Abstract
OBJECTIVE
Abnormal cellular cholesterol handling in islets may contribute to β-cell dysfunction in type 2 diabetes. β-Cell deficiency for the ATP binding cassette transporter A1 (ABCA1), which mediates the efflux of cellular cholesterol, leads to altered intracellular cholesterol homeostasis and impaired insulin secretion in mice. We aimed to assess the impact of ABCA1 dysfunction on glucose homeostasis in humans.
RESEARCH DESIGN AND METHODS
In heterozygous carriers of disruptive mutations in ABCA1 and family-based noncarriers of similar age, sex, and BMI, we performed oral glucose tolerance tests (OGTTs) (n = 15 vs. 14) and hyperglycemic clamps (n = 8 vs. 8).
RESULTS
HDL cholesterol levels in carriers were less than half those in noncarriers, but LDL cholesterol levels did not differ. Although fasting plasma glucose was similar between groups, glucose curves after an OGTT were mildly higher in carriers than in noncarriers. During hyperglycemic clamps, carriers demonstrated lower first-phase insulin secretion than noncarriers but no difference in insulin sensitivity. The disposition index (a measure of β-cell function adjusted for insulin sensitivity) of the carriers was significantly reduced in ABCA1 heterozygotes.
CONCLUSIONS
Carriers of loss-of-function mutations in ABCA1 show impaired insulin secretion without insulin resistance. Our data provide evidence that ABCA1 is important for normal β-cell function in humans.
The reasons for β-cell dysfunction in type 2 diabetes are incompletely understood. One hypothesis suggests that the accumulation of toxic lipid leads to the loss of insulin secretion characteristic of this disorder (1). Although the role of free fatty acids and triglycerides has been extensively studied (2), much less is known about the role of cholesterol in this process. The ATP-binding cassette transporter, subfamily A, member 1 (ABCA1) regulates the rate-limiting step in cholesterol transport out of cells and is thus a candidate molecule for influencing cholesterol metabolism in β-cells.
In humans, homozygosity for naturally occurring loss-of-function mutations in ABCA1 leads to Tangier disease, an extremely rare disorder characterized by near absence of HDL cholesterol in plasma as well as a 30–40% reduction in LDL cholesterol, increased risk of coronary artery disease (CAD), and accumulation of cholesterol in tissues (3). Approximately 100 cases of Tangier disease have been reported worldwide. Heterozygous carriers of loss-of-function mutations in ABCA1, generally ascertained from Tangier disease pedigrees, also exhibit decreased HDL cholesterol levels and accelerated atherogenesis due to impaired removal of cholesterol from lipid-laden macrophages in the presence of relatively normal levels of total plasma cholesterol (4).
Targeted deletion of β-cell Abca1 in mice results in accumulation of cholesterol in islets, reduced glucose-stimulated insulin secretion (GSIS), and impaired glucose tolerance (5). In addition, the common ABCA1 polymorphism R230C was shown to be associated with a fourfold increase in the occurrence of diabetes in a Mexican population (6). These novel findings imply an important role for ABCA1 in maintaining glucose-mediated insulin secretion.
Interestingly, mice lacking Abca1 specifically in β-cells have a more severe impairment in β-cell function compared with mice lacking Abca1 globally, possibly because of the higher levels of total plasma cholesterol in mice with β-cell–specific deletion of Abca1. This finding suggests that the degree of β-cell dysfunction caused by ABCA1 deficiency may relate to the level of plasma cholesterol to which the islets are exposed. To assess whether deficiency of ABCA1 function influences β-cell function in humans, we therefore chose to study carriers of heterozygous ABCA1 mutations, with severe reductions in ABCA1 function but relatively normal total plasma cholesterol levels. We performed oral glucose tolerance tests (OGTTs) and hyperglycemic clamps in ABCA1 heterozygotes and family-based control subjects. Our data indicate that ABCA1 plays a significant role in insulin secretion and β-cell function in humans.
RESEARCH DESIGN AND METHODS
During the past decade, we have used our lipid clinic network and contacts with general practitioners in the Netherlands to collect plasma and DNA from individuals with familial hypoalphalipoproteinemia, with the intent of identifying genes that control HDL cholesterol levels. We contacted heterozygous carriers of proven (7) loss-of-function mutations in ABCA1 and unaffected (family) control subjects of similar age, sex, and BMI and asked them to participate. Written informed consent was obtained after explanation of the purpose, nature, and potential risks of the study. All subjects who gave informed consent were included in the study and the final analysis. The study was approved by the institutional review board of the Academic Medical Center of the University of Amsterdam.
Genotyping
For mutation detection, genomic DNA was prepared from 10 ml of whole blood on an AutopureLS apparatus according to the manufacturer's protocol (Gentra Systems, Minneapolis, MN). Forward and reverse PCR primers, flanking each exon, were designed with Primer3 (http://frodo.wi.mit.edu). PCR amplification was performed with 50 ng genomic DNA in a 25-μl reaction volume containing 1× Taq DNA polymerase buffer (Qiagen, Hilden, Germany), 50 μmol/l concentrations of each dNTP, 0.4 μmol/l concentrations of each primer, and 1 unit of Taq DNA polymerase. The thermal cycling conditions were as follows: 96°C for 5 min, then 35 cycles of 30 s at 96°C, 30 s at 60°C, and 30 s at 72°C in a PCR apparatus (T3 Biocycler; Biometra, Göttingen, Germany). The sequence reactions were performed using fluorescently labeled dideoxy chain terminations with a BigDye terminator ABI Prism kit (Applied Biosystems, Foster City, CA) according to the manufacturer's protocol and analyzed on an automated DNA sequencer (model 370; Applied Biosystems). Sequences were analyzed with the Sequencher package (Gene Codes, Ann Arbor, MI). Subjects included in the present study were heterozygous carriers of the following extremely rare ABCA1 mutations: C1477R, M1091T, and R587W (4,7–10) and L996P and Q978X (C. Candini et al., manuscript in preparation). None of these mutations were detected in >200 control chromosomes. C1477R has been reported in nine heterozygous individuals, M1091T in four individuals, and R587W in seven individuals (11).
OGTT
Subjects were asked to discontinue any lipid-lowering medication 3 weeks before the study, except for those with a history of CAD. On the morning of the study day, fasting plasma glucose and insulin were determined after venous sampling of 4.5 ml of blood. Subsequently, subjects were asked to ingest 75 g glucose, administered as a 375-ml solution of 20% glucose in water. At t = 30, 60, 90, and 120 min another 4.5-ml blood sample was obtained for repetitive assessment of blood glucose and insulin.
Hyperglycemic clamp
We performed hyperglycemic clamps in a subset of participants, on a separate day, within 6 months of the OGTT. Subjects were instructed to maintain their usual diet preceding the study and to discontinue any lipid-lowering medication 3 weeks before the study visit (unless there was a history of CAD). On the study day at 9:00 a.m., an antecubital vein was cannulated for blood sampling. Blood glucose was measured using a bedside calibrated glucose sensor (YSI 2300 STAT S; YSI, Yellow Springs, OH). The antecubital vein of the contralateral arm was cannulated for infusions of 20% glucose and arginine hydrochloride (50 ml of 100 mg/ml). First-phase insulin secretion was determined using an intravenous glucose bolus. On the basis of the fasting baseline plasma glucose level and the subject's body weight, a 20% glucose bolus was given over 1 min, with the aim of reaching a plasma glucose level of 13 mmol/l ([weight/70 × 10 − plasma glucose] ml of a 20% glucose solution). Blood glucose levels were kept at 13 mmol/l by continuous glucose infusion. Pump settings (glucose infusion rate) were adapted on the basis of measured blood glucose levels. Blood was collected at t = 0, 2.5, 5, 7.5, 10, and 15 min for the determination of glucose, insulin, C-peptide, and glucagon. Subsequently, blood was sampled every 5 min for glucose determination. At t = 90, 100, 110, 120, 125, 130, 135, 140, 145, and 150 min blood was sampled for hormone determination. An arginine bolus (5 g) was given at t = 120 min. Volunteers remained fasting until the end of the study. Glucose administration was then tapered over 30 min, and volunteers were offered a carbohydrate-rich meal.
Plasma measurements
Total, HDL, and LDL cholesterol and triglycerides were determined in fasted plasma using standard laboratory procedures within 1 h after sampling. HDL cholesterol was determined by a homogeneous enzymatic colorimetric assay, using polyethylene glycol–modified cholesterol esterase and oxidase (Roche, Basel, Switzerland). A1C was determined by high-performance liquid chromatography. Insulin was determined on an Immulite 2000 system (Diagnostic Products, Los Angeles, CA), using a chemiluminescent immunometric assay with an intra-assay variation of 1.6–5.8%, interassay variation of 3.6–6.4%, and detection limit of 15 pmol/l. Glucagon was determined with the Linco 125I radioimmunoassay (RIA) (Linco Research, St. Charles, MO) with an intra-assay variation of 3.4–5.8%, interassay variation of 9.8–10.9%, and detection limit of 15 ng/l. C-peptide was measured by RIA (RIA-coat C-peptide; Byk-Sangtec Diagnostica, Dietzenbach, Germany) with an intra-assay variation of 6.3–9.2%, interassay variation of 7.0–14.2%, and detection limit of 50 pmol/l.
Calculations
Homeostasis model assessment (HOMA) indexes were calculated with the following formula: HOMA = insulin (picomoles)/6.945 × glucose (millimoles)/22.5. Glucose and insulin excursions during the OGTT were compared using incremental areas under the curve (AUCs) at t = 0 through t = 120. Insulin sensitivity was assessed using the composite insulin sensitivity index according to Matsuda and Defronzo, calculated with the following formula: 10,000/square root of [fasting glucose × fasting insulin] × [mean glucose × mean insulin during OGTT] (12). For hyperglycemic clamps, plasma insulin and C-peptide concentrations were taken as measures of insulin secretion, and glucagon levels was taken as a measure of α-cell function. First-phase secretion measures during the hyperglycemic clamps were compared between groups using incremental AUCs at t = 0 through t = 10. The response to arginine was compared using incremental AUCs at t = 120 through t = 150. In addition, pancreatic insulin secretion was estimated using the C-peptide deconvolution method described by Van Cauter et al. (13).
Under stable conditions of constant hyperglycemia, the amount of glucose infused (milligrams per kilogram) equals the amount of metabolized glucose (M). M was calculated as the average glucose infusion rate during the last 30 min of the clamp (t = 90 through t = 120). The M value divided by the average plasma insulin concentration (I) during the same interval, the M/I ratio, provides a measurement of tissue sensitivity to insulin (micrograms per kilogram per minute per picomole per liter) (14).
The disposition index, calculated as the product of the M/I ratio and the first-phase insulin response (incremental AUC of insulin levels at t = 0 through t = 10) is a measure of the normal β-cell response adequate for any degree of insulin resistance (14,15).
Statistical analyses
Results are reported as means ± SD or as medians (interquartile range [IQR]) unless noted otherwise. All incremental AUCs were calculated using the trapezoidal rule. Differences in continuous baseline variables between carriers and control subjects were tested using Student's t test, except for triglycerides, which are known to be nonnormally distributed. Differences in triglycerides and continuous outcome variables between carriers and control subjects were tested using the nonparametric Mann-Whitney U test. In addition, where appropriate, P values for interaction between time and genotype were calculated using two-way repeated-measures ANOVA. Differences in categorical variables were tested using a Pearson χ2 test. P < 0.05 was taken to indicate significant differences. All statistical analyses were performed with SPSS software (SPSS, Chicago, IL).
RESULTS
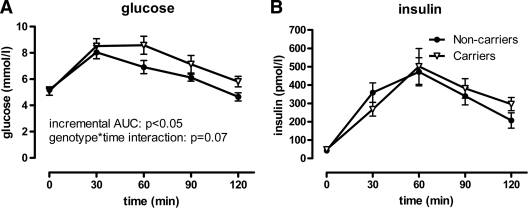
We performed OGTTs in 15 subjects heterozygous for validated loss-of-function mutations in ABCA1 and 14 control subjects. Carriers were recruited from five different families (four C1477R carriers, three M1091T carriers, three R587W carriers, four L996P carriers, and one Q978X carrier); family control subjects were siblings, cousins, or partners of a carrier. Baseline characteristics are shown in Table 1. Carriers had >50% reduced HDL cholesterol levels compared with control subjects, but, importantly, LDL cholesterol levels were comparable between carriers and noncarriers. Fasting glucose levels were not significantly different between carriers and control subjects. Glucose curves tended to be different when assessed using a genotype × time interaction (P = 0.07). However, the incremental area under the glucose tolerance curve was significantly greater for carriers compared with control subjects (245 [IQR 153–353] vs. 155 mmol · l−1 · min−1 [84–198], P < 0.05) (Fig. 1A). Insulin curves were not significantly different between groups (incremental AUC [18.5–46.2] vs. 32.1 nmol · l−1 · min−1 [18.3–48.6], P = 0.93; genotype × time interaction P = 0.31) (Fig. 1B). Composite insulin sensitivity indexes also did not differ between groups (carriers 6.6 [3.1–7.9] vs. noncarriers 6.0 [4.3–9.4], P = 0.69). These data suggest that carriers of ABCA1 mutations have mildly reduced glucose tolerance, which is not explained by increased insulin resistance.
Table 1.
Baseline characteristics of participants in the OGTT
| Noncarriers | Carriers | P | |
|---|---|---|---|
| n | 14 | 15 | |
| Age (years) | 52 ± 9 | 55 ± 13 | 0.51 |
| Men | 7 (50) | 9 (60) | 0.59 |
| BMI | 24.9 ± 2.9 | 24.7 ± 2.8 | 0.89 |
| Cholesterol (mmol/l) | |||
| Total | 4.99 ± 1.20 | 4.50 ± 0.92 | 0.23 |
| LDL | 3.09 ± 0.97 | 3.22 ± 0.83 | 0.71 |
| HDL | 1.57 ± 0.40 | 0.70 ± 0.30 | <0.001 |
| Triglycerides (mmol/l) | 0.68 [0.44–0.92] | 0.75 [0.53–2.14] | 0.17 |
| Fasting glucose (mmol/l) | 5.2 ± 0.5 | 5.1 ± 1.2 | 0.76 |
| Fasting insulin (pmol/l) | 43 ± 20 | 48 ± 35 | 0.62 |
| HOMA index | 1.44 ± 0.71 | 1.65 ± 1.30 | 0.60 |
| History of CAD | 0 (0) | 3 (20) | 0.08 |
| History of diabetes | 0 (0) | 0 (0) | N/A |
| Family history of diabetes | 1 (7) | 0 (0) | 0.29 |
| Smokers | 2 (14) | 2 (13) | 0.94 |
| Medication use | |||
| Statins | 1 (7) | 5 (33) | 0.08 |
| Hormone replacement therapy | 1 (7) | 0 (0) | 0.29 |
| Thiazides | 0 (0) | 0 (0) | N/A |
| β-Blockers | 1 (7) | 1 (7) | 0.96 |
Data are means ± SD, n (%), or median [IQR].
Figure 1.
OGTT results in carriers (△) versus controls (●). Plasma glucose and insulin curves after 75 g orally ingested glucose. A: Glucose curves were higher for carriers than for control subjects. B: Insulin curves were not different between groups. Error bars depict SEM.
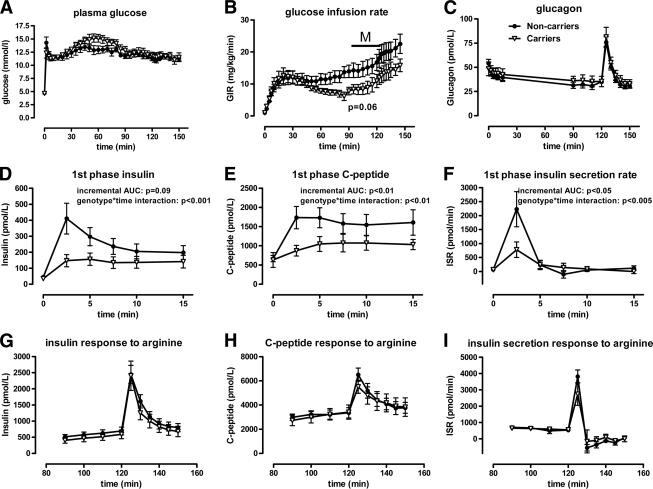
A subgroup of 16 subjects who participated in the OGTT also agreed to participate in a hyperglycemic clamp to evaluate β-cell function. This group consisted of four C1477R carriers, two M1091T carriers, one L996P carrier, one Q978X carrier, and eight control subjects. Baseline characteristics are listed supplementary Table A (available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc09-1562/DC1). Plasma glucose levels were fixed at 13 mmol/l by titrating the glucose infusion rate (Fig. 2A). Glucose infusion rates (M) tended to be lower in carriers (8.1 [IQR 6.0–12.0] vs. 12.4 mg · kg−1 · min−1 [IQR 10.8–19.1], P = 0.059) (Fig. 2B), probably reflecting reduced glucose tolerance. Incremental glucagon AUCs were comparable between groups (first phase −39 [−98 to 11] vs. −82 pmol · l−1 · min−1 [−137 to −69], P = 0.16; response to arginine 423 [161–515] vs. 331 pmol · l−1 · min−1 [299–350], P = 0.25; P values for genotype × time interactions were 0.09 and 0.99, respectively) (Fig. 2C). First-phase insulin response to an acute glucose bolus, measured by incremental AUC, tended to be lower in carriers than in control subjects (0.79 [0.42–1.58] vs. 1.53 nmol · l−1 · min−1 [1.06–3.71], P = 0.09) but was significantly lower when assessed using genotype × time interaction (P < 0.001) (Fig. 2D). Incremental AUCs of the first-phase C-peptide response were markedly lower in carriers versus control subjects (3.50 [1.09–5.02] vs. 6.62 nmol · l−1 · min−1 [4.73–11.48], P < 0.01; genotype × time interaction P < 0.01) (Fig. 2E). First-phase insulin secretion rates estimated by C-peptide deconvolution were lower in carriers as well (2.45 [1.88–3.42] vs. 3.81 nmol/m2 [2.92–6.95], P < 0.05; genotype × time interaction P < 0.005) (Fig. 2F). Together, these data indicate reduced first-phase insulin secretion in response to glucose.
Figure 2.
Hyperglycemic clamp results in carriers (△) versus controls (●). A: Plasma glucose curves during the hyperglycemic clamp were similar between carriers and noncarriers. B: The glucose infusion rate (GIR) under steady-state conditions, an approximation of the amount of glucose metabolized (M), tended to be lower in carriers, reflecting reduced glucose tolerance (P = 0.06) C: Glucagon curves, as a measure of α-cell function, did not differ between carriers and noncarriers during the clamp. D, E, and F: The first-phase insulin and C-peptide responses to an acute glucose bolus, as well as insulin secretion rates (ISR) during this period, were lower in carriers than in control subjects. G, H, and I: There were no significant differences in these measures between carriers and noncarriers in response to arginine. Error bars depict SEM.
In contrast, the insulin and C-peptide response to arginine stimulation did not differ significantly between carriers and noncarriers (insulin incremental AUC 17.3 [IQR 9.0–21.9] vs. 16.3 nmol · l−1 · min−1 [14.1–22.2], P = 0.75; genotype × time interaction P = 0.98 and C-peptide incremental AUC 26.1 [20.5–40.5] vs. 34.6 nmol · l−1 · min−1 [23.4–50.3], P = 0.40; genotype × time interaction P = 0.40, respectively) (Fig. 2G and H). Insulin secretion rates in response to arginine were also similar between groups (13.4 [8.70–17.1] vs. 14.1 nmol/m2 [7.39–16.5], P = 0.92; genotype × time interaction P = 0.36) (Fig. 2I).
To exclude insulin resistance as a possible cause for the difference in glucose tolerance, we calculated the M/I ratio as a measure of insulin sensitivity (14) and found that it was comparable between groups (22 μg · kg−1 · min−1 per pmol/l [IQR 19–33] for carriers and 22 μg · kg−1 · min−1 per pmol/l [IQR 17–41] for noncarriers; P = 0.96), indicating that insulin sensitivity is not altered in carriers of loss-of-function ABCA1 mutations, consistent with data from mice lacking Abca1 (5). In contrast, the disposition index, an index for β-cell function adjusted for insulin sensitivity (15), was significantly lower in carriers than in control subjects (407 [225–789] for carriers and 1,605 [976–2,295] for noncarriers; P < 0.02).
CONCLUSIONS
Here we show that the cholesterol transporter ABCA1 influences β-cell function in humans. Heterozygous carriers of ABCA1 mutations displayed mild hyperglycemia with no difference in insulin response after an oral glucose challenge compared with noncarriers of similar age, sex, and BMI. Using hyperglycemic clamps, we found that ABCA1 heterozygotes show a reduced first-phase insulin response to hyperglycemia and normal insulin sensitivity, resulting in a markedly decreased disposition index. Glucagon levels were similar throughout the study, suggesting that the observations were not affected by a difference in glucose disposition. Collectively, these findings point to a defect in β-cell function in ABCA1 heterozygotes.
Mice with targeted deletion of Abca1 in β-cells accumulate cholesterol in islets, leading to impaired insulin secretion both in vivo and in vitro (5). Our data extend these findings and suggest that cholesterol handling may also be a determinant of β-cell function in humans. The specific means by which ABCA1 dysfunction impairs β-cell function remain incompletely understood. Glucose enters the β-cell via a GLUT, whereupon it is modified by glucokinase in the rate-limiting step in glucose sensing. The subsequent glucose metabolism pathway results in closing of the ATP-sensitive potassium channel, membrane depolarization, calcium influx into the cell via the L-type calcium channel, and exocytosis of insulin-containing granules (16). This first-phase secretory response is augmented by a potassium channel-independent pathway, which is largely responsible for the second-phase insulin response (17). Arginine is known to stimulate insulin secretion by directly inducing membrane depolarization independent of potassium channels and thus largely independent of glucose sensing and glucose metabolism pathways (18–21).
In ABCA1 heterozygotes, the first-phase insulin response was significantly impaired, whereas insulin secretion during steady hyperglycemia (between t = 90 and t = 120) was not statistically different between groups. The secretory response to arginine was also comparable between carriers and noncarriers. These data suggest that ABCA1 dysfunction does not limit insulin secretion per se but rather the insulin release in response to an acute glucose challenge. In light of these observations, it seems unlikely that islet cholesterol accumulation due to β-cell ABCA1 dysfunction results in a substantial loss of β-cell mass. This finding is consistent with the observation that mice with a deletion of β-cell Abca1 have normal β-cell mass (5).
Our observations are best explained if ABCA1 dysfunction has a predominant impact on first-phase GSIS by affecting pathways upstream of β-cell membrane depolarization. In support of this notion, cholesterol depletion of HIT-T15 hamster insulinoma cells using methyl-β-cyclodextrin results in redistribution of voltage-gated potassium channels and a marked enhancement of GSIS (22). Similarly, the treatment of islets with methyl-β-cyclodextrin or mevastatin increases GSIS, whereas cholesterol overloading reduces GSIS (23). In the latter study, these effects were found to be related to intracellular glucose metabolism: a cholesterol-enriched lipid environment of insulin granules reduced cytoplasmic glucokinase activity indirectly by trapping glucokinase in insulin granules (23). ABCA1 heterozygotes may therefore display impaired first-phase GSIS because of excess islet cholesterol interfering with glucose sensing and downstream β-cell membrane depolarization.
Our data suggest that the cholesterol accumulation observed in β-cells of mice with β-cell-specific Abca1 deletion may also occur in humans with heterozygous mutations. However, the normal secretory response to arginine in ABCA1 heterozygotes contrasts with the impaired secretory response to arginine observed in mice with a total deficiency of β-cell Abca1 (5). This apparent discrepancy may be due to a milder ABCA1 dysfunction in our heterozygotes compared with the knockout mice, which could be overcome by the powerful secretagogue effect of arginine. In our study, none of the ABCA1 mutation carriers had diabetes, possibly indicating that carriership is a relatively mild islet susceptibility factor for diabetes by itself. In addition, the relatively small sample size of the present study, which is related to the extreme scarcity of subjects with loss-of-function mutations in ABCA1, should be considered as a limitation. At this point, insufficient data are available to make a broad statement regarding their risk of diabetes. An inherent limitation to all case-control studies is that matched groups may differ in respects other than just the trait of interest. We controlled for BMI, age, and sex, and none of the participants had diabetes. Therefore, we believe we have at least minimized the chance that the abnormalities in glucose homeostasis were due to anything else but the ABCA1 mutation status.
A recent study reported that four homozygous patients with Tangier disease had significantly greater excursion of OGTT curves after a 75-g glucose load compared with unrelated control subjects (24) in agreement with our findings in ABCA1 heterozygotes. These authors did not report additional studies of β-cell function. However, all four subjects in this study had preexisting type 2 diabetes (fasting glucose values 9.05–10.0 mmol/l), suggesting that these patients may have been subject to selection bias. It will be necessary to determine the true prevalence of type 2 diabetes in patients with Tangier disease to determine whether they are indeed at increased risk for developing type 2 diabetes.
In summary, we show that ABCA1 is important for normal β-cell function in humans. our understanding of the role of ABCA1 in β-cell function and glucose homeostasis.
Supplementary Material
Acknowledgments
This work was sponsored by a grant from the Dutch Heart Foundation (2008B070 to E.S.G.S.) and from the Canadian Institutes of Health Research (to M.R.H.). J.K.K. is supported by a fellowship from the Michael Smith Foundation for Health Research. M.R.H. is a University Killam Professor and holds a Canada Research Chair in Human Genetics.
No potential conflicts of interest relevant to this article were reported.
We give special thanks to C.A. Koch and J.F. Los for genetic fieldwork and to Dr. E. van Bavel for mathematical assistance with C-peptide deconvolution. We thank M.J. Geerlings for determination of endocrinological plasma parameters.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Prentki M, Nolan CJ. Islet β cell failure in type 2 diabetes. J Clin Invest 2006;116:1802–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wajchenberg BL. β-Cell failure in diabetes and preservation by clinical treatment. Endocr Rev 2007;28:187–218 [DOI] [PubMed] [Google Scholar]
- 3. Nofer JR, Remaley AT. Tangier disease: still more questions than answers. Cell Mol Life Sci 2005;62:2150–2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Dam MJ, de Groot E, Clee SM, Hovingh GK, Roelants R, Brooks-Wilson A, Zwinderman AH, Smit AJ, Smelt AH, Groen AK, Hayden MR, Kastelein JJ. Association between increased arterial-wall thickness and impairment in ABCA1-driven cholesterol efflux: an observational study. Lancet 2002;359:37–42 [DOI] [PubMed] [Google Scholar]
- 5. Brunham LR, Kruit JK, Pape TD, Timmins JM, Reuwer AQ, Vasanji Z, Marsh BJ, Rodrigues B, Johnson JD, Parks JS, Verchere CB, Hayden MR. β-Cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat Med 2007;13:340–347 [DOI] [PubMed] [Google Scholar]
- 6. Villarreal-Molina MT, Aguilar-Salinas CA, Rodríguez-Cruz M, Riaño D, Villalobos-Comparan M, Coral-Vazquez R, Menjivar M, Yescas-Gomez P, Königsoerg-Fainstein M, Romero-Hidalgo S, Tusie-Luna MT, Canizales-Quinteros S. The ATP-binding cassette transporter A1 R230C variant affects HDL cholesterol levels and BMI in the Mexican population: association with obesity and obesity-related comorbidities. Diabetes 2007;56:1881–1887 [DOI] [PubMed] [Google Scholar]
- 7. Singaraja RR, Visscher H, James ER, Chroni A, Coutinho JM, Brunham LR, Kang MH, Zannis VI, Chimini G, Hayden MR. Specific mutations in ABCA1 have discrete effects on ABCA1 function and lipid phenotypes both in vivo and in vitro. Circ Res 2006;99:389–397 [DOI] [PubMed] [Google Scholar]
- 8. Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO, Loubser O, Ouelette BF, Fichter K, Ashbourne-Excoffon KJ, Sensen CW, Scherer S, Mott S, Denis M, Martindale D, Frohlich J, Morgan K, Koop B, Pimstone S, Kastelein JJ, Genest J, Jr, Hayden MR. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat Genet 1999;22:336–345 [DOI] [PubMed] [Google Scholar]
- 9. Kuivenhoven JA, Hovingh GK, van Tol A, Jauhiainen M, Ehnholm C, Fruchart JC, Brinton EA, Otvos JD, Smelt AH, Brownlee A, Zwinderman AH, Hayden MR, Kastelein JJ. Heterozygosity for ABCA1 gene mutations: effects on enzymes, apolipoproteins and lipoprotein particle size. Atherosclerosis 2003;171:311–319 [DOI] [PubMed] [Google Scholar]
- 10. Marcil M, Brooks-Wilson A, Clee SM, Roomp K, Zhang LH, Yu L, Collins JA, van Dam M, Molhuizen HO, Loubster O, Francis Ouellette BF, Sensen CW, Fichter K, Mott S, Denis M, Boucher B, Pimstone S, Genest J, Kastelein JJ, Hayden MR. Mutations in the ABC 1 gene in familial HDL deficiency with defective cholesterol efflux. Lancet 1999;354:1341–1346 [DOI] [PubMed] [Google Scholar]
- 11. Brunham LR, Singaraja RR, Hayden MR. Variations on a gene: rare and common variants in ABCA1 and their impact on HDL cholesterol levels and atherosclerosis. Annu Rev Nutr 2006;26:105–129 [DOI] [PubMed] [Google Scholar]
- 12. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 13. Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992;41:368–377 [DOI] [PubMed] [Google Scholar]
- 14. Nijpels G, Boorsma W, Dekker JM, Hoeksema F, Kostense PJ, Bouter LM, Heine RJ. Absence of an acute insulin response predicts onset of type 2 diabetes in a Caucasian population with impaired glucose tolerance. J Clin Endocrinol Metab 2008;93:2633–2638 [DOI] [PubMed] [Google Scholar]
- 15. Bergman RN, Ader M, Huecking K, Van CG. Accurate assessment of β-cell function: the hyperbolic correction. Diabetes 2002;51(Suppl. 1):S212–S220 [DOI] [PubMed] [Google Scholar]
- 16. Brunham LR, Kruit JK, Verchere CB, Hayden MR. Cholesterol in islet dysfunction and type 2 diabetes. J Clin Invest 2008;118:403–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Straub SG, Sharp GW. Hypothesis: one rate-limiting step controls the magnitude of both phases of glucose-stimulated insulin secretion. Am J Physiol Cell Physiol 2004;287:C565–C571 [DOI] [PubMed] [Google Scholar]
- 18. Thams P, Capito K. l-Arginine stimulation of glucose-induced insulin secretion through membrane depolarization and independent of nitric oxide. Eur J Endocrinol 1999;140:87–93 [DOI] [PubMed] [Google Scholar]
- 19. Fajans SS, Floyd JC, Jr, Knopf RF, Guntsche EM, Rull JA, Thiffault CA, Conn JW. A difference in mechanism by which leucine and other amino acids induce insulin release. J Clin Endocrinol Metab 1967;27:1600–1606 [DOI] [PubMed] [Google Scholar]
- 20. Fajans SS, Quibrera R, Pek S, Floyd JC, Jr, Christensen HN, Conn JW. Stimulation of insulin release in the dog by a nonmetabollizable amino acid. Comparison with leucine and arginine. J Clin Endocrinol Metab 1971;33:35–41 [DOI] [PubMed] [Google Scholar]
- 21. Smith PA, Sakura H, Coles B, Gummerson N, Proks P, Ashcroft FM. Electrogenic arginine transport mediates stimulus-secretion coupling in mouse pancreatic β-cells. J Physiol 1997;499(Pt 3):625–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xia F, Gao X, Kwan E, Lam PP, Chan L, Sy K, Sheu L, Wheeler MB, Gaisano HY, Tsushima RG. Disruption of pancreatic β-cell lipid rafts modifies Kv2.1 channel gating and insulin exocytosis. J Biol Chem 2004;279:24685–24691 [DOI] [PubMed] [Google Scholar]
- 23. Hao M, Head WS, Gunawardana SC, Hasty AH, Piston DW. Direct effect of cholesterol on insulin secretion: a novel mechanism for pancreatic β-cell dysfunction. Diabetes 2007;56:2328–2338 [DOI] [PubMed] [Google Scholar]
- 24. Koseki M, Matsuyama A, Nakatani K, Inagaki M, Nakaoka H, Kawase R, Yuasa-Kawase M, Tsubakio-Yamamoto K, Masuda D, Sandoval C, Ohama T, Nakagawa-Toyama Y, Matsuura F, Nishida M, Ishigami M, Hirano KI, Sakane N, Kumon Y, Suehiro T, Nakamura T, Shimomura I, Yamashita S. Impaired insulin secretion in four Tangier disease patients with ABCA1 mutations. J Atheroscler Thromb 2009;16:292–296 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.