathological conditions such as neurodegenerative and autoimmune diseases, cancer or AIDS (Thompson, 1995; Hetts, 1998; Perry et al., 1998) have defective apoptosis in the pathogenetic route. Cell death by apoptosis is accompanied by a stereotyped and interconnected series of events that include cell collapse, formation of membrane blebs, chromatin condensation and DNA degradation. Selective degradation of intracellular substrates during apoptosis also occurs and it is mainly due to the activity of highly conserved cystein proteases, named caspases (for cysteinyl aspartate-specific proteinases; Alnemri et al., 1996; Nicholson and Thornberry, 1997). Caspases selectively cleave a set of about 100 targets, although some estimates reach a number of >200 (Nicholson, 1999).
It is well established that variations in cytosolic calcium concentration [Ca2+]c trigger key cellular functions, for example, contraction of myofilaments, secretion of hormones and neurotransmitters and modulation of metabolism, to cite a few (Berridge et al., 2003; Rizzuto and Pozzan, 2006; Clapham, 2007). Moreover, Ca2+ also has a major function in triggering mitotic division in numerous cell types (e.g., T lymphocytes and of oocytes) and, conversely, in the regulation of cell death (Giorgi et al., 2008). The notion that the cellular Ca2+ overload is highly toxic, causing massive activation of proteases and phospholipases was known to cell biologists since the late 1960s. Electron micrographs of clearly damaged cells showed swollen mitochondria full of Ca2+ phosphate precipitates in the 1960s and 1970s and the toxicity of Ca2+ ionophores in cultured cells was one of the first effects of these molecules to be described (Pressman, 1976; Fariss et al., 1985). Classically, this toxic role of Ca2+ has been associated to necrosis, that is, the catastrophic derangement of cell integrity and function following the exposure to different types of cell injury and leading to the activation of Ca2+-activated hydrolysing enzymes. Typical examples are complement-induced cell death and excitotoxicity, in which glutamate-dependent hyperstimulation leads neurons to the necrotic death (Budd and Nicholls, 1996; Nicotera and Orrenius, 1998).
More recent data, however, have suggested a function of Ca2+ also in the regulation of other types of cell death. Several studies have shown that the increases of [Ca2+]c occur, both at early and late stages of the apoptotic pathway (Kruman et al., 1998; Tombal et al., 1999; Lynch et al., 2000) and both Ca2+ release from the endoplasmic reticulum (ER) and capacitative Ca2+ influx through Ca2+ release-activated Ca2+ channels have been proposed to be apoptogenic (Pinton and Rizzuto, 2006). Thus, a common view is that while severe Ca2+ dysregulation can promote cell death through necrosis, more controlled intracellular [Ca2+] increases induced by milder insults promote cell death through apoptosis.
The question obviously arises as to how a single-signaling molecule can trigger, often in the same cell type, so vastly the different functions. The key to solving this puzzle is presumably in the unique physicochemical characteristics of Ca2+ and its capacity to establish local concentrations within cells, that in turn form a variety of recognizable signatures corresponding to specific functional effects. Indeed, a unique characteristics of Ca2+ within the cell cytoplasm is its low rate of diffusion (compared with the other classical second messengers, cyclic AMP, cyclic GMP and inositol 3-phosphate (IP3), diffusion of Ca2+ is over 100-fold slower), due to the presence of a variety of binding sites (Rizzuto and Pozzan, 2006). Also, in distinction to the other messengers, several organelles disseminated throughout the cell can sequester Ca2+ and, in response to appropriate signals, release it back into the cytoplasm. These conditions are ideal for the generation of subcellular heterogeneities in [Ca2+], for example, in the proximity of plasma membrane or organelle Ca2+ channels. The existence of subcellular domains in which [Ca2+] largely exceeds the bulk cytosolic values have been postulated for a long time, but only in the last decade, these values have been directly measured and their primary function in controlling some cell functions, beyond the classical function in promoting neurosecretion at the presynaptic membrane, has become evident (Rizzuto and Pozzan, 2006).
The aim of this review is to present and discuss the available data that link alterations in intracellular Ca2+ homeostasis to various stages of the normal or altered apoptotic signaling cascade. There are many aspects of apoptosis itself that will not be covered here; interested readers are directed to other chapters in this special issue.
The ER calcium concentration modulates the sensitivity to apoptosis
A clear impetus in the study of Ca2+ homeostasis in apoptosis came from the observation that important regulatory of apoptosis, the proteins of the Bcl-2 family, are localized in organelles deeply involved in Ca2+ handling (the mitochondria and the ER). Indeed, Bcl-2 has been detected in association with the outer mitochondrial membrane, with the ER and with the nucleus and a cytoplasmic form of Bcl-2 is also known to exist (Pinton and Rizzuto, 2006). Although most investigators agree with the idea that only Bcl-2 bound to membranes is involved in inhibiting cell death, the mechanism, the importance and function of Bcl-2 in different cellular locations are still a matter of controversy.
The first association between Bcl-2 and Ca2+ homeostasis dates back to 1993, when Bcl-2 overexpression was shown to prevent the reduction in the Ca2+ concentration of the ER ([Ca2+]ER) that was observed upon the withdrawal of interleukin-3 in hematopoietic cell lines (Baffy et al., 1993). This effect was not secondary to the antiapoptotic effect of Bcl-2 (e.g., preventing the loss of energy and thus of ER Ca2+; during apoptosis), as Bcl-2 overexpression was also reported to decrease the size of the ER Ca2+ released (Lam et al., 1994). These observations were further expanded into a comprehensive picture where targeted probes allowed a detailed subcellular analysis of Ca2+ homeostasis and the complex signals controlling mitochondrial participation at least partially unveiled. In these experiments, an ER-targeted aequorin chimera (Pinton et al., 2007b) was transiently coexpressed with Bcl-2 in HeLa cells. No toxicity due to Bcl-2 transient overexpression was observed, in distinction to the experiments in which a Bcl-2-GFP chimera was used (Wang et al., 2001). Rather, as expected, the cells overexpressing Bcl-2 displayed an enhanced survival upon ceramide treatment (Pinton et al., 2001b), in agreement with previous reports (Zhang et al., 1996; Rippo et al., 2000) Most strikingly, compared with controls, Bcl-2 overexpressing cells showed a ~30% reduction in the Ca2+ levels within both the ER and the Golgi apparatus. As a consequence, the [Ca2+] increases elicited in these cells by stimuli coupled to IP3 generation were reduced both in the cytoplasm and in the mitochondria (Pinton et al., 2000).
The capacity of antiapoptotic proteins to reduce [Ca2+]ER described in these initial studies was later confirmed and expanded in studies that employed other approaches and genetically altered cell models (Foyouzi-Youssefi et al., 2000; Palmer et al., 2004). Moreover, Korsmeyer and collegues (Danial and Korsmeyer, 2004), demonstrated not only that embryonic fibroblasts from mice (MEFs) in which the genes for the proapoptotic members of the Bcl-2 family Bax and Bak had both been deleted (double knockout MEFs) showed a major reduction in [Ca2+]ER, but also that silencing of Bcl-2 in these cells partially restored [Ca2+]ER values to control levels. These authors also showed that double knockout MEFs are markedly resistant to a variety of apoptotic stimuli (Scorrano et al., 2003). Altogether, these data support the hypothesis that Ca2+ movement from the ER to mitochondria is a key process in some apoptotic routes.
In support of this possibility, we then showed that early after overexpression of Bax in HeLa cells, [Ca2+]ER levels are higher than in controls, whereas at later stages (during progression into apoptosis), the difference from control cells becomes virtually undetectable (Chami et al., 2004). Finally, Tsien and colleagues (Palmer et al., 2004) showed that the green tea compound epigallocatechin gallate, known to bind and inactivate Bcl-2, reduced Ca2+ leakage from the ER and restored [Ca2+]ER of Bcl-2 overexpressing cells to values similar to those of control cells.
Conceptually, similar data were also obtained with other unrelated antiapoptotic proteins. The most striking example was provided by an oncogene expressed in a human hepatocarcinoma. This oncogene is generated by the integration of the hepatitis B virus genome in the gene encoding the protein SERCA1 (sarco–endoplasmic reticulum Ca2+ ATPase type 1). The viral activation was shown to cis-activate SERCA1 chimeric transcripts with splicing of exons 4 and/or 11. Splicing of exon 11 creates a frameshift and a premature stop codon in exon 12. The encoded protein lacks most of the cytosolic N and P domains and critical Ca2+-binding regions of the transmembrane region. This protein is incapable of active Ca2+ pumping (Chami et al., 2000) and is causally involved in the neoplastic phenotype. Although the molecular mechanism of this effect has not been elucidated yet, it may be speculated that the mutated SERCA could either interfere with the activity of endogenous pumps and/or could act as a Ca2+ leak pathway from the ER. These data are consistent with the observations that the overexpression of SERCA in HeLa cells increases the susceptibility of cells to apoptotic agents (Ma et al., 1999; Pinton et al., 2001a). This notion was further reinforced by the study of the antiapoptotic coxsackie viral protein 2B that was shown to reduce ER Ca2+ levels (Campanella et al., 2004).
In this context, the observation that Bcl-2 also downregulates Ca2+ influx through the plasma membrane is not surprising. In principle, depletion of the ER Ca2+ store could lead to the activation of capacitative Ca2+ influx (Putney, 1990), and thus induce a prolonged [Ca2+]c elevation (i.e., a potentiation of Ca2+-mediated effects on apoptosis). Indeed, ER depletion of comparable degree was reported to cause a substantial activation (over 50%) of store-dependent Ca2+ influx (Hofer et al., 1998). Conversely, Bcl-2 expression markedly reduces the [Ca2+]c increase induced by Ca2+ readdition to cells in which the Ca2+ store had been fully depleted by SERCA blockers (Pinton et al., 2000). This effect, that may represent a long-term adaptation to lower [Ca2+]ER levels, is most likely due to the reduction of the number of functional channels in the plasma membrane (Vanden Abeele et al., 2002). In analysing the mechanism of neuroendocrine differentiation (that incidentally is a common hallmark of a variety of carcinomas), Vanoverberghe et al. (2004) showed that in LNCaP cells (androgen-sensitive human prostate adenocarcinoma cells) the same Ca2+ signaling alteration (partial ER depletion and reduction of the capacitative Ca2+ current) was observed upon Bcl-2 expression and upon the induction of neuroendocrine differentiation, although in the latter case, different molecular mechanisms may be operative.
Different [Ca2+]ER levels imply a varied amount of Ca2+ that can be released into the cytosol, but also a different regulation of Ca2+-sensitive luminal processes of the ER (such as protein post-translational modifications and sorting). To verify which is the real target of the signaling modulation, we and others have acted on the luminal buffer, the low-affinity Ca2+-binding protein calreticulin and demonstrated that the protective effect depends on the decrease of the releasable Ca2+ pool. Indeed, in calreticulin overexpressing cells, in which the amplitude and duration of Ca2+ signals from ER lumen toward cytosol are enhanced (Bastianutto et al., 1995) without changing [Ca2+]ER (Xu et al., 2001) cell viability is drastically reduced upon ceramide treatment (Pinton et al., 2001b). Conversely, cell lines derived from calreticulin knockouts, that show a marked decrease in ER Ca2+ release upon cell stimulation, are more resistant to apoptosis (Nakamura et al., 2000).
What is the role of mitochondria in this scenario?
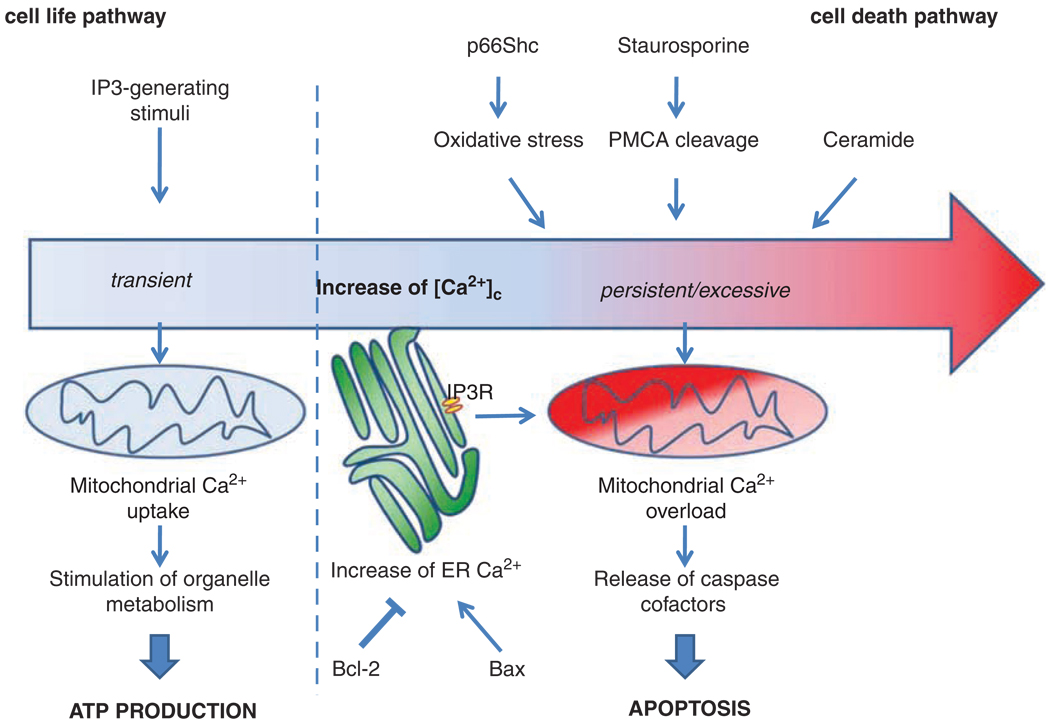
Mitochondria are the paradigm of the double-sworded effect of Ca2+ on cell life and death (Figure 1). On the one hand, work by us and other groups has shown that despite the low affinity of mitochondrial Ca2+ transporters, large Ca2+ fluxes occur across the mitochondrial membranes when a physiological stimulus elicits a [Ca2+]c rise, because these organelles are not exposed to the (lower) bulk [Ca2+]c increase, but to microdomains generated in the proximity of the open Ca2+ channels. In other words, the strategic location of mitochondria close to the source of the [Ca2+]c rise (the ER and/or the plasma membrane) allows them to be exposed to [Ca2+] that meet the affinity of their transporters and allows the rapid and large accumulation of the cation in the matrix (Pinton et al., 1998). In turn, this accumulation has an important physiological function: by stimulating intramitochondrial effectors (such as the Ca2+-dependent dehydrogenases of the Krebs cycle), it allows the prompt tuning of organelle metabolism (and hence ATP production) to the increased needs of an activated cell (Jouaville et al., 1999). Interestingly, recent works indicate that other Ca2+-dependent metabolic checkpoints are operative. Namely, the aspartate/glutamate metabolite carriers were shown to be activated by Ca2+ and in turn, recombinant expression of wild-type aspartate/glutamate metabolite carriers enhanced ATP production upon cell stimulation (Lasorsa et al., 2003). Different mechanisms can finely tune amplitude and kinetics of the mitochondrial Ca2+ responses. For example, Ca2+ uptake can be increased or decreased by protein kinases (PKs), such as protein PKC (protein kinase C; Pinton et al., 2004) or p38 mitogen-activated PKs (Montero et al., 2002).
Figure 1.
Differential decoding of Ca2+-linked stimuli evoking the activation of cell metabolism or apoptosis.
At the same time, mitochondria are important checkpoints of the apoptotic process, as they may release caspase cofactors (Kroemer et al., 2007). Indeed, the apoptotic intrinsic pathway is activated by the release of several mitochondrial proteins into the cytosol. The main player in the finely tuned apoptotic activation process is undoubtedly cytochrome c. The majority of cytochrome c is tightly bound to mitochondrial inner membrane, thanks to its electrostatic interactions with acidic phospholipids, but a small fraction probably exists loosely attached to inner mitochondrial membrane and available for mobilization. This protein is an irreplaceable component of the mitochondrial electron transport chain, shuttling electrons from complexes III to IV, and is thus essential to life: the disruption of its only gene is embryonic lethal (Garrido et al., 2006). Once released in the cytoplasm, this protein drives the assembly of a caspases activating complex together with Apaf-1 (apoptosis–protease activating factor 1) and caspase 9, the so-called ‘apoptosome’. Cytochrome c, once in the cytosol, induces the rearrangement and heptaoligomerization of Apaf-1: each of these complexes can recruit up to seven caspase molecules, leading to their proteolytic self-processing and consequent activation (Hill et al., 2003).
Mitochondria contain several other proapoptotic, intermembrane space-resident proteins, such as Smac/DIABLO, HtrA2/Omi, AIF and EndoG. DIABLO (direct inhibitor of apoptosis-binding protein with a low isoelectric point) and HtrA2 (high temperature requirement protein A2) both have an N-terminal domain that can interact and inhibit IAPs (inhibitor of apoptosis proteins). IAPs, such as XIAP, cIAP-1 and cIAP-2, are cytosolic soluble peptides that normally associate and stabilize procaspases, thus preventing their activation. Conversely, apoptosis-inducing factor and EndoG (endonuclease G) translocate from intermembrane space to the nucleus upon treatment with several apoptotic stimuli where they seem to mediate chromatin condensation and DNA fragmentation (Ravagnan et al., 2002).
We and others thus verified whether Ca2+ was involved in regulating mitochondrial morphology and release of proapoptotic proteins. In HeLa cells upon ceramide treatment, we observed Ca2+ release from the ER and loading into mitochondria. As a consequence, organelle swelling and fragmentation were detected that were paralleled by the release of cytochrome c. These changes were prevented by Bcl-2 expression as well as experimental conditions that lowered [Ca2+]ER (Pinton et al., 2001b). Mitochondrial permeability transition pore (mPTP: a large conductance channel that opens through a conformational change of its still debated protein components) opening in ceramide-dependent apoptosis was directly demonstrated by Hajnoczky and colleagues (Szalai et al., 1999) who could demonstrate that the lipid mediator facilitates PTP opening. In this case, ceramide acts as a ‘mitochondrial sensitizer’ that transforms physiological IP3-mediated Ca2+ signals into inducers of apoptosis.
The above-described intrinsic pathway of apoptosis is controlled by the Bcl-2 protein family. Proapoptotic Bax and Bak proteins exist as inactive monomers in viable cells with Bax localizing in the cytosol, loosely attached to membranes, and Bak residing in mitochondrial fraction. Upon apoptosis induction, Bax translocates to mitochondria where it homooligomerizes and inserts in the outer membrane; similarly, also Bak undergoes a conformational change, which induces its oligomerization at the outer mitochondrial membrane. Together, these events trigger mitochondrial outer membrane permeabilization, the crucial process mediating the release of intermembrane space-resident caspase cofactors into the cytoplasm (Danial and Korsmeyer, 2004).
Mitochondria also undergo a more ‘macroscopic’ remodeling of their shape during the programed cell death. Indeed, after apoptosis induction, mitochondria become largely fragmented, resulting in small, rounded and numerous organelles. This process occurs quite early in apoptotic cell death, soon after Bax/Bak oligomerization, but before caspase activation. Interestingly, the perturbation of the equilibrium between fusion and fission rates seems to correlate with cell death sensitivity. In particular, conditions in which mitochondrial fission is inhibited, such as DRP1 (dynamin-like protein 1) downregulation or mitofusins overexpression, strongly delay caspase activation and cell death induced by numerous stimuli. Similarly, stimulation of organelle fission (by DRP1 overexpression or Mfn1/2 and OPA1 inhibition) promotes apoptosis by facilitating cytochrome c release and apoptosome assembly (Youle and Karbowski, 2005). However, the relationship between mitochondrial fusion/fission and apoptosis is complex and mitochondrial fragmentation is not necessarily related to apoptosis. Indeed, mitochondrial fission per se does not increase cell death and DRP1 overexpression has been reported to protect cells from some apoptotic challenges, such those dependent on mitochondrial Ca2+ overload (Szabadkai et al., 2004).
Another hallmark of apoptosis is the loss of mitochondrial membrane potential, secondary to the opening of mPTP triggered by different pathological conditions (e.g., Ca2+ overload, ATP depletion, oxidative stress, high inorganic phosphate or fatty acid). The molecular structure of this pore is currently highly debated, but the main players in mPTP assembly seem to include the adenine nucleotide transporter (ANT) in the inner membrane, the voltage-dependent anion channel (VDAC), the peripheral benzodiazepine receptor in the outer membrane and cyclophilin D, a matrix protein (Bernardi et al., 2006). The availability of chemical mPTP inhibitors such as cyclosporine A and related compounds lacking the cytosolic inhibitory effect on calcineurin, as well as the development of cyclophilin D knockout mouse will help to clarify the role of mPTP in physiological and pathological condition and identify areas of pharmacological intervention in common disorders such as ischemia-reperfusion injury, liver diseases, neurodegenerative and muscle disorders (Baines et al., 2005; Basso et al., 2005; Nakagawa et al., 2005).
Interestingly, some of the proposed components of the mPTP participate in Ca2+ homeostasis. Indeed, transient expression of VDAC enhanced the amplitude of the agonist-dependent increases in mitochondrial matrix Ca2+ concentration by allowing the fast diffusion of Ca2+ from ER release sites to the inner mitochondrial membrane. As to the functional consequences, VDAC overexpressing cells are more susceptible to ceramide-induced cell death, thus confirming that mitochondrial Ca2+ uptake has a key function in the process of apoptosis (Rapizzi et al., 2002). ANT overexpression instead reduced the amplitude of the [Ca2+]m peak following ER Ca2+ release, and this effect was partially reversed by treating the cells with cyclosporine A, suggesting the involvement of mPTP in ER-mitochondria Ca2+ transfer (Wieckowski et al., 2006).
Moreover, mitochondria are quantitatively the most important source of intracellular reactive oxygen species and leak from the electron transfer chain is supposed to be the main route (Turrens, 2003). Recently, a totally new, unexpected pathway has emerged that involves p66Shc in mitochondrial reactive oxygen species production. Intriguingly, upon phosphorylation by PKCβ and peptidyl–prolyl cis/trans isomerase (Pin1) recognition, p66shc translocates to mitochondria (Pinton et al., 2007a) where it exerts its own oxidoreductase activity (Giorgio et al.., 2005). As a consequence, p66shc directly oxidizes cytochrome c (thus allowing electron to escape mitochondrial electron transport chain) and generates H2O2, leading to mPTP opening and in turn cell death. The existence of a protein that ‘steals’ electrons from the mitochondrial electron transport chain and produces reactive oxygen species provides direct evidence for the role of reactive oxygen species in signal transduction, that may represent the biochemical basis of the free radical theory of ageing (Pinton and Rizzuto, 2008).
Cytosolic players
Important as mitochondria may be, the role of Ca2+ in the control of the apoptotic process is by no means limited to these organelles. Indeed, the cytoplasm is endowed with numerous effectors that can efficiently decode an extracellular signal into the induction of apoptosis in a Ca2+-dependent manner. Multiple signaling cascades—critical for cell survival, differentiation or degeneration—are mediated by [Ca2+]c (Pozzan et al., 1994; Berridge et al., 2000). The signaling process in all these phenomena is dependent on the concerted activities of many intracellular factors, including PKs, phospholipases, proteases and endonucleases, and the coordinate regulation of these factors has a fundamental role in decoding the extracellular signal into the ultimate cellular event. This molecular machinery exhibits a large complexity and partial redundancy (most of the elements occur in different isoforms, with specific recruitment routes and substrate specificities) and the overall picture is far from being clarified. Thus, a detailed evaluation of the role of the various cytosolic Ca2+ effectors in apoptosis would be too lengthy to be included in a short review and, at the same time, largely incomplete. We will just focus on highlighting the possible mechanisms of action of cytosolic Ca2+ effectors, by reviewing a few possible checkpoints that received much attention in the recent years.
The signaling cascade: kinases and phosphatases
Among the various kinases directly or indirectly activated by Ca2+ signals, the PKC family has been proposed to play an important role in the Ca2+-mediated signaling of apoptosis. The term PKC identifies a family of phospholipid-dependent serine/threonine kinases that are activated by diverse intracellular factors, including diacylglycerol and Ca2+ (Parker and Murray-Rust, 2004).
Protein kinases C can have a dual function in apoptosis, that is, the activation of specific PKC isoforms may protect or induce cell death, often in a cell-type-specific manner (Lavin et al., 1996; Liu et al., 2002; Griner and Kazanietz, 2007).
In the signaling routes of apoptosis, also the Ca2+-dependent phosphatases appear to play an important role. In particular, various apoptotic routes share the activation of the Ca2+-dependent serine–threonine phosphatase calcineurin through a process blocked by Bcl-2 (Shibasaki and McKeon, 1995). In this case, defined intracellular targets of utmost importance in apoptosis have been identified: calcineurin dephosphorylates and activates the Bad protein (a proapoptotic member of the Bcl-2 family), thus enhancing its heterodimerization with Bcl-XL and promoting apoptosis (Wang et al., 1999).
The intracellular proteases
As mentioned above, many biochemical and genetic studies on apoptosis have revealed that intracellular proteases are key players in this process. In particular, early studies have pointed to the primacy of caspase proteases as mediators of the execution phase. More recent evidence, however, supports the idea that proteases other than caspases participate in apoptosis, in particular the family of Ca2+-dependent proteases known as calpains. Indeed, the most obvious direct link between [Ca2+]c elevations and the proteolysis of cellular targets (the paradigm of apoptosis) is through the activation of these cysteine proteases that are synthesized as inactive proenzymes and activated by an autocatalytic cleavage triggered by Ca2+. The family of proteins includes isozymes with different distribution (including ubiquitous and tissue-specific isoforms) and Ca2+ affinity (ranging from micromolar, for the μ-calpains, to millimolar, for the m-calpain levels; Carafoli and Molinari, 1998). Activation of calpains, that can be triggered by various pathophysiological stimuli, has a direct impact on the execution of apoptosis as calpains have been shown to cleave key elements in the apoptotic machinery, such as members of the Bcl-2 family, for example, Bcl-XL (Nakagawa and Yuan, 2000) or Bid (Mandic et al., 2002), caspase-12 (Nakagawa et al., 2000) and the XIAP (X-linked inhibitor of apoptosis) (Kobayashi et al., 2002). Parenthetically, in monocyte/macrophage cells, Ca2+ signaling is involved in nuclear factor-κB activation through the activation of calpain. Calpain inhibitors may thus be effective in inhibiting the activation of latently infected human immunodeficiency virus (Teranishi et al., 2003). An important role for calpains in the apoptotic process is also provided by human genetic disorders of skeletal muscle. The concentration of ubiquitous calpains increases in Duchenne muscular dystrophy, and null mutations of muscle-specific calpain (calpain 3) cause a form of limb-girdle muscular dystrophy (Tidball and Spencer, 2000), thus highlighting both the importance of these proteins in muscle cell death and their complex interplay.
At the same time, the main factors of the apoptotic proteolytic cascades, the caspases, have been drawn to the Ca2+ field. The first link described between caspases and Ca2+ homeostasis has been the demonstration of the Ca2+ sensitivity of a member of the caspase protease family, caspase-12. Caspase-12 is localized in the ER (Nakagawa et al., 2000) and has been reported to be activated when the ER undergoes stress (including disruption of ER Ca2+ homeostasis and accumulation of excess proteins in ER), but not by membrane- or mitochondrial-targeted apoptotic signals. Caspase-12 thus participates in the ER stress-induced apoptosis pathway (Yoneda et al., 2001).
Finally a ‘two-hit’ model for cadmium-induced apoptosis has been recently proposed. On the one side, cadmium directly or indirectly damages mitochondria, thus mediating cytochrome c release and caspase-9 activation. On the other side, cadmium-induced release of Ca2+ from ER stores and cadmium-mediated inhibition of SERCA pumps are proposed to cause a generalized alteration of Ca2+ homeostasis. The disruption in ER calcium homeostasis compromises the ER compartment, thus inducing ER stress and ER-mediated apoptosis through caspase 12 (Biagioli et al., 2008).
Proteolyzing the Ca2+ signaling machinery: an apoptotic strategy
Along with nuclear and cytoskeletal damage, disruption of cell signaling and ion homeostasis could warrant irreversibility of the cell’s commitment to death. In this respect, it is not surprising that amplification loops also involve a pleiotropic signaling route, such as that mediated by Ca2+ ions, although only very recent work has clarified molecular targets and cellular consequences.
Various components of the Ca2+ signaling machinery have been described to be cleaved by caspases, with potentially different cellular consequences. IP3 receptor type 1 (IP3R-1) has been identified as a caspase-3 substrate. Caspase-3 dependent IP3R-1 cleavage results in the inhibition of IP3-induced Ca2+ release activity. Given that Ca2+ release may act as a potentiation loop of apoptosis (Hirota et al., 1999), such an effect could represent a negative feedback mechanism. Along the same lines, Ca2+-permeable glutamate receptors of the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) subtype have also been described to be a target of caspase in neuronal apoptosis and Alzheimer’s disease (Chan and Mattson, 1999). Their inactivation would avoid excitotoxicity and Ca2+ overload in neurons destined to apoptosis (Glazner et al., 2000).
More recently, caspase-dependent cleavage of plasma membrane Ca2+ ATPase (PMCA), the most effective route allowing the rapid return of [Ca2+]c to basal levels (Camello et al., 1996; Brini et al., 2000), has also been described (Schwab et al., 2002); both the neuron-specific PMCA2 and the ubiquitous PMCA4 isoforms are cleaved by caspases. While PMCA2 is cleaved in vivo following brain ischemia and in neurons undergoing apoptosis after excitotoxic stimulation, PMCA4 is cleaved in non-neuronal cells induced to die by apoptosis by staurosporine. As a consequence, PMCA cleavage results in loss of function and aberrant intracellular Ca2+ transients (Schwab et al., 2002). Along the same lines, also the type 1 Na+/Ca2+ transporter (NCX) type 1 is cleaved by caspase-3 in cerebellar granule neurons undergoing apoptosis (Bano et al., 2005). Our own work revealed a similar mechanism in a radically different model of cell death, that is, that triggered in hepatic cells by the expression of hepatitis B virus X protein. Elevations of [Ca2+]c signals in cells overexpressing hepatitis B virus X protein trigger cell death due to caspase-3-dependent cleavage and inactivation of PMCA4 (Schwab et al., 2002; Chami et al., 2003). Amplification of the cytosolic Ca2+ signals through the impairment of Ca2+ pumps is not entirely surprising, if one takes into account the functional properties of the Ca2+ release and uptake mechanisms. Indeed, while an increased Ca2+ filling of intracellular stores does not enhance Ca2+ release, due to the Ca2+ feedback inhibition on the IP3R, impairment of PMCA is highly effective at increasing [Ca2+]c (Brini et al., 2000). As to the functional consequences, this alteration of Ca2+ signaling may represent a powerful potentiation loop, facilitating the rapid commitment of cells to death.
The endoplasmic reticulum-mitochondria cross-talk
The ER controls multiple cellular processes including translocation of soluble and membrane proteins into the secretory pathway, detoxification of metabolites and biosynthesis of lipids. The ER also serves as the principal internal store of calcium ions that mediate signaling, ATP production and apoptosis (Voeltz et al., 2002).
High-resolution 3D electron tomography reveals that the extended ER network forms close contacts with each of these secretory pathway compartments and with the mitochondria (Marsh et al., 2001). Indeed, as much as 20% of the mitochondrial surface is in direct contact with the ER, underlining the dynamic and highly regulated communication between the ER and mitochondria (Rizzuto et al., 1998). The close contacts formed between the ER and mitochondria have led to the model that ER-mitochondria communication may occur by direct transfer rather than vesicular traffic. In support of this model, biochemical studies reveal that the ER also communicates with mitochondria through mitochondria-associated membranes (MAMs) (Vance, 1990). These ER-contiguous membranes that contain multiple phospholipid- and glycosphingolipid-synthesizing enzymes, including fatty acid-CoA ligase 4 and phosphatidylserine synthase-1, and support direct transfer of lipids between the ER and mitochondria (Piccini et al., 1998; Stone and Vance, 2000).
In addition to supporting lipid transfer, the apposed ER and mitochondria also exchange Ca2+ ions, which regulate the processes ranging from ER chaperone-assisted folding of newly synthesized proteins to the regulation of mitochondria-localized dehydrogenases involved in ATP-producing Kreb’s cycle reactions and the activation of calcium-dependent enzymes that execute cell death programs (Berridge, 2002; Rimessi et al., 2008).
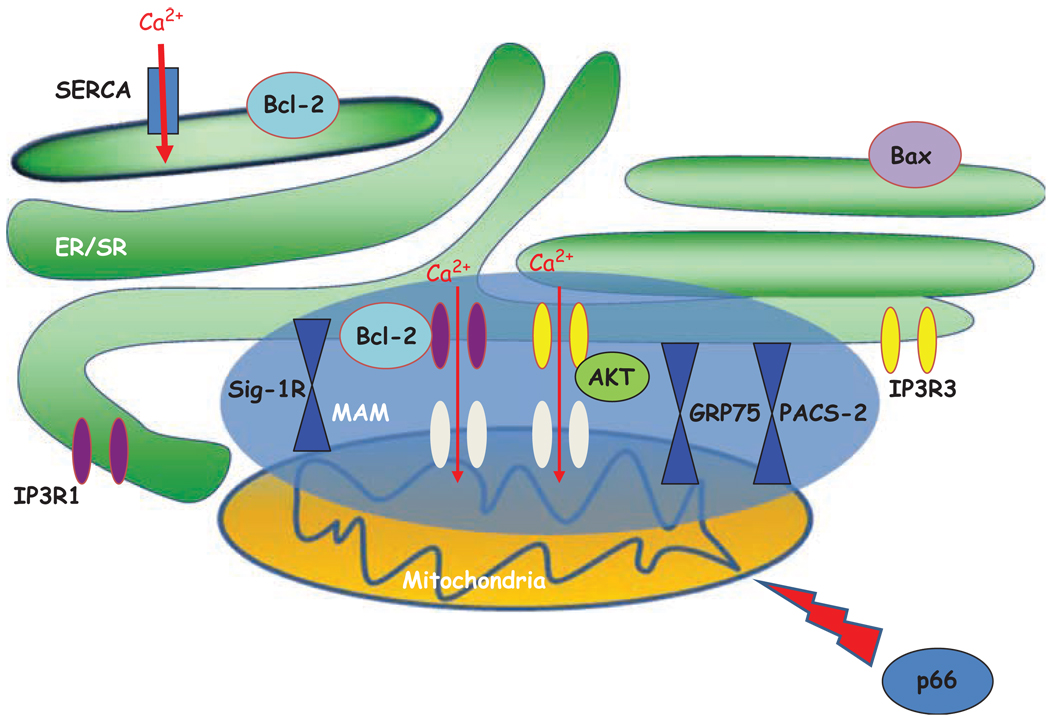
Mitochondria-associated membrane contains key Ca2+ handling proteins and Ca2+ sensing ER chaperones (Figure 2) that may participate in the fine-tuning of cellular Ca2+ signals. Specifically, Hayashi and Su (Hayashi and Su, 2007) reported that sigma-1 receptor acts as a novel ‘ligand-operated’ chaperone that specifically targets MAM. Interestingly, they found that sigma-1 receptors form a Ca2+ sensitive chaperone machinery with BiP and prolong Ca2+ signaling from ER into mitochondria by stabilizing IP3R-3s at MAM. This constitutes the first report of an ER chaperone influencing mitochondrial Ca2+ signaling from the side of the ER lumen.
Figure 2.
Mitochondria-associated membrane (MAM) machinery in cell survival and cell death: proteins involved in mitochondrial and reticular Ca2+ homeostasis and in MAM structure. SERCA: sarco–endoplasmic reticulum Ca2+ ATPase; IP3R-1: inositol 3 phosphate receptor type 1; IP3R-1: inositol 3 phosphate receptor type 3; Sig1R: σ1 receptor (reticular chaperone); GRP75: glucose-regulated protein 75 (mitochondrial chaperone); PACS-2: molecular chaperone that links ER-mitochondrial axis; p66: 66-kDa isoform of the growth factor adapter Shc.
In a recent study, we found that the mitochondrial chaperone grp75 regulates IP3R-mediated mitochondrial Ca2+ signaling (Szabadkai et al., 2006). In particular, we demonstrated that isoform 1 of VDAC is physically linked to the ER Ca2+-release channel IP3R through grp75, highlighting chaperone-mediated conformational coupling between the IP3R and the mitochondrial Ca2+ uptake machinery.
In addition, Simmen et al.(Simmen et al., 2005) demonstrated that PACS-2 is a multifunctional sorting protein that controls the ER-mitochondria axis and the role of this axis in cellular homeostasis and apoptosis. They showed that PACS-2 is required for the intimate association of mitochondria with the ER: PACS-2 depletion induces mitochondria fragmentation and uncouples this organelle from the ER raising the possibility that, in addition to mediating MAM formation, PACS-2 might also influence ER folding and calcium homeostasis (Simmen et al., 2005). Immunocytochemical studies show that regions of the ER apposed to mitochondria are enriched with IP3 receptors, identifying these zones as ‘hotspots’ of calcium transfer from the ER to the mitochondria (Rizzuto and Pozzan, 2006).
Mitochondria and ER appear thus physically and physiologically coupled, and this has a profound functional significance (Hajnoczky et al., 1995; Rizzuto et al., 1998). Regarding cell death, the release of Ca2+ from ER stores by IP3Rs has been implicated in multiple models of apoptosis as being directly responsible for mitochondrial calcium overload (Pinton et al., 2002; Hajnoczky et al., 2003), due in part to the privileged communication of the IP3R with closely adjacent mitochondria (Csordas et al., 1999). Indeed, it is becoming increasingly appreciated that ER-mitochondrial calcium signaling is crucial in several models of apoptosis (Demaurex and Distelhorst, 2003; Orrenius et al., 2003; Rizzuto et al., 2003; Scorrano et al., 2003). The requirement of IP3R for calcium-dependent cell death is exemplified by the resistance to apoptosis of cells with antisense knockdown or genetic deletion of IP3R gene (Khan et al., 1996; Jayaraman and Marks, 1997; Sugawara et al., 1997; Blackshaw et al., 2000). In this picture, the three isoforms of the IP3R appear to play distinct roles (Hirota et al., 1999; Assefa et al., 2004). Initial evidence suggested that Ca2+-dependent apoptotic death was mediated by the type 3 IP3R (Khan et al., 1996), but subsequent studies have shown that the type 1 isoform can also mediate apoptosis (Hirota et al., 1999; Boehning et al., 2003; Assefa et al., 2004). Interestingly, Korsmeyer’s group found that Bcl-2 and IP3R-1 physically interact at the ER surface and proposed a model in which Bcl-2 family members regulate IP3R-1 phosphorylation to control the rate of ER Ca2+ leak from intracellular stores and as consequence, apoptosis. In support of this view, the [Ca2+]ER reduction of Bax/Bak knockouts was reversed by siRNA silencing of IP3R-1 (Oakes et al., 2005).
Additional mechanisms, however, have been proposed. Bcl-XL was shown to directly bind to the IP3R and sensitize it to low agonist doses. Bax prevents the effect of Bcl-XL, both in terms of its binding to the IP3R and of capacity of modifying the sensitivity to IP3 (White et al., 2005). Expression of Bcl-XL reduced [Ca2+]ER in type 3 but not type 1 or 2 IP3R-expressing cells. In contrast, Bcl-XL enhanced spontaneous [Ca2+]c signaling in all three IP3R isoform-expressing cell lines. These results suggest that the modulation of [Ca2+]ER is not a specific requirement for ER-dependent antiapoptotic effects of Bcl-XL. Rather, apoptosis protection is conferred by enhanced spontaneous [Ca2+]c signaling by Bcl-XL interaction with all isoforms of the IP3R (Li et al., 2007).
In this complex scenario, recent data show that type 3 IP3Rs, localized in the MAM, have a selective function in the induction of apoptosis by preferentially transmitting apoptotic Ca2+ signals into mitochondria, whereas type 1 IP3Rs predominantly mediate cytosolic Ca2+ mobilization (Mendes et al., 2005). Accordingly, siRNA silencing of IP3R-3-blocked apoptosis, whereas transfection of IP3R-1 antisense constructs was ineffective (Blackshaw et al., 2000). Mitochondria appear to be the downstream effectors of this pathway, as knockdown of IP3R-3 significantly decreased agonist-induced mitochondrial Ca2+ uptake (Hayashi and Su, 2007).
A final crucial aspect is that, in response to survival signals, Akt/PKB interacts with and phosphorylates IP3Rs, significantly reducing their Ca2+ release activity (Khan et al., 2006; Szado et al., 2008). Moreover, phosphorylation of IP3Rs by PKB reduced cellular sensitivity to apoptotic stimuli through a mechanism that involved diminished Ca2+ flux from the ER to the mitochondria. In particular, Joseph and colleagues (Khan et al., 2006) demonstrated that all three isoforms present a consensus sequence for phosphorylation by AKT kinase and that IP3R-1 and IP3R-3 are substrate for activated AKT in vivo, but IP3R-1 phosphorylation did not affect Ca2+ homeostasis. IP3R-3 appears thus as a likely effector of the antiapoptic activity of AKT. The elucidation of the role of IP3R-3 in Ca2+ transfer from the ER to mitochondria, of its molecular mechanism and of the regulatory effect of AKT phosphorylation may reveal a novel unexplored pharmacological target in apoptosis. On this, the data are still very limited, but some important elements are starting to emerge.
Not only apoptosis: the calcium-autophagy link
Autophagy is an ubiquitous and highly conserved catabolic program through which cells in stress conditions (such as starvation, growth factor deprivation, protein aggregation and numerous anticancer treatments) degradate proteins and cytosolic components to recycle their macromolecules and to obtain nutrients (Giorgi et al., 2008). Autophagy regulation is a highly complex process involving many signaling complexes and pathways. A large set of highly conserved proteins, named autophagy-related proteins, has been discovered and many of them form complexes, which are involved in the process of autophagosome formation. According to its general definition, autophagy could be considered as a cell survival response, a mechanism to face the energetic cell emergences sustaining the basic metabolic processes in stress conditions. However, the role of autophagy in the regulation of cellular life/death is likely to be very complex, and recent evidence highlights autophagy as a cell death mechanism, that is, type II programed cell death (Baehrecke, 2005; Kroemer et al., 2005; Tsujimoto and Shimizu, 2005). Indeed, in apoptosis-deficient mammalian cells, autophagy acts as an alternative death mechanism (Lum et al., 2005) and in Bax-Bak double knockout of MEF (which are unable to perform the apoptotic program), treatment with apoptotic inducers, such as etoposide, thapsigargin or SDS, enhance autophagosomes formation. To further underline the relationship between apoptosis and autophagy, many data have been collected, which support an involvement of Bcl-2 in the regulation of autophagy. In particular, in leukemic cells, Bcl-2 deregulation increases autophagy (Saeki et al., 2000), whereas in neuronal progenitor cells and in serum deprived cerebellar granule cells, Bcl-2 overexpression inhibits autophagy through interaction with Beclin 1 (Pattingre et al., 2005). Finally, of interest to the topic covered in this review, both apoptosis and autophagy appear to be regulated by Ca2+. However, while the role of Ca2+ in apoptosis has been exhaustively investigated and to a good extent clarified, the understanding of its role in autophagy is still poorly understood. Hoyer-Hansen et al. (2007) demonstrated recently that various Ca2+ mobilizing stimuli, such as vitamin D3 compounds, ATP, thapsigargin and ionomycin, by inducing an increase in [Ca2+]c, activate the Ca2+/calmodulin-dependent protein kinase-kinase (CaMKK) and consequently inhibit mTOR (mammalian target of rapamycin). So, the final effect of a rise in [Ca2+]c is an induction of autophagy. Overall, these data show that the relationship between apoptosis and autophagy also extends to the regulatory effect of [Ca2+], but this relationship is still unclear. This notion will be fundamental in unraveling the extracellular and intracellular conditions that lead cellular stresses into death pathway and dictate the final outcome of a pathological event or a pharmacological intervention.
Acknowledgment
We are deeply indebted to past and present collaborators. This work was supported by the Italian Association for Cancer Research (AIRC), Telethon, local funds from the University of Ferrara, the Italian University Ministry, the PRRIITT program of the Emilia Romagna Region, the Italian Space Agency (ASI), NIH (Grant no.1P01AG025532-01A1) and the United Mitochondrial Disease Foundation (UMDF).
Abbreviations
- AIF
apoptosis-inducing factor
- APAF-1
apoptosis protease-activating factor-1
- Calcium concentrations: [Ca2+]c
cytosolic
- [Ca2+]m
mitochondrial
- [Ca2+]ER
in the endoplasmic reticulum
- CASPASE
cysteinyl/aspartate-specific protease
- DAG
diacylglycerol
- DRP
dynamin-like protein
- GRP75
glucose-regulated protein 75
- ER
endoplasmic reticulum
- IP3
inositol 1,4,5 trisphosphate
- IP3R
inositol 1,4,5 trisphosphate receptor
- MAM
mitochondrial-associated membrane
- Mefs
mouse embryonic fibroblasts
- mPTP
mitochondrial permeability transition pore
- PACS-2
cytosolic sorting protein-2
- PK
protein kinase
- PMCA
plasma membrane Ca2+ ATP-ase
- SERCA
sarco–endoplasmic reticulum Ca2+ ATPase
- VDAC
voltage anion-dependent channel
References
- Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, et al. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- Assefa Z, Bultynck G, Szlufcik K, Nadif KN, Vermassen E, Goris J, et al. Caspase-3-induced truncation of type 1 inositol trisphosphate receptor accelerates apoptotic cell death and induces inositol trisphosphate-independent calcium release during apoptosis. J Biol Chem. 2004;279:43227–43236. doi: 10.1074/jbc.M403872200. [DOI] [PubMed] [Google Scholar]
- Baehrecke EH. Autophagy: dual roles in life and death? Nat Rev Mol Cell Biol. 2005;6:505–510. doi: 10.1038/nrm1666. [DOI] [PubMed] [Google Scholar]
- Baffy G, Miyashita T, Williamson JR, Reed JC. Apoptosis induced by withdrawal of interleukin-3 (IL-3) from an IL-3-dependent hematopoietic cell line is associated with repartitioning of intracellular calcium and is blocked by enforced Bcl-2 oncoprotein production. J Biol Chem. 1993;268:6511–6519. [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Bano D, Young KW, Guerin CJ, Lefeuvre R, Rothwell NJ, Naldini L, et al. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell. 2005;120:275–285. doi: 10.1016/j.cell.2004.11.049. [DOI] [PubMed] [Google Scholar]
- Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of cyclophilin D. J Biol Chem. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- Bastianutto C, Clementi E, Codazzi F, Podini P, De Giorgi F, Rizzuto R, et al. Overexpression of calreticulin increases the Ca2+ capacity of rapidly exchanging Ca2+ stores and reveals aspects of their lumenal microenvironment and function. J Cell Biol. 1995;130:847–855. doi: 10.1083/jcb.130.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi P, Krauskopf A, Basso E, Petronilli V, Blachly-Dyson E, Di Lisa F, et al. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS J. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Biagioli M, Pifferi S, Ragghianti M, Bucci S, Rizzuto R, Pinton P. Endoplasmic reticulum stress and alteration in calcium homeostasis are involved in cadmium-induced apoptosis. Cell Calcium. 2008;43:184–195. doi: 10.1016/j.ceca.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Blackshaw S, Sawa A, Sharp AH, Ross CA, Snyder SH, Khan AA. Type 3 inositol 1,4,5-trisphosphate receptor modulates cell death. FASEB J. 2000;14:1375–1379. doi: 10.1096/fj.14.10.1375. [DOI] [PubMed] [Google Scholar]
- Boehning D, Patterson RL, Sedaghat L, Glebova NO, Kurosaki T, Snyder SH. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol. 2003;5:1051–1061. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- Brini M, Bano D, Manni S, Rizzuto R, Carafoli E. Effects of PMCA and SERCA pump overexpression on the kinetics of cell Ca(2+) signalling. EMBO J. 2000;19:4926–4935. doi: 10.1093/emboj/19.18.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd SL, Nicholls DG. A reevaluation of the role of mitochondria in neuronal Ca2+ homeostasis. J Neurochem. 1996;66:403–411. doi: 10.1046/j.1471-4159.1996.66010403.x. [DOI] [PubMed] [Google Scholar]
- Camello P, Gardner J, Petersen OH, Tepikin AV. Calcium dependence of calcium extrusion and calcium uptake in mouse pancreatic acinar cells. J Physiol. 1996;490(Pt 3):585–593. doi: 10.1113/jphysiol.1996.sp021169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella M, de Jong AS, Lanke KW, Melchers WJ, Willems PH, Pinton P, et al. The coxsackievirus 2B protein suppresses apoptotic host cell responses by manipulating intracellular Ca2+ homeostasis. J Biolo Chem. 2004;279:18440–18450. doi: 10.1074/jbc.M309494200. [DOI] [PubMed] [Google Scholar]
- Carafoli E, Molinari M. Calpain: a protease in search of a function? Biochem Biophys Res Commun. 1998;247:193–203. doi: 10.1006/bbrc.1998.8378. [DOI] [PubMed] [Google Scholar]
- Chami M, Ferrari D, Nicotera P, Paterlini-Brechot P, Rizzuto R. Caspase-dependent alterations of Ca2+ signaling in the induction of apoptosis by hepatitis B virus X protein. J Biol Chem. 2003;278:31745–31755. doi: 10.1074/jbc.M304202200. [DOI] [PubMed] [Google Scholar]
- Chami M, Gozuacik D, Saigo K, Capiod T, Falson P, Lecoeur H, et al. Hepatitis B virus-related insertional mutagenesis implicates SERCA1 gene in the control of apoptosis. Oncogene. 2000;19:2877–2886. doi: 10.1038/sj.onc.1203605. [DOI] [PubMed] [Google Scholar]
- Chami M, Prandini A, Campanella M, Pinton P, Szabadkai G, Reed JC, et al. Bcl-2 and Bax exert opposing effects on Ca2+signalling, which do not depend on their putative pore-forming region. J Biol Chem. 2004;279:54581–54589. doi: 10.1074/jbc.M409663200. [DOI] [PubMed] [Google Scholar]
- Chan SL, Mattson MP. Caspase and calpain substrates: roles in synaptic plasticity and cell death. J Neurosci Res. 1999;58:167–190. [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Csordas G, Thomas AP, Hajnoczky G. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J. 1999;18:96–108. doi: 10.1093/emboj/18.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Demaurex N, Distelhorst C. Cell biology. Apoptosis—the calcium connection. Science. 2003;300:65–67. doi: 10.1126/science.1083628. [DOI] [PubMed] [Google Scholar]
- Fariss MW, Pascoe GA, Reed DJ. Vitamin E reversal of the effect of extracellular calcium on chemically induced toxicity in hepatocytes. Science. 1985;227:751–754. doi: 10.1126/science.3918345. [DOI] [PubMed] [Google Scholar]
- Foyouzi-Youssefi R, Arnaudeau S, Borner C, Kelley WL, Tschopp J, Lew DP, et al. Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proc Natl Acad Sci USA. 2000;97:5723–5728. doi: 10.1073/pnas.97.11.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13:1423–1433. doi: 10.1038/sj.cdd.4401950. [DOI] [PubMed] [Google Scholar]
- Giorgi C, Romagnoli A, Pinton P, Rizzuto R. Ca2+ signaling, mitochondria and cell death. Curr Mol Med. 2008;8:119–130. doi: 10.2174/156652408783769571. [DOI] [PubMed] [Google Scholar]
- Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Glazner GW, Chan SL, Lu C, Mattson MP. Caspase-mediated degradation of AMPA receptor subunits: a mechanism for preventing excitotoxic necrosis and ensuring apoptosis. J Neurosci. 2000;20:3641–3649. doi: 10.1523/JNEUROSCI.20-10-03641.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G, Davies E, Madesh M. Calcium signaling and apoptosis. Biochem Biophys Res Commun. 2003;304:445–454. doi: 10.1016/s0006-291x(03)00616-8. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Hetts SW. To die or not to die: an overview of apoptosis and its role in disease. JAMA. 1998;279:300–307. doi: 10.1001/jama.279.4.300. [DOI] [PubMed] [Google Scholar]
- Hill MM, Adrain C, Martin SJ. Portrait of a killer: the mitochondrial apoptosome emerges from the shadows. Mol Interv. 2003;3:19–26. doi: 10.1124/mi.3.1.19. [DOI] [PubMed] [Google Scholar]
- Hirota J, Furuichi T, Mikoshiba K. Inositol 1,4,5-trisphosphate receptor type 1 is a substrate for caspase-3 and is cleaved during apoptosis in a caspase-3-dependent manner. J Biol Chem. 1999;274:34433–34437. doi: 10.1074/jbc.274.48.34433. [DOI] [PubMed] [Google Scholar]
- Hofer AM, Fasolato C, Pozzan T. Capacitative Ca2+ entry is closely linked to the filling state of internal Ca2+ stores: a study using simultaneous measurements of ICRAC and intraluminal [Ca2+] J Cell Biol. 1998;140:325–334. doi: 10.1083/jcb.140.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Jayaraman T, Marks AR. T cells deficient in inositol 1,4,5-trisphosphate receptor are resistant to apoptosis. Mol Cell Biol. 1997;17:3005–3012. doi: 10.1128/mcb.17.6.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouaville LS, Pinton P, Bastianutto C, Rutter GA, Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc Natl Acad Sci USA. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AA, Soloski MJ, Sharp AH, Schilling G, Sabatini DM, Li SH, et al. Lymphocyte apoptosis: mediation by increased type 3 inositol 1,4,5-trisphosphate receptor. Science. 1996;273:503–507. doi: 10.1126/science.273.5274.503. [DOI] [PubMed] [Google Scholar]
- Khan MT, Wagner L, Yule DI, Bhanumathy C, Joseph SK. Akt kinase phosphorylation of inositol 1,4,5-trisphosphate receptors. J Biol Chem. 2006;281:3731–3737. doi: 10.1074/jbc.M509262200. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Yamashita K, Takeoka T, Ohtsuki T, Suzuki Y, Takahashi R, et al. Calpain-mediated X-linked inhibitor of apoptosis degradation in neutrophil apoptosis and its impairment in chronic neutrophilic leukemia. J Biol Chem. 2002;277:33968–33977. doi: 10.1074/jbc.M203350200. [DOI] [PubMed] [Google Scholar]
- Kroemer G, El-Deiry WS, Golstein P, Peter ME, Vaux D, Vandenabeele P, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2005;12 Suppl 2:1463–1467. doi: 10.1038/sj.cdd.4401724. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- Kruman I, Guo Q, Mattson MP. Calcium and reactive oxygen species mediate staurosporine-induced mitochondrial dysfunction and apoptosis in PC12 cells. J Neurosci Res. 1998;51:293–308. doi: 10.1002/(SICI)1097-4547(19980201)51:3<293::AID-JNR3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Lam M, Dubyak G, Chen L, Nunez G, Miesfeld RL, Distelhorst CW. Evidence that BCL-2 represses apoptosis by regulating endoplasmic reticulum-associated Ca2+ fluxes. Proc Natl Acad Sci USA. 1994;91:6569–6573. doi: 10.1073/pnas.91.14.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasorsa FM, Pinton P, Palmieri L, Fiermonte G, Rizzuto R, Palmieri F. Recombinant expression of the Ca(2+)-sensitive aspartate/glutamate carrier increases mitochondrial ATP production in agonist-stimulated Chinese hamster ovary cells. J Biol Chem. 2003;278:38686–38692. doi: 10.1074/jbc.M304988200. [DOI] [PubMed] [Google Scholar]
- Lavin MF, Watters D, Song Q. Role of protein kinase activity in apoptosis. Experientia. 1996;52:979–994. doi: 10.1007/BF01920107. [DOI] [PubMed] [Google Scholar]
- Li C, Wang X, Vais H, Thompson CB, Foskett JK, White C. Apoptosis regulation by Bcl-x(L) modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating. Proc Natl Acad Sci USA. 2007;104:12565–12570. doi: 10.1073/pnas.0702489104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem. 2002;80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- Lynch K, Fernandez G, Pappalardo A, Peluso JJ. Basic fibroblast growth factor inhibits apoptosis of spontaneously immortalized granulosa cells by regulating intracellular free calcium levels through a protein kinase Cdelta-dependent pathway. Endocrinology. 2000;141:4209–4217. doi: 10.1210/endo.141.11.7742. [DOI] [PubMed] [Google Scholar]
- Ma TS, Mann DL, Lee JH, Gallinghouse GJ. SR compartment calcium and cell apoptosis in SERCA overexpression. Cell Calcium. 1999;26:25–36. doi: 10.1054/ceca.1999.0049. [DOI] [PubMed] [Google Scholar]
- Mandic A, Viktorsson K, Strandberg L, Heiden T, Hansson J, Linder S, et al. Calpain-mediated Bid cleavage and calpain-independent Bak modulation: two separate pathways in cisplatin-induced apoptosis. Mol Cell Biol. 2002;22:3003–3013. doi: 10.1128/MCB.22.9.3003-3013.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh BJ, Mastronarde DN, Buttle KF, Howell KE, McIntosh JR. Organellar relationships in the Golgi region of the pancreatic beta cell line, HIT-T15, visualized by high resolution electron tomography. Proc Natl Acad Sci USA. 2001;98:2399–2406. doi: 10.1073/pnas.051631998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes CC, Gomes DA, Thompson M, Souto NC, Goes TS, Goes AM, et al. The type III inositol 1,4,5-trisphosphate receptor preferentially transmits apoptotic Ca2+ signals into mitochondria. J Biol Chem. 2005;280:40892–40900. doi: 10.1074/jbc.M506623200. [DOI] [PubMed] [Google Scholar]
- Montero M, Lobaton CD, Moreno A, Alvarez J. A novel regulatory mechanism of the mitochondrial Ca2+ uniporter revealed by the p38 mitogen-activated protein kinase inhibitor SB202190. FASEB J. 2002;16:1955–1957. doi: 10.1096/fj.02-0553fje. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Bossy-Wetzel E, Burns K, Fadel MP, Lozyk M, Goping IS, et al. Changes in endoplasmic reticulum luminal environment affect cell sensitivity to apoptosis. J Cell Biol. 2000;150:731–740. doi: 10.1083/jcb.150.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6:1028–1042. doi: 10.1038/sj.cdd.4400598. [DOI] [PubMed] [Google Scholar]
- Nicholson DW, Thornberry NA. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- Nicotera P, Orrenius S. The role of calcium in apoptosis. Cell Calcium. 1998;23:173–180. doi: 10.1016/s0143-4160(98)90116-6. [DOI] [PubMed] [Google Scholar]
- Oakes SA, Scorrano L, Opferman JT, Bassik MC, Nishino M, Pozzan T, et al. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci USA. 2005;102:105–110. doi: 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- Palmer AE, Jin C, Reed JC, Tsien RY. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc Natl Acad Sci USA. 2004;101:17404–17409. doi: 10.1073/pnas.0408030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker PJ, Murray-Rust J. PKC at a glance. J Cell Sci. 2004;117:131–132. doi: 10.1242/jcs.00982. [DOI] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Perry G, Nunomura A, Lucassen P, Lassmann H, Smith MA. Apoptosis and Alzheimer’s disease. Science. 1998;282:1268–1269. doi: 10.1126/science.282.5392.1265h. [DOI] [PubMed] [Google Scholar]
- Piccini M, Vitelli F, Bruttini M, Pober BR, Jonsson JJ, Villanova M, et al. FACL4, a new gene encoding long-chain acyl-CoA synthetase 4, is deleted in a family with Alport syndrome, elliptocytosis, and mental retardation. Genomics. 1998;47:350–358. doi: 10.1006/geno.1997.5104. [DOI] [PubMed] [Google Scholar]
- Pinton P, Brini M, Bastianutto C, Tuft RA, Pozzan T, Rizzuto R. New light on mitochondrial calcium. Biofactors. 1998;8:243–253. doi: 10.1002/biof.5520080312. [DOI] [PubMed] [Google Scholar]
- Pinton P, Ferrari D, Di Virgilio F, Pozzan T, Rizzuto R. Molecular machinery and signalling events in apoptosis. Drug Dev Res. 2001a;52:558–570. [Google Scholar]
- Pinton P, Ferrari D, Magalhaes P, Schulze-Osthoff K, Di Virgilio F, Pozzan T, et al. Reduced loading of intracellular Ca(2+) stores and downregulation of capacitative Ca(2+) influx in Bcl-2-overexpressing cells. J Cell Biol. 2000;148:857–862. doi: 10.1083/jcb.148.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P, Ferrari D, Rapizzi E, Di Virgilio F, Pozzan T, Rizzuto R. A role for calcium in Bcl-2 action? Biochimie. 2002;84:195–201. doi: 10.1016/s0300-9084(02)01373-1. [DOI] [PubMed] [Google Scholar]
- Pinton P, Ferrari D, Rapizzi E, Di Virgilio FD, Pozzan T, Rizzuto R. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 2001b;20:2690–2701. doi: 10.1093/emboj/20.11.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P, Leo S, Wieckowski MR, Di Benedetto G, Rizzuto R. Long-term modulation of mitochondrial Ca2+ signals by protein kinase C isozymes. J Cell Biol. 2004;165:223–232. doi: 10.1083/jcb.200311061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P, Rimessi A, Marchi S, Orsini F, Migliaccio E, Giorgio M, et al. Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science. 2007a;315:659–663. doi: 10.1126/science.1135380. [DOI] [PubMed] [Google Scholar]
- Pinton P, Rimessi A, Romagnoli A, Prandini A, Rizzuto R. Biosensors for the detection of calcium and pH. Methods Cell Biol. 2007b;80:297–325. doi: 10.1016/S0091-679X(06)80015-4. [DOI] [PubMed] [Google Scholar]
- Pinton P, Rizzuto R. Bcl-2 and Ca2+ homeostasis in the endoplasmic reticulum. Cell Death Differ. 2006;13:1409–1418. doi: 10.1038/sj.cdd.4401960. [DOI] [PubMed] [Google Scholar]
- Pinton P, Rizzuto R. p66Shc, oxidative stress and aging: importing a lifespan determinant into mitochondria. Cell Cycle. 2008;7:304–308. doi: 10.4161/cc.7.3.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T, Rizzuto R, Volpe P, Meldolesi J. Molecular and cellular physiology of intracellular calcium stores. Physiol Rev. 1994;74:595–636. doi: 10.1152/physrev.1994.74.3.595. [DOI] [PubMed] [Google Scholar]
- Pressman BC. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Rapizzi E, Pinton P, Szabadkai G, Wieckowski MR, Vandecasteele G, Baird GS, et al. Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ micro-domains to mitochondria. J Cell Biol. 2002;159:613–624. doi: 10.1083/jcb.200205091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravagnan L, Roumier T, Kroemer G. Mitochondria, the killer organelles and their weapons. J Cell Physiol. 2002;192:131–137. doi: 10.1002/jcp.10111. [DOI] [PubMed] [Google Scholar]
- Rimessi A, Giorgi C, Pinton P, Rizzuto R. The versatility of mitochondrial calcium signals: from stimulation of cell metabolism to induction of cell death. Biochim Biophys Acta. 2008;1777:808–816. doi: 10.1016/j.bbabio.2008.05.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippo MR, Malisan F, Ravagnan L, Tomassini B, Condo I, Costantini P, et al. GD3 ganglioside directly targets mitochondria in a bcl-2-controlled fashion. FASEB J. 2000;14:2047–2054. doi: 10.1096/fj.99-1028com. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, et al. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Ferrari D, Chami M, Szabadkai G, Magalhaes PJ, et al. Calcium and apoptosis: facts and hypotheses. Oncogene. 2003;22:8619–8627. doi: 10.1038/sj.onc.1207105. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- Saeki K, Yuo A, Okuma E, Yazaki Y, Susin SA, Kroemer G, et al. Bcl-2 down-regulation causes autophagy in a caspase-independent manner in human leukemic HL60 cells. Cell Death Differ. 2000;7:1263–1269. doi: 10.1038/sj.cdd.4400759. [DOI] [PubMed] [Google Scholar]
- Schwab BL, Guerini D, Didszun C, Bano D, Ferrando-May E, Fava E, et al. Cleavage of plasma membrane calcium pumps by caspases: a link between apoptosis and necrosis. Cell Death Differ. 2002;9:818–831. doi: 10.1038/sj.cdd.4401042. [DOI] [PubMed] [Google Scholar]
- Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- Shibasaki F, McKeon F. Calcineurin functions in Ca(2+)-activated cell death in mammalian cells. J Cell Biol. 1995;131:735–743. doi: 10.1083/jcb.131.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmen T, Aslan JE, Blagoveshchenskaya AD, Thomas L, Wan L, Xiang Y, et al. PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. EMBO J. 2005;24:717–729. doi: 10.1038/sj.emboj.7600559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SJ, Vance JE. Phosphatidylserine synthase-1 and -2 are localized to mitochondria-associated membranes. J Biol Chem. 2000;275:34534–34540. doi: 10.1074/jbc.M002865200. [DOI] [PubMed] [Google Scholar]
- Sugawara H, Kurosaki M, Takata M, Kurosaki T. Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. EMBO J. 1997;16:3078–3088. doi: 10.1093/emboj/16.11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadkai G, Bianchi K, Varnai P, De Stefani D, Wieckowski MR, Cavagna D, et al. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadkai G, Simoni AM, Chami M, Wieckowski MR, Youle RJ, Rizzuto R. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol Cell. 2004;16:59–68. doi: 10.1016/j.molcel.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Szado T, Vanderheyden V, Parys JB, De Smedt H, Rietdorf K, Kotelevets L, et al. Phosphorylation of inositol 1,4,5-trisphosphate receptors by protein kinase B/Akt inhibits Ca2+ release and apoptosis. Proc Natl Acad Sci USA. 2008;105:2427–2432. doi: 10.1073/pnas.0711324105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalai G, Krishnamurthy R, Hajnoczky G. Apoptosis driven by IP(3)-linked mitochondrial calcium signals. EMBO J. 1999;18:6349–6361. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teranishi F, Liu ZQ, Kunimatsu M, Imai K, Takeyama H, Manabe T, et al. Calpain is involved in the HIV replication from the latently infected OM10.1 cells. Biochem Biophys Res Commun. 2003;303:940–946. doi: 10.1016/s0006-291x(03)00447-9. [DOI] [PubMed] [Google Scholar]
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Spencer MJ. Calpains and muscular dystrophies. Int J Biochem Cell Biol. 2000;32:1–5. doi: 10.1016/s1357-2725(99)00095-3. [DOI] [PubMed] [Google Scholar]
- Tombal B, Denmeade SR, Isaacs JT. Assessment and validation of a microinjection method for kinetic analysis of [Ca2+]i in individual cells undergoing apoptosis. Cell Calcium. 1999;25:19–28. doi: 10.1054/ceca.1998.0005. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Shimizu S. Another way to die: autophagic programmed cell death. Cell Death Differ. 2005;12 Suppl 2:1528–1534. doi: 10.1038/sj.cdd.4401777. [DOI] [PubMed] [Google Scholar]
- Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twomey C, McCarthy JV. Pathways of apoptosis and importance in development. J Cell Mol Med. 2005;9:345–359. doi: 10.1111/j.1582-4934.2005.tb00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance JE. Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem. 1990;265:7248–7256. [PubMed] [Google Scholar]
- Vanden Abeele F, Skryma R, Shuba Y, Van Coppenolle F, Slomianny C, Roudbaraki M, et al. Bcl-2-dependent modulation of Ca(2+) homeostasis and store-operated channels in prostate cancer cells. Cancer Cell. 2002;1:169–179. doi: 10.1016/s1535-6108(02)00034-x. [DOI] [PubMed] [Google Scholar]
- Vanoverberghe K, Vanden Abeele F, Mariot P, Lepage G, Roudbaraki M, Bonnal JL, et al. Ca2+ homeostasis and apoptotic resistance of neuroendocrine-differentiated prostate cancer cells. Cell Death Differ. 2004;11:321–330. doi: 10.1038/sj.cdd.4401375. [DOI] [PubMed] [Google Scholar]
- Voeltz GK, Rolls MM, Rapoport TA. Structural organization of the endoplasmic reticulum. EMBO Rep. 2002;3:944–950. doi: 10.1093/embo-reports/kvf202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, et al. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- Wang NS, Unkila MT, Reineks EZ, Distelhorst CW. Transient expression of wild-type or mitochondrially targeted Bcl-2 induces apoptosis, whereas transient expression of endoplasmic reticulum-targeted Bcl-2 is protective against Bax-induced cell death. J Biol Chem. 2001;276:44117–44128. doi: 10.1074/jbc.M101958200. [DOI] [PubMed] [Google Scholar]
- White C, Li C, Yang J, Petrenko NB, Madesh M, Thompson CB, et al. The endoplasmic reticulum gateway to apoptosis by Bcl-X(L) modulation of the InsP3R. Nat Cell Biol. 2005;7:1021–1028. doi: 10.1038/ncb1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieckowski MR, Szabadkai G, Wasilewski M, Pinton P, Duszynski J, Rizzuto R. Overexpression of adenine nucleotide translocase reduces Ca2+ signal transmission between the ER and mitochondria. Biochem Biophys Res Commun. 2006;348:393–399. doi: 10.1016/j.bbrc.2006.07.072. [DOI] [PubMed] [Google Scholar]
- Xu XZ, Moebius F, Gill DL, Montell C. Regulation of melastatin, a TRP-related protein, through interaction with a cytoplasmic isoform. Proc Natl Acad Sci USA. 2001;98:10692–10697. doi: 10.1073/pnas.191360198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda T, Imaizumi K, Oono K, Yui D, Gomi F, Katayama T, et al. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2- dependent mechanism in response to the ER stress. J Biol Chem. 2001;276:13935–13940. doi: 10.1074/jbc.M010677200. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Karbowski M. Mitochondrial fission in apoptosis. Nat Rev Mol Cell Biol. 2005;6:657–663. doi: 10.1038/nrm1697. [DOI] [PubMed] [Google Scholar]
- Zhang J, Alter N, Reed JC, Borner C, Obeid LM, Hannun YA. Bcl-2 interrupts the ceramide-mediated pathway of cell death. Proc Natl Acad Sci USA. 1996;93:5325–5328. doi: 10.1073/pnas.93.11.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]