Abstract
Summary: The increasing availability of experimentally determined binding affinities for drugs on multiple protein targets requires the design of specific mining and visualization tools that graphically integrate chemical and biological data in an efficient environment. With this aim, we developed iPHACE, an integrative web-based tool to navigate in the pharmacological space defined by small molecule drugs contained in the IUPHAR-DB, with additional interactions present in PDSP. Extending beyond traditional querying and filtering tools, iPHACE offers a means to extract knowledge from the target profile of drugs as well as from the drug profile of protein targets.
Availability: iPHACE is available at http://cgl.imim.es/iphace/ (EU site) and http://agave.health.unm.edu/iphace/ (US mirror)
Contact: jmestres@imim.es
1 INTRODUCTION
The perception that drugs bind selectively to only a few biological targets has been largely biased by the limited time and resources devoted to the systematic screening of compounds against a large panel of proteins. Recent efforts towards generating, compiling and storing pharmacological data for drugs are challenging this traditional perception, as most drugs appear to have a rich polypharmacology (Mestres et al., 2009). In this respect, data repositories such as the Psychoactive Drug Screening Program (PDSP; Roth et al., 2004), DrugBank (Wishart et al., 2008), IUPHAR-DB (Harmar et al., 2009) and PubChem (Wang et al., 2009) have become essential public resources that provide up-to-date information on the binding affinity profile of drugs.
The increasing amount of available data on drug–target interactions brings on the need to develop new interactive tools for data integration and mining that can facilitate knowledge extraction. All the above databases offer, via individual web sites, the possibility to perform some degree of querying and filtering to enable data access. In addition, other resources such as SEA (Keiser et al., 2007), SuperTarget (Günther et al., 2008), STITCH (Kuhn et al., 2008) and DrugViz (Xiong et al., 2008) provide purposely designed tools to analyse and visualize drug–target relationships. Along these lines, iPHACE represents a new integrative conceptual framework to navigate in the pharmacological space covered by drugs. The current release contains 4089 interactions between 739 drugs and 181 targets [147 G protein-coupled receptors (GPCRs) and 34 ion channels] extracted from IUPHAR-DB and PDSP.
2 IMPLEMENTATION
Database processing: database implementation in iPHACE requires that all data are provided as a standard structure data file (SDF) with pre-defined fields under a common format (e.g. TargetName, pActivity, ActivityDescriptor, AffinityType and Species). These SDFs are then processed to generate unique drug and target identifiers and to compute molecular descriptors. All information is ultimately parsed and added to a mysql database that currently contains 2122 interactions between 739 drugs and 140 targets from IUPHAR-DB, supplemented by 3009 interactions between 330 of those drugs and 121 targets from PDSP. This mysql database is managed by a php script that pre-generates all php and html pages displayed by the iPHACE navigator, as well as all chemical structure images, descriptor histograms and supplementary information resulting from filtering and searching actions. ChemAxon applets are used for chemical structure depiction and for substructure searches.
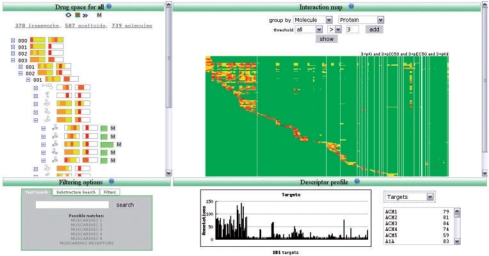
Classification schemes: drug space is organized using a six-level structural hierarchy (Cases et al., 2005): the top three levels are integers representing the number of rings in the largest ring system, the number of ring systems and the number of bonds connecting the most distant ring systems in the structure; the remaining three levels are hash-codes that uniquely identify the atomic framework, the scaffold and finally the molecule (Fig. 1). Target space is organized using standard functional classifications schemes available for GPCRs and ion channels (Garcia-Serna et al., 2006).
Fig. 1.
The four working areas of the iPHACE navigator loaded with 739 drugs extracted from IUPHAR-DB and complemented with PDSP.
Working areas: the iPHACE navigator is divided into four main working areas (Fig. 1). The top-left area contains the classification schemes used to organize Drug and Target spaces. The top-right area is used to show the Interaction map and the Structural browser. One can switch between the two using the control buttons present in the top-left area. The bottom-left area is reserved for Text and Substructure searches and to apply Filters to identify drugs with similar activity profiles within a user-defined cutoff. The similarity between the activity profiles of two drugs having affinity for N targets is obtained by computing the Euclidean distance between the respective affinity vectors relative to the maximum Euclidean distance found for a pair of drugs in the set having affinity for N targets. Finally, the bottom-right area depicts the Descriptor profile; descriptors can be selected from a pull-down menu. All four working areas are interconnected. Browsing on the Drug and Target spaces will automatically rebuild the corresponding Interaction map and Descriptor profile areas.
3 APPLICATIONS
Depending on their background, different users might find different utility for iPHACE. This is a list of potential scenarios, with common questions and how they can be addressed within iPHACE:
What is the target space associated with drugs having activity data for the Muscarinic 1 receptor? Go to Text Search and type ‘muscarinic’. Click on ‘MUSCARINIC 1’. The resulting Interaction map shows that there are 79 drugs with activity data for the muscarinic 1 receptor; for some of these drugs, there is activity data related to 87 additional targets. To focus on high-activity interactions, expressed as -log(molar), the user can change the activity threshold placed above the Interaction map.
What is the target space associated with drugs having a piperazine substructure? Go to Substructure search, click on the little rectangle, draw a piperazine, close the applet and click on the search icon. The resulting interaction map shows that there are 63 drugs containing a piperazine substructure with activity data for 74 targets. Most interestingly, 56 of those drugs (88.9%) have activity data for class A amine GPCRs. Only 43.0% of the entire drug set have activity data on those receptors, which confirms that piperazine is a privileged substructure for drugs acting on amine GPCRs (Klabunde and Hessler, 2002).
Which are the drugs having the most similar activity profile to chlorpromazine? Go to Text Search and type ‘chlorpromazine’. Mark the box next to the drug name. Go then to Filters, click on the box next to profile similarity and apply filtering. An interaction map between eight drugs and 51 targets appears, drugs being ordered (top to bottom) according to their activity profile similarity with respect to chlorpromazine. The activity profile of each drug can be accessed by clicking on the name of the drug. A right-mouse click brings you back to the Interaction map. The structures of these eight drugs can be inspected by clicking on the ‘8 molecules’ link present in the Drug space working area. Note that despite the structural variety of these drugs, they are all classified as anti-psychotics.
4 CONCLUSION
The iPHACE navigator was designed to enable a multidisciplinary team of drug discovery scientists to visually process information on chemical structures and biological activities of drugs in an efficient, organized and integrative manner. As more data on the interaction of drugs with multiple targets become available, tools such as iPHACE are likely to deepen our understanding of both the polypharmacology of drugs and the cross-pharmacology of targets.
ACKNOWLEDGEMENTS
We thank ChemAxon for its applets.
Funding: the Instituto de Salud Carlos III, the Ministerio de Ciencia e Innovación (BIO2008-02329); National Institutes of Health (5U54MH084690-02).
Conflict of Interest: none declared.
REFERENCES
- Cases M, et al. Chemical and biological profiling of an annotated compound library directed to the nuclear receptor family. Curr. Top. Med. Chem. 2005;5:763–772. doi: 10.2174/1568026054637665. [DOI] [PubMed] [Google Scholar]
- García-Serna R, et al. FCP: functional coverage of the proteome by structures. Bioinformatics. 2006;22:1792–1793. doi: 10.1093/bioinformatics/btl188. [DOI] [PubMed] [Google Scholar]
- Günther S, et al. Supertarget and Matador: resources for exploring drug-target relationships. Nucleic Acids Res. 2008;36:D919–D922. doi: 10.1093/nar/gkm862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmar AJ, et al. IUPHAR-DB: the IUPHAR database of G protein-coupled receptors and ion channels. Nucleic Acids Res. 2009;37:D680–D685. doi: 10.1093/nar/gkn728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser MJ, et al. Relating protein pharmacology by ligand chemistry. Nat. Biotechnol. 2007;25:197–206. doi: 10.1038/nbt1284. [DOI] [PubMed] [Google Scholar]
- Klabunde T, Hessler G. Drug design strategies for targeting G protein-coupled receptors. ChemBioChem. 2002;3:928–944. doi: 10.1002/1439-7633(20021004)3:10<928::AID-CBIC928>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Kuhn M, et al. STITCH: interaction networks of chemicals and proteins. Nucleic Acids Res. 2008;36:D684–D688. doi: 10.1093/nar/gkm795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestres J, et al. The topology of drug-target networks: implicit dependence on drug properties and target families. Mol. BioSyst. 2009;5:1051–1057. doi: 10.1039/b905821b. [DOI] [PubMed] [Google Scholar]
- Roth BL, et al. Magic shot guns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat. Rev. Drug Discov. 2004;3:353–359. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- Wang Y, et al. PubChem: a public information system for analyzing bioactivities of small molecules. Nucleic Acids Res. 2009;37:W623–W633. doi: 10.1093/nar/gkp456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart DS, et al. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008;36:D901–D906. doi: 10.1093/nar/gkm958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong B, et al. DrugViz: a Cytoscape plugin for visualyzing and analyzing small molecule drugs in biological networks. Bioinformatics. 2008;24:2117–2118. doi: 10.1093/bioinformatics/btn389. [DOI] [PubMed] [Google Scholar]