Abstract
Coordination between the activities of organelles and the nucleus requires the exchange of signals. Using Chlamydomonas, we provide evidence that plastid-derived chlorophyll precursors may replace light in the induction of two nuclear heat-shock genes (HSP70A and HSP70B) and thus qualify as plastidic signal. Mutants defective in the synthesis of Mg-protoporphyrin IX were no longer inducible by light. Feeding of Mg-protoporphyrin IX or its dimethyl ester to wild-type or mutant cells in the dark resulted in induction. The analysis of HSP70A promoter mutants that do or do not respond to light revealed that these chlorophyll precursors specifically activate the light signaling pathway. Activation of gene expression was not observed when protoporphyrin IX, protochlorophyllide, or chlorophyllide were added. A specific interaction of defined chlorophyll precursors with factor(s) that regulate nuclear gene expression is suggested.
In eukaryotic cells, there is a complex network of regulatory signals between the nucleus and organelles. Many of the structural and regulatory proteins necessary for organelle development and function are encoded by nuclear genes resulting in the well-known dominating role of the nucleus in biogenesis of mitochondria and plastids (1, 2). On the other hand, intact, functional organelles are a prerequisite for the expression of a subset of nuclear genes. Many of the nuclear genes, dependent on functional chloroplasts, encode photosynthesis-related proteins (3). These genes are not expressed in plants with defective plastids caused, e.g., by carotenoid deficiency leading to photodestruction of plastids (4–7) or by mutations resulting in ribosome-deficient plastids (8). The concept of a signal originating in plastids or on their surface and regulating transcription of specific nuclear genes (“plastidic signal”) was derived from these studies (9, 10). The nature of the plastidic signal remained elusive. Genetic studies have revealed that, in Arabidopsis, at least three nonallelic loci (GUN1–3) are necessary for signaling from plastids to the nucleus (11). This result raised the question whether a single or multiple compound(s) must be considered as plastidic signal(s). In addition to light, transcription, and translation occurring in the chloroplast were found to be necessary for the expression of the nuclear genes CAB, RBCS, and PETH. The plastidic signaling compounds (of unknown nature) were believed to inactivate or modify a transcription factor, most probably a repressor, that binds to the promoter region of the respective genes (12).
Intermediates of chlorophyll synthesis (Fig. 1) as a plastidic signal were first suggested to act as regulators of nuclear gene expression in Chlamydomonas. Experimental conditions that were assumed to cause accumulation of Mg-protoporphyrin monomethyl ester (MgPROTOMe) inhibited light-dependent accumulation of CAB and RBCS transcripts (13, 14). These results suggested that the accumulation of chlorophyll precursors resulted in mRNA destabilization (13, 15). Accumulation of MgPROTOMe though was not tested by the authors but hypothesized to occur in Chlamydomonas reinhardtii by analogy to higher plants. In cress seedlings, an increase in MgPROTOMe pool size was shown to cause decreased steady-state levels of light-induced mRNAs (CAB, PSI2). Run-off measurements suggested that MgPROTOMe accumulation interfered with light-dependent transcription (16, 17).
Figure 1.
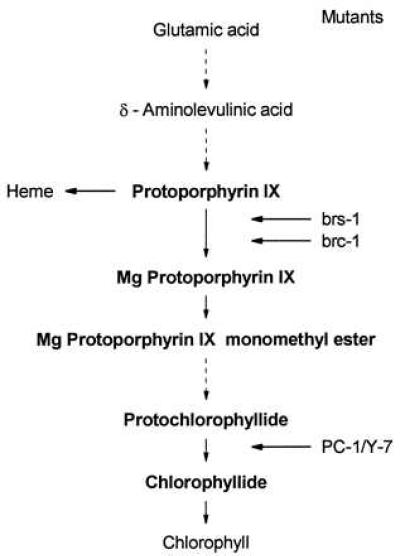
Pathway of chlorophyll synthesis. Dashed arrows represent multiple steps. Conversions blocked in mutants are indicated.
The expression of the nuclear heat-shock genes HSP70A and HSP70B, encoding cytosolic and plastid-localized heat-shock proteins, respectively, can, in addition to induction by heat stress, also be induced by light (18–20). We have shown previously (21) that, for the light induction of these genes, a pathway is utilized that is different from the one used in response to heat shock. A clue to our biological understanding of the light induction of HSP70 genes was provided by the observation that, in cells incubated in the dark and then shifted to light intensities that caused photoinhibition, photosystem II was less damaged and recovered faster when, prior to light stress, the cultures were pre-exposed to dim light for 60 min. During this preincubation, the HSP70 genes were induced. For HSP70B, the plastid-localized heat-shock protein, increased levels were detected after light induction (20). Evidence for a role for the HSP70B in the recovery of photosystem II activity from photoinhibitory damage has been obtained recently by the analysis of mutants with either reduced or elevated levels of HSP70B (M. Schroda, O. Vallon, F.-A. Wollman, and C.F.B., unpublished data). These data suggest that light induction of HSP70 genes provides the cells with increased levels of these chaperones that, by mechanisms not yet elucidated, are advantageous for the chloroplast under light stress conditions.
In the present paper, we provide evidence that MgPROTO or MgPROTOMe, added to a Chlamydomonas culture in the dark, can replace light in inducing genes HSP70A and HSP70B and thus qualify as plastidic signal.
MATERIALS AND METHODS
Algal Strains.
Strain CC-124 (wild type) of C. reinhardtii was obtained from the Chlamydomonas Genetics Center (Duke University, Durham, NC). The brs-1 and brc-1 mutants, which both accumulate PROTO in darkness, have been described (22). The double mutant PC-1/Y-7 accumulates protochlorophyllide (PCHLD) and cannot form chlorophyllide (CHLD) due to a deletion mutation in the light-dependent NADPH:PCHLD oxidoreductase (PC-1) (23) and a defect in one of the steps required for light-independent CHLD formation (Y-7) (24). The mutant strains were kindly provided by W.-Y. Wang (University of Iowa, Iowa City).
Culture Conditions.
Strain CC-124 was grown in Tris/acetate/phosphate (TAP) medium (25) with aeration and continuous irradiation (30 μmol⋅m−2⋅s−1) at 23°C to a density of 4 × 106 cells per ml (26). For light induction, the cultures were then divided into subcultures of 50 ml each, and incubation continued in the dark. After 20 h of dark incubation, these cultures were again exposed to white light (30 μmol⋅m−2⋅s−1) and samples were taken for RNA isolation (18). Mutant cultures, due to their light sensitivity, were grown in the dark and treated like wild-type cells after reaching a density of 2–4 × 106 cells per ml.
RNA Gel Blot Analyses.
Ten micrograms of total RNA per lane were separated on formaldehyde-containing agarose gels and transferred to nylon membranes (Hybond N+, Amersham). Prehybridization (3 h) and hybridization (18 h) were performed at 65°C in 0.1 M NaCl, 50 mM Tris⋅HCl (pH 7.5), 0.1% sodium pyrophosphate, 10× Denhardt solution, 1% SDS, 10% dextran sulfate, 60% formamide, and 100 μg/ml of sheared, denatured herring sperm DNA. The probes were labeled with [α-32P]dCTP (3,000 Ci/mmol; 1 Ci = 37 GBq) by the random priming protocol. After hybridization the membranes were washed twice in 2× standard saline citrate (SSC) for 5 min at room temperature and once in 2× SSC, 1% SDS for 30 min at 65°C.
Hybridization Probes.
Genomic clones of HSP70A and HSP70B were used as HSP70 probes (ref. 18; described as hsp70–2 and hsp80–35, respectively). Plasmid Ba295, containing a 4.4-kb genomic DNA fragment of the C. reinhardtii 18S and 23S rRNA genes (27) was provided by J.-D. Rochaix (University of Geneva, Switzerland).
Induction by Porphyrins.
Porphyrins were dissolved in dimethyl sulfoxide (final concentration 15 mM). For induction experiments, the compounds were added to subcultures that had been preincubated in the dark for 20 h (see above). Subsequent incubation with the compounds was performed in the dark for 1 h (unless otherwise stated) followed by cell harvest and RNA extraction (18).
Synthesis of Various Porphyrins.
For the preparation of MgPROTO, 100 mg of PROTO were dissolved in 20 ml dimethyl sulfoxide and heated to 150°C under a stream of nitrogen. Magnesium acetate (2.5 g) was added and the mixture was heated until insertion was complete, as tested by the absorption spectrum of small aliquots. The product was extracted with n-butanol. The butanol phase was thoroughly washed with water, dried with Na2SO4, and evaporated. The purity of the product was checked by HPLC. MgPROTO dimethyl ester (MgPROTOMe2) was prepared as described above using PROTOMe2 as the starting material. PCHLD a and CHLD a were prepared according to standard procedures (28).
RESULTS
Induction of HSP70A and HSP70B Does Not Occur or Is Delayed in Mutants Defective in MgPROTO Synthesis.
In Chlamydomonas, light induces increased expression of the nuclear heat-shock genes HSP70A (encoding a cytosolic protein) and HSP70B (encoding a chloroplast-localized protein) by a mechanism that is independent of the normal heat-shock response (18, 19, 21). Light induction of HSP70 genes was not observed in a mutant blocked in chlorophyll synthesis (Fig. 1). Thus, in mutant brs-1, which is unable to convert PROTO into MgPROTO and thus accumulates PROTO (22), induction of neither HSP70A nor HSP70B was detected upon light exposure (Fig. 2). Mutant brc-1, defective in the synthesis of MgPROTO only in the dark (22), accumulated these HSP70 mRNAs upon light induction only after a delay of ≈0.5 h as compared with the wild-type strain (Fig. 2). In both mutants, the HSP70 genes were normally inducible following a heat shock in the light or in the dark (data not shown). A mutant (PC-1/Y-7) blocked in the conversion of PCHLD to CHLD in the light and in the dark (24) exhibited normal light induction of the HSP70 genes (Fig. 2). From these results we conclude that either a reaction catalyzing the synthesis of MgPROTO or intermediates of chlorophyll biosynthesis after PROTO and before CHLD may be involved in light induction of HSP70 genes.
Figure 2.
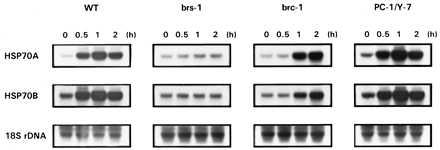
Induction of HSP70A and HSP70B expression by light in C. reinhardtii wild-type cells (WT), and mutants impaired in the synthesis of MgPROTO (brs-1 and brc-1), or in the conversion of PCHLD to CHLD (PC-1/Y-7). At time 0, cultures were shifted from dark into light (30 μmol⋅m−2⋅s−1). For Northern blot analyses, 10 μg of total RNA were hybridized with HSP70A and HSP70B genomic probes. As a control, an 18S rDNA probe was used. Conditions for hybridization, washing, and exposure to x-ray films were identical for all probes.
Addition of MgPROTO in the Dark Induces the HSP70 Genes.
If indeed intermediates of chlorophyll biosynthesis played a role in the induction pathway, the feeding of chlorophyll precursors would be expected to substitute for the light signal. This can be tested by the addition of MgPROTO to cultures in the dark. Addition of these precursors in the light caused cell death due to severe photooxidative damage (data not shown). As shown in Fig. 3, addition of MgPROTO resulted in a transient induction of the HSP70 genes. The kinetics of mRNA accumulation were similar to those observed upon dark-light shift. To further characterize the inducing activity of this chlorophyll precursor, different amounts of MgPROTO were added to cells in the dark. The results reveal a clear correlation between the degree of HSP70A mRNA accumulation and the concentration of the inducing porphyrin (Fig. 4A).
Figure 3.
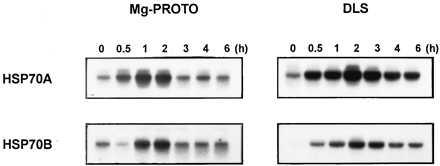
Induction of HSP70A and HSP70B by exogenous addition of MgPROTO. Northern blot analyses show the kinetics of mRNA accumulation after addition of MgPROTO at a final concentration of 4 μM in the dark (MgPROTO) or after shift from dark into white light in the absence of MgPROTO (DLS).
Figure 4.
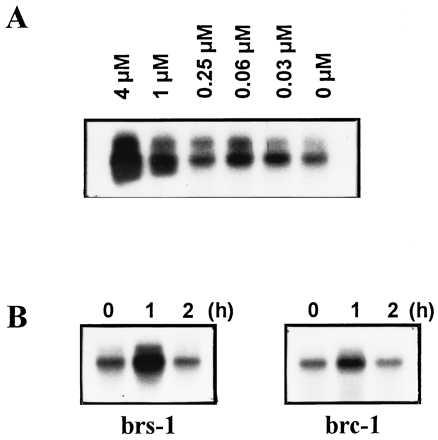
Induction of HSP70A by exogenous addition of MgPROTO. (A) Dependence of HSP70A mRNA accumulation on the concentration of MgPROTO. MgPROTO was added in the dark at the final concentrations indicated and after 1 h of incubation cells were harvested and RNA was isolated. (B) HSP70A mRNA accumulation in mutants impaired in the synthesis of MgPROTO after the addition of MgPROTO at a final concentration of 4 μM in the dark.
Since, depending on the genotype used, mutants defective in the synthesis of MgPROTO showed no or delayed light induction of the HSP70 genes (Fig. 2), supplementation of MgPROTO should restore induction. Indeed, feeding of MgPROTO to either the brs-1 or the brc-1 mutant in the dark resulted in an increased expression of HSP70A (Fig. 4B) and HSP70B (not shown).
HSP70 Gene Induction by MgPROTO Uses the Light-Specific Signaling Pathway.
Although various chlorophyll precursors are well known for their potential to cause damage to cells in the light, such effects have not been observed in the dark (29). However, the addition of MgPROTO to cells of C. reinhardtii in the dark may still elicit the general stress response. To test whether feeding of MgPROTO in the dark activates the general stress response or the light-specific signaling pathway we made use of an HSP70A promoter deletion mutation (Δ-138) that essentially abolished light induction of the HSP70A gene. This mutated promoter still normally responds to heat stress (21). As a control, a second HSP70A promoter deletion construct (Δ-209) that responds normally to light as well as heat stress was chosen. These promoter mutant constructs, fused to a tagged HSP70A gene (21), were reintroduced into C. reinhardtii cells and tested for their response to the addition of MgPROTO in the dark. Feeding of MgPROTO to Δ-209 in the dark resulted in an induction of the tagged HSP70A gene (Fig. 5). In contrast, Δ-138 responded neither to the addition of MgPROTO nor to light. Induction of the endogenous HSP70A gene in these transformants by MgPROTO, light, or heat stress turned out to be normal (Fig. 5).
Figure 5.
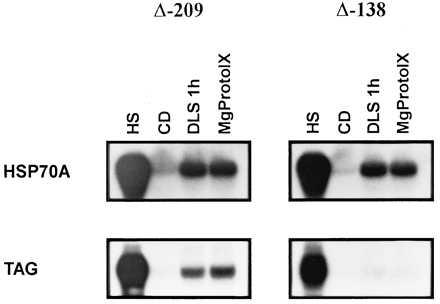
Test of HSP70A promoter deletion constructs for induction by MgPROTO. Two 5′truncations into the upstream region of the HSP70A promoter (Δ-209 and Δ-138) were converted into reporter genes by insertion of a 299 bp DNA fragment (TAG) into the 3′untranslated region of HSP70A (21) and co-transformed with a plasmid containing the ARG7 gene into an arg7 mutant. After an incubation for 20 h in the dark samples for RNA isolation were taken from subcultures that either had received MgPROTO (4 μM final concentration) for 1 h, were shifted from dark to light for 1 h (DLS), were shifted from 23°C to 40°C for 30 min (HS), or were incubated in the dark for another hour (CD). Ten micrograms of total RNA (2 μg for heat shock) was hybridized with the TAG probe, thereby specifically detecting the mRNA encoded by the promoter deletion constructs, or an HSP70A probe that hybridized to both HSP70A mRNAs.
Porphyrin Compounds That May Mediate HSP70A Induction.
Our mutant analysis has provided evidence for an involvement of porphyrin intermediates between MgPROTO and PCHLD in the light induction of HSP70 genes (Fig. 2). To identify the specific compound(s) involved, different chlorophyll precursors (Fig. 1) were used in feeding experiments. No induction was observed when PROTO was added to cultures in the dark (Fig. 6). Also, the addition of Mg2+ alone as well as in combination with PROTO did not cause induction (data not shown). Presence of Mg2+ in the tetrapyrrole ring thus appears to be required for induction. PCHLD and CHLD were also unable to induce the HSP70A gene. Since the difference between MgPROTOMe and PCHLD rests in the side groups at the third ring of the tetrapyrrole, an involvement of this part of the molecule in determining specificity may be deduced. MgPROTO and MgPROTOMe2 (used instead of the naturally occurring monomethyl ester) were thus the only chlorophyll precursors tested that had inducing potential.
Figure 6.
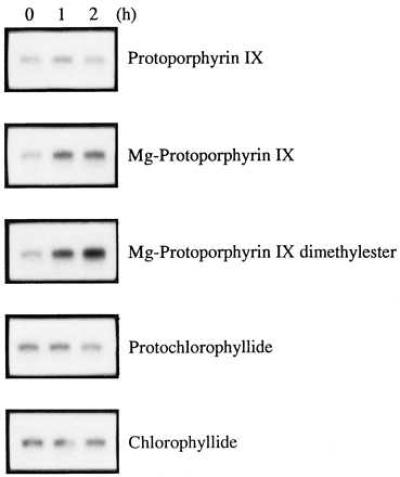
Analysis of different chlorophyll precursors for their potential to induce HSP70A mRNA accumulation. After 20 h of incubation in the dark cultures of the wild-type strain were supplemented with different chlorophyll precursors at final concentrations of 4 μM and incubation was continued in the dark. Samples for RNA isolation were taken at the time points indicated.
DISCUSSION
Here we report on experimental evidence for a role of the chlorophyll precursors MgPROTO and MgPROTOMe as intermediates in the signaling pathway by which light activates the expression of the nuclear heat-shock genes HSP70A and HSP70B. MgPROTO is the product of Mg-chelatase, the first enzyme specific for chlorophyll synthesis after the branching point between chlorophyll and heme biosyntheses (30, 31). As a “bottleneck” enzyme, it appears to play a regulatory role in chlorophyll metabolism. According to recent investigations (32), it is a chloroplast-localized, soluble stromal protein. Being synthesized in the chloroplast compartment, the reaction product, MgPROTO, must thus be considered as a true “plastidic factor,” i.e., a compound originating within the plastids.
Mutant brs-1, which is unable to form MgPROTO (22) and did not show light induction of HSP70 mRNA (Fig. 2), provided a first indication for the participation of the chloroplast in the light control of the HSP70 genes. However, the inducibility of the HSP70 genes in a mutant blocked in a later step of chlorophyll synthesis, i.e., the conversion of PCHLD to CHLD (mutant PC-1/Y-7), showed that, for light induction, neither chlorophyll nor a functional photosynthetic apparatus are needed, an observation corroborated by the competence of various photosynthetic mutants for HSP70 light induction (J.K. and C.F.B., unpublished data). The crucial role of MgPROTO and MgPROTOMe in the light signaling pathway was confirmed by the feeding of MgPROTO or MgPROTOMe2 to C. reinhardtii cells in the dark that resulted in the transient accumulation of HSP70A and HSP70B mRNAs, very similar to that observed after dark-light shift (Fig. 3). Neither earlier (PROTO) nor later (PCHLD, CHLD) chlorophyll precursors had the potential to induce the HSP70 genes. The observation that the defect in light induction of HSP70 genes in mutant brs-1 that does not synthesize MgPROTO can be restored by feeding of MgPROTO provides experimental support for the involvement of this compound in the transcriptional regulation of these genes. Thus, in contrast to previous indirect evidence (13, 14), we present here direct evidence for a regulation of nuclear genes by defined chlorophyll precursors.
Arguments against a specific role of the chlorophyll intermediates in the light induction of the HSP70 genes may be based on a possible activation of the general stress response by these compounds. Using HSP70A promoter mutants, we could demonstrate that loss of light-specific induction also abolished the induction by MgPROTO (Fig. 5). This result suggests that light and MgPROTO have the same targets in the HSP70A promoter; targets that are different from those utilized by the cell′s general stress response system (21).
As a model, we propose that the signal chain for the induction of HSP70 genes is activated by light within the chloroplast or its envelope. Following this activation, MgPROTO and/or MgPROTOMe become accessible on the cytoplasmic side of the chloroplast. We entertain two alternative hypotheses for mechanisms by which light may mediate the increase of these chlorophyll precursors in the cytoplasm (Fig. 7). Firstly, a dark-light shift may cause an increase in the levels of various chlorophyll precursors. These precursors may diffuse from the chloroplast to the cytoplasm and activate there the downstream branch of the signaling pathway. A transient 30–200-fold increase of MgPROTO and MgPROTOMe pools within 30–60 min following a dark-light shift has been observed in tobacco and barley seedlings (G. Pöpperl, U.O., and W.R., unpublished results). A similar, but smaller increase has also been observed in C. reinhardtii (G. Pöpperl, U.O., and W.R., unpublished results). However, a strict correlation between an increase in the amount of porphyrins and the induction of HSP70 genes remains yet to be established. Secondly, a light activated mechanism for the transport of MgPROTO and/or MgPROTOMe from the chloroplast to the cytoplasm could also account for an increase in cytoplasmic porphyrin levels following a dark-light shift. In the cytoplasm, MgPROTO and/or MgPROTOMe may be recognized by factor(s) that either regulate the expression of nuclear genes directly or stimulate a signaling pathway that controls gene expression. MgPROTO and MgPROTOMe thus qualify as plastidic factors involved in the communication from the chloroplast to the nucleus.
Figure 7.
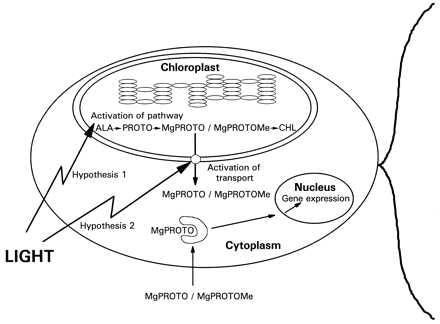
Model for the light signaling pathway from chloroplast to nucleus. The model is explained in the text. ALA, δ-aminolevulinic acid; CHL, chlorophyll.
This light regulatory system for the HSP70 genes in C. reinhardtii has certain features in common with the proposed mechanism for the regulation of nuclear genes CYC1 and CYC7, encoding iso-1-cytochrome c and iso-2-cytochrome c, respectively, in S. cerevisiae. The two yeast genes are activated by a regulatory protein, HAP. HAP1 is activated by binding to heme provided by the mitochondria and then binds to upstream activating sequences of both CYC genes (33–35).
In Chlamydomonas, the chlorophyll precursors presumably represent just one of a number of plastidic factors involved in the regulation of nuclear genes. It has been shown recently that the redox status of the plastoquinone pool in the chloroplast represents a photon-sensing system that, linked by a phosphorylation cascade, regulates CAB gene transcription in response to light intensity (36). With the identification of MgPROTO and MgPROTOMe as signaling intermediates, compounds are at hand that should facilitate the isolation of protein(s) from the cytoplasm or nucleus that bind to these porphyrins. The molecular characterization of the photoreceptor involved presents another challenge.
Acknowledgments
We thank S. Schoch for the gift of PCHLD and CHLD, W.-Y. Wang for the mutant strains, F.-A. Wollman for stimulating discussions, and A. Batschauer, R. Bock, U. Johanningmeier, and M. Schroda for their critical reading of the manuscript. This work was supported by the Deutsche Forschungsgemeinschaft.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: PROTO, protoporphyrin IX; MgPROTO, magnesium PROTO; MgPROTOMe, MgPROTO monomethyl ester; MgPROTOMe2, MgPROTO dimethyl ester; CHLD, chlorophyllide; PCHLD, protochlorophyllide; HSP, heat shock protein.
References
- 1.Whittaker P A, Danks S M. Mitochondria: Structure, Function and Assembly. New York: Longman; 1978. [Google Scholar]
- 2.Kirk J T O, Tilney-Bassett R A E. The Plastids: Their Chemistry, Structure, Growth and Inheritance. Amsterdam: Elsevier; 1978. [Google Scholar]
- 3.Kusnetsov V, Bolle C, Lübberstedt T, Sopory S, Herrmann R G, Oelmüller R. Mol Gen Genet. 1996;252:631–639. doi: 10.1007/BF02173968. [DOI] [PubMed] [Google Scholar]
- 4.Mayfield S P, Taylor W C. Eur J Biochem. 1984;144:79–84. doi: 10.1111/j.1432-1033.1984.tb08433.x. [DOI] [PubMed] [Google Scholar]
- 5.Oelmüller R, Mohr H. Planta. 1986;167:106–113. doi: 10.1007/BF00446376. [DOI] [PubMed] [Google Scholar]
- 6.Ernst D, Schefbeck K. Plant Physiol. 1988;88:255–258. doi: 10.1104/pp.88.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oelmüller R, Briggs W R. Plant Physiol. 1990;92:434–439. doi: 10.1104/pp.92.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hess W R, Müller A, Nagy F, Börner T. Mol Gen Genet. 1994;242:305–312. doi: 10.1007/BF00280420. [DOI] [PubMed] [Google Scholar]
- 9.Taylor W C. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:211–233. [Google Scholar]
- 10.Oelmüller R. Photochem Photobiol. 1989;49:229–239. [Google Scholar]
- 11.Susek R E, Ausubel F M, Chory J. Cell. 1993;74:787–799. doi: 10.1016/0092-8674(93)90459-4. [DOI] [PubMed] [Google Scholar]
- 12.Gray J C, Sornarajah R, Zabron A A, Duckett C M, Kahn M S. In: Photosynthesis: from Light to Biosphere. Mathis P, editor. Dordrecht, The Netherlands: Kluwer; 1995. pp. 543–550. [Google Scholar]
- 13.Johanningmeier U, Howell S H. J Biol Chem. 1984;259:13541–13549. [PubMed] [Google Scholar]
- 14.Johanningmeier U. Eur J Biochem. 1988;177:417–424. doi: 10.1111/j.1432-1033.1988.tb14391.x. [DOI] [PubMed] [Google Scholar]
- 15.Herrin D L, Battey J F, Greer K, Schmidt G W. J Biol Chem. 1992;267:8260–8269. [PubMed] [Google Scholar]
- 16.Kittsteiner U, Brunner H, Rüdiger W. Physiol Plant. 1991;81:190–196. [Google Scholar]
- 17.Oster U, Brunner H, Rüdiger W. J Photochem Photobiol. 1996;36:255–261. [Google Scholar]
- 18.von Gromoff E D, Treier U, Beck C F. Mol Cell Biol. 1989;9:3911–3918. doi: 10.1128/mcb.9.9.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Müller F W, Igloi G L, Beck C F. Gene. 1992;111:165–173. doi: 10.1016/0378-1119(92)90684-h. [DOI] [PubMed] [Google Scholar]
- 20.Drzymalla C, Schroda M, Beck C F. Plant Mol Biol. 1996;31:1185–1194. doi: 10.1007/BF00040835. [DOI] [PubMed] [Google Scholar]
- 21.Kropat J, von Gromoff E D, Müller F W, Beck C F. Mol Gen Genet. 1995;248:727–734. doi: 10.1007/BF02191713. [DOI] [PubMed] [Google Scholar]
- 22.Wang W-Y, Wang W L, Boynton J E, Gilham N H. J Cell Biol. 1974;63:806–823. doi: 10.1083/jcb.63.3.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Timko M P. Plant Mol Biol. 1996;30:15–37. doi: 10.1007/BF00017800. [DOI] [PubMed] [Google Scholar]
- 24.Ford C, Mitchell S, Wang W-Y. Mol Gen Genet. 1981;184:460–464. [Google Scholar]
- 25.Harris E H. The Chlamydomonas Sourcebook. San Diego: Academic; 1989. [Google Scholar]
- 26.Treier U, Fuchs S, Weber M, Wakarchuk W W, Beck C F. Arch Microbiol. 1989;152:572–577. [Google Scholar]
- 27.Marco Y, Rochaix J-D. Mol Gen Genet. 1980;177:715–723. doi: 10.1007/BF00272684. [DOI] [PubMed] [Google Scholar]
- 28.Helfrich M, Schoch S, Schäfer W, Ryberg M, Rüdiger W. J Am Chem Soc. 1996;118:2606–2611. [Google Scholar]
- 29.Kittsteiner U, Mostowska A, Rüdiger W. Physiol Plant. 1991;81:139–147. [Google Scholar]
- 30.von Wettstein D, Gough S, Kannangara C G. Plant Cell. 1995;7:1039–1057. doi: 10.1105/tpc.7.7.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rüdiger, W. (1997) Phytochemistry, in press.
- 32.Walker C J, Weinstein J D. Physiol Plant. 1995;94:419–424. [Google Scholar]
- 33.Guarente L, Mason T. Cell. 1983;32:1279–1286. doi: 10.1016/0092-8674(83)90309-4. [DOI] [PubMed] [Google Scholar]
- 34.Padmanaban G, Venkatesvar V, Rangarajan P N. Trends Biochem Sci. 1989;14:492–496. doi: 10.1016/0968-0004(89)90182-5. [DOI] [PubMed] [Google Scholar]
- 35.Pfeifer K, Kim K-S, Kogan S, Guarente L. Cell. 1989;56:291–301. doi: 10.1016/0092-8674(89)90903-3. [DOI] [PubMed] [Google Scholar]
- 36.Escoubas J-M, Lomas M, Laroche J, Falkowski P G. Proc Natl Acad Sci USA. 1995;92:10237–10241. doi: 10.1073/pnas.92.22.10237. [DOI] [PMC free article] [PubMed] [Google Scholar]


