Abstract
OBJECTIVE
This trial tested whether mycophenolate mofetil (MMF) alone or with daclizumab (DZB) could arrest the loss of insulin-producing β-cells in subjects with new-onset type 1 diabetes.
RESEARCH DESIGN AND METHODS
A multi-center, randomized, placebo-controlled, double-masked trial was initiated by Type 1 Diabetes TrialNet at 13 sites in North America and Europe. Subjects diagnosed with type 1 diabetes and with sufficient C-peptide within 3 months of diagnosis were randomized to either MMF alone, MMF plus DZB, or placebo, and then followed for 2 years. The primary outcome was the geometric mean area under the curve (AUC) C-peptide from the 2-h mixed meal tolerance test.
RESULTS
One hundred and twenty-six subjects were randomized and treated during the trial. The geometric mean C-peptide AUC at 2 years was unaffected by MMF alone or MMF plus DZB versus placebo. Adverse events were more frequent in the active therapy groups relative to the control group, but not significantly.
CONCLUSIONS
Neither MMF alone nor MMF in combination with DZB had an effect on the loss of C-peptide in subjects with new-onset type 1 diabetes. Higher doses or more targeted immunotherapies may be needed to affect the autoimmune process.
Type 1 diabetes is a chronic, slowly progressive autoimmune disease (1). Immunotherapy aimed at modifying the course of disease has been demonstrated to be successful in a number of immune conditions including rheumatoid arthritis, systemic lupus erythematosus, and multiple sclerosis. Infusion of an anti-CD3 monoclonal antibody showed preservation of β-cell function in type 1 diabetes (2–4).
The Diabetes Control and Complications Trial (DCCT) demonstrated that improved metabolic control reduces chronic complications in type 1 diabetes (5). A post hoc analysis of DCCT found that those with residual β-cell function, manifested by C-peptide values >0.2 pmol/ml, had both less hypoglycemia and fewer complications than those without residual function (6). Thus an intervention that prolongs β-cell function would be expected to improve metabolic control and reduce complications (7).
Mycophenolic acid (MPA) was discovered in 1896 and characterized in 1952. Mycophenolate mofetil (MMF) is rapidly absorbed after oral administration and hydrolyzed to MPA (8). MPA is a potent, selective, noncompetitive, reversible inhibitor of inosine monophosphate dehydrogenase that inhibits de novo guanosine nucleotide synthesis without incorporation into DNA. T- and B-lymphocytes depend on de novo synthesis of purines for their proliferation, while other cell types can use salvage pathways. Thus, MMF has potent cytostatic effects on lymphocytes. MMF is effective in autoimmune diseases (psoriasis and uveitis) (9,10), as anti-rejection therapy in transplant recipients (11), and in diabetic animal models (12,13).
Daclizumab (DZB) is a humanized monoclonal antibody that binds to CD25, the α subunit of the interleukin-2 (IL-2) receptor expressed on the surface of activated lymphocytes. DZB inhibits IL-2 binding and the progression of T-lymphocytes through the cell cycle. The Edmonton protocol used DZB induction therapy in islet transplantation in type 1 diabetes (14). It has been used in several autoimmune conditions (multiple sclerosis and uveitis) (15,16). Recent work in the DR-BB rat model demonstrated a synergistic effect of these two drugs when used together (17).
The objective of this study was to determine whether MMF alone or MMF combined with DZB could diminish progression of β-cell destruction in recent-onset type 1 diabetes.
RESEARCH DESIGN AND METHODS
This multi-center trial was conducted at 13 sites with subjects aged 8–45 years with autoimmune type 1 diabetes for less than 3 months and with evidence of β-cell function evidenced by stimulated C-peptide >0.2 pmol on a 2-h mixed meal tolerance test (MMTT). Autoimmune type 1 diabetes was defined by the presence of any of four islet autoantibodies within 14 days of diagnosis (GAD, insulinoma-associated protein 2, or islet cell autoantibodies [ICAs]). Subjects were otherwise healthy without major systemic illness nor allergic or autoimmune conditions requiring treatment with immunosuppressive agents or steroids. The protocol was approved by the Type 1 Diabetes TrialNet Steering Committee, the Data and Safety Monitoring Board (DSMB), and regulatory authorities; human subject approval was obtained at participating sites prior to study initiation. All subjects provided written, informed consent.
Study design
The study was a three-arm, randomized, double-masked, placebo-controlled clinical trial conducted by Type 1 Diabetes TrialNet. Roche Pharmaceuticals provided MMF, DZB, and placebo, but had no involvement in study management, data collection and analysis, or manuscript preparation. There were 126 subjects randomized to receive MMF alone (with DZB placebo), MMF and DZB in combination, or control (MMF placebo and DZB placebo), stratified within clinical center.
By error, among the last six sites to join the study, 12 subjects assigned to receive MMF alone inadvertently received DZB-alone, thus resulting in an imbalance in the group sample sizes. The results from these 12 subjects are not presented herein.
MMF or matched placebo was administered daily at a dose of 600 mg/m2 (maximum 2,000 mg/day) in 2–3 divided doses for 2 years. DZB or matched placebo was given by intravenous infusion at study day 0 and two weeks later at a dose of 1 mg/kg. All subjects were to be followed for at least 2 years under the intention-to-treat principle, including those who did not receive the full course of assigned therapy.
Because both drugs reduce the ability to fight viral infections, screening for cytomegalovirus (CMV), and Epstein-Barr virus (EBV) was based on close surveillance rather than active prophylaxis.
Study visits were conducted to assess safety weekly × 4, biweekly × 2. Thereafter, EBV-negative subjects were followed monthly, and EBV-positive subjects were followed at 3-month intervals. Visits included assessment of diabetes care, adverse events, and laboratory measurements to assess medication side effects. In the case of an acute infection, additional studies were performed.
All participants received intensive diabetes management with the goal of maintaining A1C levels ≤7.0%.
An independent DSMB met every 6 months and had quarterly summary safety reviews. A medical monitor, masked to treatment assignment, reviewed all adverse events. An infectious disease committee developed treatment algorithms for common infections and provided consultation as needed.
Laboratory assessments
Blood samples were analyzed at core laboratories. A 4-h MMTT was conducted at baseline and 2 years, and a 2-h MMTT at 3, 6, 12, and 18 months with timed sample collection at 15–30 min intervals. C-peptide levels were measured using a two-site immunoenzymometeric assay (Tosoh 600 II analyzer). A1C was measured quarterly using ion-exchange high performance liquid chromatography (Variant II, Bio-Rad Diagnostics). The reliability coefficient for each assay was >0.99 from split duplicate samples.
Biochemical autoantibodies (GAD-65, ICA-512, mIAA) were measured using radio-immunobinding assays; ICAs were measured using indirect immunofluorescence. Potential participants were screened for antibodies to hepatitis B surface antigen, hepatitis C, and human immunodeficiency virus using enzyme immunoassays that, if positive, resulted in exclusion from the study.
Antibodies to CMV EBV were measured using indirect immunofluorescence (anti-EBV VCA IgM) and enzyme immunoassay (anti-CMV IgG and IgM; anti-EBV VCA and EBNA IgG). CMV and EBV viral load was measured using real time quantitative PCR (Lightcycler System; Roche Applied Science). MMF peak and trough levels were determined by MPA concentrations using HPLC. Flow cytometry was used to measured T-lymphocyte subpopulations, including CD4CD25.
Statistics
The prespecified primary analyses were based on the intention-to-treat cohort that included all subjects randomized correctly to the three specified treatment groups. The primary outcome was the geometric mean difference between active- and placebo-treated subjects of the area under the stimulated C-peptide curve over the first 2 h of a 4-h MMTT conducted at the 2-year visit in an ANCOVA model adjusting for the baseline C-peptide, age, and sex. The 2-h C-peptide area under the curve (AUC) (pmol/ml/120 min) was computed using the trapezoidal rule from timed measurements of C-peptide during each MMTT (including the basal). The AUC mean (pmol/ml) equals the AUC divided by the interval of time. The log([mean C-peptide] + 1) transformation of the baseline and follow-up AUC mean was used to allow for mean C-peptide values close to zero and to normalize the distribution of the residuals (6).
Data from all 13 centers contributed to the primary and secondary effectiveness analyses of the MMF plus DZB combination and its respective control group. However, owing to the randomization error, subjects received MMF alone in only seven centers. Thus, these analyses compare the MMF alone subjects only with the concurrently randomized control subjects from these centers.
Secondary analyses include assessment of differences between groups over time in a longitudinal normal errors repeated-measures model of the log([mean C-peptide] + 1) values. The group geometric mean C–peptide was obtained using the inverse transformation. The mean rate of change over 3–24 months was estimated using a mixed effects random coefficient model (18) using the log values. The Cox proportional hazards model assessed the relative risk (hazard ratio) of the loss of the 2-h C-peptide <0.2 pmol/ml (19).
Prespecified secondary outcomes also include: differences in A1C, insulin dose, hypoglycemic episodes, rates of infection, and adverse events over time.
For assessment of safety, the two active groups are compared with the total control group enrolled. The percents of subjects with an event were compared among the three groups using Fisher exact test. The rate of events per subject was compared between groups using the Poisson model test (20).
The target sample size of 120 subjects (40 per group) provided 85% power to detect a 65% difference in the geometric mean C-peptide for any one of the three possible pairwise comparisons among the three treatment groups using a test at the 0.05 level (one-sided, adjusted for three comparisons), with 10% loss to follow-up. Owing to the randomization error, the protocol was modified to compare the MMF and DZB combination group versus all placebo subjects, and to compare the MMF only subjects versus the placebo subjects enrolled within the same clinical centers (7 of the original 13) in which the randomization was not affected, each using a test at the 0.025 level (one-sided, adjusted for two comparisons). The MMF plus DZB versus placebo comparison, with about 40 per group, provided 85% power to detect a 61% difference; and the MMF alone versus placebo comparison, with about 30 per group, provided 80% power to detect a 67% increase, each allowing for 10% losses to follow-up.
In April 2008, based on 41 and 47% of the planned total information, the DSMB recommended termination of the treatment phase of the study. At that time the conditional power of each comparison under the current trend in the data were less than 0.02% and under the original design assumptions, termination for futility led to less than a 1% increase in the probability of a type II error (21). Sites were notified on April 30, 2008, to immediately terminate treatment but continue to follow all subjects. This report is based on closed and locked data for all visits through April 30, 2008. Nominal one-sided P values (without adjustment for multiple tests) are presented for analyses of primary and secondary effectiveness outcomes; 2-sided P values for safety outcomes.
RESULTS
Subjects
Supplemental Figure A1 (available in the online appendix at http://care.diabetesjournals.org/cgi/content/full/dc09-1349) summarizes subject disposition-screening of 228 subjects, randomization of 126 subjects, and subsequent disposition. Table 1 presents baseline characteristics for each active therapy group versus its respective control group. The groups were well-matched with 30% ages 8–12 years, 31% ages 13–17 years, and 39% age ≥18 years. Mean time from diagnosis to enrollment was 76 days.
Table 1.
Baseline characteristics of the treatment groups, including all 114 subjects correctly randomized
| MMF + DZB |
MMF alone |
|||
|---|---|---|---|---|
| Active | Control | Active | Control | |
| n | 41 | 42 | 31 | 28 |
| Age (years) | 18.3 ± 9.1 | 18.8 ± 10.5 | 17.1 ± 6.7 | 15.8 ± 8.0 |
| Race (% white) | 38 (93) | 39 (93) | 30 (97) | 27 (96) |
| Non-Hispanic (%) | 40 (98) | 39 (93) | 29 (94) | 26 (93) |
| Number of Ab+ (%) | ||||
| 1 | 1 (2) | 3 (7) | 4 (13) | 1 (4) |
| 2 | 11 (27) | 8 (19) | 7 (23) | 7 (25) |
| 3 | 12 (29) | 16 (38) | 6 (19) | 11 (39) |
| 4 | 17 (41) | 15 (36) | 14 (45) | 9 (32) |
| Male sex (%) | 23 (56) | 25 (60) | 20 (65) | 16 (57) |
| 2-h C-peptide AUC means (pmol/ml) | 0.71 ± 0.36 | 0.71 ± 0.34 | 0.65 ± 0.28 | 0.73 ± 0.36 |
| Baseline A1C (%) | 7.5 ± 1.3 | 7.7 ± 1.6 | 7.4 ± 1.0 | 7.5 ± 1.5 |
| Baseline total insulin dose/kg | 0.40 ± 0.26 | 0.36 ± 0.20 | 0.35 ± 0.15 | 0.39 ± 0.22 |
| Weight (kg) | 58.9 ± 16.6 | 59.0 ± 16.3 | 61.3 ± 18.3 | 57.2 ± 16.7 |
| Height (cm) | 162.4 ± 13.5 | 162.7 ± 13.7 | 164.4 ± 15.4 | 160.9 ± 13.4 |
| BMI (kg/m2) | 22.0 ± 4.2 | 21.8 ± 3.6 | 22.1 ± 4.1 | 21.6 ± 4.0 |
| zBMI (only on subjects <20 years) | 0.44 ± 1.11 | 0.66 ± 0.79 | 0.42 ± 1.02 | 0.57 ± 0.74 |
| n | 30 | 28 | 21 | 23 |
| Mean A1C over 24 months (%) | 7.2 ± 1.2 | 7.2 ± 1.0 | 7.0 ± 1.2 | 7.3 ± 0.9 |
| Mean insulin dose/kg over 24 months | 0.56 ± 0.29 | 0.55 ± 0.32 | 0.59 ± 0.31 | 0.63 ± 0.34 |
| Mean MPA level over 24 months (mcg/ml)* | 4.5 ± 3.4 | 0.6 ± 0.6 | 5.8 ± 4.0 | 0.6 ± 0.2 |
| Received 2 full DZB infusions (%) | 40 (98)** | 42 (100) | 31 (100) | 28 (100) |
| % of subjects MMF compliant*** | 36 (88) | 41 (98) | 27 (87) | 27 (96) |
Means ± SD are presented for continuous variables.
*Limit of quantitation = 0.5 units.
**One subject did not receive the second infusion due to patient decision to continue study treatment.
***80% or greater by capsule count up through last recorded visit starting with month 3.
Safety data are presented on all 114 properly randomized subjects, excluding the 12 who received the DZB-alone instead of MMF alone. Of these, 60 completed an MMTT at 2 years and contributed to the primary outcome analysis.
All randomized subjects received study treatment, and all but one received the two planned DZB/placebo infusions. Median compliance with MMF daily capsules was estimated to be 75% in the MMF plus DZB group, 63% in the MMF alone group, and 71% in the control group over the treatment period based on capsule counts. Mean MPA trough levels over 24 months were 4.5 ± 3.4 (mcg/ml) and 5.8 ± 4.0 (mcg/ml), respectively, for MMF plus DZB and MMF alone groups with expected trough range of 1.0–3.5 μg/ml. Treatment was terminated in 23 subjects due to adverse events (5), elevated liver enzymes (2), EBV PCR-positivity (8), treatment noncompliance (3), or loss to follow-up (5) (Supplemental Figure A1).
C-peptide
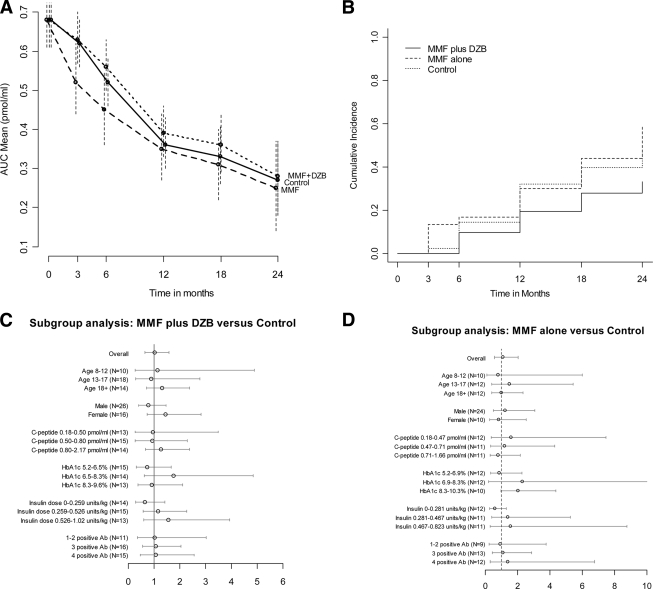
Mean AUC C-peptide at entry was 0.70 ± 0.33 pmol/ml. Control subjects lost C-peptide at a rate of 53.5% per year, and both the MMF alone and the MMF plus DZB treatment groups had comparable rates of loss, 46.4% and 48.1%, respectively. In the primary analysis (Fig. 1A) at 2 years,;the geometric mean stimulated C-peptide AUC was 0.28 pmol/ml (95% CI 0.19–0.37) in those treated with MMF plus DZB, compared with 0.27 (0.18–0.37) for their control subjects, P = 0.47; and 0.25 (0.14–0.37) in MMF alone treated subjects, compared with 0.23 (0.12–0.35) for their control subjects, P = 0.41. There was no statistical difference between treatment and control subjects over 2 years or during the early phase when DZB would have been more active. Results were similar for 4-h AUC mean C-peptide at 2 years.
Figure 1.
Effect of MMF and MMF plus DZB on C-peptide over 2 years. A) The geometric means and 95% CIs for the 2-h AUC stimulated C-peptide levels over time within each group. B) The cumulative incidence of decline in peak C-peptide to <0.2 pmol/ml within each group. The relative hazard was 0.61 (95% CI: 0.28–1.33, P = 0.11) for MMF plus DZB vs. control, and 1.05 (0.50–2.19, P = 0.83) for MMF alone vs. control. C) Ratio of geometric means for MMF plus DZB vs. control groups, with 95% CIs, within subgroups of subjects defined at baseline. D) Likewise for MMF alone vs. control (A1C 2nd tertile upper 95% confidence limit is 28.9).
During follow-up, all but eight subjects had detectable levels of C-peptide. The AUC mean C-peptide fell below 0.2 pmol/ml during follow-up in 12 MMF plus DZB, 16 MMF alone, and 17 control subjects. Cumulative incidence of decline of peak C-peptide below 0.2 pmol/ml did not differ between groups (Fig. 1B).
In the primary analysis, the geometric mean ratio for MMF plus DZB versus control subjects was 1.02 (95% CI 0.65–1.59) and for MMF alone versus control subjects was 1.08 (0.57–2.02). Figure 1C and D show that these mean ratios and confidence limits within subgroups, defined by baseline characteristics, are not nominally significantly different from 1, with the exception of the effect of MMF alone within the 10 subjects in the highest tertile of baseline A1C (P = 0.042). However, comparison of ratios among the A1C tertiles, and among all other subgroups, failed to reach significance demonstrating that variation among subgroups was within the realm of chance. Similar results applied to subgroups defined from the mean levels of A1C and insulin dose over 24 months.
DZB reduced CD4CD25 T-cell levels maximally at 4 weeks (depletion 83.9% and blocking 97.5%) and these recovered within 6–12 months. The month 24 C-peptide level was not associated with either percent reduction in CD4CD25 T-cells within the MMF plus DZB group, or MMF trough levels in either the MMF plus DZB or MMF alone groups.
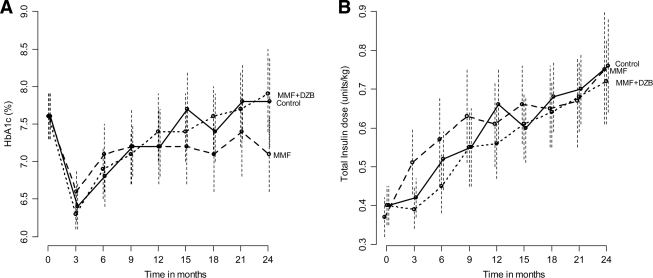
A1C and insulin dose
All groups achieved A1C of 7.2–7.3% throughout the study (Fig. 2A). Mirroring changes in C-peptide, daily insulin dose slowly rose from below 0.5 units/kg at baseline to 0.57 units/kg with MMF plus DZB versus 0.61 units/kg among control subjects (P = 0.17); and to 0.65 units/kg with MMF alone versus 0.62 units/kg among control subjects (P = 0.68) (Fig. 2B).
Figure 2.
Effect of MMF and MMF plus DZB on glycemic control over time. A) Mean A1C (%) and 95% confidence limits over time. B) Mean insulin dose and 95% confidence limits over time within each group.
Adverse events
There were 19 serious adverse events (AEs) reported for 14 subjects (34%) in the MMF and DZB group, nine in five subjects (16%) in the MMF alone group, and three in three subjects (7%) in the control group (online appendix Table 1), P < 0.01.
More grade 2 or higher AEs occurred in MMF plus DZB subjects (167 or 4.1 events/subject) compared with MMF alone (117, 3.8 events/subject) or control subjects (133, 3.2 events/subject), P = 0.09 (online appendix Table 1). Contrary to expectations, there was no difference in the occurrence of infectious or gastrointestinal events among groups. Eight individuals had asymptomatic reactivation of previous EBV infection using a sensitive PCR assay (five in MMF plus DZB, one in MMF, and two in control). Neutropenia and leukopenia, both side effects of MMF and DZB, occurred approximately equally among the three groups. A slight excess of elevated liver enzymes occurred in the MMF plus DZB group. Major hypoglycemic events were reported for 27 subjects, with an average of two each, with no difference among groups.
CONCLUSIONS
The aim of the present study was to arrest β-cell destruction in recently diagnosed type 1 diabetic subjects when preservation of existing β-cells may have a clinically meaningful effect on long-term outcomes of type 1 diabetes. We found no treatment benefit from either MMF alone or from the combination of MMF and DZB in this randomized, masked, placebo-controlled trial.
Although MMF has been effective in combination with other anti-rejection drugs (such as sirolimus and tacrolimus) in a number of transplant protocols (11), alone it may not have as much effect on effector cells, which can damage and kill islets without requiring cell division, the primary mode of MMF action. Although several studies have reported a potential negative effect of MMF on islet cell function, we did not see any greater loss of C-peptide in the MMF alone group compared with control subjects. For both MMF and DZB, we chose the lowest known effective doses of each. DZB has been shown to reduce recurrences in multiple sclerosis (15) and uveitis (16) when given monthly. Our use of two doses of DZB may not have been sufficient to affect activated effectors cells in the pancreas even with MMF, despite reasonably good depletion/coating in the peripheral circulation. Cyclosporine, which also affects the IL-2 signaling pathway, was shown to be effective in past trials if given at high doses and early enough in the course of disease (22). This, and the aforementioned effect of DZB in two other autoimmune diseases, suggests that the lower dose may have played a major part in the lack of effect of this therapy. Although higher doses may have greater therapeutic effect, this has to be measured against the increased risk of side effects. Even at the doses used in this study, there was an increase in AEs when the two drugs were used together in comparison to MMF alone or placebo.
CD4CD25+ regulatory T-cells play an important role in immune regulation and a potential problem with an anti-IL-2 receptor antibody is worsening autoimmunity rather than reducing it if the effects of the drug on the regulatory cell population outweigh its effects on the activated-effector cell population. In this study we saw no worsening of β-cell destruction or development of other autoimmune conditions with the use of DZB.
While overall compliance with the MMF and DZB regimens was high, it is possible that the need to withdraw study drug, primarily MMF, for various intervals due to AEs, may have affected the ability to demonstrate a beneficial effect. However, mechanistic assessments showed that MMF and DZB each were bioavailable and had the intended immunologic effects, but these effects were not associated with the C-peptide levels after 2 years. Modified anti-CD3 antibodies have been shown to reduce the rate of loss of C-peptide in new-onset type 1 diabetic subjects similar to those studied in this trial (2–4). MMF and anti-IL-2R are downstream of the important major histocompatibility complex-peptide/T-cell–receptor interaction, which is the driver of the autoimmune response. Therapies such as anti-CD3 and anti-CD20 as well as antigens such as GAD, oral insulin, and DiaPep277 may have the potential to alter this critical reaction and blunt the direct activation of autoreactive T-cells rather than limit their activity and division, which is where MMF and DZB are most critical. One additional difference between this study and the cyclosporine trials (22) that had been successful was that the time to treatment was 56 days with anti-CD3 versus 76 days in this study. Post hoc analysis did not reveal this to be a factor in the failure to see an effect and probably suggests that more targeted therapy at sufficient dose is necessary to arrest the diabetes process. New therapies such as DiaPep277 in adults (23) or GAD immunization (24) have recently been shown to slow the rate of loss of C-peptide, and others are under study such as anti-CD20, abatacept, and thymoglobulin.
Although we were concerned with the number of adverse events that might occur from the use of immunosuppressive agents, it is clear from our analysis that the number and type we detected were for the most part within our prestudy expectations and did not prevent study subjects from continuing treatment. The finding of asymptomatic low-level PCR reactivation of EBV in both treated and untreated patients was unexpected and may reflect the differential sensitivity of our EBV viral PCR assay as well as our rigorous screening program to ensure patient safety.
Type 1 Diabetes TrialNet is an National Institutes of Health (NIH)-sponsored multicenter trial group formed to perform intervention trials in new-onset type 1 diabetes and pre-diabetes, as well as to develop immunologic and mechanistic assays to better understand type 1 diabetes pathogenesis. There are several advantages of multicenter networks. These include consistency in study design and study outcomes allowing better comparisons between trials. Proposed studies are rigorously reviewed for scientific and ethical justification, clinical feasibility and prioritization by a diverse group of clinicians, basic scientists, statisticians, and ethicists (25). Trials are monitored by metabolic, infectious disease, and safety monitoring committees in addition to oversight by an independent DSMB.
Although this trial was unsuccessful at finding new therapies to induce clinical remission in type 1 diabetes, it showed that our network can successfully design, recruit, and conduct clinical trials of sufficient size. The use of novel agents, alone or in combination, will be facilitated by the clinical trial process developed for this first trial under the TrialNet mechanism.
Supplementary Material
Acknowledgments
This study was conducted by the Type 1 Diabetes TrialNet Study Group, a clinical trials network funded by NIH through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Institute of Child Health and Human Development, and the General Clinical Research Centers Program with support of the Juvenile Diabetes Research Foundation International and the American Diabetes Association.
P.A.G. reports receiving grants from Bayhill Therapeutics, Macrogenics, and Tolerx; D.W. reports serving on an advisory board for Genentech; H.R. reports serving on an advisory board for Genentech/Roche and receiving a grant from Macrogenics/Eli Lilly; D.A.S. reports serving on advisory boards for Genentech and Roche; J.M.L. reports receiving consulting fees from Tolerx, Bayhill Therapeutics, and Andromeda Biotech; J.S.S. reports receiving grants from Bayhill Therapeutics and Osiris Therapeutics. No other potential conflicts of interest relevant to this article were reported.
Footnotes
Clinical trial reg. no. NCT00100178, clinicaltrials.gov.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Aly T, Devendra D, Eisenbarth GS. Immunotherapeutic approaches to prevent, ameliorate, and cure type 1 diabetes. Am J Ther 2005;12:481–490 [DOI] [PubMed] [Google Scholar]
- 2. Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 2002;346:1692–1698 [DOI] [PubMed] [Google Scholar]
- 3. Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med 2005;352:2598–2608 [DOI] [PubMed] [Google Scholar]
- 4. Herold KC, Gitelman S, Greenbaum C, Puck J, Hagopian W, Gottlieb P, Sayre P, Bianchine P, Wong E, Seyfert-Margolis V, Bourcier K, Bluestone JA. Immune Tolerance Network ITN007AI Study Group. Treatment of patients with new onset type 1 diabetes with a single course of anti-CD3 mAb Teplizumab preserves insulin production for up to 5 years. Clin Immunol 2009;132:166–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DCCT Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 6. Palmer JP, Fleming GA, Greenbaum CJ, Herold KC, Jansa LD, Kolb H, Lachin JM, Polonsky KS, Pozzilli P, Skyler JS, Steffes MW. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21–22 October 2001. Diabetes 2004;53:250–264 [DOI] [PubMed] [Google Scholar]
- 7. Sjöberg S, Gjötterberg M, Berglund L, Möller E, Ostman J. Residual C-peptide excretion is associated with a better long-term glycemic control and slower progress of retinopathy in type I (insulin-dependent) diabetes mellitus. J Diabet Complications 1991;5:18–22 [DOI] [PubMed] [Google Scholar]
- 8. Brazelton TR, Morris RE. Molecular mechanisms of action of new xenobiotic immunosuppressive drugs: tacrolimus (FK506), sirolimus (rapamycin), mycophenolate mofetil and leflunomide. Curr Opin Immunol 1996;8:710–720 [DOI] [PubMed] [Google Scholar]
- 9. Epinette WW, Parker CM, Jones EL, Greist MC. Mycophenolic acid for psoriasis: a review of pharmacology, long-term efficacy, and safety. J Am Acad Dermatol 1987;17:962–971 [DOI] [PubMed] [Google Scholar]
- 10. Larkin G, Lightman S. Mycophenolate mofetil: a useful immunosuppressive in inflammatory eye disease. Ophthalmology 1998;106:370–374 [DOI] [PubMed] [Google Scholar]
- 11. Sollinger HW. Mycophenolate mofetil for the prevention of acute rejection in primary cadaveric renal allograft recipients: U.S. Renal Transplant Mycophenolate Mofetil Study Group. Transplantation 1995;60:225–232 [DOI] [PubMed] [Google Scholar]
- 12. Hao L, Calcinaro F, Gill RG, Eugui EM, Allison AC, Lafferty KJ. Facilitation of specific tolerance induction in adult mice by RS-61443. Transplantation 1992;53:590–595 [DOI] [PubMed] [Google Scholar]
- 13. Hao L, Chan SM, Lafferty KJ. Mycophenolate mofetil can prevent the development of diabetes in BB rats. Ann N Y Acad Sci 1993;696:328–332 [DOI] [PubMed] [Google Scholar]
- 14. Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type I diabetes mellitus using a glucocorticoid-free immunosupressive regimen. N Engl J Med 2000;343:230–238 [DOI] [PubMed] [Google Scholar]
- 15. Bielekova B, Richert N, Howard T, Blevins G, Markovic-Plese S, McCartin J, Frank JA, Würfel J, Ohayon J, Waldmann TA, McFarland HF, Martin R. Humanized anti-CD25 (daclizumab) inhibits disease activity in multiple sclerosis patients failing to respond to interferon beta. Proc Natl Acad Sci U S A 2004;101:8705–8708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nussenblatt RB, Peterson JS, Foster CS, Rao NA, See RF, Letko E, Buggage RR. Initial evaluation of subcutaneous daclizumab treatments for noninfectious uveitis: a multicenter noncomparative interventional case series. Ophthalmology 2005;112:764–770 [DOI] [PubMed] [Google Scholar]
- 17. Ugrasbul F, Moore WV, Tong PY, Kover KL. Prevention of diabetes: effect of mycophenolate mofetil and anti-CD25 on onset of diabetes in the DRBB rat. Pediatr Diabetes 2008;9:596–601 [DOI] [PubMed] [Google Scholar]
- 18. Demidenko E. Mixed Models: Theory and Applications. Hoboken, New Jersey, John Wiley & Sons, 2004. [Google Scholar]
- 19. Lachin J. The Assessment of Relative Risks. Hoboken, New Jersey, John Wiley and Sons, 2000. [Google Scholar]
- 20. Holm S. A simple sequentially rejective multiple test procedure. Scand J Statist 1979;6:65–70 [Google Scholar]
- 21. Lachin JM. Operating characteristics of sample size re-estimation with futility stopping based on conditional power. Statistics in Medicine 2006;25:3348–3365 [DOI] [PubMed] [Google Scholar]
- 22. Stiller CR, Dupré J, Gent M, Jenner MR, Keown PA, Laupacis A, Martell R, Rodger NW, von Graffenried B, Wolfe BM. Effects of cyclosporine immunosuppression in insulin-dependent diabetes mellitus of recent onset. Science 1984;223:1362–1367 [DOI] [PubMed] [Google Scholar]
- 23. Raz I, Avron A, Tamir M, Metzger M, Symer L, Eldor R, Cohen I, Elias D. Treatment of new-onset type 1 diabetes with peptide DiaPep277 is safe and associated with preserved beta-cell function: extension of a randomized, double-blind, phase II trial. Diabete Metab Res Rev 2007;23:292–298 [DOI] [PubMed] [Google Scholar]
- 24. Ludvigsson J, Faresjö M, Hjorth M, Axelsson S, Chéramy M, Pihl M, Vaarala O, Forsander G, Ivarsson S, Johansson C, Lindh A, Nilsson NO, Aman J, Ortqvist E, Zerhouni P, Casas R. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med 2008;359:1909–1920 [DOI] [PubMed] [Google Scholar]
- 25. Skyler JS, Greenbaum CJ, Lachin JM, Leschek E, Rafkin-Mervis L, Savage P, Spain L. Type 1 Diabetes TrialNet Study Group. Type 1 Diabetes TrialNet: an international collaborative clinical trials network. Ann N Y Acad Sci 2008;1150:14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.