Abstract
OBJECTIVE
Little is known regarding recent changes in glitazone use.
RESEARCH DESIGN AND METHODS
Interrupted time series analyses of nationally representative office-visit data using IMS Health's National Disease and Therapeutic Index.
RESULTS
From 2003 through 2005, glitazone use increased steadily. From February 2005 to January 2007, rosiglitazone use decreased by 16% (95% CI −20 to −11) annually; pioglitazone use increased at an annual rate of 14% (9–18). During a period of Food and Drug Administration (FDA) advisories, rosiglitazone use declined sharply from 0.42 million monthly treatment visits (February 2007) to 0.13 million monthly visits (May 2008). Pioglitazone use remained stable, accounting for ∼5.8 million physician visits (77% of all glitazone use) where a treatment was used during the final 12 months of observation.
CONCLUSIONS
The combined effect of scientific publications, advisories, and media exposure was associated with a substantial decrease in rosiglitazone use. Despite a class-level FDA advisory, pioglitazone use was not similarly affected.
Diabetes treatments change and evolve, and glitazones were rapidly incorporated into practice, with U.S. expenditures reaching $4.2 billion in 2007 (1). Even early evidence suggested rare but serious adverse cardiovascular events with glitazone use (2). Based on accumulating evidence (3), in May 2007 the Food and Drug Administration (FDA) issued an advisory about rosiglitazone's cardiovascular risks (4,5), followed by a class-wide advisory in August 2007 and an additional rosiglitazone advisory in November 2007 (4).
Emerging scientific evidence and the FDA advisories received considerable media coverage, and each of these factors may have been influential in affecting pioglitazone and rosiglitazone use. We examine pioglitazone and rosiglitazone use based on a nationally representative audit of office-based physicians.
RESEARCH DESIGN AND METHODS
We used the IMS National Disease and Therapeutic Index (NDTI) to obtain monthly data on oral diabetic therapies from January 2003 to June 2009 (5,6). Our outcome variable was a treatment visit, defined as a visit during which the patient was treated with a glitazone.
Each drug therapy within the NDTI is linked to a specific six-digit taxonomic code capturing information similar to the ICD-9. We queried this for diagnoses of diabetes, excluding those aged <35 years or those with type 1 diabetes. In secondary analyses, we examined whether there was evidence that decreases in glitazone use following the FDA advisories occurred differentially among individuals at higher cardiovascular risk.
We used time series regressions to examine glitazone use from 1) January 2003 through January 2005, prior to most safety signals; 2) February 2005 through January 2007, during which safety signals began to emerge; 3) February 2007 through May 2008, when the FDA communicated multiple advisories; and 4) June 2008 through June 2009. We separated the first from the second period based on a visual data inspection (Fig. 1) and JoinPoint analyses (7). For each period, we described a level of use (average number of treatment visits during period) and a slope (monthly change in the number of treatment visits). Models included a constant, a term indicating study month, a dummy variable indicating the period, and an interaction term between month and period. We used autoregressive integrated moving average (ARIMA) models to control the autocorrelated variables, varying the moving average structure of the error terms accordingly (8).
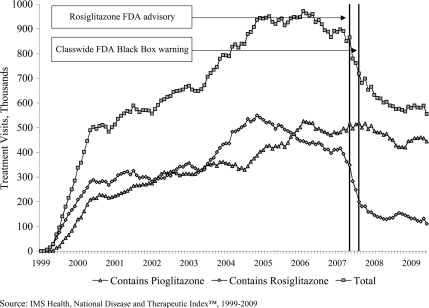
Figure 1.
Trends in monthly glitazone use among office-based physicians in the U.S., 1999–2009.
RESULTS
From January 2003 to May 2005, the annual growth rate for glitazone treatment visits was 22% (95% CI 19–25) (online appendix [available at http://care.diabetesjournals.org/cgi/content/full/dc09-1834/DC1]). At their maximum, glitazone accounted for 34% of all treatment visits (11.2 million glitazone treatment visits during 2005) for type 2 diabetes. From February 2007 through May 2008, during the period of FDA advisories, aggregate glitazone use decreased at an annual rate of 29% (−31 to −27), reaching a new level of 0.72 million (0.67–0.77) treatment visits per month during this period (Fig. 1). Use then reached a plateau, with a statistically insignificant decline of 2% (−9 to 5) during the final period of observation.
Both agents grew similarly from January 2003 through January 2005, with annual growth rates of 32% (95% CI 24–39%) and 14% (8–20) for rosiglitazone and pioglitazone, respectively. Trends diverged markedly during the period of FDA advisories, with a 60% annual reduction in use of rosiglitazone (−64 to −56) and 9% reduction in use of pioglitazone (−17 to −0.3). Following the FDA advisories, use stabilized with rosiglitazone, accounting for ∼23% of glitazone use, totaling 1.8 million (1.4–2.1) visits from June 2008 to June 2009 as compared with 5.8 million (5.0–6.5) for pioglitazone, which accounted for the remainder of glitazone use.
The percentage of patients codiagnosed with congestive heart failure, coronary heart disease, hypertension, atherosclerosis, or cerebrovascular disease on rosiglitazone or pioglitazone remained approximately a third from 2003 until June 2009, and no substantial change in the age distribution of pioglitazone and rosiglitazone users occurred (data not shown).
CONCLUSIONS
We found rapid increases in glitazone use from their market debut until 2005. As scientific evidence, FDA advisories, and media coverage of their potential cardiovascular risks accrued, there was a sharp decline in rosiglitazone use; the initial decline began up to 2 years prior to the May 2007 FDA advisory. Despite a class-wide advisory in August 2007, pioglitazone treatment visits did not similarly decline. Consistent with settings of technology adoption (9,10), decreases in rosiglitazone and pioglitazone use occurred nonselectively, rather than among those at highest cardiovascular risk (data not shown). These changes are important because glitazones were widely adopted into practice following their market debut, despite questions regarding their potential safety.
The substantial difference in market response to the FDA advisories between rosiglitazone and pioglitazone is noteworthy, and debates continue regarding the degree to which the cardiovascular risks that have been best demonstrated with rosiglitazone reflect a class-effect. Considerable evidence supports the greater safety of pioglitazone as compared with rosiglizatone (11,12), and the fact that declines in rosiglitazone began far prior to the FDA advisory suggests that clinicians, to some degree, may have heeded early safety signals (2). Nevertheless, the continuing uncertainty regarding the cardiovascular risks of these agents, as well as the degree of within-class heterogeneity in risk, suggests the importance of the routine inclusion of cardiovascular end points in studies that are used to seek FDA approval for diabetes therapies (13). The potential risks of the glitazones, relative to other available agents (e.g., sulfonyureas, biguanides), also suggests their limited role as monotherapy for diabetes or use in patients at elevated risk of congestive heart failure or ischemic heart disease.
Our study has several limitations. We examined limited outcomes and used cross-sectional data precluding examining longitudinal prescribing patterns. Nevertheless, our findings suggest a substantial decrease in the use of rosiglitazone by these office-based physicians during the FDA advisories that occurred between February 2007 and May 2008. Similarly large reductions in pioglitazone were not observed, nor did reductions in glitazone use appear to be concentrated among the elderly or those otherwise at highest risk for adverse events from these therapies. The public health impact of the changes described is not clear.
Supplementary Material
Acknowledgments
G.C.A. has career development awards from the Robert Wood Johnson Physician Foundation and the Agency for Healthcare Research and Quality (K08 HS15699-01A1). He has received grants from Pfizer and the Merck Foundation. He serves as a consultant for IMS Health, which provides information, analytics, and consulting services to the pharmaceutical industry and other health care sectors. In his capacity as a consultant, he receives fees for advising IMS on the use of their data assets for academic purposes. The funding sources had no role in study design or conduct; collection, management, analysis, or interpretation of the data; or preparation, review, or final manuscript approval. No other potential conflicts of interest relevant to this article were reported.
The statements, findings, conclusions, views, and opinions contained and expressed in this article are based in part on data obtained under license from the following IMS Health Incorporated information service(s): the National Disease and Therapeutic Index (1999–2009) and IMS Health Incorporated.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008;168:2088–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wooltorton E. Rosiglitazone (avandia) and pioglitazone (actos) and heart failure. CMAJ 2002;166:219 [PMC free article] [PubMed] [Google Scholar]
- 3. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457–2471 [DOI] [PubMed] [Google Scholar]
- 4. Federal Drug Administration, Center for Drug Evaluation and Research. Label and approval history [Internet]. Available at http://www.accessdata.fda.gov/Scripts/cder/drugsatfda/index.cfm?fuseaction=search.label_ApprovalHistory#apphist. Accessed 1 July 2009
- 5. Walton SM, Schumock GT, Lee KV, Alexander GC, Meltzer D, Stafford RS. Prioritizing medications for policy and research initiatives examining off-label prescribing. Pharmacotherapy 2008;28:1443–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment for type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008;168:2088–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19:335–351 [DOI] [PubMed] [Google Scholar]
- 8. McDowall D. Interrupted Time Series Analysis. Vol 21. Beverly Hills, CA, and London, Sage, 1980. [Google Scholar]
- 9. Dai C, Stafford RS, Alexander GC. National trends in cyclooxygenase-2 inhibitor use since market release: nonselective diffusion of a selectively cost-effective innovation. Arch Intern Med 2005;165:171–177 [DOI] [PubMed] [Google Scholar]
- 10. Berwick DM. Disseminating innovations in health care. JAMA 2003;289:1969–1975 [DOI] [PubMed] [Google Scholar]
- 11. Dormandy J, Van Troostenburg de Bruyn A, Bhattacharya M. Overall safety of pioglitazone in high-risk patients with type 2 diabetes. Drug Saf 2009;32:187–202 [DOI] [PubMed] [Google Scholar]
- 12. Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA 2007;298:1180–1188 [DOI] [PubMed] [Google Scholar]
- 13. Psaty BM, Furberg CD. The record on rosiglitazone and the risk of myocardial infarction. N Engl J Med 2007;357:67–69 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.