Abstract
OBJECTIVE
To determine the effect of total parenteral nutrition (TPN)-induced hyperglycemia on hospital outcome.
RESEARCH DESIGN AND METHODS
The study determined whether blood glucose values before, within 24 h, and during days 2–10 of TPN are predictive of hospital complications and mortality.
RESULTS
Subjects included a total of 276 patients receiving TPN for a mean duration of 15 ± 24 days (±SD). In multiple regression models adjusted for age, sex, and diabetes status, mortality was independently predicted by pre-TPN blood glucose of 121–150 mg/dl (odds ratio [OR] 2.2, 95% CI 1.1–4.4, P = 0.030), 151–180 mg/dl (3.41, 1.3–8.7, P = 0.01), and >180 mg/dl (2.2, 0.9–5.2, P = 0.077) and by blood glucose within 24 h of >180 mg/dl (2.8, 1.2–6.8, P = 0.020). A blood glucose within 24 h of >180 mg/dl was associated with increased risk of pneumonia (OR 3.1, 95% CI 1.4–7.1) and acute renal failure (2.3, 1.1–5.0).
CONCLUSIONS
Hyperglycemia is associated with increased hospital complications and mortality in patients receiving TPN.
The beneficial effect of total parenteral nutrition (TPN) in improving the nutrition status of hospitalized malnourished patients is well established (1). Recent randomized trials and meta-analyses, however, have raised questions about its safety and the increased rate of TPN-associated complications and mortality in critically ill patients (2–4). The increased risk of complications during TPN therapy can be related, among other factors, to the development of hyperglycemia, which occurs in 10–88% of hospitalized patients receiving TPN therapy (4–6). Despite the high frequency of TPN-induced hyperglycemia, it is not known if the severity of hyperglycemia and/or the timing of hyperglycemia before initiation or during TPN therapy lead to hospital complications. Accordingly, we determined 1) the impact of TPN-induced hyperglycemia on survival and 2) whether blood glucose value before, shortly after initiation (within 24 h), and/or during TPN therapy can serve as predictive markers of in-hospital complications and mortality.
RESEARCH DESIGN AND METHODS
This work involved retrospective study of medical and surgical patients receiving TPN during the period of 1 January 2006 to 31 December 2006 at Grady Memorial Hospital in Atlanta, Georgia. Patients were managed after the hospital TPN nutrition protocol aimed to provide 25–35 kcal · kg−1 · day−1 and 1–2 g protein · kg−1 · day−1. We collected information on demographics; blood glucose on admission, pre-TPN, within 24 h, and during days 2–10 of TPN; Acute Physiology and Chronic Health Evaluation (APACHE) II score; length of hospital stay; hospital complications; and mortality.
Data analysis
For comparison of baseline demographics and clinical characteristics between groups, we used two-sample Wilcoxon's tests for continuous variables and χ2 test for categorical variables with Bonferroni's corrections when applicable. Multiple logistic regression and adjusted odds ratios (ORs) were used to determine the influence of clinical characteristics on mortality and complications. A P value of 0.05 was considered significant.
RESULTS
The study population included 276 consecutive medical (33%) and surgical (65%) patients (mean age 51 ± 18 years, BMI 26 ± 7 kg/m2, known diabetes 19.2%, intensive care unit admission 78.2%). TPN was started 12 ± 12 days after admission and was given for a mean duration of 15 ± 24 days.
The mean blood glucose level on admission was 139 ± 85 mg/dl. The mean blood glucose level before TPN was 123.2 ± 33 mg/dl and increased to a mean blood glucose of 146 ± 44 mg/dl within 24 h of TPN and remained elevated (147 ± 40 mg/dl) during days 2–10 of TPN infusion (P < 0.01 from baseline).
The overall hospital mortality was 27.2%. Deceased patients were older, were more likely to be in the intensive care unit, and had higher admission APACHE II scores versus nondeceased patients (all, P < 0.01). Deceased patients had a higher pre-TPN blood glucose (129 ± 37 vs. 121 ± 32 mg/dl, P = 0.08), a higher blood glucose within 24 h (162 ± 55 vs. 139 ± 37 mg/dl, P = 0.003), and a higher blood glucose during days 2–10 of TPN (161 ± 53 vs. 142 ± 34 mg/dl, P = 0.013) than nondeceased patients.
In multiple regression models adjusted for age, sex, and history of diabetes, the likelihood of death was independently predicted by elevated pre-TPN blood glucose between 121 and 150 mg/dl (OR 2.2, 95% CI 1.1–4.4, P = 0.030), 151 and 180 mg/dl (3.41, 1.3–8.7, P = 0.010), and >180 mg/dl (2.2, 0.9–5.2, P = 0.077) or by the blood glucose within 24 h >180 mg/dl (2.8, 1.2–6.8, P = 0.020) versus patients with a mean blood glucose ≤120 mg/dl. In multivariate analysis adjusting for age, sex, and history of diabetes, the blood glucose within 24 h of TPN >180 mg/dl was associated with increased risk of pneumonia (OR 3.6, 95% CI 1.6–8.4) and acute renal failure (2.2, 1.02–4.8 1) compared with patients with blood glucose <120 mg/dl. Patients with higher blood glucose levels during TPN had a longer hospital (P = 0.011) and intensive care unit (P = 0.008) length of stay.
CONCLUSIONS
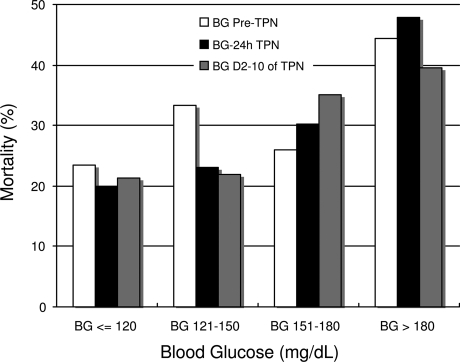
Malnutrition is reported in up to 40% of critically ill patients (1,7) and is associated with increased risk of hospital complications, longer hospital stay, and mortality (8). Despite improving the nutrition state and immunologic competence (9), TPN therapy has been associated with increased risk for infections and mortality (2,10–13). The increased risk of complications appears to be related, among other factors, to the development of hyperglycemia (4,14). Observational studies have reported a 33% mortality rate in TPN patients who developed hyperglycemia (15), as well as an increased risk of cardiac complications, infections, systemic sepsis, and acute renal failure (3,4,6). In agreement with these reports, we found a strong correlation between TPN-induced hyperglycemia and poor clinical outcome. Of interest, we observed that values before and within 24 h of initiation of TPN are better predictors of hospital mortality and complications than blood glucose during the entire duration of TPN (Fig. 1).
Figure 1.
Mean blood glucose (BG) levels and mortality rate. □, blood glucose levels pre-TPN (BG Pre-TPN), P = 0.167. ■, blood glucose levels within 24 hours of TPN (BG-24h TPN), P = 0.004. ■, blood glucose levels during days 2–10 of TPN (BG D2–10 of TPN), P = 0.060.
In multiple regression models adjusted for age, sex, and diabetes status, mortality was independently predicted by pre-TPN blood glucose values between 151 and 180 mg/dl (OR 3.41, 95% CI 1.3–8.7, P = 0.01) and >180 mg/dl (2.2, 0.9–5.2, P = 0.077), as well as by blood glucose within 24 h of TPN >180 mg/dl (2.8, 1.2–6.8, P = 0.020) versus patients without hyperglycemia. In addition, blood glucose >180 mg/dl within 24 h of initiation of TPN was associated with increased risk of pneumonia (3.1, 1.4–7.1) and acute renal failure (2.3, 1.1–5.0).
The mechanisms underlying the detrimental effects of hyperglycemia relate to alterations in immune functions and inflammatory response (16,17). Hyperglycemia impairs leukocyte function, phagocytosis, and chemotaxis (18). Hyperglycemia also increases counterregulatory hormones, inflammatory cytokines, and oxidative stress (16,17), which can lead to endothelial dysfunction and cardiovascular complications (17). In addition to hyperglycemia, the administration of Intralipid in TPN solutions may worsen clinical outcome. Intralipid infusion, a soybean oil-based emulsion rich in n-6 polyunsaturated fatty acids (19), has been associated with exaggerated inflammatory response, immunosuppression, insulin resistance, increased blood pressure, endothelial dysfunction, and oxidative stress (19).
In summary, TPN-induced hyperglycemia is associated with increased length of hospital stay, increased risk of complications, and higher mortality in hospitalized patients. Our study indicates that blood glucose values before and within 24 h of initiation of TPN are better predictors of hospital mortality and complications than the mean blood glucose during the entire duration of TPN. These results suggest that early and aggressive intervention to prevent and correct hyperglycemia may improve clinical outcome in patients receiving TPN.
Acknowledgments
We appreciate the support of the Medical Records Department staff at Grady Memorial Hospital. G.E.U. is supported by research grants from the American Diabetes Association (7-03-CR-35) and the National Institutes of Health (M01 RR-00039).
No potential conflicts of interest relevant to this article were reported.
This work was presented in abstract form at the 69th Scientific Sessions, American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Ziegler TR. Parenteral nutrition in the critically ill patient. N Engl J Med 2009;361:1088–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heyland DK, Montalvo M, MacDonald S, Keefe L, Su XY, Drover JW. Total parenteral nutrition in the surgical patient: a meta-analysis. Can J Surg 2001;44:102–111 [PubMed] [Google Scholar]
- 3. Perioperative total parenteral nutrition in surgical patients. The Veterans Affairs Total Parenteral Nutrition Cooperative Study Group. N Engl J Med 1991;325:525–532 [DOI] [PubMed] [Google Scholar]
- 4. der Voort PH, Feenstra RA, Bakker AJ, Heide L, Boerma EC, der Horst IC. Intravenous glucose intake independently related to intensive care unit and hospital mortality: an argument for glucose toxicity in critically ill patients. Clin Endocrinol (Oxf) 2006;64:141–145 [DOI] [PubMed] [Google Scholar]
- 5. Schloerb PR. Glucose in parenteral nutrition: a survey of U.S. medical centers. JPEN J Parenter Enteral Nutr 2004;28:447–452 [DOI] [PubMed] [Google Scholar]
- 6. Cheung NW, Napier B, Zaccaria C, Fletcher JP. Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition. Diabetes Care 2005;28:2367–2371 [DOI] [PubMed] [Google Scholar]
- 7. Giner M, Laviano A, Meguid MM, Gleason JR. In 1995 a correlation between malnutrition and poor outcome in critically ill patients still exists. Nutrition 1996;12:23–29 [DOI] [PubMed] [Google Scholar]
- 8. Correia MI, Waitzberg DL. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr 2003;22:235–239 [DOI] [PubMed] [Google Scholar]
- 9. McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, Ochoa JB, Napolitano L, Cresci G. A.S.P.E.N. Board of Directors, American College of Critical Care Medicine, Society of Critical Care Medicine. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2009;33:277–316 [DOI] [PubMed] [Google Scholar]
- 10. Anderson AD, Jain PK, MacFie J. Parenteral nutrition in the critically ill. Intensive Care Med 2003;29:2103 [DOI] [PubMed] [Google Scholar]
- 11. Bellantone R, Doglietto G, Bossola M, Pacelli F, Negro F, Sofo L, Crucitti F. Preoperative parenteral nutrition of malnourished surgical patients. Acta Chir Scand 1988;154:249–251 [PubMed] [Google Scholar]
- 12. Klein S, Kinney J, Jeejeebhoy K, Alpers D, Hellerstein M, Murray M, Twomey P. Nutrition support in clinical practice: review of published data and recommendations for future research directions: summary of a conference sponsored by the National Institutes of Health, American Society for Parenteral and Enteral Nutrition, and American Society for Clinical Nutrition. Am J Clin Nutr 1997;66:683–706 [DOI] [PubMed] [Google Scholar]
- 13. Marik PE. Death by total parenteral nutrition: part deux. Crit Care Med 2006;34:3062 [DOI] [PubMed] [Google Scholar]
- 14. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002;87:978–982 [DOI] [PubMed] [Google Scholar]
- 15. Valero MA, Alegre E, Gomis P, Moreno JM, Miguelez S, Leon-Sanz M. Clinical management of hyperglycaemic patients receiving total parenteral nutrition. Clin Nutr 1996;15:11–15 [DOI] [PubMed] [Google Scholar]
- 16. Kitabchi AE, Freire AX, Umpierrez GE. Evidence for strict inpatient blood glucose control: time to revise glycemic goals in hospitalized patients. Metabolism 2008;57:116–120 [DOI] [PubMed] [Google Scholar]
- 17. Umpierrez GE, Kitabchi AE. ICU care for patients with diabetes. Curr Opin Endocrinol 2004;11:75–81 [Google Scholar]
- 18. Turina M, Fry DE, Polk HC, Jr. Acute hyperglycemia and the innate immune system: clinical, cellular, and molecular aspects. Crit Care Med 2005;33:1624–1633 [DOI] [PubMed] [Google Scholar]
- 19. Umpierrez GE, Smiley D, Robalino G, Peng L, Kitabchi AE, Khan B, Le A, Quyyumi A, Brown V, Phillips LS. Intravenous intralipid-induced blood pressure elevation and endothelial dysfunction in obese African-Americans with type 2 diabetes. J Clin Endocrinol Metab 2009;94:609–614 [DOI] [PMC free article] [PubMed] [Google Scholar]