Abstract
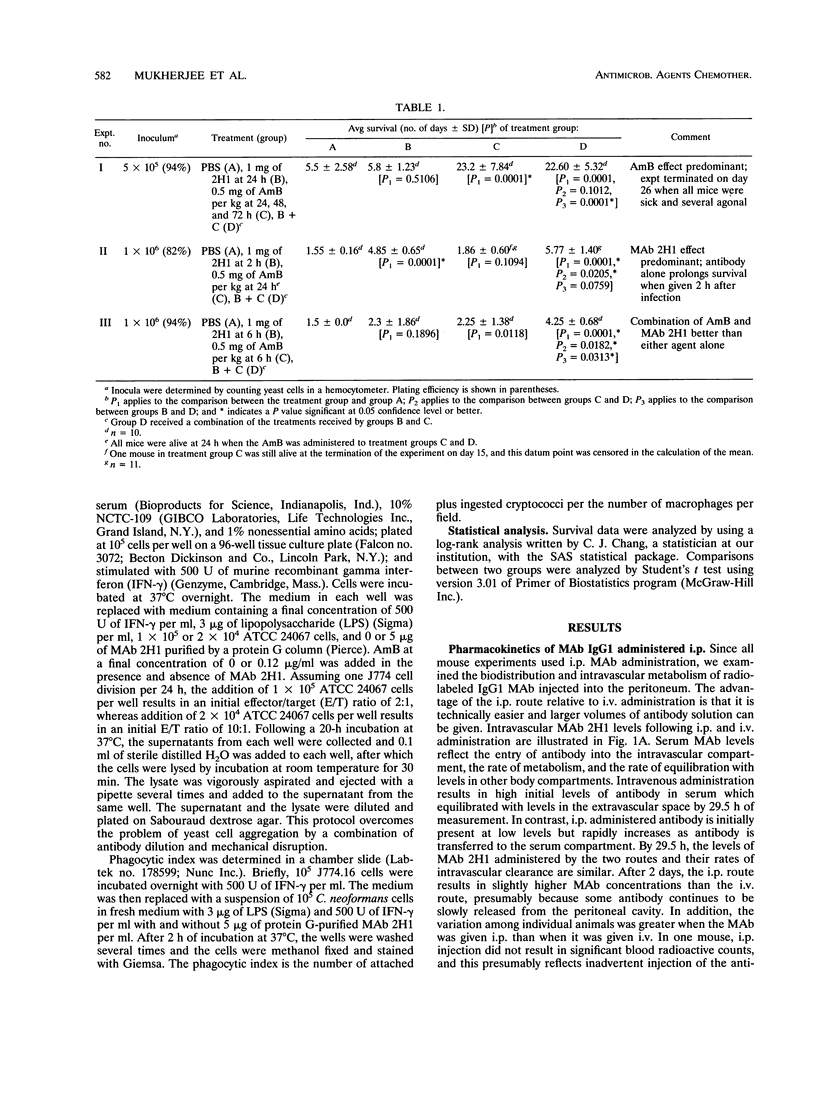
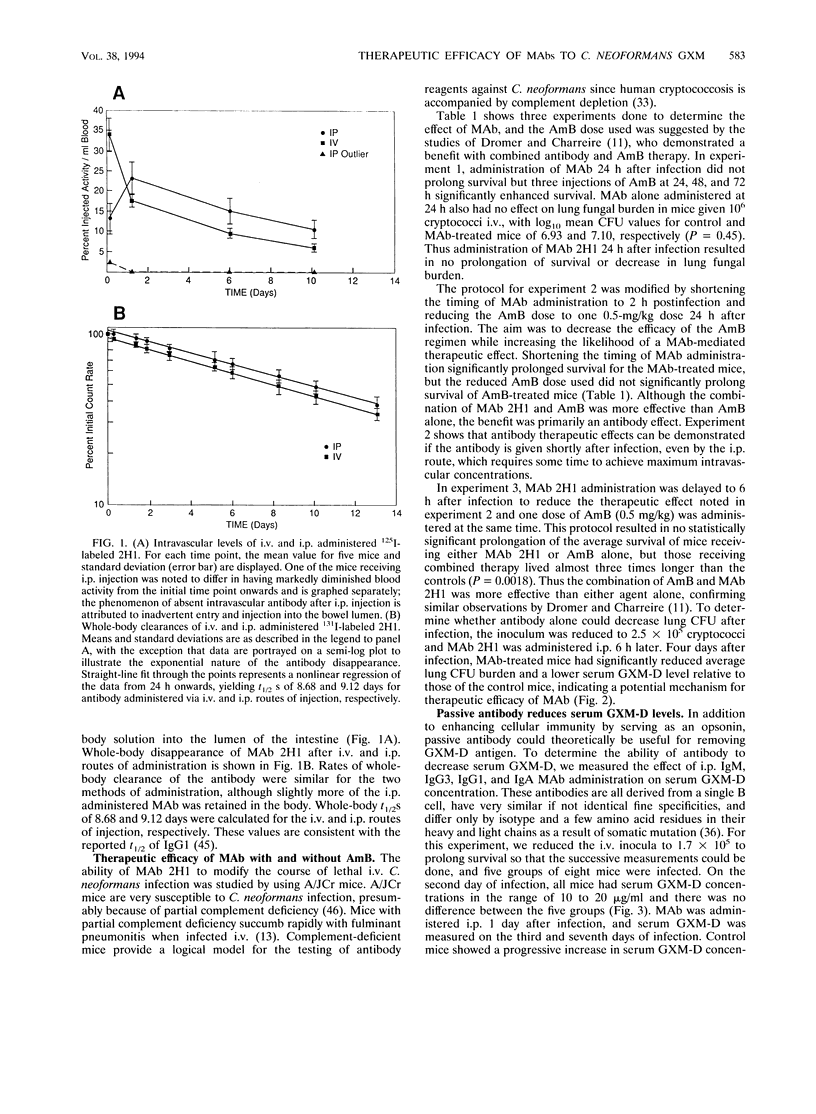
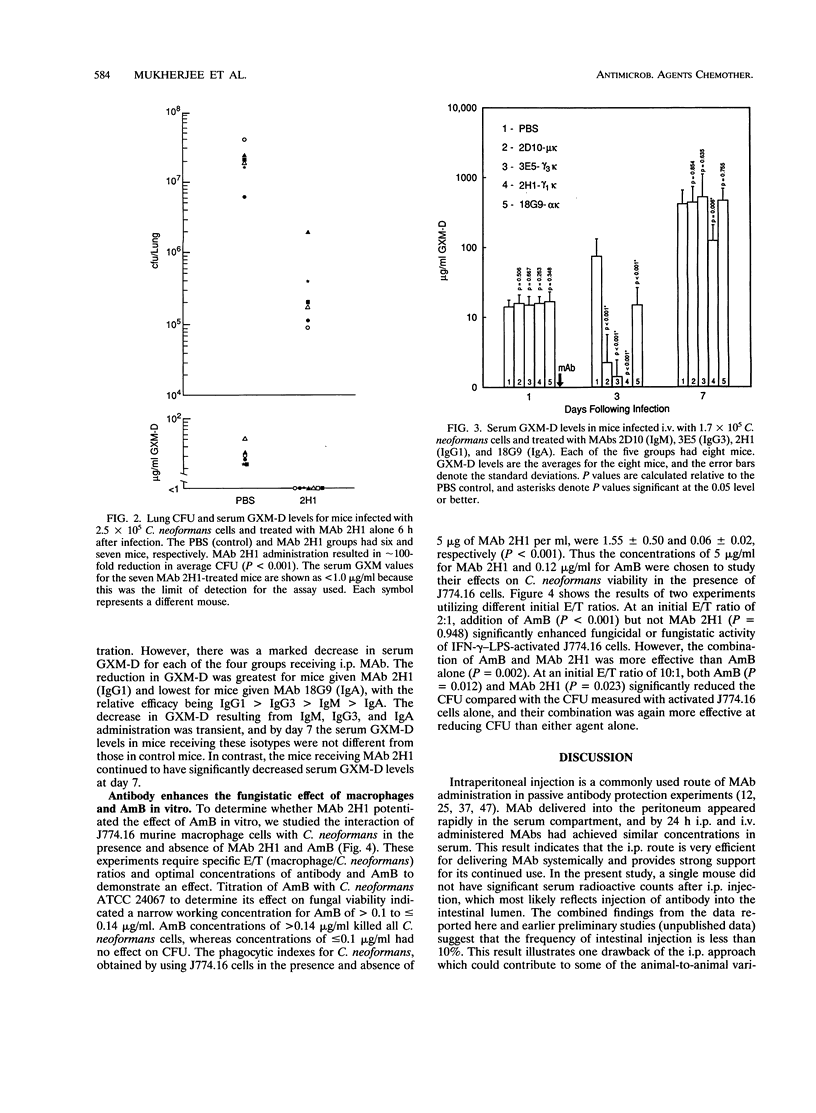
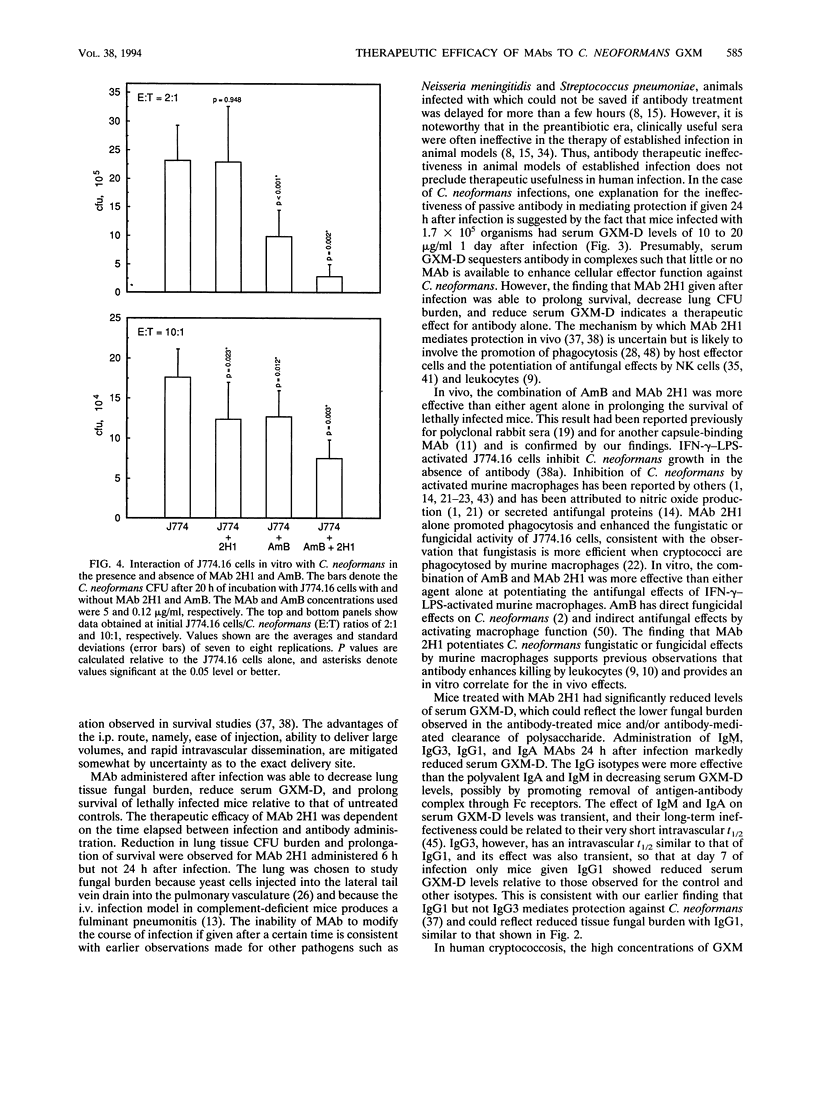
The therapeutic efficacy of the immunoglobulin G1 (IgG1) monoclonal antibody (MAb) 2H1 to the Cryptococcus neoformans capsular polysaccharide was studied with and without amphotericin B (AmB) in a murine model of intravenous (i.v.) infection. MAb and AmB were administered by intraperitoneal (i.p.) injection after i.v. infection with a C. neoformans serotype D strain. Intraperitoneal administration of MAb 2H1 resulted in rapid distribution to the intravascular compartment, and the half-lives of i.p. and i.v. administered MAb were similar. Administration of MAb 2H1 alone resulted in increased survival, decreased lung fungal burden, and reduced serum glucuronoxylomannan antigen levels when given 2 to 6 h but not 24 h after infection. In vivo, the combination of MAb 2H1 and AmB was more effective at prolonging survival than either agent alone. MAbs of IgM, IgG1, IgG3, and IgA isotypes given 1 day after infection were effective in reducing serum GXM-D levels, with their relative efficacy being IgG1 > IgG3 > IgM > IgA. In vitro, MAb 2H1 was a potent opsonin of C. neoformans and the combination of MAb 2H1 and AmB was more effective than either agent alone in decreasing C. neoformans colony counts in the presence of the murine macrophage cell line J774.16. The results confirm that capsule-binding MAbs can enhance the effect of AmB against C. neoformans and provide support for considering combined therapy in humans.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alspaugh J. A., Granger D. L. Inhibition of Cryptococcus neoformans replication by nitrogen oxides supports the role of these molecules as effectors of macrophage-mediated cytostasis. Infect Immun. 1991 Jul;59(7):2291–2296. doi: 10.1128/iai.59.7.2291-2296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brajtburg J., Powderly W. G., Kobayashi G. S., Medoff G. Amphotericin B: current understanding of mechanisms of action. Antimicrob Agents Chemother. 1990 Feb;34(2):183–188. doi: 10.1128/aac.34.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A., Mukherjee J., Scharff M. D. Monoclonal antibody based ELISAs for cryptococcal polysaccharide. J Immunol Methods. 1992 Sep 18;154(1):27–35. doi: 10.1016/0022-1759(92)90209-c. [DOI] [PubMed] [Google Scholar]
- Casadevall A., Spitzer E. D., Webb D., Rinaldi M. G. Susceptibilities of serial Cryptococcus neoformans isolates from patients with recurrent cryptococcal meningitis to amphotericin B and fluconazole. Antimicrob Agents Chemother. 1993 Jun;37(6):1383–1386. doi: 10.1128/aac.37.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J., Xing Y., Magliozzo R. S., Bloom B. R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992 Apr 1;175(4):1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Allison A. C. Nature of the effector cells responsible for antibody-dependent cell-mediated killing of Cryptococcus neoformans. Infect Immun. 1976 Sep;14(3):716–720. doi: 10.1128/iai.14.3.716-720.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dromer F., Charreire J., Contrepois A., Carbon C., Yeni P. Protection of mice against experimental cryptococcosis by anti-Cryptococcus neoformans monoclonal antibody. Infect Immun. 1987 Mar;55(3):749–752. doi: 10.1128/iai.55.3.749-752.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dromer F., Charreire J. Improved amphotericin B activity by a monoclonal anti-Cryptococcus neoformans antibody: study during murine cryptococcosis and mechanisms of action. J Infect Dis. 1991 May;163(5):1114–1120. doi: 10.1093/infdis/163.5.1114. [DOI] [PubMed] [Google Scholar]
- Dromer F., Perronne C., Barge J., Vilde J. L., Yeni P. Role of IgG and complement component C5 in the initial course of experimental cryptococcosis. Clin Exp Immunol. 1989 Dec;78(3):412–417. [PMC free article] [PubMed] [Google Scholar]
- Flesch I. E., Schwamberger G., Kaufmann S. H. Fungicidal activity of IFN-gamma-activated macrophages. Extracellular killing of Cryptococcus neoformans. J Immunol. 1989 May 1;142(9):3219–3224. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- GADEBUSCH H. H. Passive immunization against Cryptococcus neoformans. Proc Soc Exp Biol Med. 1958 Jul;98(3):611–614. doi: 10.3181/00379727-98-24123. [DOI] [PubMed] [Google Scholar]
- GORDON M. A., LAPA E. SERUM PROTEIN ENHANCEMENT OF ANTIBIOTIC THERAPY IN CRYPTOCOCCOSIS. J Infect Dis. 1964 Oct;114:373–377. doi: 10.1093/infdis/114.4.373. [DOI] [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Specific amino acid (L-arginine) requirement for the microbiostatic activity of murine macrophages. J Clin Invest. 1988 Apr;81(4):1129–1136. doi: 10.1172/JCI113427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Perfect J. R., Durack D. T. Macrophage-mediated fungistasis in vitro: requirements for intracellular and extracellular cytotoxicity. J Immunol. 1986 Jan;136(2):672–680. [PubMed] [Google Scholar]
- Granger D. L., Perfect J. R., Durack D. T. Macrophage-mediated fungistasis: requirement for a macromolecular component in serum. J Immunol. 1986 Jul 15;137(2):693–701. [PubMed] [Google Scholar]
- Gray L. D., Roberts G. D. Experience with the use of pronase to eliminate interference factors in the latex agglutination test for cryptococcal antigen. J Clin Microbiol. 1988 Nov;26(11):2450–2451. doi: 10.1128/jcm.26.11.2450-2451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybill J. R., Hague M., Drutz D. J. Passive immunization in murine cryptococcosis. Sabouraudia. 1981 Dec;19(4):237–244. doi: 10.1080/00362178185380411. [DOI] [PubMed] [Google Scholar]
- Katz S., Grosfeld J. L., Folkening W. J., Rosenthal R. S., Merkel G. J. Initial organ localisation of blood-borne Candida albicans in a rat model of disseminated candidosis. J Med Microbiol. 1992 Oct;37(4):291–295. doi: 10.1099/00222615-37-4-291. [DOI] [PubMed] [Google Scholar]
- Kozel T. R., Cazin J. Nonencapsulated Variant of Cryptococcus neoformans I. Virulence Studies and Characterization of Soluble Polysaccharide. Infect Immun. 1971 Feb;3(2):287–294. doi: 10.1128/iai.3.2.287-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Follette J. L. Opsonization of encapsulated Cryptococcus neoformans by specific anticapsular antibody. Infect Immun. 1981 Mar;31(3):978–984. doi: 10.1128/iai.31.3.978-984.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Gotschlich E. C. The capsule of cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J Immunol. 1982 Oct;129(4):1675–1680. [PubMed] [Google Scholar]
- Kozel T. R., Gulley W. F., Cazin J., Jr Immune response to Cryptococcus neoformans soluble polysaccharide: immunological unresponsiveness. Infect Immun. 1977 Dec;18(3):701–707. doi: 10.1128/iai.18.3.701-707.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., McGaw T. G. Opsonization of Cryptococcus neoformans by human immunoglobulin G: role of immunoglobulin G in phagocytosis by macrophages. Infect Immun. 1979 Jul;25(1):255–261. doi: 10.1128/iai.25.1.255-261.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTMAN M. L. Cryptococcosis (torulosis). Current concepts and therapy. Am J Med. 1959 Dec;27:976–998. doi: 10.1016/0002-9343(59)90181-0. [DOI] [PubMed] [Google Scholar]
- Macher A. M., Bennett J. E., Gadek J. E., Frank M. M. Complement depletion in cryptococcal sepsis. J Immunol. 1978 May;120(5):1686–1690. [PubMed] [Google Scholar]
- Miller M. F., Mitchell T. G., Storkus W. J., Dawson J. R. Human natural killer cells do not inhibit growth of Cryptococcus neoformans in the absence of antibody. Infect Immun. 1990 Mar;58(3):639–645. doi: 10.1128/iai.58.3.639-645.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J., Casadevall A., Scharff M. D. Molecular characterization of the humoral responses to Cryptococcus neoformans infection and glucuronoxylomannan-tetanus toxoid conjugate immunization. J Exp Med. 1993 Apr 1;177(4):1105–1116. doi: 10.1084/jem.177.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J., Pirofski L. A., Scharff M. D., Casadevall A. Antibody-mediated protection in mice with lethal intracerebral Cryptococcus neoformans infection. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3636–3640. doi: 10.1073/pnas.90.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee J., Scharff M. D., Casadevall A. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect Immun. 1992 Nov;60(11):4534–4541. doi: 10.1128/iai.60.11.4534-4541.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S., Lee S., Mukherjee J., Scharff M. D., Casadevall A. Monoclonal antibodies to Cryptococcus neoformans capsular polysaccharide modify the course of intravenous infection in mice. Infect Immun. 1994 Mar;62(3):1079–1088. doi: 10.1128/iai.62.3.1079-1088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. W., Cozad G. C. Immunological unresponsiveness induced by cryptococcal capsular polysaccharide assayed by the hemolytic plaque technique. Infect Immun. 1972 Jun;5(6):896–901. doi: 10.1128/iai.5.6.896-901.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi N., Murphy J. W. Antibody-dependent natural killer cell-mediated growth inhibition of Cryptococcus neoformans. Infect Immun. 1986 Feb;51(2):556–562. doi: 10.1128/iai.51.2.556-562.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington J. E. Newer uses of intravenous immunoglobulins as anti-infective agents. Antimicrob Agents Chemother. 1990 Aug;34(8):1463–1466. doi: 10.1128/aac.34.8.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfect J. R., Granger D. L., Durack D. T. Effects of antifungal agents and gamma interferon on macrophage cytotoxicity for fungi and tumor cells. J Infect Dis. 1987 Aug;156(2):316–323. doi: 10.1093/infdis/156.2.316. [DOI] [PubMed] [Google Scholar]
- Pettoello-Mantovani M., Casadevall A., Kollmann T. R., Rubinstein A., Goldstein H. Enhancement of HIV-1 infection by the capsular polysaccharide of Cryptococcus neoformans. Lancet. 1992 Jan 4;339(8784):21–23. doi: 10.1016/0140-6736(92)90142-p. [DOI] [PubMed] [Google Scholar]
- Pollock R. R., French D. L., Metlay J. P., Birshtein B. K., Scharff M. D. Intravascular metabolism of normal and mutant mouse immunoglobulin molecules. Eur J Immunol. 1990 Sep;20(9):2021–2027. doi: 10.1002/eji.1830200921. [DOI] [PubMed] [Google Scholar]
- Rhodes J. C., Wicker L. S., Urba W. J. Genetic control of susceptibility to Cryptococcus neoformans in mice. Infect Immun. 1980 Aug;29(2):494–499. doi: 10.1128/iai.29.2.494-499.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford J. E., Lupan D. M., Schlageter A. M., Kozel T. R. Passive immunization against Cryptococcus neoformans with an isotype-switch family of monoclonal antibodies reactive with cryptococcal polysaccharide. Infect Immun. 1990 Jun;58(6):1919–1923. doi: 10.1128/iai.58.6.1919-1923.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlageter A. M., Kozel T. R. Opsonization of Cryptococcus neoformans by a family of isotype-switch variant antibodies specific for the capsular polysaccharide. Infect Immun. 1990 Jun;58(6):1914–1918. doi: 10.1128/iai.58.6.1914-1918.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer E. D., Spitzer S. G., Freundlich L. F., Casadevall A. Persistence of initial infection in recurrent Cryptococcus neoformans meningitis. Lancet. 1993 Mar 6;341(8845):595–596. doi: 10.1016/0140-6736(93)90354-j. [DOI] [PubMed] [Google Scholar]
- Wolf J. E., Massof S. E. In vivo activation of macrophage oxidative burst activity by cytokines and amphotericin B. Infect Immun. 1990 May;58(5):1296–1300. doi: 10.1128/iai.58.5.1296-1300.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckier L. S., Rodriguez L. D., Scharff M. D. Immunologic and pharmacologic concepts of monoclonal antibodies. Semin Nucl Med. 1989 Jul;19(3):166–186. doi: 10.1016/s0001-2998(89)80012-1. [DOI] [PubMed] [Google Scholar]



