Abstract
OBJECTIVE
To determine if baseline subgroups in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial can be identified for whom intensive compared with standard glycemia treatment had different effects on all-cause mortality.
RESEARCH DESIGN AND METHODS
Exploratory post hoc intention-to-treat comparisons were made between intensive and standard glycemia groups on all-cause mortality by subgroups defined by baseline characteristics.
RESULTS
There were few significant interactions between baseline characteristics and effects of intensive versus standard glycemia treatment on mortality: self-reported history of neuropathy (hazard ratio [HR] 1.95, 95% CI 1.41–2.69) versus no history of neuropathy (0.99, 0.79–1.26; P value for interaction 0.0008), higher A1C (A1C >8.5%: HR 1.64, 95% CI 1.22–2.22; A1C 7.5–8.4%: 1.00, 0.75–1.34; A1C <7.5%: 1.00, 0.67–1.50; P value for interaction 0.04), and aspirin use (HR 1.45, 95% CI 1.13–1.85, compared with 0.96, 0.72–1.27, in nonusers; P value for interaction 0.03).
CONCLUSIONS
We found a remarkable similarity of effect from intensive compared with standard glycemia treatment on mortality across most baseline subgroups. No differential effect was found in subgroups defined by variables anticipated to have an interaction: age, duration of diabetes, and previous history of cardiovascular disease. The three baseline characteristics that defined subgroups for which there was a differential effect on mortality may help identify patients with type 2 diabetes at higher risk of mortality from intensive regimens for glycemic control. Further research is warranted.
Numerous epidemiological studies have demonstrated a relationship between elevated A1C and a greater risk of cardiovascular (CVD) events and mortality in type 2 diabetes (1–3). Therefore, it has been hypothesized that a reduction to near-normal levels of A1C in patients with type 2 diabetes would reduce the risk of these adverse outcomes. Three large randomized controlled clinical trials testing this hypothesis in individuals with longstanding type 2 diabetes reported their main results in the past 2 years (4–6).
The Data Safety Monitoring Board of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial discontinued the intensive glycemia arm because of an increase in all-cause mortality in the intensive glycemia arm compared with the standard glycemia arm. The finding of excess mortality in the intensive arm of the ACCORD trial has led to controversy about implementation of intensive glucose control in patients with type 2 diabetes (7,8). Adding to the controversy were results of the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) and Veterans Affairs Diabetes Trial (VADT), demonstrating that although there was no significant reduction in the primary end point of CVD events, there was no increase in mortality with the intensive glycemia arm compared with the standard glycemia arm (4,6), which has raised questions about reasons for these discrepancies (9–12).
A critical question relates to the applicability and generalizability of the conclusions of the ACCORD trial to the broader population or to specific subgroups of patients with type 2 diabetes. Indeed, prespecified subgroup analyses in ACCORD did suggest a significant benefit of intensive glycemic control on CVD events in those participants with lower A1C at entry or absence of CVD event by history, but there was no suggestion of a differential effect on mortality (5). However, these observations are based on only a few subgroup analyses at the time of the primary publication. The effect on mortality of intensive compared with standard glycemia treatment may have been modified by other possible characteristics of patients at entry. We have therefore carried out exploratory post hoc analyses of the effects of intensive compared with standard glycemia treatment in ACCORD participants categorized by various baseline characteristics on all-cause mortality at the time of discontinuation of the intensive glycemia treatment of ACCORD, with the goal to determine if particular subgroups at higher or lower risk from the intensive intervention can be identified.
RESEARCH DESIGN AND METHODS
ACCORD is a multicenter randomized clinical trial testing the effect of very tight control of blood glucose in patients with type 2 diabetes compared with standard therapy on a composite outcome of CVD death, nonfatal MI, and nonfatal stroke. The factorally designed trial is also testing effects of intensive blood pressure control compared with standard (the Blood Pressure trial) and use of fenofibrate plus statin compared with placebo plus statin (the Lipid trial).
The treatment goal for the intensive arm was an A1C of <6%, whereas the treatment goal for the standard arm was A1C of 7–7.9%, with the expectation that the mean A1C for the standard arm would be ∼7.5%. The mean duration of the trial was expected to be 5 years, but the intensive glycemia arm of the study was discontinued on 5 February 2008 because of excess mortality in the intensive group, and all participants were transitioned to the standard glycemia treatment protocol. Complete details of the study design and conduct have been previously published (13,14).
The dataset for the current analyses is the same as that for the 2008 main results paper, which includes all randomized participants from enrollment until 10 December 2007, an average trial follow-up of 3.5 years (5). Subgroup definitions are indicated in Table 1; baseline characteristics to define the subgroups were divided into four categories: 1) demographic and anthropometric characteristics, 2) medical history characteristics, 3) medication use, and 4) laboratory variables. Analyses were conducted using intention-to-treat comparisons between the intensive glycemia and standard groups on the mortality rate within each subgroup as defined in Table 1 above (15). Analyses were conducted at the ACCORD coordinating center using SAS 9.2 (SAS Institute, Cary, NC). Cox proportional-hazards regression analysis was used to examine the risk of all-cause mortality by glycemia arm within each subgroup. A test of the interaction between the baseline variable and treatment arm was done to assess homogeneity of the treatment effect across levels of subgroups, i.e., to determine if effects on mortality of intensive versus standard glycemia treatments differed between the subgroup categories. All analyses were adjusted for the study stratification factors: 1) history of CVD (except for the analysis of CVD variables), 2) assignment to the Lipid or Blood Pressure trial (each trial had different eligibility criteria), 3) assignment to the Lipid trial and randomized to fenofibrate, and 4) assignment to the Blood Pressure trial and randomized to the intensive blood pressure intervention. Where possible, categorical variables were also assessed as continuous variables to determine if the results differed from the categorical analysis. Multiple Cox regression analyses were performed to determine if any observed trends persist after adjusting for multiple baseline characteristics. Because these were exploratory analyses, no P value adjustment was made for multiple comparisons.
Table 1.
Baseline variables
| Demographics | Medical history | Medications | Lab tests |
|---|---|---|---|
| Age, race/ethnicity, sex, lives alone, clinical network, BMI, waist circumference, education, year randomization | Prior CVD event, prior coronary heart failure, diabetes duration, history of neuropathy, peripheral neuropathy, retinal laser/surgery, visual acuity, smoking, depression, blood pressure, electrocardiogram | Sulfonylureas, metformin, thiazolidinediones, any insulin HCTZ, ACE inhibitors, β-blockers, calcium-channel blockers, fibrates, statins, aspirin, antidepressants | A1C, LDL, HDL, triglyceride, serum creatinine, glomerular filtration rate (modified diet renal disease), urinary albumin-to-creatinine ratio |
Demographics: age (<65, 65–69, 70–74, ≥75 years), race/ethnicity (Hispanic, white, black, or other), lives alone (vs. with others), clinical center network (7 CCNs), BMI (<30, 30–34, ≥35 kg/m2), waist circumference (<96.7, 96.7–106.6, 106.7–116, ≥116.1 cm), education (<high school, high-school graduate, attended some college or technical school, college graduate), year of randomization into ACCORD (2001–2005). Medical history: diabetes duration (0–5, 6–10, 11–15, ≥16 years), history of neuropathy/nerve problems (“Has the participant ever been told by a physician that he or she has neuropathy/nerve problems”: yes/no), peripheral neuropathy (pedal amputation or a score >2 on the clinical examination portion of the MNSI), visual acuity (<20/40, 20/20–20/40, ≥20/20), smoking status (current, former, never), blood pressure (<135, ≥135), electrocardiogram selected variables (any gross abnormalities, evidence of prior infarction, Q-T interval on electrocardiogram corrected for heart rate [QTc]). Medication use: yes/no at baseline. Laboratory variables: A1C (<7.5, 7.5–8.4, ≥8.5%), LDL cholesterol (<100, 100–119, ≥120 mg/dl), HDL cholesterol (quartiles, mg/dl), triglycerides (<300, ≥300 mg/dl), serum creatinine (0.1–1.0, >1 mg/dl), glomerular filtration rate (modified diet renal disease) (quartiles ml/min), urinary albumin-to-creatinine ratio (<30, 30–300, ≥300 mg/g). HCTZ, hydrochlorotiazide.
RESULTS
Overall, 257 participants experienced the end point of all-cause mortality in the intensive glycemia arm and 203 participants experienced the end point of all-cause mortality in the standard glycemia arm, as previously reported (5.0 vs. 4.0%; hazard ratio (HR) 1.22; 95% CI 1.01–1.46; P = 0.04) (10).
Tables of all the subgroup analyses with all the variables examined are in the online appendix (available at http://care.diabetesjournals.org/cgi/content/full/dc09-1471/DC1). Here we specifically note those of significance and borderline significance.
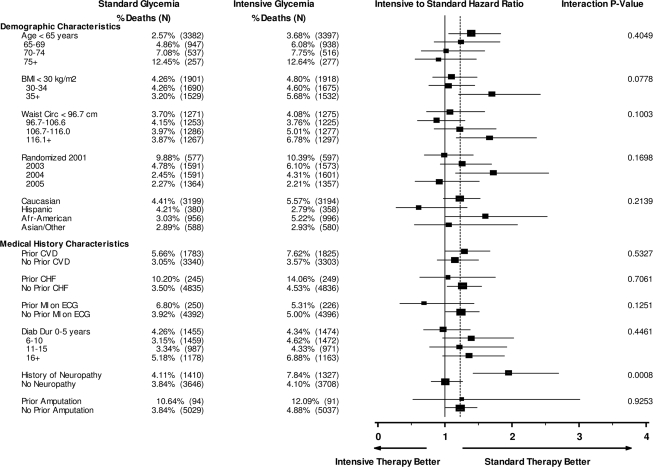
None of the baseline demographic and anthropometric characteristics had a statistically significant interaction with glycemia group assignment on mortality (Fig. 1). The highest BMI category (≥35 kg/m2) showed the highest HR for intensive versus standard glycemia (HR 1.70, 95% CI 1.20–2.41) compared with BMI <30–34 kg/m2 (1.05, 0.76–1.44) and BMI <30 kg/m2 (1.01, 0.81–1.48; P value for interaction = 0.078). Similarly, the two highest quartiles for waist circumference also showed the highest HR for intensive versus standard glycemia (P value for interaction = 0.10). We did not find significant interactions for age, sex, race, living alone, clinical network, and education level or randomization year.
Figure 1.
Demographic characteristics and medical history. HRs are shown for all-cause mortality in intensive versus standard glycemia groups within demographic and medical history subgroups, adjusted for the study stratification factors: 1) history of CVD (except for the analysis of CVD variables), 2) assignment to the Lipid or Blood Pressure trial (each trial had different eligibility criteria), 3) assignment to Lipid trial and randomized to fenofibrate, and 4) assignment to the Blood Pressure trial and randomized to the intensive blood pressure intervention.
Among the baseline medical history subgroups (Fig. 1), participants with a self-reported history of neuropathy/nerve problems had a greater risk of mortality in the intensive glycemia arm compared with the standard glycemia arm (P value for interaction = 0.0008). Moreover, the interaction between self-reported history of neuropathy/nerve problems and glycemia arm continued to remain highly significant (P = 0.006) after adjusting for age, sex, waist circumference, smoking, diabetes duration, hypoglycemic agents used, history of retinopathy, history of amputation, and systolic blood pressure. However, the presence of peripheral neuropathy at baseline, as documented by pedal amputation or a score >2 on the clinical examination portion of the Michigan Neuropathy Screening Instrument (MNSI) was not associated with increased mortality in the intensive arm over the standard arm (Fig. 1). We did not find a predictive association for excess mortality in the intensive group for prior CVD disease, duration of diabetes, history of retinal surgery, smoking, depression, systolic blood pressure, electrocardiogram variables, or prior amputation.
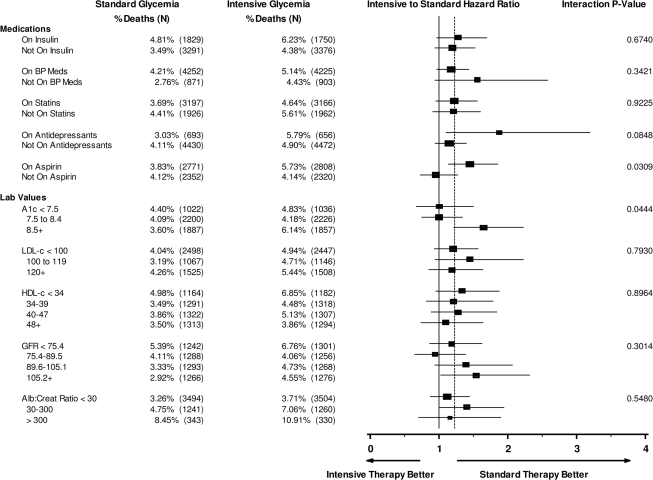
The analysis of medications reported at baseline (Fig. 2) found that use of aspirin was associated with excess mortality in the intensive group (HR 1.45, 95% CI 1.13–1.85) compared with nonusers (0.96, 0.72–1.27; P value for interaction = 0.03). A similar but not statistically significant differential effect occurred for use of antidepressant medications at baseline (HR 1.87, 95% CI 1.10–3.20, for users compared with 1.15, 0.94–1.40, for nonusers; P value for interaction = 0.08). We did not find any diabetes medication or combination of diabetes medications at baseline to be predictive of higher mortality in the intensive group versus the standard group. Furthermore, no hypolipidemic agent or antihypertensive medication had an interaction with group assignment in predicting mortality.
Figure 2.
Medication and laboratory tests. HRs are shown for all-cause mortality in intensive versus standard glycemia groups within the medication and laboratory tests subgroups adjusted for the study stratification factors: 1) history of CVD (except for the analysis of CVD variables), 2) assignment to the Lipid or Blood Pressure trial (each trial had different eligibility criteria), 3) assignment to Lipid trial and randomized to fenofibrate, and 4) assignment to the Blood Pressure trial and randomized to the intensive blood pressure intervention.
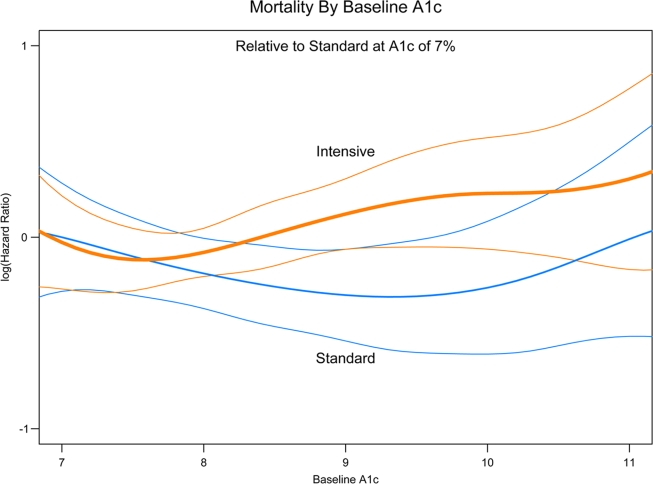
Laboratory test results at baseline (Fig. 2) found that participants with baseline blood levels of A1C >8.5% in the intensive glycemia arm had a higher risk of death than the participants with the same baseline levels in the standard arm (HR 1.64, 95% CI 1.22–2.22), whereas this increased risk was not apparent if baseline A1C was <7.5% (1.00, 0.67–1.50) or between 7.8 and 8.4% (1.00, 0.75–1.34) (P value for interaction = 0.04). None of the other lab values examined showed a statistically significant interaction with the glycemia trial arm, including LDL or HDL cholesterol, triglycerides, serum creatinine, estimated glomerular filtration rate, or albumin-to-creatinine ratio. Figure 3 shows the log of the HR (compared with the standard arm at baseline A1C of 7.0) for all-cause mortality by treatment group. A visual review indicates that after ∼8.2, the higher the A1C at baseline in the intensive glycemia group, the higher the participant's risk. No such relationship is seen in the standard glycemia group.
Figure 3.
A1C and all-cause mortality. Spline curves displaying the log of the HR for all-cause mortality by treatment and baseline A1C are shown. All HRs are with respect to standard glycemia with baseline A1C of 7.0. The bold line represents the intensive treatment group, the finer line the standard group, and the colored lines the 95% CIs.
Multiple Cox regression analysis including the three variables (self-reported history of neuropathy, A1C, and aspirin use) found to have statistically significant interactions with the glycemia arm at the 0.05 level lend support to the univariate finding (A1C >8.5%, HR 1.64, 95% CI 1.22–2.22, P value for interaction = 0.0012), self-reported history of neuropathy/nerve problems at baseline (1.95, 1.41–2.7, P value for interaction <0.0001), and use of aspirin (1.45, 1.13–1.85, P value for interaction = 0.0032).
CONCLUSIONS
In these analyses from ACCORD, we found a remarkable similarity of effect on mortality of intensive compared with standard glycemia arm assignment across numerous post hoc baseline subgroups. Importantly, no differential effect of intensive versus standard treatment on mortality was found in subgroups defined by variables anticipated to have such an interaction: age, duration of diabetes, previous history of CVD disease (Figs. 1–2). Nevertheless, we did find that the intensive treatment had a higher effect on mortality in subgroups defined by three baseline clinical variables: A1C ≥8.5% (interaction P = 0.044), aspirin use (interaction P = 0.03), and self-reported history of neuropathy/nerve problems (interaction P = 0.0008). However, because of the multiple comparisons and the post hoc nature of these analyses, the interaction P values for A1C and aspirin use should only be considered suggestive. A marginal differential effect also was found in subgroups with higher BMI and higher waist circumference at baseline.
Consistent with the prevailing epidemiologic evidence of the relationship between glycemic control and mortality (1–3,16,17), the increase in mortality in the intensive arm was observed among those participants with higher (≥8.5%) compared with lower (<8.5%) A1C at baseline. This effect was evident when A1C was examined as a categorical variable but was seen to a lesser extent when we examined A1C as a continuous variable. The spline plot of HRs across levels of baseline A1C imply an increasingly higher risk with higher baseline A1C in the intensive arm, but not in the standard arm. Individuals with higher A1C at baseline may represent individuals with more difficult disease to manage and/or individuals who may adhere less to therapy. Whether individuals at highest risk ultimately achieved or failed to achieve more intensive treatment targets will require additional analysis.
Baseline participant report of neuropathy/nerve problems defined a subgroup in which the intensive approach for glycemia treatment had a differential effect on mortality, with a highly significant P value of 0.0008. Peripheral neuropathy is a common complication of diabetes, and excess mortality has been related to diminished peripheral sensation (16,18), foot ulceration, and history of lower-extremity amputation (19). Yet in our cohort, neither MNSI-documented peripheral neuropathy nor history of amputation was associated with a differential effect on mortality from intensive compared with standard glycemia treatment (although very few participants with an amputation entered the study). The discrepancy suggests the two methods of detecting neuropathy may identify different populations. Indeed, the MNSI detected peripheral neuropathy in 4,357 participants in contrast to 2,737 participants who reported a history of neuropathy, of whom 61% had also an MNSI score indicative of neuropathy. The discordance among various indexes of neuropathy in their strength for predicting outcomes (symptoms versus physical findings) was also apparent in the DIAD study (20), where a significant relation to CVD outcomes was found with one symptom (numbness) and one sign (“absent sensation”), but it was borderline for a different symptom (pain) and had no relation to yet another symptom (tingling) and two physical signs (absent vibration, absent reflex). The discrepancy does not diminish the possible importance of neuropathy as a predictor for worse outcomes from intensive glycemia treatment, or even as an etiologic agent of CVD outcomes or mortality; this issue deserves further study.
Lastly, there was a differential effect on mortality of intensive compared with standard treatment by use of aspirin at baseline. Although aspirin use is recommended for patients with type 2 diabetes (21), the evidence supporting its benefit is now being reconsidered (22). Recent clinical trials including or limited to those with type 2 diabetes (23–25) have not shown a significant reduction in either CVD mortality or overall mortality in patients with type 2 diabetes, although these studies often included populations considered to be at lower risk of CVD disease than the population recruited for the ACCORD trial. A possible explanation could be that aspirin use is merely a proxy for those at higher risk of CVD disease, although we did not see an increased risk among those with a history of CVD disease at baseline.
There are a number of important limitations to the analyses conducted for this article. First, we cannot exclude the possibility that the findings of significant interactions are due to chance alone. For a set of 38 independent variables, we would expect to find P < 0.05 for at least 1.9 variables by chance alone, suggesting the results for A1C and aspirin use should be interpreted with caution, since P values were very close to the 0.05 level. Nonetheless, self-report of neuropathy had a very strong statistical significance. Data from other sources will be required for confirmation of the findings presented. Many of the baseline characteristics were self-reported by participants; although a certain amount of misclassification may be expected, we would not expect this to differ by study arm. Finally, these analyses should be considered exploratory and hypothesis generating, since the subgroups were not defined a priori and ACCORD was not powered to look at specific subgroups—nor was the study powered for an analysis of mortality, since the prespecified primary outcome was the rate of major CVD events.
In summary, we have identified three baseline characteristics that defined subgroups in which there was a differential effect on mortality of the intensive compared with standard glycemia treatment in ACCORD: A1C ≥8.5%, self-reported history of neuropathy, and aspirin use at study entry. The interactions in the A1C and aspirin use were of marginal significance and can only be considered suggestive and will require additional study. Of note is that the most significant association found was with self-report of neuropathy. Importantly, other baseline characteristics that were hypothesized to identify subgroups with differential effects on mortality by treatment arm did not have such interaction, including increasing age, duration of diabetes, and history of CVD disease. Further analysis of the role of neuropathy on mortality in type 2 diabetes is warranted. The ACCORD study group is currently performing additional analyses to define the possible role of autonomic neuropathy—as well as other factors—in the outcomes observed.
Supplementary Material
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
Clinical trial reg. no.: NCT00000620, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Lowe LP, Liu K, Greenland P, Metzger BE, Dyer AR, Stamler J. Diabetes, asymptomatic hyperglycemia, and 22-year mortality in black and white men: the Chicago Heart Association Detection Project in Industry Study. Diabetes Care 1997;20:163–169 [DOI] [PubMed] [Google Scholar]
- 2. Andersson DK, Svärdsudd K. Long-term glycemic control relates to mortality in type II diabetes. Diabetes Care 1995;18:1534–1543 [DOI] [PubMed] [Google Scholar]
- 3. Damsgaard EM, Frøland A, Jørgensen OD, Mogensen CE. Eight to nine year mortality in known non-insulin dependent diabetics and controls. Kidney Int 1992;41:731–735 [DOI] [PubMed] [Google Scholar]
- 4. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 5. Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 7. Havas S. The ACCORD Trial and control of blood glucose level in type 2 diabetes mellitus: time to challenge conventional wisdom. Arch Intern Med 2009;169:150–154 [DOI] [PubMed] [Google Scholar]
- 8. Montori VM, Fernández-Balsells M. Glycemic control in type 2 diabetes: time for an evidence-based about-face? Ann Intern Med 2009;150:803–808 [DOI] [PubMed] [Google Scholar]
- 9. Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, Howard BV, Kirkman MS, Kosiborod M, Reaven P, Sherwin RS; American Diabetes Association; American College of Cardiology Foundation; American Heart Association. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009;32:187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cefalu WT, Watson K. Intensive glycemic control and cardiovascular disease observations from the ACCORD study: now what can a clinician possibly think? Diabetes 2008;57:1163–1165 [DOI] [PubMed] [Google Scholar]
- 11. Dluhy RG, McMahon GT. Intensive glycemic control in the ACCORD and ADVANCE trials. N Engl J Med 2008;358: 2630–2633 [DOI] [PubMed] [Google Scholar]
- 12. Nissen SE. ENHANCE and ACCORD: controversy over surrogate end points. Curr Cardiol Rep 2008;10:159–161 [DOI] [PubMed] [Google Scholar]
- 13. ACCORD Study Group. Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, Genuth S, Gerstein HC, Ginsberg HN, Goff DC, Jr, Grimm RH, Jr, Margolis KL, Probstfield JL, Simons-Morton DG, Sullivan MD. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol 2007;99:21i–33i [DOI] [PubMed] [Google Scholar]
- 14. Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994;17:1281–1289 [DOI] [PubMed] [Google Scholar]
- 15. Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine–reporting of subgroup analyses in clinical trials. N Engl J Med 2007;357:2189–2194 [DOI] [PubMed] [Google Scholar]
- 16. Cusick M, Meleth AD, Agrón E, Fisher MR, Reed GF, Knatterud GL, Barton FB, Davis MD, Ferris FL, 3rd, Chew EY; Early Treatment Diabetic Retinopathy Study Research Group. Associations of mortality and diabetes complications in patients with type 1 and type 2 diabetes: early treatment diabetic retinopathy study report no. 27. Diabetes Care 2005;28:617–625 [DOI] [PubMed] [Google Scholar]
- 17. Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–234 [DOI] [PubMed] [Google Scholar]
- 18. Coppini DV, Bowtell PA, Weng C, Young PJ, Sönksen PH. Showing neuropathy is related to increased mortality in diabetic patients: a survival analysis using an accelerated failure time model. J Clin Epidemiol 2000;53:519–523 [DOI] [PubMed] [Google Scholar]
- 19. Lavery LA, van Houtum WH, Armstrong DG, Harkless LB, Ashry HR, Walker SC. Mortality following lower extremity amputation in minorities with diabetes mellitus. Diabetes Res Clin Pract 1997;37:41–47 [DOI] [PubMed] [Google Scholar]
- 20. Young LH, Wackers FJ, Chyun DA, Davey JA, Barrett EJ, Taillefer R, Heller GV, Iskandrian AE, Wittlin SD, Filipchuk N, Ratner RE, Inzucchi SE; DIAD Investigators. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009;301:1547–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. American Diabetes Association. Standards of medical care in diabetes–2009. Diabetes Care 2009;32(Suppl. 1):S13–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sirois C, Poirier P, Moisan J, Grégoire JP. The benefit of aspirin therapy in type 2 diabetes: what is the evidence? Int J Cardiol 2008;129:172–17 [DOI] [PubMed] [Google Scholar]
- 23. Sacco M, Pellegrini F, Roncaglioni MC, Avanzini F, Tognoni G, Nicolucci A; PPP Collaborative Group. Primary prevention of cardiovascular events with low-dose aspirin and vitamin E in type 2 diabetic patients: results of the Primary Prevention Project (PPP) trial. Diabetes Care 2003;26:3264–3272 [DOI] [PubMed] [Google Scholar]
- 24. Belch J, MacCuish A, Campbell I, Cobbe S, Taylor R, Prescott R, Lee R, Bancroft J, MacEwan S, Shepherd J, Macfarlane P, Morris A, Jung R, Kelly C, Connacher A, Peden N, Jamieson A, Matthews D, Leese G, McKnight J, O'Brien I, Semple C, Petrie J, Gordon D, Pringle S, MacWalter R. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 2008;337:a1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ogawa H, Nakayama M, Morimoto T, Uemura S, Kanauchi M, Doi N, Jinnouchi H, Sugiyama S, Saito Y; Japanese Primary Prevention of Atherosclerosis With Aspirin for Diabetes (JPAD) Trial Investigators. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA 2008;300:2134–2141 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.