Abstract
Rifabutin, a semisynthetic derivative of rifamycin S, was examined alone and in combination with other drugs for activity in treatment of systemic toxoplasmosis and toxoplasmic encephalitis in murine models. One hundred percent of the mice infected with a lethal inoculum of tachyzoites or cysts of Toxoplasma gondii were protected against death by treatment with doses of 400 or 300 mg of rifabutin per kg administered alone for 10 days. Doses of 200 mg/kg protected at least 80% of the mice, and doses of 100 mg/kg protected 10 to 40% of the infected mice against death. Doses of 50 mg/kg were not protective but caused a delay in time to death. Combination of nonprotective (50-mg/kg) or slightly protective (100-mg/kg) doses of rifabutin with doses of sulfadiazine, pyrimethamine, clindamycin, or atovaquone that did not confer any protection against death from toxoplasmosis when administered alone resulted in remarkable enhancement of the in vivo activities of all of these drugs. Seventy-five percent of the infected mice survived when treated with 100 mg of rifabutin per kg per day combined with the ineffective dose of 10 mg of pyrimethamine per kg. A dose of 50 mg of rifabutin per kg in combination with the ineffective dosages of clindamycin (25 mg/kg/day), atovoquone (5 mg/kg/day), and sulfadiazine (80 mg per liter of drinking water) protected at least 80, 60, and 60% of the mice against death, respectively. The inflammatory responses in the brains of mice treated for 30 days with 200 mg of rifabutin per kg per day were significantly reduced compared with those in the brains of untreated controls. These observations suggest that clinical trials with rifabutin for treatment and prevention of human toxoplasmosis may be justified, particularly when the drug is used in combination with other drugs with activity against T. gondii.
Full text
PDF
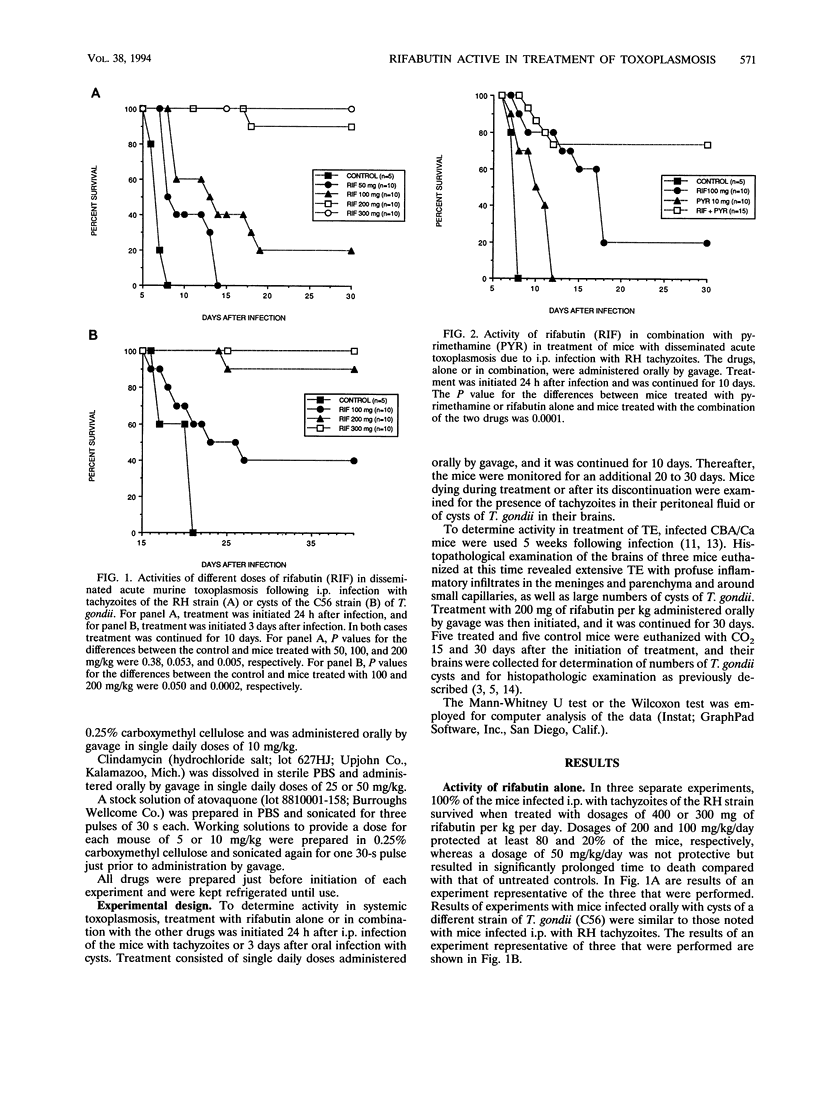
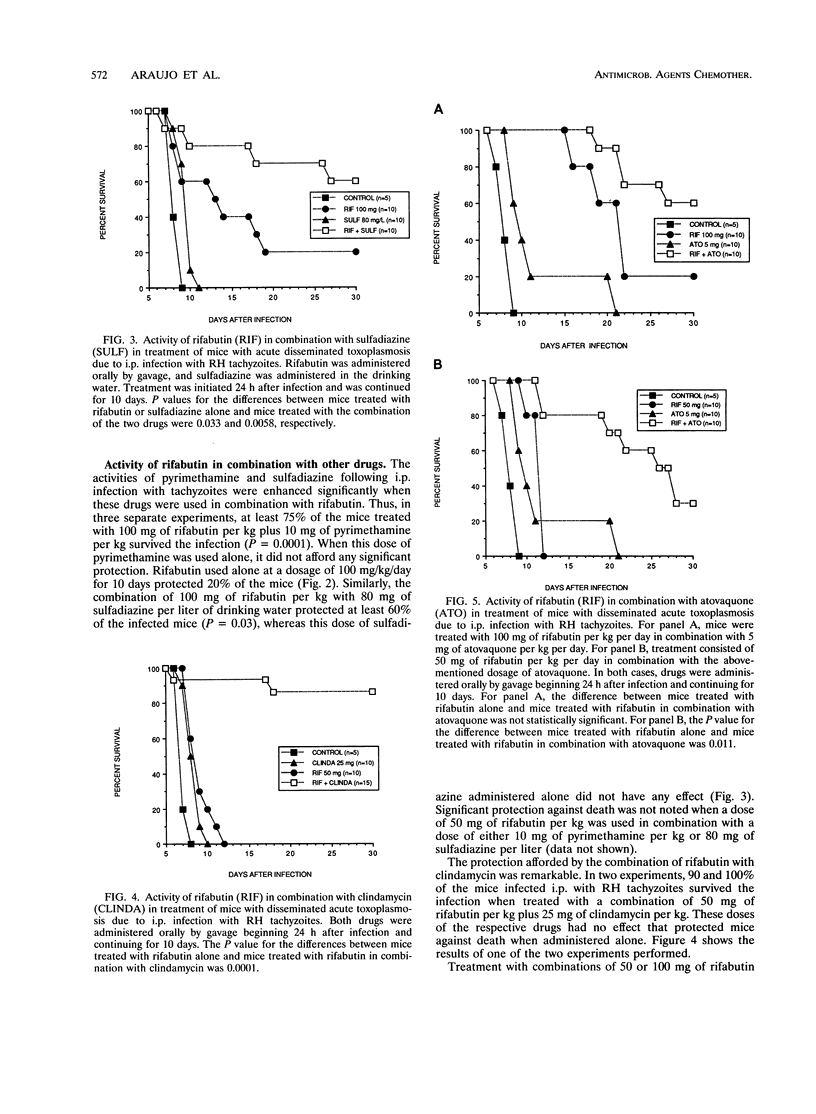
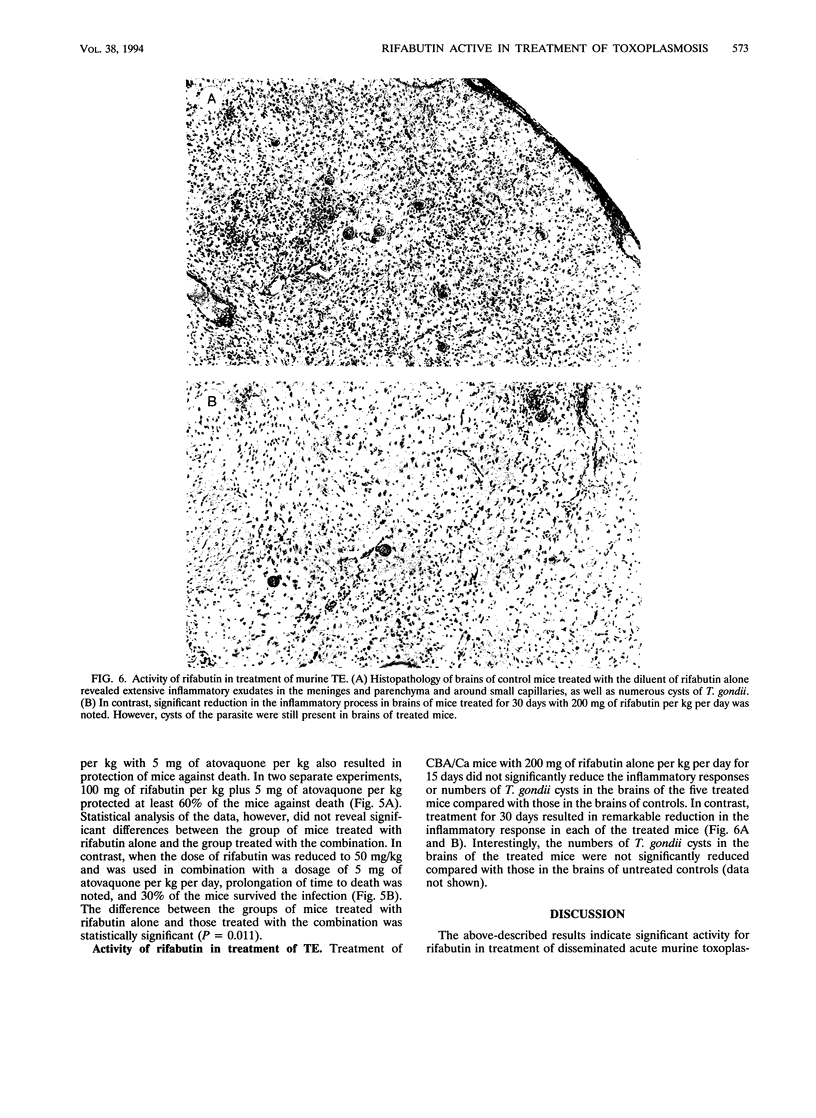


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araujo F. G. Depletion of CD4+ T cells but not inhibition of the protective activity of IFN-gamma prevents cure of toxoplasmosis mediated by drug therapy in mice. J Immunol. 1992 Nov 1;149(9):3003–3007. [PubMed] [Google Scholar]
- Araujo F. G., Huskinson-Mark J., Gutteridge W. E., Remington J. S. In vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against the cyst form of Toxoplasma gondii. Antimicrob Agents Chemother. 1992 Feb;36(2):326–330. doi: 10.1128/aac.36.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo F. G., Huskinson J., Remington J. S. Remarkable in vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against tachyzoites and tissue cysts of Toxoplasma gondii. Antimicrob Agents Chemother. 1991 Feb;35(2):293–299. doi: 10.1128/aac.35.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo F. G., Lin T., Remington J. S. The activity of atovaquone (566C80) in murine toxoplasmosis is markedly augmented when used in combination with pyrimethamine or sulfadiazine. J Infect Dis. 1993 Feb;167(2):494–497. doi: 10.1093/infdis/167.2.494. [DOI] [PubMed] [Google Scholar]
- Araujo F. G., Prokocimer P., Remington J. S. Clarithromycin-minocycline is synergistic in a murine model of toxoplasmosis. J Infect Dis. 1992 Apr;165(4):788–788. doi: 10.1093/infdis/165.4.788. [DOI] [PubMed] [Google Scholar]
- Araujo F. G., Shepard R. M., Remington J. S. In vivo activity of the macrolide antibiotics azithromycin, roxithromycin and spiramycin against Toxoplasma gondii. Eur J Clin Microbiol Infect Dis. 1991 Jun;10(6):519–524. doi: 10.1007/BF01963942. [DOI] [PubMed] [Google Scholar]
- Chiodini R. J., Kreeger J. M., Thayer W. R. Use of rifabutin in treatment of systemic Mycobacterium paratuberculosis infection in mice. Antimicrob Agents Chemother. 1993 Aug;37(8):1645–1648. doi: 10.1128/aac.37.8.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannemann B. R., Israelski D. M., Remington J. S. Treatment of toxoplasmic encephalitis with intravenous clindamycin. Arch Intern Med. 1988 Nov;148(11):2477–2482. [PubMed] [Google Scholar]
- Dannemann B., McCutchan J. A., Israelski D., Antoniskis D., Leport C., Luft B., Nussbaum J., Clumeck N., Morlat P., Chiu J. Treatment of toxoplasmic encephalitis in patients with AIDS. A randomized trial comparing pyrimethamine plus clindamycin to pyrimethamine plus sulfadiazine. The California Collaborative Treatment Group. Ann Intern Med. 1992 Jan 1;116(1):33–43. doi: 10.7326/0003-4819-116-1-33. [DOI] [PubMed] [Google Scholar]
- Ellner J. J., Goldberger M. J., Parenti D. M. Mycobacterium avium infection and AIDS: a therapeutic dilemma in rapid evolution. J Infect Dis. 1991 Jun;163(6):1326–1335. doi: 10.1093/infdis/163.6.1326. [DOI] [PubMed] [Google Scholar]
- Horsburgh C. R., Jr Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991 May 9;324(19):1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- Hughes W. T., Kennedy W., Shenep J. L., Flynn P. M., Hetherington S. V., Fullen G., Lancaster D. J., Stein D. S., Palte S., Rosenbaum D. Safety and pharmacokinetics of 566C80, a hydroxynaphthoquinone with anti-Pneumocystis carinii activity: a phase I study in human immunodeficiency virus (HIV)-infected men. J Infect Dis. 1991 Apr;163(4):843–848. doi: 10.1093/infdis/163.4.843. [DOI] [PubMed] [Google Scholar]
- Israelski D. M., Araujo F. G., Conley F. K., Suzuki Y., Sharma S., Remington J. S. Treatment with anti-L3T4 (CD4) monoclonal antibody reduces the inflammatory response in toxoplasmic encephalitis. J Immunol. 1989 Feb 1;142(3):954–958. [PubMed] [Google Scholar]
- Narang P. K., Lewis R. C., Bianchine J. R. Rifabutin absorption in humans: relative bioavailability and food effect. Clin Pharmacol Ther. 1992 Oct;52(4):335–341. doi: 10.1038/clpt.1992.152. [DOI] [PubMed] [Google Scholar]
- Remington J. S., Yagura T., Robinson W. S. The effect of rifampin on Toxoplasma gondii. Proc Soc Exp Biol Med. 1970 Oct;135(1):167–172. doi: 10.3181/00379727-135-35011. [DOI] [PubMed] [Google Scholar]




