Abstract
Mutations in the human OCRL gene, which encodes a phosphatidylinositol(4,5)bisphosphate 5-phosphatase, result in the X-linked oculocerebrorenal syndrome of Lowe. Mice with a targeted disruption of Ocrl have no phenotypic abnormalities. Targeted disruption of its closest paralog, Inpp5b, causes male infertility in the 129S6 background. Mice with disruptions of both genes are lost in utero prior to 9.5-10.5 dpc, indicating that there is a functional overlap between the two paralogs early in development. We analyzed the pattern of X-inactivation in four tissues of distinct embryonic origin from Ocrl wt/−;Inpp5b −/− females to explore the timing and tissue distribution of the functional overlap. X-inactivation was strongly skewed against the disrupted Ocrl − allele being on the active X chromosome in all four tissues tested, indicating that there is early selection against cell lineages lacking both Ocrl and Inpp5b. Extraembryonic tissue was also involved in the lethality because there were never any live-born Ocrl wt/−;Inpp5b −/− females when the functional Ocrl wt allele was on the paternal X chromosome, which is preferentially inactivated in trophoblast-derived extraembryonic tissues. Live-born Ocrl wt/−;Inpp5b −/− females were found when the functional Ocrl wt allele was maternal, although in fewer numbers than expected. The importance of the extraembryonic tissues in the early embryonic lethality of embryos lacking both Ocrl and Inpp5b is reinforced by the successful isolation of a viable 40,XX Ocrl −/−;Inpp5b −/− embryonic stem cell from the inner cell mass of a 3.5-dpc blastocyst prior to implantation. These results indicate a functional overlap of Ocrl and Inpp5b in most cell lineages, especially in extraembryonic tissues.
Introduction
The oculocerebrorenal syndrome of Lowe (OCRL; OMIM #309000) is a rare X-linked disorder characterized by congenital cataracts, mental retardation, behavioral abnormalities, and a proximal renal tubular dysfunction that includes low-molecular-weight proteinuria and variable aminoaciduria, phosphaturia, and bicarbonaturia (Suchy and Nussbaum 2009). The disorder is caused by mutations in a gene encoding a phosphatidylinositol(4,5)bisphosphate 5-phosphatase [PI(4,5) 5-phosphatase] that was originally named OCRL because it was identified by positional cloning from Lowe syndrome patients (Attree et al. 1992). An attempt to develop a mouse model for Lowe syndrome failed when it was found that mice with targeted disruption of the Ocrl gene failed to have the phenotype of the Lowe syndrome, or any other discernible phenotype (Janne et al. 1998). We hypothesized that Ocrl − mice might be protected from disease by a compensating enzymatic activity. Ocrl is highly homologous to an autosomal paralog, Inpp5b (Jefferson and Majerus 1995; Zhang et al. 1995), which has a similar enzymatic function (Ross et al. 1991), and we hypothesized that Inpp5b might serve to compensate for loss of Ocrl in mice. Mice with targeted disruptions of Inpp5b have a mild phenotype of male infertility in the inbred 129S6 background but no signs of Lowe syndrome (Hellsten et al. 2001, 2002), while mice deficient in both enzyme activities appear to die during embryogenesis, prior to E9.5–10.5, indicating that the two genes do encode proteins with overlapping function (Janne et al. 1998).
In this report, we used mouse crosses, X chromosome inactivation (XCI) analysis, and cell culture experiments to further characterize the timing of the embryonic lethality in doubly deficient embryos and to determine which subsets of embryonic tissues require that one or the other of these two PI(4,5) 5-phosphatases be present. We show that the functional overlap between these two enzymes occurs in all three embryonic layers as well as in the trophoblast-derived extraembryonic tissues during the critical period of implantation and early embryonic development between E3.5 and E9.5.
Materials and methods
Mouse breeding
All mouse experiments were approved by the National Human Genome Research Institute Animal Care and Use Committee and followed the National Institutes of Health guidelines. The mouse strains carrying disrupted Ocrl and Inpp5b were previously published (Hellsten et al. 2001; Janne et al. 1998). The Ocrl mutant strain had a mixed genetic background from the 129S4 (J1 ES cell line) founder mice outcrossed to C57Bl/6J. The Inpp5b mutant strain had a mixed background from 129S6 (TC1 ES cell line) founder mice bred to NIH Black Swiss in order to maintain fertility in this line. B6.Cg-Pgk1 a/J mice were from the Jackson Laboratory (stock number 000827). Thus, the test and control mice, with differential Pgk1 alleles marking the two X chromosomes, were on a mixed genetic background of 129, C57Bl/6, and NIH Black Swiss.
Allele-specific marking of the X chromosome
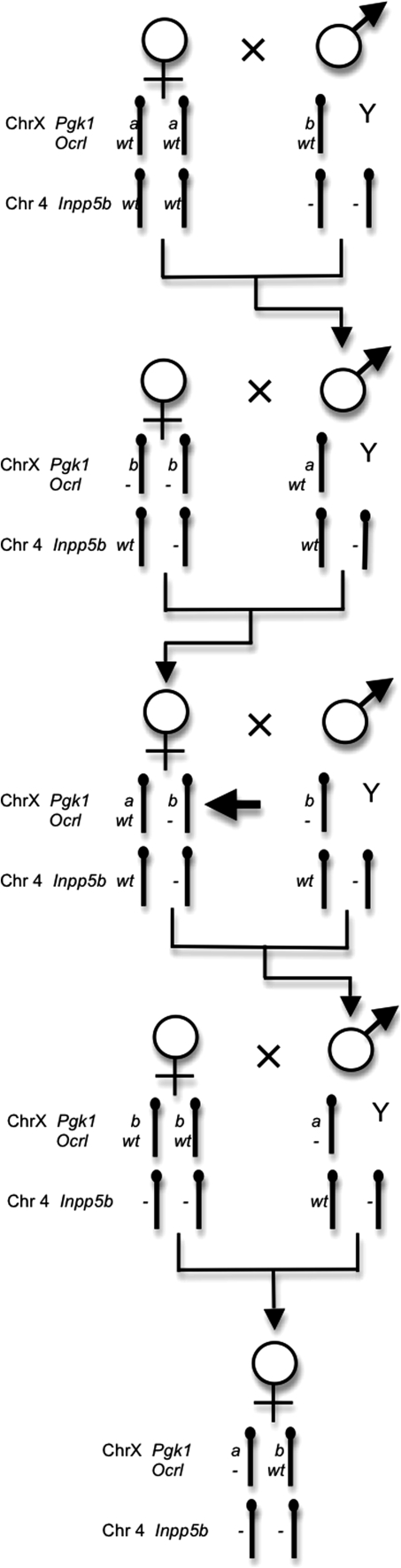
The disrupted Ocrl allele was originally engineered in mice carrying the X-linked phosphoglycerate kinase 1 (Pgk1) b allele. To differentially mark the two X chromosomes in an Ocrl wt/− female for allele-specific expression analysis, we carried out a four-step breeding scheme (Fig. 1) to ultimately generate female mice heterozygous for disruption of Ocrl in which the Pgk1 a allele was always in coupling phase with the disrupted Ocrl allele and the Pgk1 b allele was in coupling with the wild-type Ocrl allele, all in the background of homozygous disrupted for Inpp5b.
Fig. 1.
Breeding scheme used to generate female mice heterozygous for the previously described disrupted allele of Ocrl and for the two Pgk1 alleles, a and b, putting the Pgk1 a allele in coupling phase with the disrupted Ocrl allele. The mice are also homozygous for the previously described disrupted allele of Inpp5b. Arrow indicates meioses in which recombination brought the Pgk1 a allele into coupling phase with the disrupted Ocrl allele. The final breeding step results in fewer Pgk1 a/b;Ocrl wt/−;Inpp5b −/− females than expected
Genotyping
Ocrl and Inpp5b mice were genotyped either by Southern blotting (Janne et al. 1998) or PCR as follows:
Ocrl wild-type: The forward primer 5′-CCCTTTTCATCTGTTAGGAGAAATC-3′ overlaps the junction of intron 18 and the 5′ end of exon 19. The reverse primer 5′-GCATGGTTAAACGCACTATGTGG-3′ is located in intron 19 which is deleted in the Ocrl knockout (KO) line. These primers yield a product 227 bp in length using an annealing temperature (T A) of 58°C.
Ocrl knockout: The Ocrl KO forward primer has the sequence 5′-GCCCTTTGATTCTAATCCCTTTTCATC-3′ and is located in the intron just prior to exon 19. The reverse primer is located in the PGK promoter, which is part of the targeting construct and has a sequence of 5′-TCTGAGCCCAGAAAGCGAA-3′. At T A = 58°C, these primers yield a product of about 480 bp.
Inpp5b wild-type: Primers flank the Neo selection cassette in the targeted allele but will not amplify through the Neo gene with the 1-min extension time. The forward primer, 5′-CTTGTGGCTGGGAGACCT-3′, is located in exon 14 and the reverse primer, 5′-AGAGAAATGCAGACGGAATG -3′, is located in intron 14. At T A = 52°C, these primers yield a product of 234 bp.
Inpp5b knockout: The reverse primer is the same as the Ocrl KO reverse primer, 5′-TCTGAGCCCAGAAAGCGAA-3′. The forward primer is the same as the wild-type primer, 5′-CTTGTGGCTGGGAGACCT-3′. Together these primers yield a 500-bp product at T A = 52°C.
Pgk1 alleles: The Pgk1 alleles were genotyped by a method previously described (Shanmugam et al. 1996). Briefly, a fragment of the Pgk1 gene was amplified by PCR using forward primer 5′-TTCTCCTCTTCCTCATCTCC-3′ and reverse primer 5′-TAAAGGACACATTTGGTCGC-3′. The PCR product is then digested at a polymorphic site with the restriction endonuclease Tth111I. The Pgk1 a allele is resistant to Tth111I digestion while the Pgk1 b allele is cleaved into two fragments of 272 and 72 bp.
DNA extraction
Mouse tail biopsies were incubated with proteinase K (Invitrogen) at 50°C overnight. DNA was purified using either a high-salt method (Thomas et al. 1992) or phenol/chloroform extraction and ethanol precipitation.
RNA extraction
Tissues were homogenized using a 6-ml Dounce homogenizer and Trizol (Invitrogen), following the manufacturer’s protocol. RNA concentration was determined by OD260 UV absorption on a Beckman DU640 spectrophotometer. DNA contamination was eliminated by treatment with Amplification Grade DNase (Invitrogen).
RT-PCR
Reverse transcriptase PCR was performed using the Superscript II RT-PCR kit (Invitrogen) according to manufacturer’s instructions. The gene-specific primer Pgk1 RT (5′-GTAAAGGCCATTCCACCACCAA-3′) was used to synthesize the first strand followed by amplification using the nested reverse primer Pgk1-R2 (5′-TTTAGCGCCTCCCAAGATAGC-3′) and forward primer Pgk1-F296 (5′-GTGTGGGCCCAGAAGTCG-3′). Products were separated from primers and dNTPs on 1% SeaPlaque low-melt agarose (FMC Bioproducts). Bands were visualized using ethidium bromide and excised from the gel using a clean razor blade. Agarose was removed by treatment with ß agarase (New England BioLabs). The final product was ethanol precipitated and resuspended in 25 μl of TE, and the concentration determined by densitometry of ethidium bromide gel electrophoretograms using NIH Image (http://rsb.info.nih.gov/nih-image/Default.html).
Single-nucleotide polymerase extension reaction (SNuPE)
A modification of the SNuPE reaction was employed (Singer-Sam et al. 1992). Briefly, a slightly longer 25-bp PAGE-purified SNuPE primer, 5′-GATGCTTTCCGAGCCTCACTGTCCA-3′, was designed which sits one base 5′ of the single base polymorphism at position 489 in the Pgk1 mRNA (GenBank ID# gi66792290). Both nonradioactive dCTP (0.2 μM final concentration) and 2 μCi 32P ddATP (Amersham) were added to a single tube containing either 10 or 7 ng of the purified RT-PCR product, 0.25 μM PAGE-purified Pgk1 SNuPE primer, 2 units Thermosequenase polymerase (Amersham), and 1 × Thermosequenase reaction buffer (Amersham) in a final volume of 20 μl. One cycle of 95°C for 2 min, 60°C for 20 s, 72°C for 15 s was performed in a PTC-100 thermocycler (MJ Research Inc.). Pgk1 b alleles incorporated the chain terminating 32P dideoxyATP for a 26-bp product. Pgk1 a alleles added the dCTP followed by the chain terminating 32P dideoxyATP for a product 27 bp in length. Samples were diluted with an equal volume of loading buffer (90% formamide, 0.5 × TBE, 0.025% bromophenol blue, 0.025% xylene cyanole), denatured for 3-5 min at 95°C, and immediately plunged into ice prior to loading 14 μl per lane in a 31 cm × 38.5 cm × 0.4 mm-thick denaturing 18% polyacrylamide [Accugel 19:1 acrylamide:bisacrylamide (National Diagnostics)] gel. Samples were run overnight on an S2 sequencing apparatus (Life Technologies) at a constant 4-6 W at room temperature until the leading dye ran off the gel. Oligonucleotide-sizing markers (Amersham Pharmacia Biotech) were labeled with γ32P ATP as per the manufacturer’s protocol, diluted in an equal volume of loading buffer, denatured at 90°C for 3 min, plunged into ice, and loaded at 3 μl per lane on the denaturing PAGE gel. The gel was dried (Bio-Rad model 583 gel dryer), exposed to a phosphoimager cassette, and analyzed on a Typhoon 8600 phosphoimager (Amersham Pharmacia Biotech). SNuPE reactions were performed in triplicate on tissues derived from 10-11 mice in the test population and 7 mice in the control populations. The control mice ranged in age from 10 to 16 weeks old (mean ± SD = 12.0 ± 2.3 weeks), while the test mice were 8–28 weeks old (mean ± SD 17.4 ± 9.1 weeks).
Phosphoimager analysis
Densitometry was performed using the Typhoon 8600 Phosphoimager using manufacturer-supplied software (Amersham Pharmacia Biotech). Background correction was calculated using the local average function. For each sample, each of the two individual alleles was measured separately and the sum of the two alleles was defined as 100% expression. Percent expression for a single allele in a sample was calculated as the densitometry value of the single allele divided by the sum of both alleles in that sample multiplied by 100%. Triplicate reactions were performed for each sample, averaged, and used to calculate standard deviation using Microsoft Excel spreadsheet software. Probability calculations reported are from t-test analysis, two-tailed, equal variance from the Microsoft Excel software.
Isolation of ES cell line
An embryonic stem (ES) cell line, lacking both Ocrl and Inpp5b, was generated as published (Hogan et al. 1994). Briefly, 3.5-day post coitum (dpc) blastocysts were harvested from naturally mated mice, individually plated into one well of a 24-well plate containing mouse fibroblast feeder layers, and cultured for 5 days. The inner cell mass-derived cells were scraped from the plate, trypsinized, and dissociated with a finely drawn glass capillary. The cells were replated and grown until ES cell-like colonies appeared. DNA was isolated from the individual colonies and subjected to karyotyping (Heng and Tsui 1993; Schweizer 1980, 1981) and genotyping by PCR and Southern blot.
Statistical analysis
Tests of significance in mouse-breeding experiments were either by the χ2 test or by exact calculation from the binomial distribution. Tests of significance on SNuPE measurements were by Student’s t test.
Results
We sought to generate Ocrl wt/−;Inpp5b −/− females, in which X-inactivation would theoretically produce functional mosaicism, with some cells deficient in both Ocrl and Inpp5b and the rest deficient only in Inpp5b. We then sought to determine whether the tissues of Ocrl wt/−;Inpp5b −/− mice would actually demonstrate such mosaicism or would show complete skewing of X-inactivation with exclusive inactivation of the X chromosome carrying the mutant Ocrl allele.
We mated Ocrl −/y;Inpp5b wt/− males to Ocrl wt/wt;Inpp5b −/− females. In this cross, the functional Ocrl allele in Ocrl wt/−;Inpp5b −/− females is maternal in origin. Since the X chromosome of paternal origin is exclusively and nonrandomly inactivated in the trophoblast-derived extraembryonic tissues of Ocrl wt/−;Inpp5b −/− embryos as they develop (Takagi and Sasaki 1975), the extraembryonic tissue lineage of Ocrl wt/−;Inpp5b −/− zygotes in which the Ocrl wt allele is maternal is not mosaic and retains Ocrl function.
We succeeded in generating mice with the Ocrl wt/−;Inpp5b −/− genotype. No obvious phenotype was apparent among the live-born Ocrl wt/−;Inpp5b −/− females. Our success in generating mice of the correct genotype from this cross indicates that Ocrl −;Inpp5b − sperm are capable of fertilization. However, instead of the expected 1:1 ratio of Ocrl wt/−;Inpp5b −/− to Ocrl wt/−;Inpp5 wt/− females, only 36 females of the Ocrl wt/−;Inpp5b −/− genotype were produced compared to 82 Ocrl wt/−;Inpp5b wt/− females, a significantly different ratio approaching 1:2 (P = 2.3 × 10−5, χ2 test) (Table 1). Since the X chromosome of maternal origin is exclusively and nonrandomly activated in the trophoblast-derived extraembryonic tissues of Ocrl wt/−;Inpp5b −/− embryos as they develop (Takagi and Sasaki 1975), these tissues were nonmosaic and contained a functioning Ocrl wt allele on the active maternal X chromosome. The 50% underrepresentation of Ocrl wt/−;Inpp5b −/− females therefore must represent an abnormality in development of the embryo proper or the embryonic mesoderm-derived extraembryonic tissues.
Table 1.
Females resulting from Ocrl wt/wt;Inpp5b −/− female × Ocrl −/y;Inpp5b wt/−male cross
| Gamete genotype | Live-born genotype | # Females observed | # Females expected | |
|---|---|---|---|---|
| Egg | Sperm | |||
| Ocrl wt;Inpp5b − | Ocrl −;Inpp5b wt | Ocrl wt/−;Inpp5b wt/− | 82 | 59 |
| Ocrl wt;Inpp5b − | Ocrl −;Inpp5b − | Ocrl wt/−;Inpp5b −/− | 36* | 59 |
| Total | 118 | 118 | ||
* P = 0.000023 (χ2 test)
In order to determine the X-inactivation pattern in tissues of Ocrl wt/−;Inpp5b −/− females, we took advantage of an expressed single-nucleotide polymorphism in the coding region of the X-linked housekeeping gene phosphoglycerate kinase (Pgk1) (Boer et al. 1990). This gene undergoes X-inactivation and the expressed polymorphism is therefore useful for determining which of the two X chromosomes is active in Ocrl wt/−;Inpp5b −/− females heterozygous for the Pgk1 polymorphism by analyzing the Pgk1 mRNA. We mated B6.Cg-Pgk1 a/J females to male Ocrl −/y;Inpp5b wt/− mice that were hemizygous for the more common Pgk1 b allele in order to introduce the Pgk1 a allele from Danish feral mice (Nielsen and Chapman 1977) into the strains carrying a disrupted Ocrl allele (Fig. 1). The Pgk1 and Ocrl loci recombined 22% of the time to bring the a allele into coupling phase with the targeted mutant Ocrl – allele. Once recombinant males were obtained that carried the Ocrl – allele in phase with Pgk1 a, the phase was maintained by carrying this recombinant chromosome in the males to avoid the potential for meiotic recombination in females. The more common domestic mouse Pgk1 b allele was kept in phase with the Ocrl wild-type allele.
We designed a modified single-nucleotide polymerase extension (SNuPE) assay of the expressed Pgk1 SNP that differentially marked the two X chromosomes to measure allele-specific expression of the Pgk1 gene. A 25-bp primer was annealed directly adjacent to the site of the SNP that distinguishes the Pgk1 a allele from the Pgk1 b allele. dCTP and the chain-terminating radioactive dideoxyATP were the only nucleotides added to the single-cycle polymerase extension reaction. The Pgk1 b allele extension product incorporates just the chain-terminating dideoxyATP, resulting in a 26-base product, while the Pgk1 a allele extension product incorporates a dCTP followed by the chain-terminating dideoxyATP, resulting in a 27-base product.
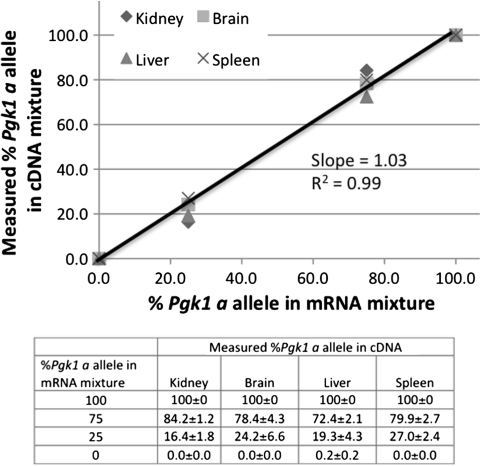
The SNUPE assay was calibrated by mixing cDNA made from mRNA from C57BL/6J (Pgk1 b allele) and the congenic strain B6.Cg-Pgk1 a /J in fixed ratios to mimic 100, 75, 25, and 0% of each allele. These results demonstrated the quantitative accuracy of the SNuPE reaction and densitometry analysis (Fig. 2), with a slope in the linear regression very close to 1 and R 2 for the regression of 0.99.
Fig. 2.
Calibration of SNuPE assay using various mixtures of Pgk1 a/a and Pgk1 b/b DNA consisting of 100% Pgk1 a/a, 75% Pgk1 a/a, 75% Pgk1 b/b, or 100% Pgk1 b/b. The actual numbers with standard deviations are given in the table at the bottom of the figure
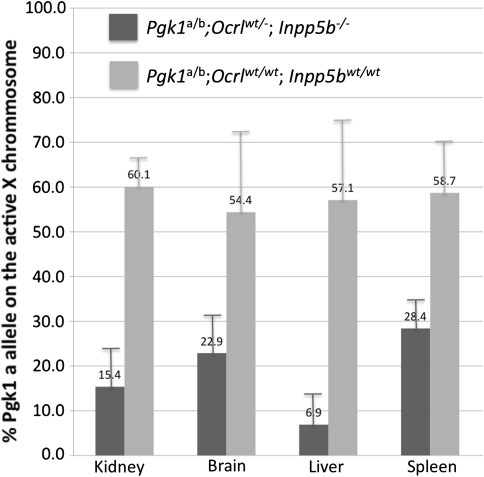
We then performed the SNuPE assay on RNA isolated from four tissues, liver, spleen, kidney, and brain, from 11 female Ocrl wt/−;Inpp5b −/− mice 8–28 weeks old. As controls, we also examined the X-inactivation pattern in RNA from tissues from seven females between 10 and 16 weeks old that were wild type for both Ocrl and Inpp5b but heterozygous for the two Pgk1 alleles. In the Ocrl wt/−;Inpp5b −/− mice, XCI was significantly skewed (P << 0.0001, two-sided Student’s t test) from the expected 70:30 under the null hypothesis of random inactivation in X controlling element (Xce) Xce a/c heterozygotes (Fig. 3) (Johnston and Cattanach 1981). The X chromosomes carrying the Pgk1 a and Ocrl − alleles were preferentially, but not exclusively, inactivated in Ocrl wt/−;Inpp5b −/− mice, despite carrying the somewhat stronger Xce c on the X chromosome carrying the Ocrl − allele. Liver demonstrated the most severe skewing: less than 6.9 ± 6.5% (mean ± SD) of the X chromosomes that carried the mutant Ocrl were active (P = 9.9 × 10−7, Student’s two-tailed t test). Kidney and brain had only 15.4 ± 8.6% and 22.9 ± 8.4%, respectively, of the mutant Ocrl chromosomes remaining active (for kidney tissue, P = 4.4 × 10−9; for brain tissue, P = 1.6 × 10−4, Student’s t test). Spleen showed the least amount of skewing of the four tissues examined, with the mutant X chromosome active in 28.4 ± 6.1% of chromosomes (P = 1.4 × 10−6, Student’s t test). The X-inactivation pattern in mRNA derived from the same four tissues from seven females that were wild type for both Ocrl and Inpp5b but heterozygous for the two Pgk1 alleles demonstrated that the Pgk1 a allele was on the active X chromosome 57.1 ± 18.8% SD of the time in liver, 60.1 ± 7.3% SD in kidney, 54.4 ± 18.8% SD in brain, and 58.7 ± 11.3% SD in spleen (Fig. 3). A fraction greater than 50% of cells with the Pgk1 a on their active X chromosome is as expected given that an X chromosome carrying the c allele of the X controlling element (Xce c), which is tightly linked to and in phase with the Pgk1 a allele, is more likely to be active in a female heterozygous for the a and c alleles (Xce a/c) (Cattanach and Papworth 1981; Krietsch et al. 1986). The more common Xce a allele is in phase with the Pgk1 b allele derived from the laboratory strain 129S4 (Courtier et al. 1995), the strain in which the disrupted Ocrl allele was originally made.
Fig. 3.
X-inactivation analysis by SNuPE assay. Results are shown as mean + 1 standard deviation of the expression for the Pgk1 a allele in kidney, brain, liver, and spleen, expressed as % of total label incorporated into SNuPE extension products. Measurements were made in tissues from Pgk1 a/b;Ocrl wt/−;Inpp5b −/− mice, where the Pgk1 a allele is in coupling phase with the disrupted Ocrl allele (dark shaded bars). X-inactivation analysis was performed in kidney, brain, spleen (11 individual mice), and liver (10 individual mice) from Pgk1 a/b;Ocrl wt/−;Inpp5b −/− mice. These same four tissues were analyzed in seven control Pgk1 a/b;Ocrl wt/wt;Inpp5b wt/wt mice (light shaded bars). P values for Pgk1 allele a expression in Pgk1 a/b;Ocrl wt/−;Inpp5b −/− mice compared to Pgk1 a/b;Ocrl wt/wt;Inpp5b wt/wt mice (controls) are P = 4.4 × 10−9 in kidney, 1.6 × 10−4 in brain, 9.9 × 10−7 in liver, and 1.4 × 10−6 in spleen (Student’s two-sided t test)
We also attempted a different breeding strategy to generate Ocrl wt/−;Inpp5b −/− mice in which we utilized Ocrl −/−;Inpp5b wt/− females, deficient in Ocrl and heterozygous for the targeted mutant Inpp5b, and crossed them to Ocrl wt/y;Inpp5b wt/− males, which expressed Ocrl and were heterozygous for the Inpp5b-targeted mutation. One-fourth of the resulting female zygotes would be expected to be Ocrl wt/−;Inpp5b −/−, with a functional Ocrl wt allele of paternal origin. In fact, we saw no live-born Ocrl wt/−;Inpp5b −/− females among a total of 53 female offspring from this cross (Table 2). This result differs significantly from an expected approximately 13 females according to the expected Mendelian ratio of 1:2:1 (P = 2.39 × 10−7, exact binomial test). Since the X chromosome of paternal origin is exclusively and nonrandomly inactivated in the trophoblast-derived extraembryonic tissues of Ocrl wt/−;Inpp5b −/− embryos as they develop (Takagi and Sasaki 1975), we conclude that these extraembryonic tissue lineages of Ocrl wt/−;Inpp5b −/− zygotes in which the Ocrl wt allele is paternal lacks both Ocrl and Inpp5b and is therefore unable to support embryonic development.
Table 2.
Females resulting from Ocrl −/−;Inpp5b wt/− female × Ocrl wt/y;Inpp5b wt/− male cross
| Gamete genotype | Live-born genotype | # Females observed | # Females expected | |
|---|---|---|---|---|
| Egg | Sperm | |||
| Ocrl −;Inpp5b wt | Ocrl wt;Inpp5b wt | Ocrl wt/−;Inpp5b wt/wt | 15 | 13.25 |
| Ocrl −;Inpp5b wt or Ocrl −;Inpp5b − | Ocrl wt;Inpp5b − or Ocrl wt;Inpp5b wt | Ocrl wt/−;Inpp5b wt/− | 38 | 26.5 |
| Ocrl −;Inpp5b − | Ocrl wt;Inpp5b − | Ocrl wt/−;Inpp5b −/− | 0* | 13.25 |
| Total | 53 | 53 | ||
* P = 2.39 × 10−7 (exact binomial test)
Despite the lack of females of the desired genotype from this cross, many Ocrl −/−;Inpp5b wt/− mice were produced, indicating that Ocrl −;Inpp5b − ova are generated, are capable of being fertilized, and can contribute to implantation and development of live mice, but only if the fertilizing sperm are functionally competent for Inpp5b.
We set up crosses between female Ocrl −/−;Inpp5b wt/− and male Ocrl −/y;Inpp5b wt/− and explanted 3.5-dpc blastocysts onto feeder layers to isolate embryonic stem cells. From these crosses we successfully isolated an embryonic stem cell clone from the inner cell mass of a Ocrl −/−;Inpp5b −/− 3.5-dpc blastocyst. These cells had a 40,XX karyotype and could be propagated with repeated passages. The very fact that we could isolate such a cell line indicates that zygotes deficient in both enzyme activities can progress at least to the epiblast stage and can develop an inner cell mass. These cells could also be propagated in culture; any attempt, however, to induce these cells to differentiate, such as by removing them from feeder cells, resulted in rapid cell death (data not shown).
Discussion
In a previous report we demonstrated that Ocrl −/−;Inpp5b −/− mice die prior to day E9.5 of embryogenesis (Janne et al. 1998). To explore this lethality, we attempted to generate Ocrl wt/−;Inpp5b −/− females in order to examine the pattern of X-inactivation since the cells in these mice that inactivated the X carrying the wild-type Ocrl allele would be deficient in both Ocrl and Inpp5b. When the crosses were designed so that the wild-type Ocrl allele was paternal in origin, there was a total absence of the desired Ocrl wt/−;Inpp5b −/− females at birth and the only embryos to survive were those rescued by the functional autosomal Inpp5b. In developing mouse embryos, X chromosome inactivation occurs through a random process in the embryo proper and in the primitive streak mesoderm-derived extraembryonic tissues such as the allantois, amnion, and mesoderm layer of the visceral yolk sac (Nagy 2003). However, X chromosome inactivation is imprinted by parent of origin in the trophoblast-derived extraembryonic tissues: the paternal X chromosome is preferentially inactivated in the extraembryonic tissues of the trophoblast, visceral endoderm, and parietal endoderm (Nagy 2003; Takagi and Sasaki 1975). We conclude that either the Ocrl PI(4,5)P2 5-phosphatase or the Inpp5b PI(4,5)P2 5-phosphatase must be present in the trophoblast-derived extraembryonic tissue of the developing mouse embryo in order for development to proceed, providing direct evidence that Ocrl and Inpp5b encode proteins with compensating and overlapping function in the placenta.
When the functional Ocrl in an Ocrl wt/−;Inpp5b −/− zygote was maternal in origin, live-born Ocrl wt/−;Inpp5b −/− female offspring were obtained and displayed no overt phenotype. This lack of phenotype can be explained by the preferential use of the maternal X chromosome with a wild-type Ocrl allele as the active chromosome. However, approximately half the expected number of Ocrl wt/−;Inpp5b −/− female offspring were seen. Assuming that X chromosome inactivation in these females is random, we hypothesize that the approximately 45% of female embryos in which the X chromosome coding for functional Ocrl and carrying the somewhat weaker Xce a allele associated with Pgk1 b is inactivated early in embryogenesis, do not survive. Those females that did survive with one copy of functional Ocrl of maternal origin but are deficient in Inpp5b all showed highly skewed XCI in all four tissues tested: liver, kidney, brain, and spleen. These tissues are derived from all three of the primary embryonic layers: endoderm, mesoderm, and ectoderm. Liver demonstrated the most severely skewed XCI pattern of the four tissues tested, with the mutant X active in only 6.9 ± 6.5% SD of the cells. Mouse liver is a tissue with low to moderate Ocrl expression but high Inpp5b expression (Janne et al. 1998). The lack of Inpp5b in liver tissue of Inpp5b −/− mice, which normally has high levels of Inpp5b, may greatly reduce liver cell survival such that the remaining moderate Ocrl expression from the X active chromosome might be a strong survival factor and thus explain the severely skewed X active coding for functional Ocrl. Mouse spleen expresses Ocrl at low levels and Inpp5b at low to moderate levels (Janne et al. 1998). However, we demonstrated that spleen also undergoes XCI skewing but less severely than the other tissues tested, allowing the mutant X to be active in 28.4 ± 6.1% SD of the cells. Ocrl expression is high in mouse brain and moderate in kidney, while Inpp5b expression is moderate in brain but high in kidney. In our experiments, kidney demonstrates more severe skewing than brain, correlating with the level of Inpp5b expression. We conclude from our experiments that tissues with normally high levels of expression of Inpp5b demonstrate the most severely skewed XCI.
It should be noted, however, that the skewing in these four tissues was not absolute. There are two possibilities to explain this observation. One is that most or all of the cell types in each of these organs can tolerate a small percentage of cells that are deficient in both enzymes. The second possibility is that these organs are made of a number of different cell types and certain types of cells in these organs, such as cells of hematopoietic origin, may be less subject to a deficiency of both enzymes. Detailed cytological methods would be needed to distinguish between these possibilities.
The XCI skewing in the control (Ocrl wt/wt;Inpp5b wt/wt) Xce a/c heterozygous mice used in this study ranged from approximately 40:60 to approximately 46:54 and was significantly less skewed than the 30:70 ratio found by others using Xce a/c mice (Johnston and Cattanach 1981). However, the parent of origin as well as the continued mixing of the genetic backgrounds from one generation to another can affect the Xce strength and degree of X chromosome skewing in mice heterozygous at the Xce locus (Chadwick and Willard 2005). We suspect that the highly mixed genetic background of the control mice used in our experiment, a mixture of 129S6, C57Bl/6J, and NIH Black Swiss, and the multiple generations of breeding used to produce them have influenced the XCI ratio as measured in our control population.
Of the two breeding schemes we used to generate Ocrl wt/−;Inpp5b −/− mice, as described in Tables 1 and 2, the first was successful and the second was not. However, both schemes were useful for demonstrating that doubly deficient Ocrl −;Inpp5b − gametes either from males or females are functional and capable of participating in fertilization. In particular, homozygous Inpp5b −/− male mice in the inbred 129S6 demonstrate a fertility defect (Hellsten et al. 2001, 2002); the defect is complemented in a mixed genetic background that includes FVB/N (Nussbaum, unpublished observations). The breeding scheme shown in Table 1 shows that the doubly deficient male gametes lacking both Ocrl and Inpp5b function in a mixed genetic background are indeed capable of fertilizing ova, which develop into embryos and give rise to live births. Although we suspect that the XCI is the key to the loss of a portion of our Ocrl wt/−;Inpp5b −/− females, we cannot entirely discount an effect from decreased production or fitness of doubly deficient male gametes.
By utilizing random X chromosome inactivation in embryos and nonrandom inactivation of the male X chromosome in trophoblast-derived extraembryonic tissues as an analytical tool, we have probed the basis for our previous observations on the prenatal lethality of doubly deficient Ocrl −/−;Inpp5b −/− or Ocrl −/y;Inpp5b −/− mice. We have shown that the two paralogs indeed have overlapping function in all three of the primary layers of the embryo and in the extraembryonic tissues and that the embryonic lethal effect of a double deficiency of Ocrl and Inpp5b occurs between 3.5 and 9.5 dpc.
Acknowledgments
This work was supported by the Division of Intramural Research of the National Human Genome Research Institute/NIH and by the Department of Medicine, University of California School of Medicine.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Attree O, Olivos IM, Okabe I, Bailey LC, Nelson DL, et al. The Lowe oculocerebrorenal syndrome gene encodes a novel protein highly homologous to inositol polyphosphate-5-phosphatase. Nature. 1992;358:239–242. doi: 10.1038/358239a0. [DOI] [PubMed] [Google Scholar]
- Boer PH, Potten H, Adra CN, Jardine K, Mullhofer G, et al. Polymorphisms in the coding and noncoding regions of murine Pgk-1 alleles. Biochem Genet. 1990;28(5/6):299–308. doi: 10.1007/BF02401419. [DOI] [PubMed] [Google Scholar]
- Cattanach BM, Papworth D. Controlling elements in the mouse. V. Linkage tests with X-linked genes. Genet Res. 1981;38:57–70. doi: 10.1017/S0016672300020401. [DOI] [PubMed] [Google Scholar]
- Chadwick LH, Willard HF. Genetic and parent-of-origin influences on X chromosome choice in Xce heterozygous mice. Mamm Genome. 2005;16:691–699. doi: 10.1007/s00335-005-0059-2. [DOI] [PubMed] [Google Scholar]
- Courtier B, Heard E, Avner P. Xce haplotypes show modified methylation in a region of the active X chromosome lying 3′ to Xist. Proc Natl Acad Sci USA. 1995;92:3531–3535. doi: 10.1073/pnas.92.8.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellsten E, Evans JP, Bernard DJ, Janne PA, Nussbaum RL. Disrupted sperm function and fertilin beta processing in mice deficient in the inositol polyphosphate 5-phosphatase Inpp5b. Dev Biol. 2001;240:641–653. doi: 10.1006/dbio.2001.0476. [DOI] [PubMed] [Google Scholar]
- Hellsten E, Bernard DJ, Owens JW, Eckhaus M, Suchy SF, et al. Sertoli cell vacuolization and abnormal germ cell adhesion in mice deficient in an inositol polyphosphate 5-phosphatase. Biol Reprod. 2002;66:1522–1530. doi: 10.1095/biolreprod66.5.1522. [DOI] [PubMed] [Google Scholar]
- Heng HH, Tsui LC. Modes of DAPI banding and simultaneous in situ hybridization. Chromosoma. 1993;102:325–332. doi: 10.1007/BF00661275. [DOI] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the mouse embryo: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- Janne PA, Suchy SF, Bernard DJ, McDonald M, Crawley J, et al. Functional overlap betweeen murine Inpp5b and Ocrl1 may explain why deficiency of the murine ortholog for OCRL1 does not cause Lowe syndrome in mice. J Clin Invest. 1998;101(10):2042–2053. doi: 10.1172/JCI2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson AB, Majerus PW. Properties of type II inositol polyphosphate 5-phosphatase. J Biol Chem. 1995;270:9370–9377. doi: 10.1074/jbc.270.16.9370. [DOI] [PubMed] [Google Scholar]
- Johnston PG, Cattanach BM. Controlling elements in the mouse. IV. Evidence of non-random X-inactivation. Genet Res. 1981;37:151–160. doi: 10.1017/S0016672300020127. [DOI] [PubMed] [Google Scholar]
- Krietsch WK, Fehlau M, Renner P, Bucher T, Fundele R. Expression of X-linked phosphoglycerate kinase in early mouse embryos homozygous at the Xce locus. Differentiation. 1986;31:50–54. doi: 10.1111/j.1432-0436.1986.tb00382.x. [DOI] [PubMed] [Google Scholar]
- Nagy A. Manipulating the mouse embryo: a laboratory manual. 3. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2003. [Google Scholar]
- Nielsen JT, Chapman VM. Electrophoretic variation for X-chromosome-linked phosphoglycerate kinase (Pgk-1) in the mouse. Genetics. 1977;87:319–325. doi: 10.1093/genetics/87.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross TS, Jefferson AB, Mitchell CA, Majerus PW. Cloning and expression of human 75-kDa inositol polyphosphate-5-phosphatase. J Biol Chem. 1991;266(30):20283–20289. [PubMed] [Google Scholar]
- Schweizer D. Simultaneous fluorescent staining of R bands and specific heterochromatic regions (DA-DAPI bands) in human chromosomes. Cytogenet Cell Genet. 1980;27:190–193. doi: 10.1159/000131482. [DOI] [PubMed] [Google Scholar]
- Schweizer D. Counterstain-enhanced chromosome banding. Hum Genet. 1981;57:1–14. [PubMed] [Google Scholar]
- Shanmugam V, Chapman VM, Sell KW, Saha BK. A novel Tth111I restriction fragment length polymorphism (RFLP) allows tracing of X-chromosome inactivation in the (Xid) heterozygote. Biochem Genet. 1996;34:17–29. doi: 10.1007/BF02396237. [DOI] [PubMed] [Google Scholar]
- Singer-Sam J, LeBon JM, Dai A, Riggs AD. A sensitive, quantitative assay for measurement of allele-specific transcripts differing by a single nucleotide. PCR Methods Appl. 1992;1:160–163. doi: 10.1101/gr.1.3.160. [DOI] [PubMed] [Google Scholar]
- Suchy SF, Nussbaum RL. Oculocerebrorenal syndrome of Lowe. In: Valle D, Beaudet AL, Vogelstein B, Kinzer K, Antonorakis S, Ballabio A, editors. The online metabolic and molecular bases of inherited disease. New York: MacMillan; 2009. [Google Scholar]
- Takagi N, Sasaki M. Preferential inactivation of the paternally derived X chromosome in the extraembryonic membranes of the mouse. Nature. 1975;256:640–642. doi: 10.1038/256640a0. [DOI] [PubMed] [Google Scholar]
- Thomas KR, Deng C, Capecchi MR. High-fidelity gene targeting in embryonic stem cells by using sequence replacement vectors. Mol Cell Biol. 1992;12:2919–2923. doi: 10.1128/mcb.12.7.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Jefferson AB, Auethavekiat V, Majerus PW. The protein deficient in Lowe syndrome is a phosphatidylinositol-4,5-bisphosphate 5-phosphatase. Proc Natl Acad Sci USA. 1995;92:4853–4856. doi: 10.1073/pnas.92.11.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]