Abstract
OBJECTIVE
To evaluate A1C for screening and diagnosis of undiagnosed type 2 diabetes defined by oral glucose tolerance testing in clinical and general populations.
RESEARCH DESIGN AND METHODS
A1C cut offs (≤5.5% to rule out diabetes; ≥7.0% to rule in diabetes) were derived from a clinical group (Melbourne Pathology [MP] group: n = 2,494; undiagnosed diabetes 34.6%) and then evaluated in a population-based sample (AusDiab group: n = 6,015; undiagnosed diabetes 4.6%).
RESULTS
For diabetes in the MP and AusDiab groups, A1C at 5.5% gave sensitivities of 98.7 and 83.5%, while A1C at 7.0% gave specificities of 98.2 and 100%, respectively. Many (61.9–69.3%) with impaired A1C (5.6–6.9%) in both populations had abnormal glucose status.
CONCLUSIONS
A1C ≤5.5% and ≥7.0% predicts absence or presence of type 2 diabetes, respectively, while at A1C 6.5–6.9% diabetes is highly probable in clinical and population settings. A high proportion of people with impaired A1C have abnormal glucose status requiring follow-up.
With current screening tools (fasting plasma glucose [FPG] with or without oral glucose tolerance test [OGTT]), the prevalence of undiagnosed diabetes in Australia remains high (1). A1C provides a practical alternative for screening (2,3). It is more convenient and reproducible than is blood glucose (3,4). As optimal cut offs are still in debate, we explore here A1C levels that confidently rule out and rule in diabetes in two different Australian populations.
RESEARCH DESIGN AND METHODS
We studied two populations. The clinical population, Melbourne Pathology (MP) group, included all patients referred by medical practitioners for an OGTT in 2003–2008 to a state-wide private pathology service (MP Services, Australia); the AusDiab population comes from a national population-based study (2004–2005 AusDiab follow-up) (5). Only people with concurrent A1C and OGTT results are included here (MP group: n = 2,494; AusDiab: n = 6,014). Glucose status was classified by American Diabetes Association criteria for OGTT (6).
A1C was determined either by Diabetes Control and Complications Trial (DCCT)-aligned (7) cation-exchange chromatography (MP population) or by boronate affinity chromatography with values converted to DCCT-aligned A1C (5) (AusDiab). Plasma glucose was measured using hexokinase.
RESULTS
A1C cut offs defined from the MP population
Among those with undiagnosed diabetes (34.6%) by OGTT criteria in the MP population, A1C at the 2.5th percentile was 5.6%. A1C ≤5.5% was thus chosen to rule out diabetes. For those without diabetes (65.4%), A1C at the 97.5th percentile was 6.9%. A1C ≥7.0% was thus chosen to rule in (diagnose) diabetes.
Applying A1C cut offs to the MP population (34.6% undiagnosed diabetes)
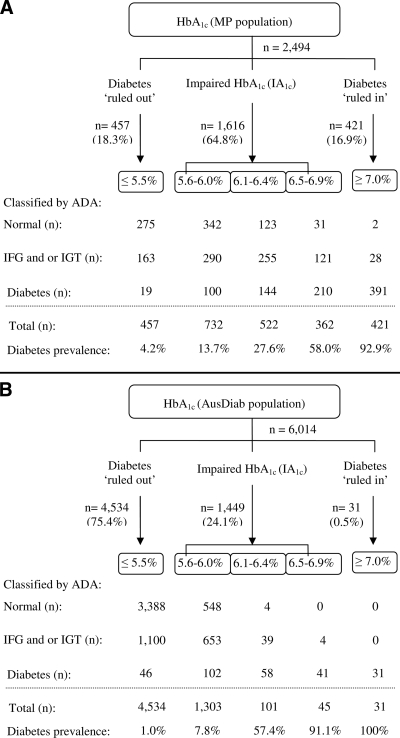
Applying the above cut offs, a total 35.2% of the MP population had diabetes ruled in or ruled out (Fig. 1A), while the remaining 64.8% had an impaired A1C of 5.6–6.9%. From those with impaired A1C, 61.9% had abnormal glucose status. For diabetes, A1C at 5.5% provided high sensitivity (97.8%) and high negative predictive value (NPV) (95.8%), while A1C at 7.0% gave high specificity (98.2%) and high positive predictive value (PPV) (92.9%). Using the recommended cut off of 6.5% (3), specificity decreased to 88.8% and the PPV decreased to 76.8%.
Figure 1.
Application of the A1C cut offs to screen or diagnose diabetes in a clinical group (MP population, n = 2,494, undiagnosed diabetes 34.6%) (A) and in a national population-based group (AusDiab population, n = 6,014, undiagnosed diabetes 4.6%) (B).
Applying A1C cut offs to the AusDiab population (4.6% undiagnosed diabetes)
Applying the same cut offs, a total 75.9% of the AusDiab population had diabetes ruled in or ruled out (Fig. 1B), while the remaining 24.1% had impaired A1C. From those with impaired A1C, 69.3% had abnormal glucose status. For diabetes, A1C at 5.5% provided moderate sensitivity (83.5%) but high NPV (99.0%), since diabetes prevalence was lower in the AusDiab than in the MP population. A1C at 7.0% gave 100% specificity and 100% PPV. By dropping the cut off to 6.5%, specificity remained 99.9%, with PPV near 100%.
CONCLUSIONS
Our study supports recommendations to use A1C for diabetes screening and diagnosis (2,3). Using two, rather than one, cut off values for A1C achieved high sensitivity for screening plus optimal specificity for diabetes diagnosis. We also show the high probability that those with impaired A1C have abnormal glucose status.
Single A1C cut offs have limited clinical utility in identifying those people with abnormal blood glucose levels. The most commonly reported single A1C cut off obtained from reported receiver-operated characteristic (ROC) curves was ∼6.1%, which gives sensitivities of 78–81%, with specificities of 79–84% (8). In our MP population, the ROC curve–identified optimal A1C of 6.2% gave a sensitivity of 82.2% and specificity of 78.8% (Z.X.L, unpublished data) but yielded a reduced PPV (67.2%) and NPV (89.3%).
We therefore apply two proposed cut offs. The lower was chosen for its high NPV, as diabetes is ruled out with high confidence (with A1C ≤5.5%, NPV was >95% in both clinical and population settings). Applying an A1C of 5.5% to National Health and Nutrition Examination Survey (NHANES) data generated a sensitivity of ∼92% and specificity of ∼30% from the published ROC curve (9). This gave an NPV of ∼99% (assuming 3.4% undiagnosed diabetes) and agrees with our finding that diabetes is very unlikely in individuals with A1C ≤5.5%. Our 7.0% cut off for diabetes diagnosis is higher than the 6.5% recommended cut off (2,3) but was chosen to optimize specificity.
For those with impaired A1C, the prevalence of diabetes increases as A1C increases. A1C is a continuous variable for diabetes and any cut off values chosen are somewhat arbitrary. From our data, people with A1C 5.6–6.0% were more likely to have either normoglycemia or pre-diabetes (impaired fasting glucose and/or impaired glucose tolerance) than diabetes, consistent with NHANES, where A1C 5.5–6.0% excluded diabetes in moderate but not high-risk individuals (10). Thus, those with an A1C of 5.6–6.0% would probably require education and lifestyle modification to prevent progression to diabetes (11) plus retesting every 6–12 months. Also in our study, people with an A1C of 6.1–6.4% were more likely to have pre-diabetes or diabetes than normoglycemia, while among those with a A1C of 6.5–6.9%, diabetes was highly probable. Thus, individuals with an A1C of 6.1–6.9% may require an OGTT to confirm their glycemic status plus lifestyle education and regular monitoring as for people with pre-diabetes. For those with an A1C ≥6.5%, screening for retinopathy is also necessary (2).
A1C as a screening/diagnostic tool has some limitations (3). The main issues are method bias, which is now being addressed by Internation Federation of Clinical Chemistry standardization (12) and certain confounding medical conditions (hemoglobinopathies and anemia). Most new A1C methods can identify or are unaffected by hemoglobinopathies. Anemia is also readily identifiable.
The cost of A1C has also been raised as a concern. While A1C analysis, per se, is more expensive than for glucose, the overall differences are small once the costs for blood collection are accounted for. From our own estimates, total costs are AUD $10.20 for A1C compared with AUD $8.80 for FPG and AUD $12.10 for a two-point collection of OGTT. These cost comparisons are consistent with reports from other countries (13–15). Further, the time and inconvenience to patients in having to fast for an OGTT cannot be ignored.
A1C ≤5.5% and ≥7.0% predicts with 97.5% confidence the absence or presence of type 2 diabetes using the OGTT as a reference. Many with impaired A1C have pre-diabetes, while diabetes is highly probable when A1C reaches 6.5–6.9%. Impaired A1C thus requires follow-up and lifestyle modification. Although the cost of A1C is slightly higher than for FPG, the overall efficiency of using A1C as a first line for diabetes screening may facilitate early diagnosis and reduce the health burden associated with diabetes complications.
Acknowledgments
The AusDiab study, co-coordinated by the Baker IDI Heart and Diabetes Institute, gratefully acknowledges the generous support given by the National Health and Medical Research Council (Grant no. 233200); the Australian Government Department of Health and Ageing; Abbott Australasia; Alphapharm; AstraZeneca; Bristol-Myers-Squibb; City Health Centre, Diabetes Service, Canberra; the Department of Health and Community Services, Northern Territory; the Department of Health and Human Services, Tasmania; the Department of Health, New South Wales; the Department of Health, Western Australia; the Department of Health, South Australia; the Department of Human Services, Victoria; Diabetes Australia; Diabetes Australia Northern Territory; Eli Lilly Australia; the estate of the late Edward Wilson; GlaxoSmithKline; the Jack Brockhoff Foundation; Janssen-Cilag; Kidney Health Australia; the Marian & FH Flack Trust; the Menzies Research Institute; Merck Sharp & Dohme; Novartis Pharmaceuticals; Novo Nordisk Pharmaceuticals; Pfizer; the Pratt Foundation; Queensland Health; Roche Diagnostics Australia; Royal Prince Alfred Hospital, Sydney; Sanofi-Aventis; and Sanofi Synthelabo.
No other potential conflicts of interest relevant to this article were reported.
The following individuals also made an invaluable contribution: A. Allman, B. Atkins, S. Bennett, A. Bonney, S. Chadban, M. de Courten, M. Dalton, D. Dunstan, T. Dwyer, H. Jahangir, D. Jolley, D. McCarty, A. Meehan, N. Meinig, S. Murray, K. O'Dea, K. Polkinghorne, P. Phillips, C. Reid, A. Stewart, R. Tapp, H. Taylor, T. Whalen, F. Wilson, and P. Zimmet.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Dunstan D, Zimmet P, Welborn T, de Courten M, Cameron A, Sicree R, Dwyer T, Colagiuri S, Jolley D, Knuiman M, Atkins R, Shaw JE. The rising prevalence of diabetes and impaired glucose tolerance: the Australian Diabetes, Obesity and Lifestyle Study. Diabetes Care 2002;25:829–834 [DOI] [PubMed] [Google Scholar]
- 2. International Expert Committee. International expert committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009;32:1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saudek CD, Herman WH, Sacks DB, Bergenstal RM, Edelman D, Davidson MB. A new look at screening and diagnosing diabetes mellitus. J Clin Endocrinol Metab 2008;93:2447–2453 [DOI] [PubMed] [Google Scholar]
- 4. Balion CM, Raina PS, Gerstein HC, Santaguida PL, Morrison KM, Booker L, Hunt DL. Reproducibility of impaired glucose tolerance (IGT) and impaired fasting glucose (IFG) classification: a systematic review. Clin Chem Lab Med 2007;45:1180–1185 [DOI] [PubMed] [Google Scholar]
- 5. Magliano DJ, Barr EL, Zimmet PZ, Cameron AJ, Dunstan DW, Colagiuri S, Jolley D, Owen N, Phillips P, Tapp RJ, Welborn TA, Shaw JE. Glucose indices, health behaviors, and incidence of diabetes in Australia. Diabetes Care 2008;21:267–272 [DOI] [PubMed] [Google Scholar]
- 6. American Diabetes Association. Standards of medical care in diabetes, 2008. Diabetes Care 2008;31(Suppl. 1):S12–S54 [DOI] [PubMed] [Google Scholar]
- 7. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long term complications in insulin dependent diabetes mellitus. N Engl J Med 1993;329: 977–986 [DOI] [PubMed] [Google Scholar]
- 8. Bennett CM, Guo M, Dharmage SC. HbA(1c) as a screening tool for detection of type 2 diabetes: a systematic review. Diabet Med 2007;24:333–343 [DOI] [PubMed] [Google Scholar]
- 9. Buell C, Kermah D, Davidson MB. Utility of A1C for diabetes screening in the 1999–2004 NHANES population. Diabetes Care 2007;30:2233–2235 [DOI] [PubMed] [Google Scholar]
- 10. Ginde AA, Cagliero E, Nathan DM, Camargo CA, Jr. Value of risk stratification to increase the predictive validity of HbA1c in screening for undiagnosed diabetes in the US population. J Gen Intern Med 2008;23:1346–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gillies CL, Abrams KR, Lambert PC, Cooper NJ, Sutton AJ, Hsu RT, Khunti K. Pharmocological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ 2007;334:299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weykamp C, John WG, Mosca A, Hoshino T, Little R, Jeppsson JO, Goodall I, Miedema K, Myers G, Reinauer H, Sacks DB, Slingerland R, Siebelder C. The IFCC reference measurement system for HbA1c: a 6-year progress report. Clin Chem 2008;54:240–248 [DOI] [PubMed] [Google Scholar]
- 13. Shirasaya K, Miyakawa M, Yoshida K, Takahashi E, Shimada N, Kondo T. Economic evaluation of alternative indicators for screening for diabetes mellitus. Prev Med 1999;29:79–78 [DOI] [PubMed] [Google Scholar]
- 14. Icks A, Haastert B, Gandjour A, John J, Löwel H, Holle R, Giani G, Rathmann W. Cost-effectiveness analysis of different screening procedures for type 2 diabetes: the KORA survey 2000. Diabetes Care 2004;27:2120–2128 [DOI] [PubMed] [Google Scholar]
- 15. Waugh N, Scotland G, McNamee P, Gillett M, Brennan A, Goyder E, Williams R, John A. Screening for type 2 diabetes: literature review and economic modelling. Health Technol Assess 2007;11:1–144 [DOI] [PubMed] [Google Scholar]