Abstract
OBJECTIVE
To individuate a novel sex-specific index, based on waist circumference, BMI, triglycerides, and HDL cholesterol, indirectly expressing visceral fat function.
RESEARCH DESIGN AND METHODS
Visceral adiposity index (VAI) was first modeled on 315 nonobese healthy subjects. Using two multiple logistic regression models, VAI was retrospectively validated in 1,498 primary care patients in comparison to classical cardio- and cerebrovascular risk factors.
RESULTS
All components of metabolic syndrome increased significantly across VAI quintiles. VAI was independently associated with both cardiovascular (odd ratio [OR] 2.45; 95% CI 1.52–3.95; P < 0.001) and cerebrovascular (1.63; 1.06–2.50; P = 0.025) events. VAI also showed significant inverse correlation with insulin sensitivity during euglycemic-hyperinsulinemic clamp in a subgroup of patients (Rs = −0.721; P < 0.001). By contrast, no correlations were found for waist circumference and BMI.
CONCLUSIONS
Our study suggests VAI is a valuable indicator of “visceral adipose function” and insulin sensitivity, and its increase is strongly associated with cardiometabolic risk.
Visceral obesity (1) is associated with increased adipocytokine production, proinflammatory activity (2), deterioration of insulin sensitivity (3), increased risk of developing diabetes, “high-triglyceride/low–HDL cholesterol dyslipidemia,” hypertension, atherosclerosis, and higher mortality rate (4–7). The identification of a routinely applicable indicator for the evaluation of visceral adipose function, with higher sensitivity and specificity than classical parameters (such as waist circumference [WC], BMI, and lipids), could be useful for cardiometabolic risk assessment. We here extrapolate a novel sex-specific index based on WC, BMI, triglycerides (TGs), and HDL (visceral adiposity index [VAI]), able to estimate the visceral adiposity dysfunction associated with cardiometabolic risk.
RESEARCH DESIGN AND METHODS
The study was approved by the Institutional Review Board, University of Palermo.
Subjects
The Alkam Metabolic Syndrome (AlkaMeSy) Study database including 13,195 primary-care patients was used (see online Appendix 1, available at http://care.diabetesjournals.org/cgi/content/full/dc09-1825/DC1). A total of 1,498 subjects were selected based on availability of full information listed in Table 1. Because data were recorded anonymously, no individual informed consent was needed.
Table 1.
Maximum likelihood estimates of logistic regression function related to the dichotomic dependent variables “coronary heart disease and/or myocardial infarction” and “transient ischemic attack and/or ischemic stroke”
| Regression coefficient | SE | Wald | P | OR | 95% CI for OR | |
|---|---|---|---|---|---|---|
| Coronary heart disease and/or myocardial infarction | ||||||
| Intercept | −10.661 | 1.990 | — | <0.001 | — | — |
| Presence of metabolic syndrome* | 0.390 | 0.697 | 0.314 | 0.575 | 1.47 | 0.37–5.79 |
| Total cholesterol | 0.004 | 0.006 | 0.523 | 0.470 | 1.00 | 0.99–1.01 |
| Diabetes or fasting glucose ≥5.6 mmol/l† | −0.904 | 0.604 | 2.241 | 0.134 | 0.40 | 0.12–1.32 |
| High blood pressure* | 0.720 | 0.503 | 2.089 | 0.148 | 2.07 | 0.77–5.54 |
| Current or former smoker | 1.400 | 0.479 | 8.545 | 0.003 | 4.05 | 1.58–10.36 |
| Male sex | 1.388 | 0.571 | 5.899 | 0.015 | 4.00 | 1.30–12.28 |
| Age at the time of event | 0.053 | 0.014 | 14.426 | <0.001 | 1.05 | 1.02–1.08 |
| VAI | 0.899 | 0.243 | 13.728 | <0.001 | 2.45 | 1.52–3.95 |
| BMI | 0.005 | 0.092 | 0.003 | 0.957 | 1.00 | 0.84–1.20 |
| WC | 0.000 | 0.031 | 0.000 | 0.994 | 1.00 | 0.94–1.06 |
| TG-to-HDL ratio | −0.605 | 0.370 | 2.678 | 0.102 | 0.54 | 0.26–1.12 |
| Transient ischemic attack and/or ischemic stroke | ||||||
| Intercept | −6.540 | 1.863 | — | <0.001 | — | — |
| Presence of metabolic syndrome* | −0.556 | 0.617 | 0.811 | 0.368 | 0.57 | 0.17–1.92 |
| Total cholesterol | −0.004 | 0.005 | 0.785 | 0.376 | 0.99 | 0.98–1.00 |
| Diabetes or fasting glucose ≥5.6 mmol/l† | 0.456 | 0.471 | 0.936 | 0.333 | 1.57 | 0.62–3.97 |
| High blood pressure* | 0.631 | 0.410 | 2.377 | 0.123 | 1.88 | 0.84–4.19 |
| Current or former smoker | 0.518 | 0.410 | 1.595 | 0.207 | 1.67 | 0.75–3.74 |
| Male sex | 0.904 | 0.474 | 3.638 | 0.056 | 2.47 | 0.97–6.25 |
| Age at the time of event | 0.085 | 0.013 | 44.53 | <0.001 | 1.08 | 1.06–1.11 |
| VAI | 0.489 | 0.218 | 5.022 | 0.025 | 1.63 | 1.06–2.50 |
| BMI | 0.039 | 0.087 | 0.204 | 0.652 | 1.04 | 0.87–1.23 |
| WC | −0.049 | 0.028 | 2.914 | 0.088 | 0.95 | 0.90–1.00 |
| TG-to-HDL ratio | 0.120 | 0.347 | 0.120 | 0.729 | 1.12 | 0.57–2.22 |
*According to Adult Treatment Panel (ATP) III criteria.
†According to current American Diabetes Association criteria. Concerning WC, BMI, TGs, and HDL, the mean values of the 6 months before the beginning of the study were considered. For patients who experienced cardio- and/or cerebrovascular accident, we used WC, BMI, TGs, and HDL recorded in the 6 months before the event. Independent variables showing P value <0.10 in univariate analyses were entered in one single step. Dichotomic variables analyzed through χ2 test or Fisher exact test were as follows: metabolic syndrome, diabetes/fasting glucose ≥5.6 mmol/l, high blood pressure, current/former smoker, and male sex. Quantitative variables analyzed through the Student's t test were as follows: total cholesterol, age at the time of event, BMI, WC, and TG-to-HDL ratio.
Model of adipose distribution and VAI formulas
A total of 315 healthy subjects with BMI between 20 and 30 kg/m2 were further selected from the 1,498 primary-care patients to calculate a model of adipose distribution (MOAD). To correct MOAD for fat function, TG (mmol/l) and HDL (mmol/l) levels were introduced in the formula. This was defined as VAI:
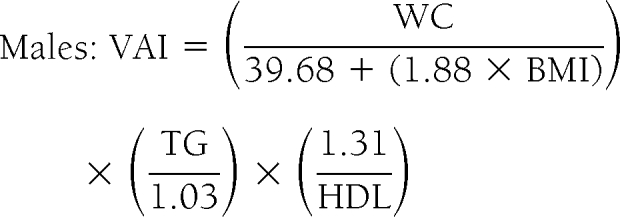 |
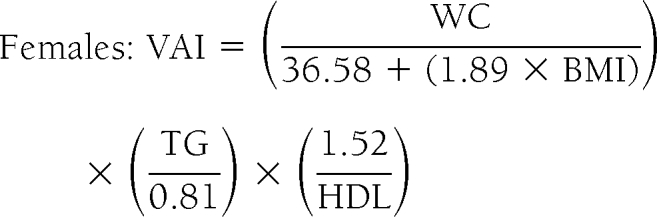 |
assuming VAI = 1 in healthy nonobese subjects with normal adipose distribution and normal TG and HDL levels (supplemental Appendix 2).
Magnetic resonance imaging
To validate MOAD, magnetic resonance imaging (MRI) was prospectically performed on 26 metabolically healthy patients (supplemental Appendix 3).
Euglycemic-hyperinsulinemic clamp
Euglycemic-hyperinsulinemic clamp was prospectically performed in 74 patients (supplemental Fig. 1, available in the online appendix). Rate of peripheral glucose utilization (M value) was calculated by dividing glucose infused during the last 40′ by body weight measured in kilograms (milligrams per kilogram per minute) (8).
Statistical analysis
Data were analyzed using SPSS 13.0 for Windows. Univariate correlations were performed using the nonparametric test (Spearman, Rs). Binary logistic regression was performed to explore possible determinants of cardio- and cerebrovascular events: “coronary heart disease and/or myocardial infarction” and “transient ischemic attack and/or ischemic stroke.” Independent variables showing a P value <0.10 in a univariate analysis were entered in one single step. Receiver-operating characteristic (ROC) analysis was used to assess whether VAI associates with cardio- and cerebrovascular events in comparison to WC, BMI, and TG-to-HDL ratio, considered separately. Difference among C statistics of VAI, BMI, WC, and TG-to-HDL ratio was calculated by Hanley and McNeil's method.
RESULTS
To verify whether MOAD, WC, and BMI correlated with visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT), we validated each index with abdominal MRI. MOAD showed significant correlation with VAT, both regarding area (Rs = 0.437, P = 0.025) and volume (Rs = 0.744, P < 0.001), but not with SAT.
The 1,498 primary-care patients were further subdivided into VAI quintiles. Age and number of patients with metabolic syndrome, diabetes, high blood pressure, low HDL, high TGs, LDL cholesterol ≥3.37 mmol/l, coronary heart disease and/or myocardial infarction, and transient ischemic attack and/or ischemic stroke increased significantly across VAI quintiles (supplemental Table 1).
Binary logistic regression models showed VAI is an indicator of visceral fat dysfunction independently associated to coronary heart disease and/or myocardial infarction and transient ischemic attack and/or ischemic stroke. Among all variables examined, only VAI, age at the time of event, smoking, and male sex were independently correlated to cardiovascular events. VAI and age at the time of event were independently associated with cerebrovascular events (Table 1).
To verify the possible relationship between insulin sensitivity and VAI, WC, and BMI, data from euglycemic-hyperinsulinemic clamps were retrospectively analyzed. M values during the clamp showed significant inverse correlation with VAI (Rs = −0.721; P < 0.001). No correlation was found between M value and either WC or BMI (supplemental Fig. 1).
Concerning cardiovascular risk, ROC curve analysis showed significant differences in C statistics between VAI and BMI (P = 0.032; SE 0.06; 95% CI 0.01–0.26), WC (P = 0.031; SE 0.06; 95% CI 0.01–0.26), and TG-to-HDL ratio (P = 0.005; SE 0.04; 95% CI 0.03–0.19). Regarding cerebrovascular risk, significant differences were found between VAI and BMI (P < 0.001; SE 0.05; 95% CI 0.14–0.36) and WC (P < 0.001; SE 0.05; 95% CI 0.15–0.36), but not TG-to-HDL ratio (P = 0.396; SE 0.03; 95% CI 0.03–0.09) (supplemental Fig. 2).
CONCLUSIONS
WC is a major clinical parameter used for the indirect evaluation of increased visceral fat (9). Nevertheless, WC alone does not help in distinguishing between subcutaneous and visceral fat mass (10). This is a considerable drawback, given that VAT and not SAT plays a decisive role in the genesis of cardiovascular sequelae (9,11–13). Here we identified an index that could be used as a surrogate marker of “adipose tissue dysfunction.” VAI was significantly correlated to all metabolic syndrome factors and cardio- and cerebrovascular events. This trend was particularly apparent from the third VAI quintile on, being moderate for the fourth and severe for the fifth quintile.
Interestingly, VAI was independently associated to cardiovascular events, along with age at the time of event, smoking, and male sex. This was not observed for WC, BMI, and the other classical cardiovascular risk factors analyzed. Furthermore, VAI and age at the time of event were the only independent risk factors for cerebrovascular events. These findings might be explained by the fact that VAI includes both physical and metabolic parameters, perhaps indirectly reflecting other nonclassical risk factors, such as altered production of adipocytokines, increased lipolysis, and plasma free fatty acids, which are not signified by BMI, WC, TGs, and HDL separately. Therefore, VAI might be a valuable index of both fat distribution and function. This is also corroborated by the correlation between MOAD and VAT, and between VAI and insulin sensitivity, evaluated by the hyperinsulinemic-euglycemic clamp.
Noteworthy, VAI shows an association with M value that is not detected by WC or BMI alone. This observation confirms that reduction in insulin sensitivity is associated not only with increased visceral fat mass, but it is also influenced by functional factors, indirectly expressed by TGs and HDL and by altered VAT-to-SAT ratio (14). Indeed, this condition characterized by visceral obesity and “high-triglyceride/low–HDL cholesterol dyslipidemia” has been associated with physiological age-linked leptin resistance, muscle, and liver insulin resistance, lipotoxic cardiomyopathy, and generalized endothelial dysfunction (15).
In conclusion, although VAI is not a diagnostic tool for cardiovascular and cerebrovascular events, the simplicity of WC and BMI measurement and TG and HDL assessment, make it an easily applicable index for the evaluation of visceral fat dysfunction. VAI might therefore be a useful tool in daily clinical practice and in population studies for the assessment of cardiometabolic risk associated with visceral obesity.
Supplementary Material
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Pascot A, Lemieux S, Lemieux I, Prud'homme D, Tremblay A, Bouchard C, Nadeau A, Couillard C, Tchernof A, Bergeron J, Després JP. Age-related increase in visceral adipose tissue and body fat and the metabolic risk profile of premenopausal women. Diabetes Care 1999;22:1471–1478 [DOI] [PubMed] [Google Scholar]
- 2. Bruunsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol Allergy 2003;23:15–39 [DOI] [PubMed] [Google Scholar]
- 3. DeNino WF, Tchernof A, Dionne IJ, Toth MJ, Ades PA, Sites CK, Poehlman ET. Contribution of abdominal adiposity to age-related differences in insulin sensitivity and plasma lipids in healthy nonobese women. Diabetes Care 2001;24:925–932 [DOI] [PubMed] [Google Scholar]
- 4. Ohlson LO, Larsson B, Svärdsudd K, Welin L, Eriksson H, Wilhelmsen L, Björntorp P, Tibblin G. The influence of body fat distribution on the incidence of diabetes mellitus: 13.5 years of follow-up of the participants in the study of men born in 1913. Diabetes 1985;34:1055–1058 [DOI] [PubMed] [Google Scholar]
- 5. Rader DJ. Effect of insulin resistance, dyslipidemia, and intra-abdominal adiposity on the development of cardiovascular disease and diabetes mellitus. Am J Med 2007;120(Suppl. 1):S12–S18 [DOI] [PubMed] [Google Scholar]
- 6. Després JP. Intra-abdominal obesity: an untreated risk factor for type 2 diabetes and cardiovascular disease. J Endocrinol Invest 2006;29(Suppl. 3):77–82 [PubMed] [Google Scholar]
- 7. Sironi AM, Gastaldelli A, Mari A, Ciociaro D, Positano V, Postano V, Buzzigoli E, Ghione S, Turchi S, Lombardi M, Ferrannini E. Visceral fat in hypertension: influence on insulin resistance and beta-cell function. Hypertension 2004;44:127–133 [DOI] [PubMed] [Google Scholar]
- 8. De Fronzo RA, Tobin JD, Andres R. The glucose clamp technique: a method for the quantification of beta cell sensitivity to glucose and of tissue sensitivity to insulin. Am J Physiol 1979;237:214–223 [Google Scholar]
- 9. Mathieu P, Pibarot P, Larose E, Poirier P, Marette A, Després JP. Visceral obesity and the heart. Int J Biochem Cell Biol 2008;40:821–836 [DOI] [PubMed] [Google Scholar]
- 10. Pouliot MC, Després JP, Lemieux S, Moorjani S, Bouchard C, Tremblay A, Nadeau A, Lupien PJ. Waist circumference and abdominal saggital diameter: best simple anthropometric indices of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol 1994;73:460–468 [DOI] [PubMed] [Google Scholar]
- 11. Taksali SE, Caprio S, Dziura J, Dufour S, Calí AM, Goodman TR, Papademetris X, Burgert TS, Pierpont BM, Savoye M, Shaw M, Seyal AA, Weiss R. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: a determinant of an adverse metabolic phenotype. Diabetes 2008;57:367–371 [DOI] [PubMed] [Google Scholar]
- 12. Kelley DE, Thaete FL, Troost F, Huwe T, Goodpaster BH. Subdivisions of subcutaneous abdominal adipose tissue and insulin resistance. Am J Physiol Endocrinol Metab 2000;278:E941–E948 [DOI] [PubMed] [Google Scholar]
- 13. Després JP. Is visceral obesity the cause of the metabolic syndrome? Ann Med 2006;38:52–63 [DOI] [PubMed] [Google Scholar]
- 14. Björntorp P. Body fat distribution, insulin resistance, and metabolic diseases. Nutrition 1997;13:795–803 [DOI] [PubMed] [Google Scholar]
- 15. Unger RH. Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology 2003;144:5159–5165 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


