Abstract
OBJECTIVE
Individuals with type 2 diabetes have a myriad of metabolic aberrations including increased inflammation, increasing their cardiovascular risk. Toll-like receptors (TLRs) and their ligands play a key role in insulin resistance and atherosclerosis. However, there is a paucity of data examining the expression and activity of TLRs in type 2 diabetes. Thus, in the present study, we examined TLR2 and TLR4 mRNA and protein expression, their ligands, and signaling in monocytes of recently diagnosed type 2 diabetic patients.
RESEARCH DESIGN AND METHODS
TLR mRNA, protein expression, TLR ligands, and TLR signaling were measured in freshly isolated monocytes from healthy human control subjects (n = 23) and type 2 diabetic subjects (n = 23) using real-time RT-PCR, Western blot, and flow cytometric assays.
RESULTS
Type 2 diabetic subjects had significantly increased TLR2, TLR4 mRNA, and protein in monocytes compared with control subjects (P < 0.05). Increased TLR2 and TLR4 expression correlated with BMI, homeostasis model assessment–insulin resistance (HOMA-IR), glucose, A1C, Nε-(carboxymethyl) lysine (CML), and free fatty acid (FFA). Ligands of TLR2 and TLR4, namely, HSP60, HSP70, HMGB1, endotoxin, and hyaluronan levels, were elevated in type 2 diabetic subjects and positively correlated with TLR2 and TLR4. Type 2 diabetic subjects showed increased MyD88, phosphorylated IRAK-1, Trif, TICAM-1, IRF-3, and NF-κB p65 expression in monocytes compared with control subjects. Furthermore, TLR-MyD88-NF-κB signaling resulted in elevated levels of cytokines (P < 0.05), but increased interleukin (IL)-1β, interferon (IFN)-γ, and endotoxin were not significant when adjusted for BMI.
CONCLUSIONS
In this comprehensive study, we make the novel observation that TLR2 and TLR4 expression and their ligands, signaling, and functional activation are increased in recently diagnosed type 2 diabetes and contribute to the proinflammatory state.
Type 2 diabetes constitutes a group of metabolic aberrations including hyperglycemia, inflammation, and insulin resistance that increase the risk of cardiovascular disease (1). The mechanisms by which these metabolic abnormalities cause cardiovascular disease are not clear. Considerable evidence indicates that the harmful effects of elevated glucose are mediated by receptors leading to increased inflammation and oxidative stress (2,3). Recent studies demonstrating associations between elevated levels of circulating C-reactive protein, proinflammatory cytokines, and homeostasis model assessment–insulin resistance (HOMA-IR) suggest that inflammation is an important etiological factor in the development of both insulin resistance and type 2 diabetes (4). Moreover, inflammatory markers link the pathology of insulin resistance and type 2 diabetes. Activation of the innate immune system via toll-like receptors (TLRs) is implicated in the pathogenesis of insulin resistance, diabetes, and atherosclerosis (5–7). Complimentary genetic studies link TLR2 and TLR4 polymorphisms to type 2 diabetes, suggesting a casual relationship between TLR function and diabetes and its complications (8).
TLRs are evolutionarily preserved pattern-recognition receptors (9), expressed on several cell types including monocytes, predominant cells of the innate immune system that are pivotal in diabetes and atherogenesis (10). TLRs play an important role in the activation and regulation of the innate immune system and inflammation (9). Each TLR family member recognizes a specific pathogen component, upon activation, triggers a signaling cascade leading to cytokine production and adaptive immune response (9). Among the TLRs, TLR2 and TLR4 play a critical role in the pathogenesis of insulin resistance, diabetes, and atherosclerosis in both clinical and experimental conditions (5–7,11). Ligands for TLR2 and TLR4 include high-mobility group B1 protein (HMGB1), heat shock protein (HSP) 60, HSP70, endotoxin, hyaluronan, advanced glycation end products, and extracellular matrix components (12). TLR2 and TLR4 bind to components of the gram-positive and -negative bacteria, respectively (12). TLR2 has broad ligand recognition specificity because of its ability to form heterodimers with TLR1, TLR6, and sometimes with CD14 and CD36 (13). TLR4 does not recognize endotoxin without the cofactor MD-2 (14). Collectively, these ligands and cofactors markedly increase TLR functionality and have not been studied in type 2 diabetes.
TLR2 and TLR4 expression have been shown to be increased in conventional insulin resistance target tissues like skeletal muscle and adipose tissue of type 2 diabetic subjects (15,16). These important studies implicating TLRs in type 2 diabetes were derived using small sample size, and the association with respective TLR2/TLR4 ligands, cofactors, downstream signaling, and functional activation remains to be properly addressed. Moreover, systemic inflammation plays a significant role in the etiology and comorbidities of type 2 diabetes, and the role of TLR2 and TLR4 in this setting is not clear. Experimental evidence in mice demonstrates that TLR2 and TLR4 activation and downstream cytokine production lead to the development of diabetes (17,18). More recently, TLR4 has been indicated as a molecular link between free fatty acids (FFAs), inflammation, and the innate immune system (5). Also, Song et al. (19) reported increased TLR4 mRNA expression in differentiating adipose tissue of db/db mice.
While these important observations from animal and human tissue data suggest a role for TLR2 and TLR4 in type 2 diabetes, it remains unknown whether alterations in TLR2 and TLR4 pathway activation contribute to systemic inflammation in diabetic human subjects. Therefore, the purpose of this study was to undertake a comprehensive analysis of TLR2 and TLR4 activation, ligands, cofactors, and downstream signaling in monocytes isolated from recently diagnosed patients with type 2 diabetes and matched control subjects.
RESEARCH DESIGN AND METHODS
Type 2 diabetic patients (n = 23) (with mean duration of diabetes = 29 months; age >35 years) were recruited from the Diabetes and Pediatric Clinics at University of California Davis Medical Center. None of the patients were on thiazolidinediones, ACE inhibitors, angiotensin receptor blockers, insulin, or statins, since they interfere with TLR expression and activation; six type 2 diabetic subjects were on metformin. Healthy control subjects (n = 23), age <35 years, with normal complete blood count; no family history of diabetes or other chronic diseases; normal kidney, liver, and thyroid function; and fasting plasma glucose <100 mg/dl were included in the study. Healthy control subjects and type 2 diabetic patients were matched for age (within 5–10 years), sex, and race. Subjects with mean A1C over the last year >10%; inflammatory disorders; microvascular and macrovascular complications; abnormal liver, renal, or thyroid function; steroid therapy, anti-inflammatory, antihypertensive, or hypolipidemic drugs; antioxidant supplements in the past 3 months; pregnancy; smoking; abnormal complete blood count; alcohol consumption >1 oz/day; consumption of omega-3 polyunsaturated fatty acid capsules (>1 g/day); and chronic high-intensity exercisers were excluded. Informed consent was obtained from all the subjects, and the study was approved by the University of California Davis Institutional Review Board. After history and physical examination, fasting blood (30 ml) was obtained. All the type 2 diabetic patients had family history of diabetes. Of the subjects, 60% were Caucasian and the remaining 40% were Hispanic and African American.
Complete blood count, lipid and lipoprotein profile, glucose, A1C, C-peptide, and C-reactive protein in all the enrolled subjects were assayed by standard laboratory techniques (11). FFA levels were assayed using reagents from Wako Chemicals (Richmond). Fasting plasma insulin concentrations were measured using an enzyme-linked immunosorbent assay (ELISA) (Linco Research). Insulin sensitivity was estimated by HOMA-IR (20).
Human monocytes
Human monocytes were isolated by gradient density centrifugation of peripheral blood using Histopaque (Sigma) and magnetic separation (Miltenyi Biotech) as reported previously (3,11). More than 88% of cells were identified as monocytes by CD14 staining, and viability was found to be >92%. Monocytes were examined before and after activation with synthetic lipoprotein Pam3Cys-Ser-(Lys) 4 (TLR2 ligand; Pam3CSK4, 170 ng/ml; Invitrogen) and lipopolysaccharide (LPS) (TLR4 ligand; 160 ng/ml; E. coli 026:B6; Sigma) overnight (12 h), with suitable control subjects (3,11). After treatment, cells were incubated with TLR2, TLR4, or isotype-matched IgG (eBioscience) depending on the cell treatment; 10,000 events were analyzed with the Becton-Dickinson Fluorescence-Activated Cell Sorter Bioanalyzer as described previously (11) after gating for CD14. Intra-assay and interassay coefficient of variation (CV) for TLR2 and TLR4 expression was >8 and >12%, respectively (3,11).
Serum and cell supernatants were used for measuring interleukin (IL)-1β, IL-6, IL-8, interferon (IFN)-γ, monocyte chemoattractant protein (MCP)-1, and tumor necrosis factor (TNF)-α with a Multiplex assay (Millipore) as a functional readout of TLR activation. IFN-β (Antigenix America), HSP60, HSP70 (Assay Designs), HMGB1 (Shino-Test, Japan), hyaluronan (Echelon), and Nε-(carboxymethyl) lysine (CML) (CycLex, Japan) were determined using ELISA in the serum of all study subjects. Nuclear extracts were used to verify activation of NF-κB (Active Motif) in study subjects, indicating increased inflammation, as described previously (3,11). Intra- and interassay CV for all the assays were determined to be between 7 and 12% (21). All the reagents used in the study were tested for endotoxin (<100 ELISA unit [EU]/ml) using a Limulus ameobocyte lysate assay (Lonza), allowing precise TLR expression measurements devoid of endotoxin (3,11).
Real-time RT-PCR
RNA was isolated from monocytes using TRI reagent (Invitrogen). cDNA was synthesized using 1 μg total RNA, and 50 ng was amplified using primer probe sets for TLR1, TLR2, TLR4, TLR6, MD-2, and 18s (SA Bioscience) following manufacturer's cycling parameters. Data are calculated using the 2−ΔΔCT method (3,11).
Western blots and coimmunoprecipitation
Monocyte cell lysates were subjected to electrophoresis and transfer, as reported earlier (3,11). Blots were probed with human myeloid differentiation factor-88 (MyD88), IL receptor–associated protein kinase-1 (IRAK-1), toll–IL-1 receptor domain (TIR)-containing adaptor molecule (TICAM-2), IFN regulatory factor-3 (IRF-3), NF-κB (NF-κBp65; Santa Cruz Biotechnology, Santa Cruz, CA), TIR-containing adapter-inducing IFN-β (Trif; Abcam), and antibodies with respective secondary IgG antibodies and developed. In all assays, β-actin and total nonphosphorylated proteins were used as internal control. Besides, cell lysates (100 μg) were immunoprecipitated with TLR2 or TLR4 antibody overnight at 4°C as reported previously (3) and blotted with TLR6, HMGB1, HSP60 (Santa Cruz), and hyaluronan (Ray Biotech) antibodies as depicted in Fig. 2F and G. Densitometric ratios were determined as reported earlier from four independent assays (3,11).
Figure 2.
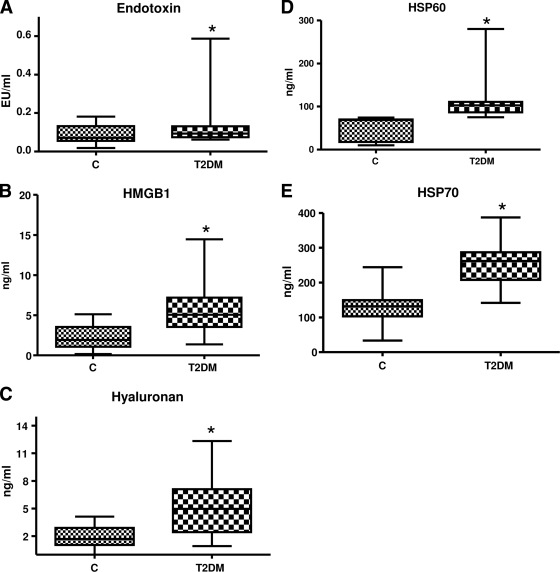
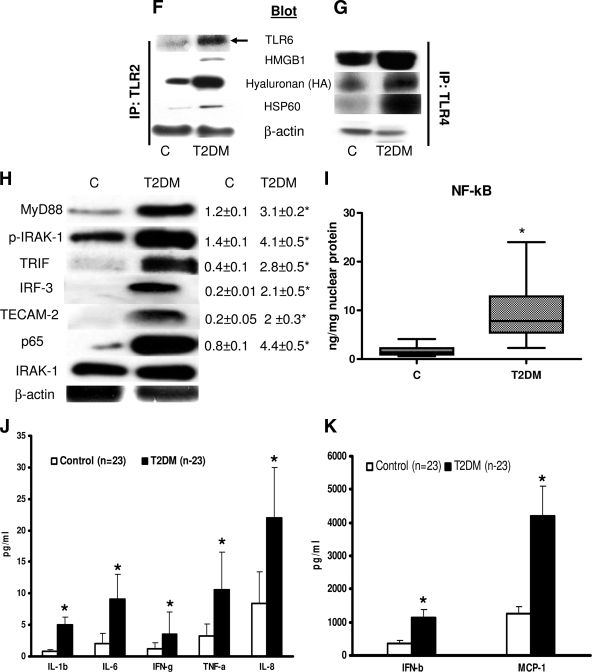
A: Endotoxin concentration in control (n = 23) and type 2 diabetic (n = 23) subjects was measured using Limulus Ameobocyte lysate assay as described in research design and methods. Values are expressed as EU/ml. *P < 0.05 vs. control. C, control; T2DM, type 2 diabetes. B: HMGB1 concentration in control (n = 23) and type 2 diabetic (n = 23) subjects was measured using ELISA as described in research design and methods. Values are expressed as ng/ml. *P < 0.0001 vs. control. C: Hyaluronan concentration in control (n = 23) and type 2 diabetic (n = 23) subjects was measured using ELISA as described in research design and methods. Values are expressed as ng/ml. *P < 0.0001 vs. control. D: HSP60 concentration in control (n = 23) and type 2 diabetes (n = 23) subjects was measured using ELISA as described in research design and methods. Values are expressed as ng/ml. *P < 0.0001 vs. control. E: HSP70 concentration in control (n = 23) and type 2 diabetic (n = 23) subjects was measured using ELISA as described in research design and methods. Values are expressed as ng/ml. *P < 0.0001 vs. control. F: Association of TLR2 with its ligands and TLR6. Representative Western blot showing enhanced expression of TLR6, HMGB1, hyaluronan, and HSP60 in basal control and type 2 diabetes monocyte cell lysates immunoprecipitated with TLR2 antibody as detailed in research design and methods. β-Actin was used as a loading control. Each assay is repeated four times. IP, immunoprecipitation. G: Association of TLR4 with its ligands. Representative Western blot showing enhanced expression of HMGB1, hyaluronan, and HSP60 in basal control and type 2 diabetes monocyte cell lysates immunoprecipitated with TLR4 antibody as detailed in research design and methods. β-Actin was used as a loading control. Each assay is repeated four times. H: Monocyte TLR signaling in the basal state. TLR downstream signaling proteins MyD88, pIRAK-1, Trif, IRF-3, TECAM-2, and NF-κB p65 were performed using specific antibodies to the respective (phospho) proteins, as described in research design and methods using β-actin as a loading and internal control for MyD88, Trif, IRF-3, TECAM-2, and NF-κB p65 and IRAK for pIRAK-1. Each blot is repeated four times with pooled monocytes from three subjects. Densitometric ratios corroborate the data. *P < 0.05 vs. control. I: The DNA binding activity of nuclear NF-κB p65 in control (n = 23) and type 2 diabetes (n = 23) monocytes was assessed by ELISA as detailed in research design and methods in the basal state. Values are normalized to milligram nuclear protein and expressed as mean ± SD. *P < 0.05 vs. control. J: Serum concentration of cytokines/chemokines in study subjects were measured using Multiplex assays as described in research design and methods. Values are expressed as picograms per milliliter. *P < 0.0001 vs. control. K: Serum concentration of cytokines/chemokines in study subjects were measured using Multiplex assays as described in research design and methods. Values are expressed as picograms per milliliter. *P < 0.0001 vs. control.
Statistical analysis
Statistical analyses were performed using SAS software (SAS Institute, Cary, NC). Data are expressed as means ± SD for all data. Parametric data were analyzed using paired, two-tailed t tests and nonparametric data using Wilcoxon's signed-rank tests. Level of significance was set at P < 0.05. Spearman's rank correlation was computed to assess association between variables.
RESULTS
Baseline characteristics of the study subjects
Subject characteristics are detailed in Table 1. There were no significant differences in age and lipid profile between control and type 2 diabetes groups. Levels of glucose, A1C, FFAs, and HOMA-IR were significantly higher in type 2 diabetic subjects than in control subjects. C-peptide and advanced glycation end product and CML levels were significantly increased in type 2 diabetic subjects compared with control subjects. C-reactive protein, a prototypic atherosclerotic marker, is increased in type 2 diabetic subjects compared with control subjects.
Table 1.
Clinical and laboratory characteristics
| Control | Type 2 diabetes | P | |
|---|---|---|---|
| n | 23 | 23 | |
| Sex (M/F) | 11/12 | 9/16 | |
| Age (years) | 46 ± 12 | 51 ± 10 | NS |
| BMI (kg/m2) | 25 ± 7 | 34 ± 9 | 0.001 |
| Fasting glucose (mmol/l) | 4.9 ± 0.5 | 8.9 ± 3.1 | 0.0001 |
| Fasting insulin (pmol/l) | 79 ± 17 | 190 ± 120 | 0.018 |
| HOMA-IR | 1.5 ± 0.4 | 3.7 ± 1.8 | 0.001 |
| Fasting FFAs (mEq/l) | 0.3 ± 0.2 | 0.9 ± 0.2 | 0.0001 |
| A1C (%) | 5.2 ± 0.2 | 8 ± 2.1 | 0.05 |
| C-peptide (ng/ml) | 1.2 ± 1 | 2.5 ± 0.6 | 0.001 |
| C-reactive peptide (mg/l) | 2.9 ± 4 | 4.7 ± 4 | 0.002 |
| Total cholesterol (mg/dl) | 184 ± 36 | 190 ± 36 | NS |
| LDL cholesterol (mg/dl) | 115 ± 26 | 120 ± 29 | NS |
| HDL cholesterol (mg/dl) | 50 ± 11 | 39 ± 15 | 0.3 |
| Triglycerides (mg/dl) | 100 ± 62 | 145 ± 67 | 0.45 |
| CML (ng/ml) | 1.9 ± 1.1 | 5.4 ± 3.1 | 0.001 |
Data are means ± SD. P values correspond to the differences between control and type 2 diabetes.
TLR2 and TLR4 mRNA and protein expression in type 2 diabetes monocytes
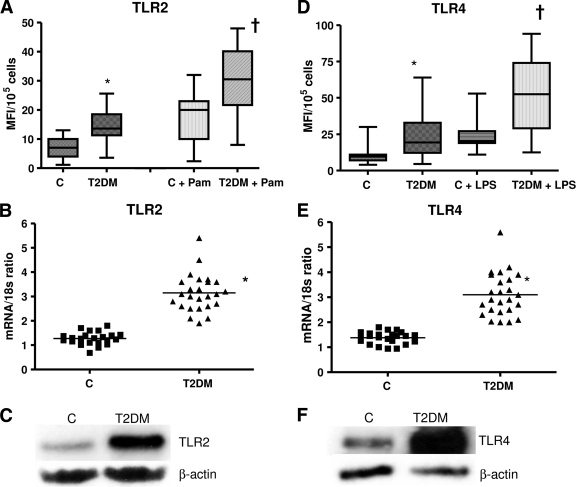
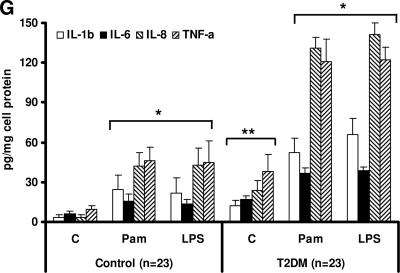
We first determined the levels of TLR2 and TLR4 expression by flow cytometric analysis in control and type 2 diabetic subjects. Monocyte surface expression of TLR2 and TLR4 was significantly elevated in type 2 diabetes compared with control in both the resting and activated state (Pam3CSK4 or LPS) (Fig. 1A and D, P < 0.005). Next, to determine if the increase in monocyte TLR2 and TLR4 expression in type 2 diabetes resulted from increased mRNA expression, we investigated the TLR2 and TLR4 mRNA levels by real-time RT-PCR. Figure 1B and E depict the corresponding increased TLR2 and TLR4 mRNA expression in type 2 diabetes compared with control subjects. We further confirmed the increased monocyte TLR2 and TLR4 protein content using Western blot assay (Fig. 1C and F). We measured downstream functional readouts (IL-1β, IL-6, IL-8, and TNF-α) of TLR2/4 ligation in resting and activated monocytes (Fig. 1G). There was a significant upregulation of proinflammatory cytokines in type 2 diabetic patients compared with control subjects in both the basal and activated state.
Figure 1.
TLR protein and mRNA content. A: TLR2 surface protein expression was measured in monocytes after Pam3CSK4 (Pam) challenge in control (n = 23) and type 2 diabetic (n = 23) subjects by flow cytometry as described in research design and methods. Values are expressed as mean fluorescence intensity units (MFI)/105 cells. *P < 0.005 vs. control; †P < 0.05 versus C+Pam. C, control; T2DM, type 2 diabetes. B: Monocyte TLR2 mRNA expression ratios in control (n = 23) and type 2 diabetes (n = 23) subjects by real-time RT-PCR as described in research design and methods. 18s mRNA is used as the housekeeping gene. Values are expressed as mean ratio ± SD. *P < 0.001 vs. control. C: Representative Western blot depicting the TLR2 protein expression in pooled resting monocytes from three control subjects and three type 2 diabetic subjects. β-Actin was used as a loading control as described in research design and methods. Each assay is repeated four times. D: TLR4 surface protein expression was measured in monocytes after LPS challenge in control (n = 23) and type 2 diabetic (n = 23) subjects by flow cytometry as described in research design and methods. Values are expressed as mean fluorescence intensity units (MFI)/105 cells. *P < 0.005 vs. control; †P < 0.05 vs. C + LPS. E: Monocyte TLR4 mRNA expression ratios in control (n = 23) and type 2 diabetic (n = 23) subjects by real-time RT-PCR as described in research design and methods. 18s mRNA is used as the housekeeping gene. Values are expressed as mean ratio ± SD. *P < 0.005 vs. control. F: Representative Western blot depicting the TLR4 protein expression in pooled resting monocytes from three control and three type 2 diabetic subjects. β-Actin was used as a loading control as described in research design and methods. Each assay is repeated four times. G: Release of cytokines in resting and activated monocyte cell culture supernatants from control and type 2 diabetic subjects using Multiplex assay as described in research design and methods. Values are expressed as pg/mg cell protein. *P < 0.001 vs. untreated C; **P < 0.005 vs. control (healthy control). C, untreated; LPS, lipopolysaccharide, TLR4 ligand; Pam, Pam3CSK4, synthetic TLR2 ligand.
TLR2 and TLR4 ligand and cofactor expression in type 2 diabetes
Identification of TLR2 and TLR4 ligands and cofactors is a key element in TLR activation and is unclear in type 2 diabetic subjects. Beyond the established microbial ligands of TLR2 and TLR4, several molecules of endogenous origin have been suggested to act as TLR2 and TLR4 ligands, notably HMGB1, HSP60, HSP70, and hyaluronan fragments. Accordingly, we measured concentration of endotoxin, HMGB1, hyaluronan, HSP60, and HSP70 in the serum of type 2 diabetic subjects and control subjects using ELISAs. Figure 2 shows the significant increased levels of these ligands in type 2 diabetic subjects. In addition, coimmunoprecipitation with TLR2 and TLR4 and blotting for HMGB1, HSP60, and hyaluronan showed strong association with both TLR2 and TLR4 in type 2 diabetes compared with control subjects (Fig. 2F and G).
It has been previously shown that TLR2 dimerizes with TLR1 or TLR6 and results in receptor activation and downstream signaling upon saturated fatty acid challenge. To determine whether TLR1 or TLR6 is required for the activation of TLR2, we measured TLR1 and TLR6 mRNA expression in monocytes of type 2 diabetes and control subjects. TLR6 mRNA levels significantly increased in type 2 diabetes (3.8 ± 0.4 vs. 1.3 ± 0.2 mRNA/18s ratio; P < 0.05) compared with control subjects, whereas TLR1 mRNA levels remained unchanged (control: 1.5 ± 0.4, type 2 diabetes: 1.9 ± 0.5 mRNA/18s ratio), and coimmunoprecipitation showed an increased TLR2/TLR6 association in type 2 diabetes (Fig. 2F). Further, MD-2 mRNA expression was also significantly increased (control 1.2 ± 0.2 vs. 2.9 ± 0.3 mRNA/18s ratio) in type 2 diabetes monocytes. These data are in line with our earlier observation that under hyperglycemic conditions, TLR2 heterodimerizes with TLR6 in monocytes (3).
TLR2 and TLR4 activation results in MyD88-dependent and -independent signaling in type 2 diabetes monocytes
We examined the TLR-mediated MyD88-dependent signaling pathway using Western blot technique. TLR2 and TLR4 both use MyD88 and activate NF-κB, common downstream signaling components for all TLRs except TLR3 (3,9). Therefore, activation of MyD88-dependent and -independent pathways was used to interpret the activation of TLR2 and TLR4.
Phosphorylation of IRAK-1, MyD88, TRIF, TECAM-2, IRF-3 protein expression in cytoplasmic extract, and p65 protein levels in nuclear extracts of monocytes were significantly increased in type 2 diabetes compared with control subjects, with no change in total protein and β-actin levels (Fig. 2H), suggesting activation of TLR-mediated signaling cascade (both MyD88-dependent and -independent) in type 2 diabetes. In addition, densitometry ratios further corroborate the data (Fig. 2H).
Increased TLR2 and TLR4 expression results in increased inflammation mediated by NF-κB
To further investigate TLR2- and TLR4-mediated inflammation in type 2 diabetes, we measured NF-κB activity in the nuclear extracts of type 2 diabetes and control subject monocytes. Type 2 diabetes showed increased NF-κB p65-dependent DNA binding activity compared with control subjects (P < 0.01) (Fig. 2I). Increased NF-κB activity corresponded to increased systemic inflammation. We measured IL-1β, IL-6, IL-8, MCP-1, IFN-β, and TNF-α serum concentration in type 2 diabetes and control subjects as a functional readout of TLR activation. There was a significant increase in all the proinflammatory mediators in type 2 diabetic subjects compared with control subjects (Fig. 2J and K).
Increased TLR expression correlates with BMI, glucose, HOMA-IR, and inflammation
Additionally, there was a significant correlation between BMI and TLR2 expression (r = 0.5; P < 0.05) and TLR4 expression (r = 0.43; P < 0.01) and significant correlation between TLR expression and glucose (TLR2: r = 0.7; TLR4, r = 0.64, P < 0.001), FFA levels (TLR2: r = 0.66; TLR4, r = 0.66, P < 0.05), HOMA-IR (TLR2: r = 0.64; TLR4, r = 0.54, P < 0.005), and A1C (TLR2: r = 0.66; TLR4, r = 0.62, P < 0.001). In addition, HSP60, HSP70, HMGB1, and endotoxin levels significantly correlated with TLR2 expression (r = 0.43, r = 0.64, r = 0.48, and r = 0.16; P < 0.05) and TLR4 expression (r = 0.43, r = 0.65, r = 0.41, and r = 0.21; P < 0.001), respectively. There was a significant correlation between TLR2 expression and IL-1β, TNF-α (r = 0.5, r = 0.5; P < 0.001), MCP-1, and NF-κB (r = 0.42, r = 0.35; P < 0.05) and between TLR4 and IL-1β, TNF-α (r = 0. 56, r = 0.55, P < 0.001), IFN-β, and NF-κB (r = 0.46, r = 0.4, P < 0.05). Because BMI was significantly different between control and type 2 diabetes, additional statistical analyses using BMI as a covariant were performed. TLR2, TLR4 (with and without Pam3CSK4 or LPS), NF-κB, endogenous ligands, CML, and cytokines were significantly higher in type 2 diabetes compared with control subjects (P < 0.001), whereas IL-1β (P = 0.09), IFN-γ (P = 0.16), and endotoxin (P = 0.11) were no longer significant.
CONCLUSIONS
The interactions among inflammation, hyperglycemia, insulin resistance, and type 2 diabetes have clear implications for atherosclerosis via the innate immune system. Besides being activators of inflammation under hyperglycemia and insulin resistance (3,5–7), both TLR2 and TLR4 are critical in atherosclerosis (7). In this study, we provided key evidence for increased monocyte TLR2 and TLR4 expression, activation, cofactor expression, ligands, and downstream signaling contributing to systemic inflammation seen in type 2 diabetic subjects. Our observations significantly add to the emerging role of TLRs in atherosclerosis and diabetes and are consistent with other studies in this context (3,7,11). Increased TLR2 and TLR4 expression is demonstrated in atherosclerotic plaque macrophages and in animal models of atherosclerosis. TLR2/4 knockout mice on C57BL6, ApoE−/−, and LDLR−/− background and MyD88 knockout mice show reduced lesion size, lipid content, and macrophage infiltration in plaques and reduced inflammation (22,23).
In two recent studies, we showed increased TLR2 and TLR4 expression, TLR ligands, intracellular signaling, and TLR-mediated inflammation in monocytes with significant correlation to A1C levels in type 1 diabetic patients (11,21). Moreover, TLR2/4 expression and activation is increased in human monocytes under hyperglycemia conditions (3). Reyna et al. (15) have shown abnormal TLR4 expression in skeletal muscle of a small number of insulin-resistant subjects, with little information on the TLR4-MyD88 signaling, levels of TLR4 cofactors (CD14/MD-2), TLR4 ligand (endotoxin), and its correlation to TLR4 expression. Moreover, the in vitro experiments in this article with myotubes and FFAs lacked appropriate endotoxin controls, and it is not clear if the study subjects were on any cholesterol-lowering medications, adding complexity to the conclusions. Creely et al. (16) showed increased TLR2 expression in the adipose tissue of type 2 diabetes with strong correlates to endotoxin levels, with no change in TLR4 expression. This study is inconclusive in terms of the lack of TLR4 expression, even under high endotoxin levels, smaller sample size, and minimal patient medication details. Du et al. (24) in a descriptive study showed that monocytes from LADA, (latent autoimmune diabetes in adults) type 2 diabetic patients on sulfonylurea therapy have significantly higher TLR4 and CD14 expression than healthy control subjects, with no mechanistic details. In this context, our study fills in all the above gaps sequentially: First, we provide data on both TLR2 and TLR4 mRNA/protein levels and critical downstream signaling events that follow their ligation in type 2 diabetes; second, we determined cofactors required for TLR2/4 activation; third, we demonstrated ligands in use for TLR2/4 activation; fourth, notably, we and others have shown that statins, thiazolidinediones, and angiotensin receptor blockers downregulate TLRs and our type 2 diabetic patients are not on any of these drugs; fifth, our TLR data showed significant correlation with major clinical estimates of type 2 diabetes, namely adjusted BMI, glucose, HOMA-IR, FFAs, ligands, cytokines; and finally, data are shown for both MyD88-dependent and -independent signaling pathways, which may be acting collectively.
In addition to the well-characterized microbial ligands, several molecules of the host origin (endogenous) have been proposed to act as ligands for TLR2 and TLR4, including HMGB1, HSPs, and hyaluronan (25), and were not examined in type 2 diabetes. HMGB1 is considered as a “late” proinflammatory mediator in sepsis. It induces activation of intracellular signaling pathways via TLR2, TLR4, and the receptor for advanced glycation end products (RAGEs), thus acting as an extracellular alarmin. HSPs also act as endogenous ligands of TLR2 and TLR4. HSP60 and HSP70 induce the production of proinflammatory cytokines via activation of TLR2 and TLR4. The inflammatory effects of recombinant human HSP60 were shown to be TLR4 dependent, suggesting that HSP60 may be a TLR4 ligand. Low–molecular weight degradation products of hyaluronan elicit proinflammatory responses in murine alveolar macrophages in rheumatoid arthritis models and other chronic inflammatory conditions. Endotoxin is the most important ligand required for TLR4 activation (14), and its levels are significantly increased in type 2 diabetic patients. In the present study, we show that type 2 diabetic patients have high circulating levels of HMGB1, HSP60, HSP70, and hyaluronan, which could trigger TLR2 and TLR4 activation, leading to a proinflammatory state by the activation of TLR2/4, synergistically with glucose, FFAs, and endotoxin. Our coimmunoprecipitation studies further suggest an association between TLR2, TLR4, HMGB1, and HSP60.
Dimerization is a major event in the functional activation of TLRs and results in cytokine production (3). TLR2 activity requires heterodimerization with TLR1 or TLR6 to recognize ligands. Using luciferase reporter assays and real-time RT-PCR, we showed that high glucose induces TLR2 and TLR6 heterodimerization, resulting in NF-κB activation and cytokine production (3). MD-2 is required for TLR4 ligation with endotoxin (14). Here, we demonstrate that both TLR6 and MD-2 mRNA expression is increased in type 2 diabetic patients, suggesting their requirement for TLR ligation in type 2 diabetic patients.
TLRs mainly signal through the adapter protein MyD88 via activation of NF-κB, resulting in the increased transcription of inflammation-related genes, such as those encoding indicated cytokines (3,9,11). In addition, a MyD88-independent pathway involving Trif is essential to TLR3 and TLR4 signaling and induces IFN-β. In two recent studies, we showed that activation of the MyD88 pathway is increased in monocytes exposed to high glucose and in type 1 diabetic patients (3,9,11). In the present study, we examined MyD88-dependent and -independent TLR activation, by determining IRF-3 and IFN-β levels as a biological indicator of MyD88-independent activation.
Taken together, the novel findings of this comprehensive study suggest that there is significant elevation of TLR2 and TLR4 protein, mRNA, endogenous ligands, and cofactors in type 2 diabetic patients, which, in concert with hyperglycemia, contributes to the increase in TLR2 and TLR4 signaling that results in the proinflammatory state of type 2 diabetes. In future studies, we will address the mechanism of synergistic effects of hyperglycemia, FFAs, and endogenous ligands on TLR2 and TLR4 expression and signaling.
Acknowledgments
This study was supported by American Diabetes Association Grant 7-07-JF-16 to M.R.D. and National Institutes of Health Grant K24 AT 00596 to I.J. S.D. was supported by NCRR-UL1-RR024146.
No potential conflicts of interest relevant to this article were reported.
We thank all the study participants for their time and efforts. We thank Rohan Jialal for help with blood draws (phlebotomy). We thank Catherine Duncan-Staley for assistance with HSP70 ELISA assay and Manpreet Kaur for editorial assistance.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–234 [DOI] [PubMed] [Google Scholar]
- 2. Yan SF, Ramasamy R, Schmidt AM. Mechanisms of disease: advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat Clin Pract Endocrinol Metab 2008;4:285–293 [DOI] [PubMed] [Google Scholar]
- 3. Dasu MR, Devaraj S, Zhao L, Hwang DH, Jialal I. High glucose induces toll-like receptor expression in human monocytes: mechanism of activation. Diabetes 2008;57:3090–3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest 2005;115:1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 2006;116:3015–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wong FS, Wen L. Toll-like receptors and diabetes. Ann N Y Acad Sci 2008;1150:123–132 [DOI] [PubMed] [Google Scholar]
- 7. Curtiss LK, Tobias PS. Emerging role of Toll-like receptors in atherosclerosis. J Lipid Res 2009;50(Suppl.):S340–S345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bagarolli RA, Saad MJ, Saad ST. Toll-like receptor 4 and inducible nitric oxide synthase gene polymorphisms are associated with type 2 diabetes. J Diabetes Complications 2009. April 21 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 9. Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol 2003;21:335–376 [DOI] [PubMed] [Google Scholar]
- 10. Hill H, Hogan N, Rallison M, Santos JI, Charette RP, Kitahara M. Functional and metabolic abnormalities of diabetic monocytes. Adv Expt Med Biol 1980;69:621–627 [DOI] [PubMed] [Google Scholar]
- 11. Devaraj S, Dasu MR, Rockwood J, Winter W, Griffen SC, Jialal I. Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J Clin Endocrinol Metab 2008;93:578–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J Leukocyte Biol 2004;76:514–519 [DOI] [PubMed] [Google Scholar]
- 13. Drage MG, Pecora ND, Hise AG, Febbraio M, Silverstein RL, Golenbock DT, Boom WH, Harding CV. TLR2 and its co-receptors determine responses of macrophages and dendritic cells to lipoproteins of Mycobacterium tuberculosis. Cell Immunol 2009;258:29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Akashi S, Shimazu R, Ogata H, Nagai Y, Takeda K, Kimoto M, Miyake K. Cutting edge: cell surface expression and lipopolysaccharide signaling via the toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. J Immunol 2000;164:3471–3475 [DOI] [PubMed] [Google Scholar]
- 15. Reyna SM, Ghosh S, Tantiwong P, Meka CS, Eagan P, Jenkinson CP, Cers osimo E, Defronzo RA, Coletta DK, Sriwijitkamol A, Musi N. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes 2008;57:2595–2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Creely SJ, McTernan PG, Kusminski CM, Fisher M, Da Silva NF, Khanolkar M, Evans M, Harte AL, Kumar S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am J Physiol Endocrinol Metab 2007;292:E740–E747 [DOI] [PubMed] [Google Scholar]
- 17. Mohammad MK, Morran M, Slotterbeck B, Leaman DW, Sun Y, Grafenstein H, Hong SC, McInerney MF. Dysregulated Toll-like receptor expression and signaling in bone marrow-derived macrophages at the onset of diabetes in the non-obese diabetic mouse. Int Immunol 2006;18:1101–1113 [DOI] [PubMed] [Google Scholar]
- 18. Kim HS, Han MS, Chung KW, Kim S, Kim E, Kim MJ, Jang E, Lee HA, Youn J, Akira S, Lee MS. Toll-like receptor 2 senses beta-cell death and contributes to the initiation of autoimmune diabetes. Immunity 2007;27:321–333 [DOI] [PubMed] [Google Scholar]
- 19. Song MJ, Kim KH, Yoon JM, Kim JB. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem Biophys Res Commun 2006;346:739–745 [DOI] [PubMed] [Google Scholar]
- 20. Devaraj S, Siegel D, Jialal I. Simvastatin (40 mg/day), adiponectin levels, insulin sensitivity, in subjects with the metabolic syndrome. Am J Cardiol 2007;100:1397–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Devaraj S, Dasu MR, Park S, Jialal I. Increased levels of ligands of TLR2 and TLR4 in type 1 diabetes. Diabetologia 2009;52:1665–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu X, Ukai T, Yumoto H, Davey M, Goswami S, Gibson FC, 3rd, Genco CA. Toll-like receptor 2 plays a critical role in the progression of atherosclerosis that is independent of dietary lipids. Atherosclerosis 2007;196:146–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shinohara M, Hirata K, Yamashita T, Takaya T, Sasaki N, Shiraki R, Ueyama T, Emoto N, Inoue N, Yokoyama M, Kawashima S. Local overexpression of Toll-like receptors at the vessel wall induces atherosclerotic lesion formation: synergism of TLR2 and TLR4. Arterioscler Thromb Vasc Biol 2007;27:2384–2391 [DOI] [PubMed] [Google Scholar]
- 24. Du T, Zhou ZG, You S, Huang G, Lin J, Yang L, Li X, Zhou WD, Chao C. Modulation of monocyte hyperresponsiveness to TLR ligands by 1,25-dihydroxy-vitamin D3 from LADA and T2DM. Diabetes Res Clin Pract 2009;83:208–214 [DOI] [PubMed] [Google Scholar]
- 25. Wagner H. Endogenous TLR ligands and autoimmunity. Adv Immunol 2006;91:159–173 [DOI] [PubMed] [Google Scholar]