Abstract
OBJECTIVE
To compare the sensitivity and specificity of luciferase immunoprecipitation (LIPS) with radioimmunoprecipitation (RIP) for the measurement of autoantibodies to the type 1 diabetes autoantigens glutamic acid decarboxylase 65 (GAD65) and insulinoma-associated protein (IA)-2β.
RESEARCH DESIGN AND METHODS
Sera from 49 type 1 diabetic patients and 100 nondiabetic control subjects from Diabetes Antibody Standardization Program 2007 were used to screen for autoantibodies to GAD65. An additional 200 type 1 diabetic patients and 200 nondiabetic control subjects were used to validate the GAD65 results and screen for autoantibodies to IA-2β.
RESULTS
LIPS showed equal sensitivity and specificity to RIP for detecting autoantibodies to GAD65 and IA-2β. Receiver-operating characteristic analysis revealed that the detection of autoantibodies to GAD65 and IA-2β by LIPS and RIP were not statistically different.
CONCLUSIONS
The LIPS assay does not require the use of radioisotopes or in vitro transcription/translation and is a practical alternative at the clinical level for the RIP assay.
Autoantibodies to glutamic acid decarboxylase 65 (GAD65), insulinoma-associated protein (IA)-2, and IA-2β are major diagnostic and predictive markers in type 1 diabetes (1,2). Autoantibodies to these proteins, which appear years before the development of clinical disease and in combination with certain HLA haplotypes, are being used to enter subjects into therapeutic intervention trials (3). The radioimmunoprecipitation (RIP) assay has been used extensively to detect these autoantibodies. Recently, we showed that luciferase immunoprecipitation (LIPS) displayed equal sensitivity and specificity to RIP for detecting IA-2 autoantibodies (4). The present experiments were initiated to see whether LIPS could be used to measure autoantibodies to GAD65 and IA-2β with a sensitivity and specificity equal to that of RIP.
RESEARCH DESIGN AND METHODS
For the LIPS assay, full-length GAD65 or the intracellular portion of IA-2β (aa 662–1,033) (5) was cloned into the pREN2 vector downstream of the Renilla luciferase reporter, and extracts were prepared from transfected Cos1 cells as described (4–6). For the RIP assay, GAD65 and IA-2β were cloned into pTNT and pGBKT7 vectors, respectively, and the [35S] methionine–labeled proteins were produced by in vitro transcription/translation (7). Autoantibodies to GAD65 and IA-2β were detected by liquid-phase immunoprecipitation using 1.0 × 107 light units (LU) of cell extracts in LIPS and ∼40,000 counts per minute (cpm) of radiolabeled protein for RIP.
Sera from 100 control and 49 type 1 diabetic patients were obtained from the 2007 Diabetes Antibody Standardization Program (DASP) (8) and used to measure autoantibodies to GAD65. In the 2007 serum exchange, sensitivity and specificity for autoantibodies to GAD65 were 82 and 96%, respectively. Control subjects from the DASP included some samples with high levels of islet autoantibodies, presumably because they were from subjects who were at high risk of developing type 1 diabetes. Neither the DASP patients with type 1 diabetes nor the control subjects are representative of the type 1 diabetic population or the general public. Additional sera from 200 age-matched nondiabetic control subjects and 200 type 1 diabetic subjects (Malmö Diabetes Study) (9) were used to validate the GAD65 findings and measure autoantibodies to IA-2β. A serum was positive if the precipitated cpm or LU exceeded the mean + 3 SD of the control subjects. MedCalc Software (Mariakerke, Belgium) was used for statistical analyses. Signal-to-noise ratios of autoantibodies for RIP and LIPS in the type 1 diabetic samples were determined as described (10).
RESULTS
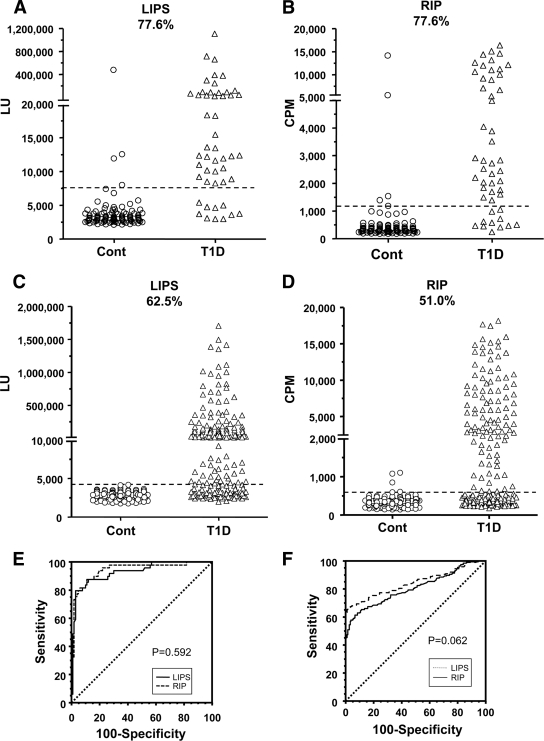
Anti-GAD65 autoantibodies determined by LIPS showed that only 3 of 100 nondiabetic control subjects were positive (Fig. 1A). In contrast, 77.6% (38 of 49) of the sera from patients with type 1 diabetes were positive by LIPS with a specificity of 97%. In RIP, 5 of 100 nondiabetic control subjects were autoantibody positive, whereas 77.6% (38 of 49) of the sera from subjects with type 1 diabetes were autoantibody positive (Fig. 1B), with a specificity of 95%. The coefficient of variation (CV) for duplicate samples was 13.5% for LIPS and 3.1% for RIP (supplemental Fig. 1A [available at http://care.diabetesjournals.org/cgi/content/full/dc09-1938/DC1). Comparison of the assays revealed a high coefficient of determination with an R2 of 0.778 (supplemental Fig. 1C). Receiver-operating characteristic (ROC) analysis showed that the area under the curves for autoantibodies to GAD65 by LIPS and RIP were not statistically different (P = 0.592) (Fig. 1E). Validation studies for GAD65 autoantibodies with 200 type 1 diabetic sera revealed 53.5% positivity by LIPS and 49.3% positivity by RIP (data not shown).
Figure 1.
GAD65 autoantibodies as determined by LIPS (A) and RIP (B) and IA-2β autoantibodies as determined by LIPS (C) and RIP (D). Dotted lines represent 3 SDs above the mean of the nondiabetic control sera. E: ROC analysis showing the area under the curve for GAD autoantibodies by LIPS (0.929 [95% CI 0.875–0.964]) and by RIP (0.941 [0.891–0.973]). There was no statistical difference (P = 0.592). F: ROC analysis showing the area under the curve for IA-2β autoantibodies by LIPS (0.844 [0.804– 0.879]) and by RIP (0.807 [0.763–0.849]). There was no statistical difference (P = 0.062).
LIPS profiling of IA-2β autoantibodies revealed that none of the 200 nondiabetic control sera were positive (Fig. 1C). In contrast, 62.5% (125 of 200) of type 1 diabetic subjects were autoantibody positive with a specificity of 100%. In RIP, 3 of 200 nondiabetic control sera were positive, whereas 51.0% (102 of 200) of the sera from subjects with type 1 diabetes were autoantibody positive (Fig. 1D) with a 97.5% specificity. The CV for duplicate samples was 10.9% for LIPS and 6.8% for RIP (supplemental Fig. 1B). Comparison of the assays revealed a high coefficient of determination, with an R2 of 0.904 (supplemental Fig. 1D). ROC analysis showed that the area under the curves for autoantibodies to IA-2β by LIPS and RIP were not statistically different (P = 0.062) (Fig. 1F). However, the signal-to-noise ratio for detecting autoantibodies to GAD65 and IA-2β was higher as determined by LIPS than by RIP (Fig. 1A–D and supplemental Fig. 1E and F).
CONCLUSIONS
In nondiabetic subjects, the presence of autoantibodies to more than one of the major diabetes-associated autoantigens is a better predictor of the development of clinical diabetes than the presence of any single autoantibody (3). Initially, autoantibodies directed against islet cells (ICAs) were detected by immunofluorescence. In recent years, the ICA technique has been replaced by the quantitative RIP assay. In the present report on autoantibodies to GAD65 and IA-2β and in our recent report on autoantibodies to IA-2 (4), we showed that the liquid-phase LIPS assay is equal in sensitivity and specificity to the liquid-phase RIP assay, in that the two assays have a high correlation coefficient and that, by ROC analysis, the areas under the curves are not statistically different.
Although further documentation is needed, our current study shows that the sensitivity for detecting autoantibodies to IA-2β by LIPS is not only equal to that of RIP but may be slightly higher (62.5 vs. 51.0%). Also, the signal-to-noise ratio is higher for LIPS compared with RIP, but the clinical significance of the very high autoantibody-positive sera detected in LIPS is not known at this time. Moreover, we have no concrete evidence that the LIPS assay is more reliable or sensitive than the RIP assay for discriminating borderline autoantibody-positive and -negative sera. It is the failure to reproducibly distinguish between positive and negative signals at the borderline that is responsible for much of the variation in routine autoantibody assays.
Although LIPS and RIP appear to be equal in sensitivity and specificity, LIPS has the advantage for a clinical laboratory of not requiring the use of radioisotopes, avoids the time and expense of in vitro transcription/translation, and offers the potential of detecting mammalian cell posttranslational modifications, which would not be found in a bacterial expression system or by in vitro transcription/translation. Use of a mixture of Renilla luciferase–tagged antigen with firefly luciferase–tagged antigen may also allow detection of autoantibodies to two different autoantigens at the same time.
Supplementary Material
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Dental and Craniofacial Research, the National Institutes of Health.
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Achenbach P, Bonifacio E, Williams AJ, Ziegler AG, Gale EA, Bingley PJ. Autoantibodies to IA-2beta improve diabetes risk assessment in high-risk relatives. Diabetologia 2008;51:488–492 [DOI] [PubMed] [Google Scholar]
- 2. Notkins AL, Lernmark A. Autoimmune type 1 diabetes: resolved and unresolved issues. J Clin Invest 2001;108:1247–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Notkins AL. New predictors of disease: molecules called predictive autoantibodies appear in the blood years before people show symptoms of various disorders: tests that detected these molecules could warn of the need to take preventive action. Sci Am 2007;296:72–79 [PubMed] [Google Scholar]
- 4. Burbelo PD, Hirai H, Leahy H, Lernmark A, Ivarsson SA, Iadarola MJ, Notkins AL. A new luminescence assay for autoantibodies to mammalian cell-prepared insulinoma-associated protein 2. Diabetes Care 2008;31:1824–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li Q, Borovitskaya AE, DeSilva MG, Wasserfall C, Maclaren NK, Notkins AL, Lan MS. Autoantigens in insulin-dependent diabetes mellitus: molecular cloning and characterization of human IA-2 beta. Proc Assoc Am Physicians 1997;109:429–439 [PubMed] [Google Scholar]
- 6. Burbelo PD, Ching KH, Klimavicz CM, Iadarola MJ. Antibody profiling by luciferase immunoprecipitation systems (LIPS). J Vis Exp 2009;7:32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hirai H, Miura J, Hu Y, Larsson H, Larsson K, Lernmark A, Ivarsson SA, Wu T, Kingman A, Tzioufas AG, Notkins AL. Selective screening of secretory vesicle-associated proteins for autoantigens in type 1 diabetes: VAMP2 and NPY are new minor autoantigens. Clin Immunol 2008;127:366–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Torn C, Mueller PW, Schlosser M, Bonifacio E, Bingley PJ. Diabetes antibody standardization program: evaluation of assays for autoantibodies to glutamic acid decarboxylase and islet antigen-2. Diabetologia 2008;51:846–852 [DOI] [PubMed] [Google Scholar]
- 9. Lindberg B, Ahlfors K, Carlsson A, Ericsson UB, Landin-Olsson M, Lernmark A, Ludvigsson J, Sundkvist G, Ivarsson SA. Previous exposure to measles, mumps, and rubella—but not vaccination during adolescence—correlates to the prevalence of pancreatic and thyroid autoantibodies. Pediatrics 1999;104:e12 [DOI] [PubMed] [Google Scholar]
- 10. Li M, Yu L, Tiberti C, Bonamico M, Taki I, Miao D, Murray JA, Rewers MJ, Hoffenberg EJ, Agardh D, Mueller P, Stern M, Bonifacio E, Liu E. A report on the International Transglutaminase Autoantibody Workshop for Celiac Disease. Am J Gastroenterol 2009;104:154–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.