Abstract
Diabetic retinopathy is the leading cause of vision loss among working-age people in the United States. The hallmark of diabetic retinopathy is vascular compromise. Increased vascular permeability leads to the development of diabetic macular edema, which is the major cause of vision loss in patients with diabetic retinopathy. Vascular occlusion causes retinal ischemia and subsequent angiogenesis (proliferative diabetic retinopathy), which increases the risk for vitreous hemorrhage and retinal detachment. Over the past 30 years our understanding of the pathophysiology of diabetic retinopathy has evolved greatly and has fostered the development of many novel treatments for this condition. This article will review promising new local and systemic pharmacologic treatments for diabetic macular edema and proliferative diabetic retinopathy.
Keywords: Corticosteroid, Diabetes, Diabetic retinopathy, Macular edema, Proliferative diabetic retinopathy, Vascular endothelial growth factor
Introduction
Diabetic retinopathy is the leading cause of visual loss among working-age people in the United States (1). The prevalence of diabetic retinopathy increases with the duration and severity of diabetes (2, 3) and with the degree of hypertension and hyperlipidemia (4). The hallmark of diabetic retinopathy is vascular injury. Increased vascular permeability leads to the development of retinal hemorrhages and fluid accumulation in the macula, which is referred to as diabetic macular edema. Vascular occlusion causes retinal ischemia and subsequent angiogenesis, which defines proliferative diabetic retinopathy.
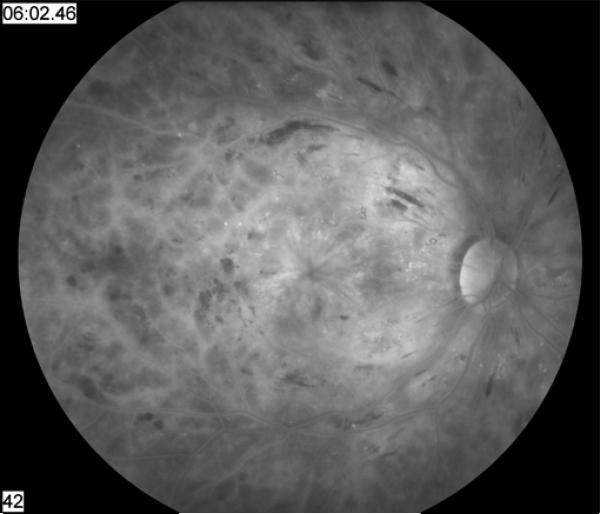
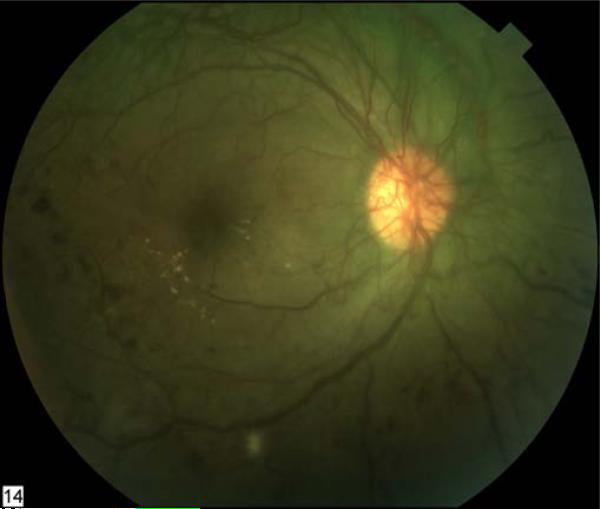
Diabetic macular edema (Figure 1) is the principal cause of visual loss in persons with diabetes (3). Thirty years ago, laser photocoagulation was demonstrated effective for limiting vision loss in patients with diabetic macular edema (5) and proliferative diabetic retinopathy (Figure 2) (6). However, some patients continue to lose vision despite intervention. Since that time, our understanding of the pathophysiology of diabetic retinopathy has evolved greatly and has fostered the development of many novel treatments for this condition. This article will review promising new local and systemic pharmacologic treatments for diabetic macular edema and proliferative diabetic retinopathy.
Figure 1. Florid diabetic macular edema in the right eye of a young patient with type I diabetes demonstrated by hyperfluorescent macular leakage late in the fluorescein angiogram.
Note the increased vascular permeability of the retinal vessels as a result of increased levels of vascular endothelial growth factor.
Figure 2. Advanced proliferative diabetic retinopathy with neovascularization of the disc.
Scattered retinal hemorrhages, macular exudates, and a cotton-wool spot are present. This fundus image of the right eye appears green because of the increased permeability of the abnormal vessels to fluorescein following an angiogram.
Local Therapies
Anti-inflammatory agents
Intravitreal triamcinolone acetonide (IVTA) has potent anti-inflammatory effects and yields short-term anatomic and visual improvements in diabetic macular edema (7-11). Given its anti-angiogenic properties, IVTA also could be a valuable adjunct to treating proliferative diabetic retinopathy (12). Branded preservative-free formulations of triamcinolone acetonide, Triesence (Alcon, Fort Worth, Texas, US) and Trivaris (Allergan, Irvine, California, US) were developed to in an effort to lessen the incidence of noninfectious endophthalmitis and other complications.
The Diabetic Retinopathy Clinical Research (DRCR) Network investigated the anatomic and visual outcomes of two doses (1mg and 4mg) of Trivaris vs. macular photocoagulation for the treatment of diabetic macular edema in a large, multicenter randomized clinical trial (RCT). After 3 years of follow-up, treatment with macular photocoagulation was associated with better vision and fewer complications. Two of the major complications of IVTA are cataract formation and ocular hypertension. In the above study, the 3 year cumulative probability of cataract surgery was 31% in the laser group, 46% in the 1mg IVTA group and 83% in the 4mg IVTA group. Among patients followed for 3 years, 4 % of the laser group, 18% of the 1mg group and 33% of the 4mg group experienced an intraocular pressure increase of greater than 10mmHg. Four patients (all in the 4mg IVTA group) required a glaucoma procedure (13). The rate of endophthalmitis in patients enrolled in the DRCRnet and SCORE (Standard care versus COrticosteroid for REtinal vein occlusion study) trials was 1/2009 injections (0.05%) (14).
Given the chronic nature of diabetic macular edema, there may be a role for sustained-release corticosteroid therapy. Several sustained-release delivery systems are currently in clinical trials. The triamcinolone-eluting intravitreal implant, I-vation (Surmodics, Inc., Eden Prairie, Minnesota, US) has been studied for the treatment of diabetic macular edema, and a Phase II clinical trial is planned. A fluocinolone acetonide intravitreal implant, Retisert (Bausch & Lomb, Rochester, New York, US) is FDA-approved for the treatment of chronic, non-infectious uveitis. A phase III clinical trial conducted in patients with DME reported high rates of cataract and glaucoma. A phase III clinical trial is underway for a smaller fluocinolone acetonide intravitreal implant, Iluvien (Alimera Sciences, Alpharetta, Georgia, United States) that can be administered in an office setting. Ozurdex (Allergan, Irvine, California, US) is an extended release biodegradable dexamethasone intravitreal implant that has recently received FDA approval in treating macular edema secondary to retinal vein occlusions. Promising results from the Phase II trial involving DME patients have led to the Phase III trial which is underway.
Nepafenac (Nevanac, Alcon, Fort Worth Texas, US) is an FDA-approved topical non-steroidal anti-inflammatory drug that has demonstrated efficacy against DME in one case report (15).
Etanercept (Enbrel, Amgen, Inc., Thousand Oaks, California, US and Wyeth, Madison, New Jersey, US) is a recombinant fusion protein with activity against TNF-α and is FDA-approved for the treatment of psoriasis (16). A small series of patients with refractory DME were treated with intravitreal etanercept with no statistically significant improvement (17). Infliximab (Remicade, Centocor, Horsham, PA, US) is another TNF-α antagonist that is FDA-approved to treat Crohn's disease. An investigation of systemic treatment of DME with infliximab (18) has led a study of administration via intravitreal injection.
Anti-Vascular Endothelial Growth Factor (VEGF) agents
Bevacizumab (Avastin, Genentech, Inc., South San Francisco, California US) is a full-length recombinant humanized antibody against VEGF-A and is an FDA-approved systemic treatment for metastatic colon cancer. Several prospective RCTs of intravitreal bevacizumab have demonstrated favorable anatomic and visual outcomes in patients with DME, including a Phase II clinical trial from the DRCR network (19-21). In two small, comparative trials, intravitreal triamcinolone was associated with better efficacy and longer duration of action in the treatment of DME than was bevacizumab (22, 23). Avery et al (2006) reported regression/resolution of iris and retinal neovascularization following intravitreal Avastin. However, recurrence of neovascularization was noticed as early as two weeks after treatment, which is its major shortcoming when compared to panretinal photocoagulation (24). Bevacizumab is gaining popularity as a clinical adjunct to panretinal photocoagulation in certain patients with PDR (25). Of note, patients have reportedly experienced traction retinal detachments following treatment with bevacizumab for PDR (26).
Ranibizumab (Lucentis, Genentech, Inc., South San Francisco, California US) is a recombinant humanized antibody fragment against VEGF-A and is FDA-approved for the treatment of exudative age-related macular degeneration (27, 28). Ranibizumab demonstrates some efficacy in the treatment of DME (29). The READ-1 study was a prospective, nonrandomized case series of 10 patients with chronic DME who were treated with ranibizumab at baseline and months 1, 2, 4, and 6. An improvement in visual acuity and mean foveal thickness was noted at month 7 (30). A larger phase II trial is currently underway (READ-2) that will compare laser photocoagulation and a combination of ranibizumab plus laser for DME. RESOLVE is a randomized double-masked, multicenter, phase II study that will assess the safety and efficacy of two concentrations of intravitreal ranibizumab compared with nontreatment control for the treatment of center-involving DME. Phase III trials (RISE and RIDE) will compare intravitreal ranibizumab to laser photocoagulation for DME. The DRCR network has several studies in progress that investigate the role of ranibizumab in the treatment of diabetic retinopathy, including IVTA vs. ranibizumab as an adjunct to panretinal photocoagulation for PDR and ranibizumab vs. IVTA in combination with laser for DME.
Anti-VEGF agents, like bevacizumab and ranibizumab, are appealing alternative treatments given their lower risks of intraocular pressure elevation and cataract formation when compared to intravitreal corticosteroids. The cumulative rates of endophthalmitis in the major ranibizumab trials, where scheduled dosing was monthly for two years, was 5/477 (1.0%) (27) and 2/277 (0.7%) (28) per eye over the course of the studies. The per-injection rates of endophthalmitis were much lower, about 0.05% per injection.
VEGF Trap-Eye (Regeneron, Tarrytown, New York, US) is a potential treatment for diabetic retinopathy that has demonstrated anti-VEGF activity (Regeneron, Tarrytown, New York, US). VEGF Trap-Eye is a recombinant fusion protein active against VEGF-A and placental growth factor. Results from the phase I trial show short-term efficacy for the treatment of DME (31).
Sirolimus, also known as rapamycin (Rapamune, Wyeth, Madison, New Jersey, US) is a macrolide with immunosuppressive and anti-VEGF activity. Systemic rapamycin has been shown to inhibit choroidal neovascularization in mice (32). A National Eye Institute sponsored pilot trial of intravitreal sirolimus is currently recruiting patients (http://clinicalstudies.info.nih.gov/detail/A_2008-EI-0175.html).
Vitreolytic agents
Intravitreal purified ovine hyaluronidase (Vitrase, ISTA Pharmaceuticals, Irvine, California, US) has shown efficacy and safety in a Phase III clinical trial to investigate its promotion of the clearance of vitreous hemorrhage from PDR, (33,34) although the agent is not FDA-approved for this purpose. The induction of a posterior vitreous detachment also could be beneficial in the treatment of DME and PDR (35). A multicenter study to compare multiple doses of intravitreal microplasmin versus sham injection for treatment of patients with DME (MIVI-II) is currently underway.
Systemic Therapies
Ruboxistaurin
Ruboxistaurin (Arxxant, Eli Lilly and Company, Indianapolis, Indiana, US) is an oral antagonist of the beta subunit of protein kinase C which may be important in the pathogenesis of diabetic retinopathy (36, 37). Therapy with ruboxistaurin is associated with a reduction in the progression of DME and a reduction in the rate of vision loss in patients with DME, (38, 39) although ruboxistaurin has not received FDA approval.
Fenofibrate
Fenofibrate is most commonly used as a treatment for hyperlipidemia. Patients treated with fenofibrate have been shown to require less photocoagulation for PDR and DME (40). This result was unrelated to serum lipid levels, which were statistically similar in both the group treated with fenofibrate and the control group (41).
Somatostatin analogues
Somatostatin is an endogenous growth hormone inhibitor with anti-angiogeneic properties. The somatostatin analogue octreotide (Sandostatin, Novartis, Basel, Switzerland) has been associated with decreased rates of progression to high-risk PDR, (42) vitreous hemorrhage and the need for vitrectomy (43) in patients with at least severe nonproliferative diabetic retinopathy (NPDR).
Conclusion
Laser photocoagulation partnered with improved glycemic, blood pressure, and cholesterol control are the standard treatments for diabetic retinopathy. However, diabetic macular edema can be chronic and refractory to standard treatments. Vitreous hemorrhage, dense cataract, and neovascular glaucoma may prevent timely laser treatment of proliferative diabetic retinopathy. For these reasons, alternative and adjunctive therapies are being explored. Over the next few years, more information will be gained regarding efficacy and safety of investigational treatments. We await the clarification of their role in our future treatment algorithms.
Acknowledgement
Partially supported by NIH center grant P30-EY014801 and by an unrestricted grant to the University of Miami from Research to Prevent Blindness, New York, NY.
Footnotes
Conflicts of Interest
Stephen G. Schwartz, M.D., M.B.A. has previously received research funding from Genentech, owns equity in Pfizer, and is co-holder of a patent pending entitled “Molecular targets for modulating intraocular pressure and differentiation of steroid responders versus nonresponders.” Jaclyn L. Kovach, M.D. is a consultant for Allergan.
References
- 1.US Centers for Disease Control and Prevention . National diabetes fact sheet:general information and national estimates on diabetes in the United States, 2005. Centers for Disease Control and Prevention; Atlanta: 2005. [Google Scholar]
- 2.Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy and associated risk factors in type I diabetes. Ophthalmology. 1998;105:1801–15. doi: 10.1016/S0161-6420(98)91020-X. [DOI] [PubMed] [Google Scholar]
- 3.Diabetes Control and Complication Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 4.UK Prespective Diabetes Study Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type II diabetes. UKPDS 33. Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 5.Early Treatment Diabetic Retinopathy Study Resesarch Group Photocoagulation for diabetic macular edema: Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103:1796–806. [PubMed] [Google Scholar]
- 6.The Diabetic Retinopathy Study Research Group Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS report number 8. The Diabetic Retinopathy Study Research Group. Ophthalmology. 1981;88:583–600. [PubMed] [Google Scholar]
- 7.Gillies MC, Sutter FK, Simpson JM, et al. Intravitreal triamcinolone for refractory diabetic macular edema: two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology. 2006;113:1533–8. doi: 10.1016/j.ophtha.2006.02.065. [DOI] [PubMed] [Google Scholar]
- 8.Lam DS, Chan CK, Mohamed S, et al. A prospective randomized trial of different doses of intravitreal triamcinolone for diabetic macular edema. Br J Ophthalmol. 2007;91:199–203. doi: 10.1136/bjo.2006.102848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam DS, Chan CK, Mohamed S, et al. Intravitreal triamcinolone plus sequential grid laser versus triamcinolone or laser alone for treating diabetic macular edema:six month outcomes. Ophthalmology. 2007;114:2162–7. doi: 10.1016/j.ophtha.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Kim JE, Pollack JS, Miller DG, et al. Isis Study group ISIS-DME: a prospective, randomized, dose-escalation intravitreal steroid injection study for refractory diabetic macular edema. Retina. 2008;28:735–40. doi: 10.1097/IAE.0b013e318163194c. [DOI] [PubMed] [Google Scholar]
- 11.Dehghan MH, Ahmadieh H, Ramezani A, et al. A randomized, placebo-controlled clinical trial of intravitreal triamcinolone for refractory diabetic macular edema. Int Ophthalmol. 2008;28:7–17. doi: 10.1007/s10792-007-9097-y. [DOI] [PubMed] [Google Scholar]
- 12.Bandello F, Polito A, Pognuz DR, et al. Triamcinolone as adjunctive treatment to laser photocoagulation for proliferative diabetic retinopathy. Arch Ophthalmol. 2006;124:643–50. doi: 10.1001/archopht.124.5.643. [DOI] [PubMed] [Google Scholar]
- 13.Diabetic Retinopathy Clinical Research Network (DRCR.net) Beck RW, Edwards AR, Aiello LP, et al. Three year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol. Mar. 2009;127(3):245–51. doi: 10.1001/archophthalmol.2008.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhavsar AR, Ip MS, Glassman AR, DRCRnet and the SCORE Study groups Am J Ophthalmol. 2007 Sep;144(3):454–6. doi: 10.1016/j.ajo.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hariprasad SM, Callanan D, Gainey S, et al. Cystoid and diabetic macular edema treated with nepafanac 0.1%. J Ocul Pharmacol Ther. 2007;23:585–90. doi: 10.1089/jop.2007.0062. [DOI] [PubMed] [Google Scholar]
- 16.Ducharme E, Weinberg JM. Etanercept. Expert Opin Biol Ther. 2008;8:491–502. doi: 10.1517/14712598.8.4.491. [DOI] [PubMed] [Google Scholar]
- 17.Tsilimbaris MK, Panagiotoglou TD, CHarisis SK, et al. The use of intravitreal etanercept in diabetic macular edema. Semin Ophthalmol. 2007;22:75–9. doi: 10.1080/08820530701418243. [DOI] [PubMed] [Google Scholar]
- 18.Sfikais PP, Markomichelakis N, Theodossiadis PG, et al. Regression of sight-threatening macular edema in type 2 diabetes following treatment with the anti-tumor necrosis factor monoclonal antibody infliximab. Diabetes Care. 2005;28:445–7. doi: 10.2337/diacare.28.2.445. [DOI] [PubMed] [Google Scholar]
- 19.Ahmadieh H, Ramezani A, Shoeibi N, et al. Intravitreal bevacizumab with or without triamcinolone for refractory diabetic macular edema; a placebo controlled trial. Graefes Arch Clin Exp Ophthalmol. 2008;246:483–9. doi: 10.1007/s00417-007-0688-0. [DOI] [PubMed] [Google Scholar]
- 20.Scott IU, Edwards AR, et al. Diabetic Retinopathy Clinical Research Network, A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007;114:186–7. doi: 10.1016/j.ophtha.2007.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soheilian M, Ramezani A, Bijanzadeh B, et al. Intravitreal bevacizumab (Avastin) injection alone or combined with triamcinolone versus macular photocoagulation as primary treatment of diabetic macular edema. Retina. 2007;27:1187–95. doi: 10.1097/IAE.0b013e31815ec261. [DOI] [PubMed] [Google Scholar]
- 22.Paccola L, Costa RA, Folgosa MS, et al. Intravitreal triamcinolone versus bevacizumab for treatment of refractory diabetic macular edema (IBEME study). Br J Ophthalmol. 2008;92:76–80. doi: 10.1136/bjo.2007.129122. [DOI] [PubMed] [Google Scholar]
- 23.Shimura M, Nakazawa T, Yasuda K, et al. Comparative therapy evaluation of intravitreal bevacizumab and triamcinolone acetonide on persistent diffuse diabetic macular edema. Am J Ophthalmol. 2008;145:854–61. doi: 10.1016/j.ajo.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 24.Avery RL, Pearlman J, Pierimici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113(10):1695–1705. e1–15. doi: 10.1016/j.ophtha.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 25.Mirshahi A, Roohipoor R, Lashay A, et al. Bevacizumab-augmented retinal laser photocoagulation in proliferative diabetic retinopathy:a randomized double-masked clinical trial. Eur J Ophthalmol. 2008;18:263–9. doi: 10.1177/112067210801800215. [DOI] [PubMed] [Google Scholar]
- 26.Arevalo JF, Maia M, Flynn HW, Jr, et al. Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol. 2008;92:213–6. doi: 10.1136/bjo.2007.127142. [DOI] [PubMed] [Google Scholar]
- 27.Rosenfeld PR, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 28.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 29.Chun DW, Heier JS, Topping TM, et al. A pilot study of multiple intravitreal injections of ranibizumab in patients with center-involving clinically significant diabetic macular edema. Ophthalmology. 2006;113:1706–12. doi: 10.1016/j.ophtha.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen QD, Tatlipinar S, Shah SM, et al. Vascular endothelial growth factor is a critical stimulus for diabetic macular edema. Am J Opthalmol. 2006;142:961–969. doi: 10.1016/j.ajo.2006.06.068. [DOI] [PubMed] [Google Scholar]
- 31.Do DV, Nguyen Qd, Browning DJ, et al. An exploratory study of the safety, tolerability and biological effect of a single intravitreal administration of vascular endothelial growth factor trap-eye in patients with diabetic macular edema. Br J Ophthalmol. 2009;93:144–9. doi: 10.1136/bjo.2008.138271. [DOI] [PubMed] [Google Scholar]
- 32.Dejneka NS, Kuroki AM, Fosnot J, et al. Systemic rapamycin inhibits retinal and choroidal neovascularization in mice. Mol Vis. 2004;10:964–72. [PubMed] [Google Scholar]
- 33.Kupperman BD, Thomas EL, de Smet MD, Grillone LR. Vitrase for Vitreous Hemorrhage Study Groups. Pooled efficacy results from two multinational randomized controlled clinical trials of a single intravitreous injection of highly purified ovine hyaluronidase (Vitrase) for the management of vitreous hemorrhage. Am J Ophthalmol. 2005;140:573–84. doi: 10.1016/j.ajo.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 34.Kupperman BD, Thomas EL, de Smet MD, Grillone LR. Vitrase for Vitreous Hemorrhage Study Groups. Safety results of two phase III trials of an intravitreous injection of highly purified ovine hyaluronidase (Vitrase) for the management of vitreous hemorrhage. Am J Ophthalmol. 2005;140:585–97. doi: 10.1016/j.ajo.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 35.Lopez-Lopez F, Rodriguez-Blanco M, Gomez-Ulla F, Marticorena J. Enzymatic vitreolysis. Curr Diabetes Rev. 2009;5:57–62. doi: 10.2174/157339909787314220. [DOI] [PubMed] [Google Scholar]
- 36.Aiello LP. The potential role of PKC β in diabetic retinopathy and macular edema. Surv Ophthalmol. 2002;47(Suppl 2):S263–9. doi: 10.1016/s0039-6257(02)00391-0. [DOI] [PubMed] [Google Scholar]
- 37.Donnelly R, Idris I, Forrester JV. Protein kinase C inhibition and diabetic retinopathy: a shot in the dark at translational research. Br J Ophthalmol. 2004;88:145–51. doi: 10.1136/bjo.88.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.PKC-DMES Study Group Effect of ruboxistaurin in patients with diabetic macular edema: thirty-month results of the randomized PKC-DMES clinical trial. Arch Ophthalmol. 2007;125:318–24. doi: 10.1001/archopht.125.3.318. [DOI] [PubMed] [Google Scholar]
- 39.Davis MD, Sheetz MJ, Aiello LP, et al. Effect of ruboxistaurin on the visual acuity decline associated with longstanding diabetic macular edema. Invest Ophthalmol Vis Sci. 2009;50:1–4. doi: 10.1167/iovs.08-2473. [DOI] [PubMed] [Google Scholar]
- 40.Keech AC, Mitchell P, Summanen PA, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomized controlled trial. Lancet. 2007;370:1687–97. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 41.Keech A, SImes RJ, Parter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study):randomized controlled trial. Lancet. 2005;366:1849–61. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 42.Grant MB, Mames RN, Fitzgerald C, et al. The efficacy of octreotide in the therapy of severe nonproliferative and early proliferative diabetic retinopathy:a randomized controlled study. Diabetes Care. 2000;23:504–9. doi: 10.2337/diacare.23.4.504. [DOI] [PubMed] [Google Scholar]
- 43.Boehm BO, Lang GK, Jehle PM, et al. Octreotide reduces vitreous hemorrhage and loss of visual acuity risk in patients with high-risk proliferative diabetic retinopathy. Horm Metab Res. 2001;33:300–6. doi: 10.1055/s-2001-15282. [DOI] [PubMed] [Google Scholar]