Abstract
There is a high prevalence rate (30-50%) of Alzheimer's disease (AD) and depression comorbidity. Depression can be a risk factor for the development of AD or it can be developed secondary to the neurodegenerative process. There are numerous documented diagnosis and treatment challenges for the patients who suffer comorbidity between these two diseases. Meta analysis studies have provided evidence for the safety and efficacy of antidepressants in treatment of depression in AD patients. Preclinical and clinical studies show the positive role of chronic administration of selective serotonin reuptake inhibitor (SSRI) antidepressants in hindering the progression of the AD and improving patient performance. A number of clinical studies suggest a beneficial role of combinatorial therapies that pair antidepressants with FDA approved AD drugs. Preclinical studies also demonstrate a favorable effect of natural antidepressants for AD patients. Based on the preclinical studies there are a number of plausible antidepressants effects that may modulate the progression of AD. These effects include an increase in neurogenesis, improvement in learning and memory, elevation in the levels of neurotrophic factors and pCREB and a reduction of amyloid peptide burden. Based on this preclinical and clinical evidence, antidepressants represent a rational complimentary strategy for the treatment of AD patients with depression comorbidity.
1. Classes of antidepressants
The monoamine hypothesis postulates that depletion in the levels of serotonin, norepinephrine, and/or dopamine in the central nervous system are the pathophysiologic basis of depression. There are five major classes of antidepressants that are categorized according to their mechanism of action on brain amines.
1.1 Non selective monoamine reuptake inhibitors (NSRI)
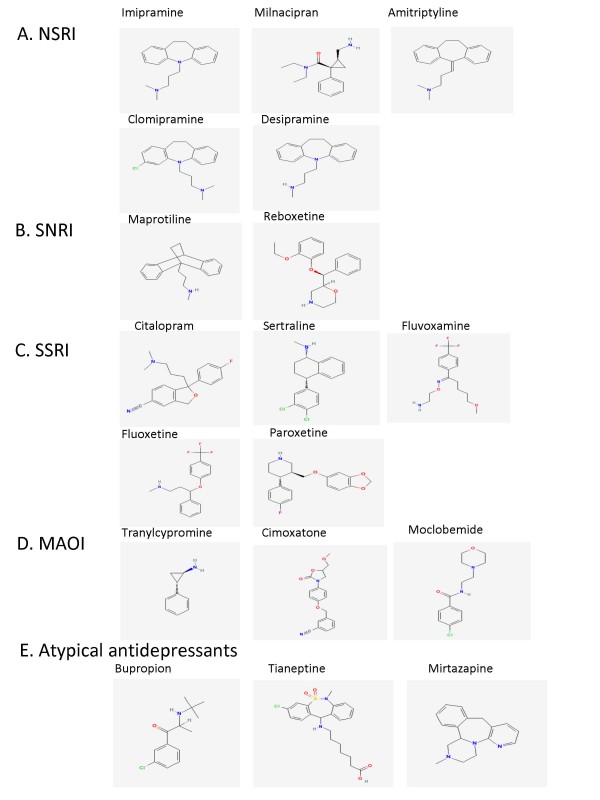
The nonselective monoamine reuptake inhibitor (NSRI) class of antidepressants includes the tricyclic antidepressants (TCA), a group of antidepressants introduced in the 1950s that inhibit the reuptake of both serotonin and noradrenalin. Examples of this class are imipramine, clomipramine, amitriptyline and despiramine (Fig. 1A) [1]. Some reports suggest that dual inhibitors may have superior efficacy and earlier response than selective reuptake inhibitors for a single monoamine [2,3]. In terms of the chemical structure, some TCAs, such as imipramine and amitriptyline, have a tertiary amine structure and are metabolized to secondary amines (Fig. 1A). Other TCAs, such as despiramine and nortriptyline, are secondary amines (Fig. 1A)[4]. In addition to their therapeutic effects; TCAs also have a number of unwanted side effects including antihistaminic, cardiotoxic and anticholinergic effects. These side effects are due to the action of TCAs on adrenergic receptors (α1), Na+, Ca2+ cardiac channels, histamine (H1) and muscarinic receptors [5-7]. The prescription of TCAs has declined due to these unwanted side effects and the advantage of new antidepressants with a better tolerability profile [4].
Figure 1.
A: Represents examples of non-selective monoamine reuptake inhibitor (NSRI) antidepressants. B: Represents examples of selective nor epinephrine reuptake inhibitor (SNRI) antidepressants. C: Represents examples of selective serotonin reuptake inhibitor (SSRI) antidepressants. D: Represents examples of Monoamine oxidase inhibitor (MAOI) antidepressants. E: Represents examples of atypical antidepressants. All the structures are downloaded from PubChem Substance http://pubchem.ncbi.nlm.nih.gov/.
There are other non-selective monoamine inhibitors that are structurally different from TCAs but share a similar mechanism of action. Examples of these agents are venlafaxine, duloxetine and milnacipran. Venalafaxine is a derivative of bicyclic phenethylamine and is a more potent inhibitor of serotonin reuptake than norepinephrine reuptake, in addition to low dopamine reuptake inhibition [6,8]. Milnacipran inhibits the reuptake of serotonin and norepinephrine with a similar potency and a negligible effect on dopamine reuptake (Fig. 1A) [9]. Clinical trials of duloxetine in the United States have demonstrated its efficacy in major depressive disorders, particularly those associated with physical pain [10,11].
1.2 Selective noradrenaline reuptake inhibitors (SNRI)
The selective norepinephrine reuptake inhibitor (SNRI) class of antidepressants selectively inhibits the reuptake of noradrenaline. Examples of this class are maprotiline and reboxetine (Fig. 1B) [1]. Maprotiline causes side effects similar to those of TCAs including dry mouth, fatigue and weight gain. Reboxetine formulations typically consist of a racemic mixture where the (S) enantiomer is 20 times more potent than the (R) enantiomer [12,13]. The primary unwanted side effects of reboxetine are cardiovascular and urinary effects.
1.3 Selective serotonin reuptake inhibitors (SSRI)
The selective serotonin reuptake inhibitor (SSRI) class includes antidepressants that selectively inhibit the reuptake of serotonin and subsequently increase the amount of serotonin available to bind to the postsynaptic receptor. SSRIs are the most commonly prescribed class of antidepressants. Examples of this class are citalopram, sertaline, fluvoxamine, fluoxetine and paroxetine (Fig. 1C) [1]. Though these compounds have different pharmacokinetic profiles and chemical structures, they are all metabolized primarily by oxidation prior to excretion [14]. In terms of chemical structure (Fig. 2), fluoxetine has a side chain of propylamine similar to TCAs while citalopram has a dimethyl aminopropyl side chain (Fig. 1C). Paroxetine, sertraline and fluvoxamine are derived from phenylpiperidine, tetrahydronaphthalene and arylketone respectively (Fig. 1C). The major advantage of the introduction of SSRIs in the 1980s was their good safety and tolerability profiles. These favorable profiles are attributed to the low affinity of SSRIs to histamine, muscarinic and α adrenergic receptors. Although SSRIs have a good safety profile, it is important to note probable drug-drug interactions due to an inhibitory effect by some SSRIs on the P450/2D6 cytochrome enzyme [15]. Additionally, reports show that SSRIs have a similar effect as TCAs on K+, Ca2+ and Na+ cardiovascular channels, which may contribute to the cardiovascular effects reported in some patients [7,15,16]. Also, sexual dysfunction is a significant side effect that has been reported for SSRIs [17].
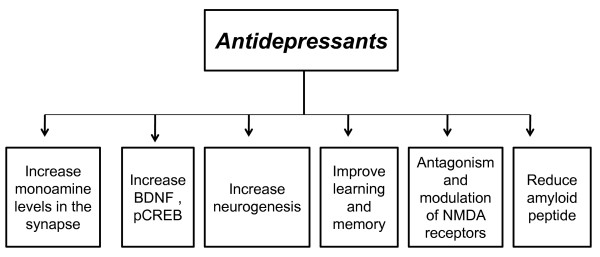
Figure 2.
Summary of different actions of antidepressants that can modulate the pathological features of Alzheimer's disease.
1.4 Monoamine oxidase inhibitors (MAOI)
The monoamine oxidase inhibitor (MAOI) class of antidepressants inhibits monoamine oxidase (MAO), the enzyme responsible for the metabolism of monoamines. An example of this class is tranylcypromine (Fig. 1D) [1]. Tranylcypromine irreversibly and nonselectively binds to MAO-A and MAO-B. There is a high tendency for hypertensive crisis associated with the use of irreversible and nonselective MAOIs with the concomitant ingestion of tyramine [4]. The development of selective and reversible inhibitors of MAO-A has provided a better safety profile [4]. MAO-A metabolizes the amines that play a major role in depression etiology. Examples of new agents selective for MAO-A are cimoxatone and moclobemide, derived from benzonitrile and benzamide respectively (Fig. 1D). Due to dangerous dietary and drug interactions, the use of MAOIs is generally reserved for patients who don't respond well to other antidepressants or suffer from atypical depression [18,19].
1.5 Atypical antidepressants
Atypical antidepressant agents produce an antidepressant effect, but their mechanism of action is not based on the monoamine hypothesis. Examples of this class are bupropion, tianeptine and mirtazapine (Fig. 1E). These compounds have well characterized mechanisms of action, but these mechanisms may not necessarily account for the antidepressant effects [1]. Bupropion inhibits the reuptake of dopamine, tianeptine stimulates the uptake of monoamines, and mirtazapine antagonizes α2 adrenergic receptors [1].
There are also many other classes of antidepressants that have been developed recently that are beyond the scope of this review. These drugs have different targets such as the dopaminergic system, serotonin receptors, adrenergic receptors and neuropeptide receptors (for review see [4]).
2. Depression and Alzheimer's disease comorbidity
Substantial evidence suggests that depression can be considered both a cause and consequence of a number of neurologic disorders, but the biological link between these disorders has not been determined yet [20]. Depression is considered causative because it is a risk factor for AD [21] particularly if a depressive episode is evident within two years of a dementia diagnosis. In such cases, the depressive episode is considered an early symptom of dementia [22-24]. Depressive symptoms are commonly detected before AD patients manifest cognitive deterioration or are clinically diagnosed [25-27]. Depression instigates a number of complications for AD patients including an increase in mortality, compromise of cognitive function [28] and hindrance in daily living activities [29].
The prevalence rate of depression and AD comorbidity is estimated to be 30-50% [30]. The comorbidity between these two diseases is heterogeneous and is consequently divided into more descriptive subtypes [30]. This categorization takes into consideration the fact that depression can be a risk factor for the development of AD [31] or it can be secondary to the neurodegenerative process [32]. Several pathological events have been discovered that provide a mechanistic link between these two diseases. Comorbidity may be due to depletion of the central superior raphe nucleus [33] or locus coeruleus neurons [34]. Additionally, high levels of glucocorticoids are secreted during depressive episodes that may later have dramatic effects on the hippocampus and lead to dementia symptoms [35].
There are also factors identified that increase the risk of depression development in AD patients. These factors include AD onset at a young age, a family history of mood disorders or depressive symptoms, and female sex [36]. Strong evidence suggests that depressive episodes can be a predictive measure for cognition loss among elderly people who suffer from moderate cognitive impairment [28].
The comorbidity between these diseases poses an impact not only on the patient but also on the caregiver who may suffer higher levels of stress due to the disturbances in the behavior of patients as a result of depression [37]. Caregiver depression is related to patient depression, a consequence that leads to hindrance in the delivery of adequate patient care [38]. It is possible to alleviate the depressive symptoms in both the patient and caregiver groups using certain behavioral interventions that target the patient and involve caregiver participation [39].
A clinical study on the homebound elderly reported that elderly who are non-ApoE4 allele carriers with depression symptoms exhibit lower levels of Aβ 42 and consequently higher plasma ratio of Aβ40/Aβ42 in comparison to non-ApoE4 carriers without depressive symptoms. Because a high Aβ40/Aβ42 ratio is considered to be a risk factor for AD, depression is thought to be a risk factor in the absence of ApoE4 [40]. Another study of geriatric depression reported preliminary findings of high levels of Aβ42 and a high ratio of Aβ42/Aβ40 in patients with late onset depression [41,42]. The exact relationship between declines in cognitive function and plasma levels of amyloid peptide remains to be determined in patients who suffer from AD and depression comorbidity.
2.1 Diagnosis challenges
Diagnosis of depression itself is challenging due to the absence of objective diagnostic tests. There is a shortage in the available knowledge of the neuronal circuitry that is involved in depression, and it is unclear where a biopsy should be taken from depressed patients. The heterogeneity of depression adds to the complexity of the diagnosis as well since multiple brain regions are likely to be involved [1].
Diagnosing depression associated with neurological disorders poses further challenges. These diseases frequently have overlapping symptoms and exhibit a similar etiology. Aphasia is an example of an overlapping symptom, which interferes with the patient and physician's ability to communicate regarding the emotional state [43]. Additionally, a reduction in the levels of neurotransmitters such as serotonin and norepinephrine is a similar etiology between AD and depression [44-47]. There is an urgent need for standardized protocols for the diagnosis of depression associated with AD [30]. This need is reflected by an ongoing effort by the National Institute of Mental Health (NIMH) to develop a standard protocol for the diagnosis of depression in AD [48].
2.2 Treatment challenges
One of the major treatment challenges is the lack of a clear treatment guide in these patients. The research methodology and the diagnostic criteria heterogeneity confound the clinical results. Another challenge is the strong placebo effect recorded for antidepressant treatment as seen in clinical trials with the TCAs clomipramine [4] and imipramine [49]. The major treatment goal for depression comorbid with other neurological disorders is to relieve the depressive symptoms and improve the coping resources for the neurologic disorder. Research that addresses the depression comorbidity with AD will lead to better treatment outcomes and may also lead to a better understanding of the neuroanatomy of depression [43].
3. Preclinical studies on mechanisms of the antidepressants in relation to Alzheimer's disease
3.1 Antidepressants stimulate neurogenesis
Recently several groups have demonstrated that neurogenesis exists in the adult brain mainly in two regions. These regions are the subventricular zone (SVZ) and subgranular zone (SGZ) of the dentate gyrus in the hippocampus, an area of the brain that is known to have an important role in learning and memory [50-53]. Reduction in neurogenesis in the SGZ is related to impairment of cognition associated with the aging process and AD, and it may greatly affect the progression of AD [50,54]. Reduction in neurogenesis is implicated in the early symptoms of AD such as impairments in acquiring information and eventually storing it [55]. This is particularly evident in some AD animal models such as mice with the APP and presenilin mutations, which have impairment in dentate gyrus neurogenesis [56-61]. This impairment has led to the introduction of endogenous neuronal precursors as a therapeutic strategy for AD [61-66]. The triple transgenic (3×Tg-AD) AD mouse model that carries mutations in the amyloid precursor protein (APPswe), taup301L and presenilin 1PS1M146V exhibits amyloid peptide and tau pathology resembling the human AD brain [67,68]. The triple transgenic (3×Tg-AD) mouse model exhibits an age dependent reduction in adult neurogenesis. At 9 months of age, male 3×Tg-AD mice have approximately a 73% reduction in the generation of new neurons; after 12 months neurogenesis is completely diminished. The reduction in neurogenesis has been correlated to the existence of amyloid peptide plaques and elevation in the number of hippocampal neurons containing amyloid peptide [69]. This study highlights the importance of early intervention to rescue neurogenesis in AD patients, which may then delay the progression of cognitive impairment. There are new strategies to replenish neuronal loss in AD by stimulating endogenous neurogenesis and transplanting neuronal progenitors (NP) [70].
Depression and stress may also decrease neurogenesis and chronic treatment with antidepressants can antagonize this effect and increase neurogenesis in the hippocampus [64,71]. Interestingly, the effects of antidepressants on neurogenesis are evident across different classes including the SNRIs, SSRIs, MAOIs and atypical antidepressants. This neurogenic effect requires chronic administration between 14-21 days, and includes an increase in the proliferation rate and new neuron survival [72]. The underlying mechanisms that mediate the neurogenic effects of antidepressants have not been identified, but there is strong evidence that neurotrophic factors such as fibroblast growth factor-2, insulin-like growth factor-1 (IGF-1) and brain derived neurotrophic factor (BDNF) are important for this effect [4]. It has been reported that the increase in the new neuron survival rate but not the proliferation rate is dependent on BDNF [73]. Antidepressant activation of the CREB pathway has also been implicated as an important component underlying the neurogenic effect [74]. Fluoxetine (SSRI) treatment for as short as 5 days can increase synaptic density in the hippocampus as determined by electron microscope [75]. In contrast, amitriptyline (TCA) treatment does not increase the number of synapses but reduces declines in synaptic density as a result of olfactory bulbectomy, a well established animal model for depression [76]. Chronic tiapentine (atypical antidepressant) treatment prevents reduction of dendrite length as a result of chronic stress [77]. Behavioral studies imply that the neurogenic effect of antidepressants is required to mediate antidepressant action. In a study by Santarelli et al., cell proliferation was inhibited by irradiation and subsequently blocked antidepressant action in chronic unpredictable stress and novelty suppressed feeding [78]. Chronic unpredictable stress and novelty suppressed feeding are depression animal models that require long term treatment with antidepressants to produce antidepressant action and are particularly relevant when the role of the neurogenesis is investigated [79]. In contrast, the tail suspension and forced swim tests require acute administration of antidepressants to produce antidepressant action. Chronic administration of antidepressants corresponds to the time frame that is required for the maturation and differentiation of new neurons to functional ones [80].
It is important to mention that acute versus chronic administration is considered a variability factor in the forced swim test [81]. The forced swim testis utilized to screen for acute antidepressant effects, although a chronic time course is required for the clinical effects [81]. A study addressing the effect of fluoxetine on the forced swim test after different dose intervals demonstrated that chronic administration can enhance the effects seen at acute or subchronic dosing [82]. This study calls attention to the weak face validity of the forced swim test [81].
Based on these animal studies where a reduction in neurogenesis was demonstrated to lead to cognitive impairment and the ability of antidepressants to stimulate adult neurogenesis, antidepressant treatment may provide AD patients with an advantage. Concurrent antidepressant treatment may increase the proliferation and survival of new neurons, particularly if the treatment is started early when depressive symptoms appear as a risk factor.
3.2 Antidepressants stimulate learning and memory
After 65 years of age, the elderly must cope with alterations in memory as a part of the normal aging process. This is evident in recognition memory changes [83] and impairment of spatial memory [84,85]. Also, hippocampal dysfunction may underlie alterations in memory during the aging process, and has been consistently observed across different species [83]. Given that age is an important risk factor for AD where up to 40% of elderly people over 65 years suffer from AD [86], drugs that stimulate learning and memory carry important benefits to AD patients. There are major changes in the hippocampus associated with the aging process such as electrophysiological silence in synapses as a result of reduction in the post synaptic density, difficulty in encoding and retaining information as evident by reduction in long term potentiation (LTP) and elevation in long term depression (LTD) and synaptic contact loss [87]. The main endophenotype of major depression is impairment in cognition [88]. This is clinically evident by difficulty in concentration and attention due to abnormalities and neuropathological changes in dorsolateral prefrontal cortex that is critical to these capacities [89-91]. Interestingly, preclinical studies in animals report that chronic treatment with antidepressants increase LTP and field potential baseline in dentate gyrus in a similar way to chronic electromagnetic stimulation [92,93]. It is speculated that the increase in newborn granule cell number in the dentate gyrus underlies the potential neuroplastic effect [94,95]. On the other hand, earlier reports showed that tricyclic antidepressants reduce LTP in CA1 pyramidal cells [96,97]. The reduction of LTP can be attributed to anticholenergic effects of the TCA that counteract their effects on neuroplasticity [80]. As a proof of concept, chronic treatment with atypical antidepressant (tiapentine) or SSRIs that have less anticholinergic properties in comparison to TCAs increase LTP and prevent stress induced reduction in LTP [98,99]. Another report confirms the beneficial effects of chronic fluoxetine and tiapentine treatment in preventing stress induced reduction of LTP in hippocampus-prefrontal cortex circuitry [100]. Based on these studies, it is evident that chronic administration of SSRIs increases cellular plasticity in dentate gyrus and CA1 pyramidal cells and prevents the harmful effects of stress in hippocampal neurons. Additionally, the anticholinergic properties of TCAs may counteract their neuroplastic effects.
There are conflicting reports on how SSRI treatment affects performance in the Morris water maze, a typical model for spatial learning and memory. Reports demonstrated an improvement in Morris water maze performance after chronic treatment with venlafaxine or fluoxetine [101-103]. Another study reports that fluoxetine does not affect performance in the Morris water maze [93]. Chronic treatment with the atypical antidepressant tiapentine does not affect performance in the Morris water maze [102] but improves performance in the radial maze discrimination task [104]. Chronic treatment with the TCA amitriptyline blocked age induced deterioration of learning and memory [105]. In contrast to SSRIs and amytriptyline, imipramine does not affect performance in the Morris water maze [101] and even worsens spatial working memory in the radial arm maze test [106]. The fact that TCAs impair cognitive function has also been reported in some clinical trials [49,107]. These preclinical studies raise awareness about selection of the proper antidepressant for AD patients. Based on the reports that have shown some antidepressants can cause memory impairment, close attention should be paid to antidepressants prescribed to AD patient [108].
3.3 Antidepressants and N-methyl-D-aspartate (NMDA) receptors
Mounting evidence supports the hypothesis that inadequate stimulation of NMDA receptors is a pathophysiological component of both depression and AD. The NMDA receptor represents an interesting treatment target due to the comorbidity between these two diseases [109-112]. Under normal physiological conditions, the synaptic activity of NMDA receptor modulates APP processing towards a direction that favors non-amyloidogenic α-secretase processing of amyloid precursor protein [113]. APP processing by α-secretase is reduced as a result of chronic NMDA receptor stimulation and leads to an increase in amyloid peptide production in the cortical neurons that resembles the pathophysiological conditions of AD [114]. Stress induced hippocampal neuronal atrophy and reduction in neurogenesis can be blocked by NMDA receptor antagonist treatment [115].
A number of studies report that chronic antidepressant treatment can modulate the expression of specific NMDA receptors subunits and ultimately NMDA receptor function [116-120]. NMDA receptor function is reduced after treatment with antidepressants [121-123]. Tricyclic antidepressants inhibit the NMDA receptor directly [124,125]. Milnacipran is a serotonin and norepinephrine reuptake inhibitor that antagonizes the NMDA receptor noncompetitively [126]. The SSRI fluoxetine inhibits the NMDA receptor directly [127].
Additionally, NMDA receptor antagonists such as memantine, 2-amino-7-phosphoheptanoic acid (AP-7), eliprodil, 1-aminocyclopropancarboxylic acid (ACPC), MK-801 and fenprodil have antidepressant-like effects [128-130]. Memantine and MK-801 are noncompetitive NMDA receptor antagonists, AP-7 is a competitive NMDA receptor antagonist, ACPC is partial agonist on the glycine site, and eliprodil and fenprodil work on the polyamine binding site of the NMDA receptor [128-130]. Interestingly, a case report for the antiviral agent amantadine which has NMDA receptor antagonistic activity provides clinical evidence for its efficacy in depression [131]. Ketamine is another NMDA receptor antagonist, which has antidepressant effects after a single dose administration in depressed patients [132]. Ketamine also exhibits antidepressant and anxiolytic effects in animal models of depression [133].
Antidepressant treatment can serve a dual role in patients who suffer from AD and depression. It can treat the depressive symptoms in addition to targeting NMDA receptor activity in AD patients. Of note is the fact that inhibition of NMDA activity is evident in SSRI agents such as fluoxetine, which have a better tolerability profile in comparison to TCAs.
3.4 Antidepressants, serotonin, BDNF and pCREB
Serotonin signaling pathways are implicated in the pathology of AD since the death of the neurons and the dysfunction of the synapse can be a result of reduction in the activation of serotonin coupled signaling pathways [134]. Amyloid peptide deposition, a major pathological feature of AD, interferes with the phosphorylation of cAMP-response element-binding protein (CREB) [135]. Intracellular amyloid peptide load affects this signaling pathway differently. Moderate elevation in levels of intracellular amyloid peptide load leads to over expression in CREB responsive genes such as BDNF, presenilin 1 and presenilin 2. High levels of intracellular amyloid peptide lead to persistent CREB hyperphosphorylation and block its translocation to the nucleus resulting in inhibition of cyclic AMP-response (CRE) directed gene expression [136]. The authors speculate that inhibition of CREB translocation causes early synaptic dysfunction prior to the extracellular accumulation of amyloid peptide [136].
Chronic treatment with antidepressants increases the synaptic concentrations of noradrenaline and/or serotonin. These increased levels then lead to activation of G-protein coupled receptors, stimulation of adenyl cyclase, and eventually upregulation of the cAMP cascade. This cascade results in increases of CREB and BDNF expression and increases in the levels of cAMP-dependent protein kinase (PKA) [4,115,137-139]. Serotonin enhancement of synaptic plasticity is mediated by activation of CREB and increases in BDNF levels [140].
Given the high prevalence rate of comorbidity between depression and AD, it is important to screen AD animal models for depressive symptoms. R406 W transgenic mice are an AD animal model with tau hyperphosphorylation, deposition of neurofibrillary tangles in forebrain and impairment in associative memory [141,142]. Interestingly, R406 W transgenic mice have been evaluated in the forced swim test and have been demonstrated to exhibit a longer immobility time than non-transgenic mice [141]. Fluvoxamine not desipramine treatment of R406 W transgenic mice restores immobility time in the forced swim test to wild type levels. This study implies that R406 W transgenic mice demonstrate depressive behaviors and provide evidence for the involvement of serotonin in these depressive symptoms. Indeed, these mice exhibited low levels of 5-hydroxyindoleaceticacid (5-HIAA) and serotonin, and fluoxamine treatment restores serotonin levels comparably to control group. This study raises speculations that the R406W mutation affects serotonergic neurons [141]. Postmortem AD brains show reductions in the levels of serotonin and its metabolites [44,143], which highlight the advantage of prescribing SSRIs to AD patients versus other antidepressants.
There is an association between reduced levels of neurotrophic factors and depressive symptoms, and mounting evidence supports the hypothesis that part of antidepressant action involves increasing levels of neurotrophic factors to compensate for their reduced levels in depressed patients [144,145]. There is a family of structurally related trophic factors that includes BDNF, neurotrophin-3, neurotrophin-4 and nerve growth factor (NGF). Generally, the production of BDNF mRNA results from the stimulation of 5-HT receptor and β-adrenoceptor coupled signaling pathways. The growth and function of serotonergic neurons are greatly increased by BDNF [145,146]. BDNF also reduces mRNA and protein levels of NMDA receptor subunits and reduces NMDA stimulated Ca2+ increase [147]. BDNF and NGF specifically have important effects on hippocampal neurons that are involved in the pathogenesis and clinical features of AD [66,148,149]. It has been reported that the amyloidogenic pathway is activated as a result of NGF deprivation [150] and that BDNF or NGF signaling interruption leads to cell death and accumulation of Aβ aggregates intracellularly and extracellularly [151]. A recent study demonstrated that BDNF gene delivery significantly restored learning and memory, reversed synaptic loss, partially normalized inappropriate gene expression and improved cell signaling in transgenic mice even after disease onset [152]. Neurotrophic factors have now entered clinical trials as both a preventative measure and as a treatment to reduce neuronal loss and stimulate neurogenesis [153,154]. These studies demonstrate that BDNF is likely a key player in mediating the beneficial effects of antidepressants in AD patients.
3.5 Antidepressants and amyloid peptide
The effect of antidepressants on amyloid peptide has particular importance. The high prevalence rate of comorbidity between depression and AD warrants the investigation of the possible dual role for antidepressants in modulating these two diseases. Additionally, antidepressants activate similar signaling pathways as the ones activated by dietary restriction and environmental enrichment, both of which have been demonstrated to reduce amyloid peptide burden in transgenic mice [134,155,156].
Chronic treatment with paroxetine for 5 months in 3×TgAD mice significantly reduces the levels of amyloid peptide 1-40 in the hippocampus and cerebral cortex [157]. Tau immunoreactivity is also significantly reduced in the hippocampus and amygdala in paroxetine treated mice [157]. Although the underlying mechanism for the action of paroxetine in reducing amyloid peptide burden and tau pathology is undetermined, there is speculation that the effect is due to enhancement of serotonin signaling and elevation of BDNF expression levels [134,158]. To investigate whether the effect of antidepressants on amyloid peptide is limited to the SSRI class, we examined the effect of increasing concentrations of antidepressants on Aβ expressing N2a neuroblastoma cells by Western blotting. The tested antidepressants include the SSRIs fluoxetine and paroxetine, the selective noradrenaline reuptake inhibitor maprotiline and the nonselective monoamine reuptake inhibitor imipramine. Interestingly, fluoxetine and paroxetine at 10 μM significantly decrease Aβ oligomers, but do not affect the levels of extracellular amyloid peptide (unpublished data). Based on these results, fluoxetine and paroxetine are likely to be beneficial to AD patients due to their role in modulating Aβ metabolism. This effect may also explain some of the beneficial effects of SSRIs in AD patients. In a screening assay for small molecules that can interact with Aβ fibrils, fluoxetine does not show potential to interact with Aβ fibrils directly [159].
Targeting amyloid precursor protein (APP) gene expression is a major anti-amyloid strategy in the treatment of AD. Desferrioxamine and phenserine target the 5' untranslated part of APP and ultimately inhibit APP translation [160]. Interestingly, paroxetine was one of the APP 5'UTR lead directed compounds based on a screening study from a 1,200 compound library [161]. Paroxetine treatment for 48 hours in B3 lens epithelial cells reduces the levels of Aβ secreted into the medium [161,162]. B3 lens epithelial cells were specifically used in this study due to high baseline levels of amyloid peptide [163]. TgCRND8 mice treated with paroxetine for three months had reduced levels of Aβ (1-40) and APP levels in brain homogenate. TgCRND8 mice were selected for this study because the APP gene open reading frame is over expressed in these mice, providing a proof of concept for the APP 5'UTR targeting strategy [163,164].
Another in vitro study addressed the effect of TCAs and SSRIs on APP processing in rat primary basal forebrain cultures [165]. Imipramine at 100 μM significantly reduced intracellular levels of APP after two hours of treatment. Imipramine and citalopram significantly increased the levels of secreted APP in the medium of the treated primary cultures [165]. Interestingly, serotonin and muscarinic agonists also increase APP secretion [166-168]. It is anticipated that the increase in APP secretion is accompanied by a decrease in intracellular APP levels. Presumably, the secreted APP will not be available for processing by β and γ secretases [165]. Whether the effect of antidepressants on APP processing and amyloid peptide are a class effect or whether these effects relate to pharmacological mechanisms individual antidepressant agents has not been determined.
3.6. Natural antidepressants and AD (Ginkgo, St. John's wort, flavonoids, and curcumin)
St. John's wort (Hypericum perforatum) extract (HPE) is well known for its antidepressant effects [169-171]. Hyperforin is considered to be the major active constituent that contributes to the neuroprotective effect of HPE [172]. The antidepressant action of hyperforin is primarily attributed to monoamine reuptake inhibition [173]. Other components in HPE have also been identified to have an important contribution to the antidepressant effect of HPE such as flavonoids [174], pseudohypericin and hypericin [175].
HPE extract has been demonstrated to exhibit neuroprotective properties by preventing the toxic effect of amyloid peptide (25-35) in the hippocampal neurons of the rat. HPE reduced lipid peroxidation, cell death and dendritic lesions [176]. In another study, pretreatment of a microglial cell line with HPE showed a dose dependent reduction in amyloid peptide induced cell death [177]. To study the effect of individual components of HPE on cell viability, individual constituents of the HPE mixture were incubated with the microglial cell line. Some flavonols such as (-)-epicatechin and (+)-catechin increased the viability of the cells but other flavonols and glycosides such as quercitrin, quercetin, hyperosid and rutin had no effect [177]. The antioxidant properties of the flavonoids resulted in reduced reactive oxygen species (ROS) production induced by amyloid peptide in the microglia [177]. Hyperforin in particular has been demonstrated to enhance memory in rodents [178]. Another study reported that hyperforin improved spatial memory by reduction of reactive astrocytes, activation of microglia and promotion of amyloid peptide deposit fragmentation [179]. Hyperforin also protects cells against the neurotoxic effect of amyloid peptide oligomers and fibrils and reduces the production of ROS [179]. An in vitro study demonstrated that hyperforin promotes the dissociation of amyloid peptide deposits dose and time dependently and converts the fibrils to protofibrils [179]. These studies provide evidence for the role of hyperforin in improving the memory by reduction of neurotoxic amyloid peptides.
Ginkgo biloba leaves are a common herbal remedy in traditional Chinese medicine. Extract of Ginkgo biloba leaves (EGB) demonstrated antidepressant action in forced swim test and tail suspension test [180]. The roles of individual constituents in EGB that relate to the antidepressant activity have not been determined. It is likely that terpenoids which represent 6.5% of EGB [181] play a role in antidepressant action based on the reported action in the central nervous system [63,182]. Another study demonstrated antidepressant activity of Ginkgo biloba lipophylic extract in learned helplessness and behavioral despair animal models[183]. It 6-alkylsalicylates have also been implicated as active constituents related to the antidepressant activity of the Ginkgo biloba lipophylic extract [183].
Ginkgo biloba leaves exhibits a number of beneficial effects for AD patients such as cognition and mood improvements and resolution of mild to moderate dementia symptoms [184-189]. Although a recent Ginkgo trial failed to demonstrate prevention of memory impairment, the authors discuss the possibility that the extract was given too late to see a preventive effect [190]. In preclinical studies, Ginkgo biloba extract (EGB 761) blocked the production of amyloid beta peptide and amyloid precursor protein in aged rodents [191]. EGB 761 also inhibits the aggregation of amyloid peptide and apoptosis by blocking the activation of caspase-3 in a neuroblastoma cell line [192]. EGB 761 has also been demonstrated to inhibit amyloid peptide induced hippocampal cell death [193] and increase the levels of phosphorylation of CREB that are reduced as a result of conditioned medium treatment to wild type neuroblastoma cells [194]. A study from our laboratory also demonstrated the neurogenic potential of EGB761 in an AD mouse model where it induced an increase in cell proliferation and neuronal precursor cells numbers in hippocampus [195].
Flavonoids are class of compounds that are derived from different plants such as tea, Ginkgo biloba and citrus [196]. Accumulating evidence supports the antidepressant activity of flavonoids in depression animal models [197-199]. Given the fact that depression and AD share common pathophysiological abnormalities of CREB- BDNF signaling pathway, citrus and green tea flavonoids may increase the phosphorylation of CREB and improve the memory [200,201]. Recently we have reported that Ginkgo flavonols activate signaling pathways, which are heavily implicated in depression including the BDNF/pCREB pathway. Additionally, Ginkgo flavonols also reduced amyloid peptide burden in double transgenic (TgAPPswe/PSe9) mouse hippocampal neurons [202].
4. Clinical studies of antidepressants on cognitive function in AD patients
4.1 Antidepressant clinical studies
In general SSRIs have a better tolerability and safety profile when compared to TCAs. Citalopram is an SSRI that has been shown to significantly improve the score of depressed patients in the Hamilton Rating Score (HAM-D), the Clinical Global Impression Scale, and the Montgomery Asberg Depression Scale (MADRS) [203]. Citalopram also significantly improves emotional and cognitive function in a subgroup of patients who suffer from dementia based on the Gottfries-Brane-Steen Dementia Rating Scale [203].
The SSRI sertraline was tested in an 8-week trial in 31 female patients diagnosed with late stage AD to determine its efficacy. Using objective rating scales, including the Cornell Scale for Depression in Dementia and others, sertraline and placebo improve ratings similarly but sertraline treatment showed a better improvement in "knit brow" facial behavior [204]. "Knit brow" is facial behavior where the brows are somewhat lowered and pulled together. It is a robust index of dysphoria in advanced stage dementia [204]. Another clinical study with sertraline treatment that lasted 12 weeks involving 22 patients who suffer from major depression and AD showed that sertaline reduced depressive symptoms significantly in comparison to placebo. Interestingly, sertraline treated patients do not show any significant change in daily living activities according to the Psychogeriatric Dependency Rating Scale in comparison to the placebo group where there was a significant decline in daily activities at weeks 9 and 12 [205].
A meta analysis study for the safety and efficacy of antidepressants in treatment of depression in AD found that antidepressants are efficacious in treatment of depression in AD patients and have a similar dropout rate as placebo [206].
4.2 Combination studies
There are number of reasons for the initiation of combinatorial studies that include antidepressants and other FDA approved drugs for the treatment of AD. First, impairment in the cholinergic system does not fully account for age-associated cognitive declines [65,207]. AchEIs also improve behavioral and non-cognitive aspects [208,209]. Secondly, there is evidence of oxidative stress, neuroinflammation in the postmortem brain of AD patients. It has been suggested that other neurotransmitter systems are involved such as the glutamatergic and serotonergic systems [210]. Abnormalities in monoaminergic systems have been reported in AD patients [211,212] and AD patients have lower levels of the neurotransmitter serotonin (5-HT) and its metabolites [213,214]. Accumulating evidence emphasizes the positive role that serotonin plays in cognitive function [215]. Improvements in both immediate and delayed verbal memory after treatment with SSRIs indicate an enhancement in hippocampal function [62,216]. Also, the efficacy of memantine for treatment of moderate-to-server dementia of AD patient supports the notion that cholinergic impairment do not fully account for age-associated cognitive decline. These factors provide a rational argument for the potential beneficial effects for combinatorial studies between antidepressants and other FDA approved drugs for the treatment of AD.
To test if the addition of an SSRI (fluoxetine) to an acetylcholinesterase inhibitor (rivastigmine) treatment regimen could benefit AD patients, a double blind placebo controlled study was conducted for 12 weeks in patients with mild to moderate dementia between the ages of 55-85 years. This study included 122 patients divided into three treatment groups: placebo, rivastigmine only and rivastigmine plus fluoxetine. The results of this report showed that there were improvements in cognition and memory in rivastigmine treated and rivastigmine plus fluoxetine treated groups but without a significant difference between these two groups. Interestingly, the rivastigmine plus fluoxetine treatment group had better performance in daily life activities and overall function which highlights the benefits that may be obtained by adding a serotonin regimen to FDA approved drugs for AD patients [216]. Another study also reported the beneficial outcome of combining sertaline (SSRI) with donepzil treatment especially for AD patients with moderate to severe dementia [217].
The interplay between the cholinergic and the serotonergic systems has an important relevance to AD as suggested by a number of studies. There is neurochemical and neuroanatomical evidence for the role of cholinergic system in modulating the serotonergic one and the potential synergism between them in improving memory function [218-223]. A recent study reports that the acute administration of citalopram reduced glucose metabolism in the brain while the concomitant administration of acetylcholinesterase inhibitor and nicotinic receptor modulator (galantamine) and citalopram have increased glucose metabolism. These data suggest a beneficial interplay between the cholinergic and serotonergic systems for AD patients [222].
These clinical studies in addition to preclinical evidence support the positive role of chronic administration of SSRIs in hindering the disease progression and improving AD patient clinical outcomes [157,224].
Conclusion
In this review, we highlighted the high prevalence rate of comorbidity between AD and depression and summarize different potential targets for antidepressant drugs that may relate to the AD pathology (see Table 1). Neurodegeneration associated with AD involves different neurotransmitter systems such as the glutamenergic, serotonergic, peptidergic and cholinergic systems [225]. There are myriad of reasons to consider antidepressants as an adjunct treatment to AD patients, several of which were discussed in this review. Additionally, we summarized some of the clinical evidence that demonstrated the beneficial effects of SSRIs in AD patients either alone or in combination with other FDA approved acetylcholine esterase inhibitors. The preclinical studies present potential targets that may underlie antidepressants mechanisms of action in AD pathology including neurogenic effects, stimulation of learning and memory, antagonism of NMDA receptors, reduction of amyloid peptide burden and upregulation of neurotrophic factors.
Table 1.
Summary of potential targets of antidepressant drugs in relate to AD pathology
| Antidepressants | Neurogenesis | Aβ | Learning & memory | NMDA Receptors |
|---|---|---|---|---|
| Fluoxetine (SSRI) |
Increase synaptic density in hippocampus[75] | Does not interact with Aβ fibrils [159]. | Protects hippocampal LTP [100]. Performance improvement in Morris water maze after chronic treatment [102]. | Inhibit NMDA receptor directly [127]. |
| Amitriptyline (NSRI) |
Does not increase synapse number but reduce decline in synaptic density [76]. | Blocks age --induced deterioration of learning and memory [105]. | ||
| Tiapentine (atypical) |
Prevents the reduction of dendrites length as a result of chronic stress [77]. | Protects hippocampal LTP [99,100]. No effects on animal performance in Morris water maze[102] but improve animal performance is radial maze discrimination task [104]. | ||
| TCA | Reduce LTP in CA1 pyramidal cells [96,97]. | Inhibit NMDA receptor directly [124,125]. | ||
| Venlafaxine (SNRI) |
Performance improvement in Morris water maze after chronic treatment [101,103]. | |||
| Imipramine (NSRI) |
Increase secreted APP, reduces intracellular APP in culture [165]. | No effect on animal performance in Morris water maze [101] and even worsen spatial working memory in radial arm maze test [106]. | Changes in binding to NMDAR [118,120]and expression of NMDAR in brain [116] | |
| Citalopram (SSRI) |
Increase the levels of secreted APP in the medium of the treated neurons [165]. | Adaptation of NMDAR complex [117]. Changes in expression of NMDAR [116]. | ||
| Clomipramine (NSRI) | Chronic administration changes the regulation of NMDA receptor control on the release of dopamine [119]. | |||
| Milnacipran (NSRI) |
Antagonize NMDA receptor uncompetitively [126]. | |||
| Paroxetine (SSRI) |
Reduces levels of Aβ and tau in Tg mice and cells [157,161-164] | |||
List of Abbreviations
NSRI: non selective monoamine reuptake inhibitor; SNRI: selective norepinerphrine reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor; AD: Alzheimer's disease; TCA: Tricyclic antidepressants; MAO: Monoamine oxidase; MAOI: M MAOI: Monoamine oxidase inhibitor; HAM-D: Hamilton Rating Score; MADS: Montgomery Asberg Depression Scale; NP: Neuronal progenitors; IGF-1: Insulin-like growth factor-1; BDNF: Brain derived neurotrophic factor; LTD: Long term depression; LTP: Long term potentiation; NMDA: N-methyl-D-aspartate; AP-7:2-amino-7-phosphoheptanoic acid; ACPC: 1-aminocyclopropancarboxylic acid; CREB: cAMP-response element-binding protein; 5-HIAA: 5-hydroxyindoleaceticacid; NGF: Nerve growth factor; APP: Amyloid precursor protein (APP); HPE: Hypericum perforatum extract; EGB: Extract of Ginkgo biloba leaves
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MA searched literature, wrote the draft and revised the manuscript; LD added additional information, edited and revised the manuscript; YL provided editing and financial support. All authors read and approved the final manuscript.
Contributor Information
Marwa Aboukhatwa, Email: mabou003@umaryland.edu.
Laura Dosanjh, Email: lauraedosanjh@gmail.com.
Yuan Luo, Email: yluo@rx.umaryland.edu.
Acknowledgements
This study was supported by NIH grant RO1AT001928-03A1 (YL) from the National Center for Complementary and Alternative Medicine.
References
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nature reviews. Neuroscience. 2006;7(2):137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- Thase ME, Nierenberg AA, Keller MB, Panagides J. Group RPS. Efficacy of mirtazapine for prevention of depressive relapse: a placebo-controlled double-blind trial of recently remitted high-risk patients. The Journal of clinical psychiatry. 2001;62(10):782–788. doi: 10.4088/jcp.v62n1006. [DOI] [PubMed] [Google Scholar]
- Thompson C. Onset of action of antidepressants: results of different analyses. Human psychopharmacology. 2002;17(Suppl 1):S27–32. doi: 10.1002/hup.386. [DOI] [PubMed] [Google Scholar]
- Pacher P, Kecskemeti V. Trends in the development of new antidepressants. Is there a light at the end of the tunnel? Current medicinal chemistry. 2004;11(7):925–943. doi: 10.2174/0929867043455594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassman AH. Cardiovascular effects of tricyclic antidepressants. Annual Review of Medicine. 1984;35:503–511. doi: 10.1146/annurev.me.35.020184.002443. [DOI] [PubMed] [Google Scholar]
- Goodman LS, Hardman JG, Limbird LE, Gilman AG. Goodman & Gilman's the pharmacological basis of therapeutics. 10. New York: McGraw-Hill; 2001. p. 2148. [Google Scholar]
- Pacher P, Ungvari Z, Nanasi PP, Furst S, Kecskemeti V. Speculations on difference between tricyclic and selective serotonin reuptake inhibitor antidepressants on their cardiac effects. Is there any? Current medicinal chemistry. 1999;6(6):469–480. [PubMed] [Google Scholar]
- Muth EA, Haskins JT, Moyer JA, Husbands GE, Nielsen ST, Sigg EB. Antidepressant biochemical profile of the novel bicyclic compound Wy-45,030, an ethyl cyclohexanol derivative. Biochemical pharmacology. 1986;35(24):4493–4497. doi: 10.1016/0006-2952(86)90769-0. [DOI] [PubMed] [Google Scholar]
- Briley M, Prost JF, Moret C. Preclinical pharmacology of milnacipran. International clinical psychopharmacology. 1996;11(Suppl 4):9–14. doi: 10.1097/00004850-199609004-00002. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Lu Y, Goldstein DJ, Hayes JR, Demitrack MA. Duloxetine, 60 mg once daily, for major depressive disorder: a randomized double-blind placebo-controlled trial. The Journal of clinical psychiatry. 2002;63(4):308–315. doi: 10.4088/jcp.v63n0407. [DOI] [PubMed] [Google Scholar]
- Pitsikas N. Duloxetine Eli Lilly & Co. Current opinion in investigational drugs (London, England: 2000) 2000;1(1):116–121. [PubMed] [Google Scholar]
- Cocchiara G, Battaglia R, Pevarello P, Strolin Benedetti M. Comparison of the disposition and of the metabolic pattern of Reboxetine, a new antidepressant, in the rat, dog, monkey and man. European journal of drug metabolism and pharmacokinetics. 1991;16(3):231–239. doi: 10.1007/BF03189965. [DOI] [PubMed] [Google Scholar]
- Dostert P, Benedetti MS, Poggesi I. Review of the pharmacokinetics and metabolism of reboxetine, a selective noradrenaline reuptake inhibitor. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 1997;7(Suppl 1):S23–35. doi: 10.1016/S0924-977X(97)00417-3. discussion S71-3. [DOI] [PubMed] [Google Scholar]
- Spinks D, Spinks G. Serotonin reuptake inhibition: an update on current research strategies. Current medicinal chemistry. 2002;9(8):799–810. doi: 10.2174/0929867024606795. [DOI] [PubMed] [Google Scholar]
- Pacher P, Ungvari Z, Kecskemeti V, Furst S. Review of cardiovascular effects of fluoxetine, a selective serotonin reuptake inhibitor, compared to tricyclic antidepressants. Current medicinal chemistry. 1998;5(5):381–390. [PubMed] [Google Scholar]
- Rodriguez de la Torre B, Dreher J, Malevany I, Bagli M, Kolbinger M, Omran H, Luderitz B, Rao ML. Serum levels and cardiovascular effects of tricyclic antidepressants and selective serotonin reuptake inhibitors in depressed patients. Therapeutic drug monitoring. 2001;23(4):435–440. doi: 10.1097/00007691-200108000-00019. [DOI] [PubMed] [Google Scholar]
- Rosen RC, Lane RM, Menza M. Effects of SSRIs on sexual function: a critical review. Journal of clinical psychopharmacology. 1999;19(1):67–85. doi: 10.1097/00004714-199902000-00013. [DOI] [PubMed] [Google Scholar]
- McGrath PJ, Stewart JW, Nunes EV, Ocepek-Welikson K, Rabkin JG, Quitkin FM, Klein DF. A double-blind crossover trial of imipramine and phenelzine for outpatients with treatment-refractory depression. The American Journal of Psychiatry. 1993;150(1):118–123. doi: 10.1176/ajp.150.1.118. [DOI] [PubMed] [Google Scholar]
- Stewart JW, Tricamo E, McGrath PJ, Quitkin FM. Prophylactic efficacy of phenelzine and imipramine in chronic atypical depression: likelihood of recurrence on discontinuation after 6 months' remission. The American Journal of Psychiatry. 1997;154(1):31–36. doi: 10.1176/ajp.154.1.31. [DOI] [PubMed] [Google Scholar]
- Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, Nemeroff CB, Bremner JD, Carney RM, Coyne JC, Delong MR, Frasure-Smith N, Glassman AH, Gold PW, Grant I, Gwyther L, Ironson G, Johnson RL, Kanner AM, Katon WJ, Kaufmann PG, Keefe FJ, Ketter T, Laughren TP, Leserman J, Lyketsos CG, McDonald WM, McEwen BS, Miller AH, Musselman D, O'Connor C, Petitto JM, Pollock BG, Robinson RG, Roose SP, Rowland J, Sheline Y, Sheps DS, Simon G, Spiegel D, Stunkard A, Sunderland T, Tibbits P Jr, Valvo WJ. Mood disorders in the medically ill: scientific review and recommendations. Biological psychiatry. 2005;58(3):175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Archives of General Psychiatry. 2006;63(5):530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareri P, De Fazio P, De Sarro G. Neuropharmacology of depression in aging and age-related diseases. Ageing research reviews. 2002;1(1):113–134. doi: 10.1016/S0047-6374(01)00370-0. [DOI] [PubMed] [Google Scholar]
- Steffens DC, Plassman BL, Helms MJ, Welsh-Bohmer KA, Saunders AM, Breitner JC. A twin study of late-onset depression and apolipoprotein E epsilon 4 as risk factors for Alzheimer's disease. Biological psychiatry. 1997;41(8):851–856. doi: 10.1016/S0006-3223(96)00247-8. [DOI] [PubMed] [Google Scholar]
- Thorpe L, Groulx B. Research CCfCC. Depressive syndromes in dementia. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 2001;28(Suppl 1):S83–95. doi: 10.1017/s0317167100001256. [DOI] [PubMed] [Google Scholar]
- Danion JM. Antidepressive agents and memory. L'Encephale. 1993;19(Spec No 2):417–422. [PubMed] [Google Scholar]
- Geerlings MI, Schoevers RA, Beekman AT, Jonker C, Deeg DJ, Schmand B, Ader HJ, Bouter LM, Van Tilburg W. Depression and risk of cognitive decline and Alzheimer's disease. Results of two prospective community-based studies in The Netherlands. The British journal of psychiatry: the journal of mental science. 2000;176:568–575. doi: 10.1192/bjp.176.6.568. [DOI] [PubMed] [Google Scholar]
- Visser PJ, Verhey FR, Ponds RW, Kester A, Jolles J. Distinction between preclinical Alzheimer's disease and depression. Journal of the American Geriatrics Society. 2000;48(5):479–484. doi: 10.1111/j.1532-5415.2000.tb04992.x. [DOI] [PubMed] [Google Scholar]
- Bassuk SS, Berkman LF, Wypij D. Depressive symptomatology and incident cognitive decline in an elderly community sample. Archives of General Psychiatry. 1998;55(12):1073–1081. doi: 10.1001/archpsyc.55.12.1073. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Steele C, Baker L, Galik E, Kopunek S, Steinberg M, Warren A. Major and minor depression in Alzheimer's disease: prevalence and impact. The Journal of neuropsychiatry and clinical neurosciences. 1997;9(4):556–561. doi: 10.1176/jnp.9.4.556. [DOI] [PubMed] [Google Scholar]
- Lee HB, Lyketsos CG. Depression in Alzheimer's disease: heterogeneity and related issues. Biological psychiatry. 2003;54(3):353–362. doi: 10.1016/S0006-3223(03)00543-2. [DOI] [PubMed] [Google Scholar]
- Green RC, Cupples LA, Kurz A, Auerbach S, Go R, Sadovnick D, Duara R, Kukull WA, Chui H, Edeki T, Griffith PA, Friedland RP, Bachman D, Farrer L. Depression as a risk factor for Alzheimer disease: the MIRAGE Study. Archives of Neurology. 2003;60(5):753–759. doi: 10.1001/archneur.60.5.753. [DOI] [PubMed] [Google Scholar]
- Zubenko GS, Zubenko WN, McPherson S, Spoor E, Marin DB, Farlow MR, Smith GE, Geda YE, Cummings JL, Petersen RC, Sunderland T. A collaborative study of the emergence and clinical features of the major depressive syndrome of Alzheimer's disease. The American Journal of Psychiatry. 2003;160(5):857–866. doi: 10.1176/appi.ajp.160.5.857. [DOI] [PubMed] [Google Scholar]
- Zweig RM, Ross CA, Hedreen JC, Steele C, Cardillo JE, Whitehouse PJ, Folstein MF, Price DL. The neuropathology of aminergic nuclei in Alzheimer's disease. Annals of Neurology. 1988;24(2):233–242. doi: 10.1002/ana.410240210. [DOI] [PubMed] [Google Scholar]
- Zubenko GS, Moossy J. Major depression in primary dementia. Clinical and neuropathologic correlates. Archives of Neurology. 1988;45(11):1182–1186. doi: 10.1001/archneur.1988.00520350020008. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Archives of General Psychiatry. 2000;57(10):925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, lin J. Depression in Alzheimer's disease: overview and treatment. Biological psychiatry. 2002;52(3):243–252. doi: 10.1016/S0006-3223(02)01348-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Salvador MT, Arango C, Lyketsos CG, Barba AC. The stress and psychological morbidity of the Alzheimer patient caregiver. International journal of geriatric psychiatry. 1999;14(9):701–710. doi: 10.1002/(SICI)1099-1166(199909)14:9<701::AID-GPS5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Teri L. Behavior and caregiver burden: behavioral problems in patients with Alzheimer disease and its association with caregiver distress. Alzheimer Disease and Associated Disorders. 1997;11(Suppl 4):S35–8. [PubMed] [Google Scholar]
- Logsdon RG, McCurry SM, Moore AL, Teri L. Family and Caregiver Issues in the Treatment of Patients With Alzheimer's Disease. Seminars in clinical neuropsychiatry. 1997;2(2):138–151. doi: 10.1053/SCNP00200138. [DOI] [PubMed] [Google Scholar]
- Sun X, Chiu CC, Liebson E, Crivello NA, Wang L, Claunch J, Folstein M, Rosenberg I, Mwamburi DM, Peter I, Qiu WQ. Depression and plasma amyloid beta peptides in the elderly with and without the apolipoprotein E4 allele. Alzheimer Dis Assoc Disord. 2009;23(3):238–44. doi: 10.1097/WAD.0b013e31819cb3ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu WQ, Sun X, Selkoe DJ, Mwamburi DM, Huang T, Bhadela R, Bergethon P, Scott TM, Summergrad P, Wang L, Rosenberg I, Folstein M. Depression is associated with low plasma Abeta42 independently of cardiovascular disease in the homebound elderly. Int J Geriatr Psychiatry. 2007;22(6):536–42. doi: 10.1002/gps.1710. [DOI] [PubMed] [Google Scholar]
- Pomara N, Doraiswamy PM, Willoughby LM, Roth AE, Mulsant BH, Sidtis JJ, Mehta PD, Reynolds CF III, Pollock BG. Elevation in plasma Abeta42 in geriatric depression: a pilot study. Neurochem Res. 2006;31(3):341–9. doi: 10.1007/s11064-005-9029-z. [DOI] [PubMed] [Google Scholar]
- Raskind MA. Diagnosis and treatment of depression comorbid with neurologic disorders. The American Journal of Medicine. 2008;121(11 Suppl 2):S28–37. doi: 10.1016/j.amjmed.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Gottfries CG. Disturbance of the 5-hydroxytryptamine metabolism in brains from patients with Alzheimer's dementia. Journal of neural transmission. Supplementum. 1990;30:33–43. doi: 10.1007/978-3-7091-3345-3_4. [DOI] [PubMed] [Google Scholar]
- Gsell W, Jungkunz G, Riederer P. Functional neurochemistry of Alzheimer's disease. Current pharmaceutical design. 2004;10(3):265–293. doi: 10.2174/1381612043386473. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455(7215):894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer CC, Smith G, DeKosky ST, Pollock BG, Mathis CA, Moore RY, Kupfer DJ, Reynolds CF III. Serotonin in aging, late-life depression, and Alzheimer's disease: the emerging role of functional imaging. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 1998;18(6):407–430. doi: 10.1016/S0893-133X(97)00194-2. [DOI] [PubMed] [Google Scholar]
- Olin JT, Schneider LS, Katz IR, Meyers BS, Alexopoulos GS, Breitner JC, Bruce ML, Caine ED, Cummings JL, Devanand DP, Krishnan KR, Lyketsos CG, Lyness JM, Rabins PV, Reynolds CF III, Rovner BW, Steffens DC, Tariot PN, Lebowitz BD. Provisional diagnostic criteria for depression of Alzheimer disease. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry. 2002;10(2):125–128. [PubMed] [Google Scholar]
- Reifler BV, Teri L, Raskind M, Veith R, Barnes R, White E, McLean P. Double-blind trial of imipramine in Alzheimer's disease patients with and without depression. The American Journal of Psychiatry. 1989;146(1):45–49. doi: 10.1176/ajp.146.1.45. [DOI] [PubMed] [Google Scholar]
- Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiological Reviews. 2005;85(2):523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- Levison SW, Goldman JE. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron. 1993;10(2):201–212. doi: 10.1016/0896-6273(93)90311-E. [DOI] [PubMed] [Google Scholar]
- Paterson JA, Privat A, Ling EA, Leblond CP. Investigation of glial cells in semithin sections. 3. Transformation of subependymal cells into glial cells, as shown by radioautography after 3 H-thymidine injection into the lateral ventricle of the brain of young rats. The Journal of comparative neurology. 1973;149(1):83–102. doi: 10.1002/cne.901490106. [DOI] [PubMed] [Google Scholar]
- Taupin P, Gage FH. Adult neurogenesis and neural stem cells of the central nervous system in mammals. Journal of neuroscience research. 2002;69(6):745–749. doi: 10.1002/jnr.10378. [DOI] [PubMed] [Google Scholar]
- Tatebayashi Y, Lee MH, Li L, Iqbal K, Grundke-Iqbal I. The dentate gyrus neurogenesis: a therapeutic target for Alzheimer's disease. Acta Neuropathologica. 2003;105(3):225–232. doi: 10.1007/s00401-002-0636-3. [DOI] [PubMed] [Google Scholar]
- Verret L, Jankowsky JL, Xu GM, Borchelt DR, Rampon C. Alzheimer's-type amyloidosis in transgenic mice impairs survival of newborn neurons derived from adult hippocampal neurogenesis. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27(25):6771–6780. doi: 10.1523/JNEUROSCI.5564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier NL, Soriano S, Kang DE, Masliah E, Hu G, Koo EH. Perturbed neurogenesis in the adult hippocampus associated with presenilin-1 A246E mutation. The American journal of pathology. 2005;167(1):151–159. doi: 10.1016/S0002-9440(10)62962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience. 2004;127(3):601–609. doi: 10.1016/j.neuroscience.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Donovan MH, Yazdani U, Norris RD, Games D, German DC, Eisch AJ. Decreased adult hippocampal neurogenesis in the PDAPP mouse model of Alzheimer's disease. The Journal of comparative neurology. 2006;495(1):70–83. doi: 10.1002/cne.20840. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Liu D, Nath A, Borchard AC, Mattson MP. Disruption of neurogenesis in the subventricular zone of adult mice, and in human cortical neuronal precursor cells in culture, by amyloid beta-peptide: implications for the pathogenesis of Alzheimer's disease. Neuromolecular medicine. 2002;1(2):125–135. doi: 10.1385/NMM:1:2:125. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Nath A, Chan SL, Borchard AC, Rao MS, Mattson MP. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer's disease. Journal of neurochemistry. 2002;83(6):1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- Wen PH, Shao X, Shao Z, Hof PR, Wisniewski T, Kelley K, Friedrich VL Jr, Ho L, Pasinetti GM, Shioi J, Robakis NK, Elder GA. Overexpression of wild type but not an FAD mutant presenilin-1 promotes neurogenesis in the hippocampus of adult mice. Neurobiology of disease. 2002;10(1):8–19. doi: 10.1006/nbdi.2002.0490. [DOI] [PubMed] [Google Scholar]
- Vythilingam M, Vermetten E, Anderson GM, Luckenbaugh D, Anderson ER, Snow J, Staib LH, Charney DS, Bremner JD. Hippocampal volume, memory, and cortisol status in major depressive disorder: effects of treatment. Biological psychiatry. 2004;56(2):101–112. doi: 10.1016/j.biopsych.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Wang SJ, Chen HH. Ginkgolide B, a constituent of Ginkgo biloba, facilitates glutamate exocytosis from rat hippocampal nerve terminals. European journal of pharmacology. 2005;514(2-3):141–149. doi: 10.1016/j.ejphar.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16(3):239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Pierce DJ, Struble RG, Price DL, Cork LC. Age-related changes in multiple neurotransmitter systems in the monkey brain. Neurobiology of aging. 1989;10(1):11–19. doi: 10.1016/S0197-4580(89)80005-3. [DOI] [PubMed] [Google Scholar]
- Williams BJ, Eriksdotter-Jonhagen M, Granholm AC. Nerve growth factor in treatment and pathogenesis of Alzheimer's disease. Progress in neurobiology. 2006;80(3):114–128. doi: 10.1016/j.pneurobio.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer's disease. Neurobiology of aging. 2003;24(8):1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39(3):409–421. doi: 10.1016/S0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Jones VC, Tabuchi M, Allan SM, Knight EM, LaFerla FM, Oddo S, Verkhratsky A. Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer's disease. PloS one. 2008;3(8):e2935. doi: 10.1371/journal.pone.0002935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugaya K, Brannen CL. Stem cell strategies for neuroreplacement therapy in Alzheimer's disease. Medical hypotheses. 2001;57(6):697–700. doi: 10.1054/mehy.2001.1424. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20(24):9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C, Zhang YJ, Nestler EJ, Duman RS. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22(9):3673–3682. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairanen M, Lucas G, Ernfors P, Castren M, Castren E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25(5):1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Chen J, Fujioka T, Malberg J, Tsuji S, Duman RS. Localization of phosphorylated cAMP response element-binding protein in immature neurons of adult hippocampus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22(22):9868–9876. doi: 10.1523/JNEUROSCI.22-22-09868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ, Leranth C. Short-term treatment with the antidepressant fluoxetine triggers pyramidal dendritic spine synapse formation in rat hippocampus. The European journal of neuroscience. 2005;21(5):1299–1303. doi: 10.1111/j.1460-9568.2005.03968.x. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Ouimet CC. Altered dendritic spine density in animal models of depression and in response to antidepressant treatment. Synapse (New York, N.Y.) 2001;42(3):151–163. doi: 10.1002/syn.10006. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Deslandes A, McEwen BS. Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. European journal of pharmacology. 1999;371(2-3):113–122. doi: 10.1016/S0014-2999(99)00163-6. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science (New York, N.Y.) 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends in pharmacological sciences. 2002;23(5):238–245. doi: 10.1016/S0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2008;33(1):88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology (Berl) 2005;177(3):245–55. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29(7):1321–30. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- Erickson CA, Barnes CA. The neurobiology of memory changes in normal aging. Exp Gerontol. 2003;38(1-2):61–9. doi: 10.1016/S0531-5565(02)00160-2. [DOI] [PubMed] [Google Scholar]
- Uttl B, Graf P. Episodic spatial memory in adulthood. Psychol Aging. 1993;8(2):257–73. doi: 10.1037/0882-7974.8.2.257. [DOI] [PubMed] [Google Scholar]
- Wilkniss SM, Jones MG, Korol DL, Gold PE, Manning CA. Age-related differences in an ecologically based study of route learning. Psychol Aging. 1997;12(2):372–5. doi: 10.1037/0882-7974.12.2.372. [DOI] [PubMed] [Google Scholar]
- Small GW, Rabins PV, Barry PP, Buckholtz NS, DeKosky ST, Ferris SH, Finkel SI, Gwyther LP, Khachaturian ZS, Lebowitz BD, McRae TD, Morris JC, Oakley F, Schneider LS, Streim JE, Sunderland T, Teri LA, Tune LE. Diagnosis and treatment of Alzheimer disease and related disorders. Consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer's Association, and the American Geriatrics Society. JAMA: the journal of the American Medical Association. 1997;278(16):1363–1371. doi: 10.1001/jama.278.16.1363. [DOI] [PubMed] [Google Scholar]
- Lister JP, Barnes CA. Neurobiological changes in the hippocampus during normative aging. Archives of Neurology. 2009;66(7):829–833. doi: 10.1001/archneurol.2009.125. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2004;29(10):1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(24):13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PO, Fossati P, Pochon JB, Levy R, Lebastard G, Lehericy S, Allilaire JF, Dubois B. Cognitive control and brain resources in major depression: an fMRI study using the n-back task. NeuroImage. 2005;26(3):860–869. doi: 10.1016/j.neuroimage.2005.02.048. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, O'Dwyer G, Teleki Z, Stockmeier CA, Miguel-Hidalgo JJ. GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2007;32(2):471–482. doi: 10.1038/sj.npp.1301234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levkovitz Y, Grisaru N, Segal M. Transcranial magnetic stimulation and antidepressive drugs share similar cellular effects in rat hippocampus. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2001;24(6):608–616. doi: 10.1016/S0893-133X(00)00244-X. [DOI] [PubMed] [Google Scholar]
- Stewart CA, Reid IC. Repeated ECS and fluoxetine administration have equivalent effects on hippocampal synaptic plasticity. Psychopharmacology. 2000;148(3):217–223. doi: 10.1007/s002130050045. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27(12):3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, van Praag H, Martone ME, Ellisman MH, Gage FH. Synapse formation on neurons born in the adult hippocampus. Nature neuroscience. 2007;10(6):727–734. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- Massicotte G, Bernard J, Ohayon M. Chronic effects of trimipramine, an antidepressant, on hippocampal synaptic plasticity. Behavioral and neural biology. 1993;59(2):100–106. doi: 10.1016/0163-1047(93)90808-U. [DOI] [PubMed] [Google Scholar]
- Von Frijtag JC, Kamal A, Reijmers LG, Schrama LH, Bos R van den, Spruijt BM. Chronic imipramine treatment partially reverses the long-term changes of hippocampal synaptic plasticity in socially stressed rats. Neuroscience letters. 2001;309(3):153–156. doi: 10.1016/S0304-3940(01)02062-6. [DOI] [PubMed] [Google Scholar]
- Holderbach R, Clark K, Moreau JL, Bischofberger J, Normann C. Enhanced long-term synaptic depression in an animal model of depression. Biological psychiatry. 2007;62(1):92–100. doi: 10.1016/j.biopsych.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Vouimba RM, Munoz C, Diamond DM. Differential effects of predator stress and the antidepressant tianeptine on physiological plasticity in the hippocampus and basolateral amygdala. Stress (Amsterdam, Netherlands) 2006;9(1):29–40. doi: 10.1080/10253890600610973. [DOI] [PubMed] [Google Scholar]
- Rocher C, Spedding M, Munoz C, Jay TM. Acute stress-induced changes in hippocampal/prefrontal circuits in rats: effects of antidepressants. Cerebral cortex (New York, N.Y.: 1991) 2004;14(2):224–229. doi: 10.1093/cercor/bhg122. [DOI] [PubMed] [Google Scholar]
- Nowakowska E, Kus K, Chodera A. Comparison of behavioural effects of venlafaxine and imipramine in rats. Arzneimittel-Forschung. 2003;53(4):237–242. doi: 10.1055/s-0031-1297102. [DOI] [PubMed] [Google Scholar]
- Nowakowska E, Kus K, Chodera A, Rybakowski J. Behavioural effects of fluoxetine and tianeptine, two antidepressants with opposite action mechanisms, in rats. Arzneimittel-Forschung. 2000;50(1):5–10. doi: 10.1055/s-0031-1300156. [DOI] [PubMed] [Google Scholar]
- Nowakowska E, Kus K, Florek E, Czubak A, Jodynis-Liebert J. The influence of tobacco smoke and nicotine on antidepressant and memory-improving effects of venlafaxine. Human & experimental toxicology. 2006;25(4):199–209. doi: 10.1191/0960327106ht611oa. [DOI] [PubMed] [Google Scholar]
- Jaffard R, Mocaer E, Poignant JC, Micheau J, Marighetto A, Meunier M, Beracochea D. Effects of tianeptine on spontaneous alternation, simple and concurrent spatial discrimination learning and on alcohol-induced alternation deficits in mice. Behavioural pharmacology. 1991;2(1):37–46. doi: 10.1097/00008877-199102000-00006. [DOI] [PubMed] [Google Scholar]
- Yau JL, Noble J, Hibberd C, Rowe WB, Meaney MJ, Morris RG, Seckl JR. Chronic treatment with the antidepressant amitriptyline prevents impairments in water maze learning in aging rats. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22(4):1436–1442. doi: 10.1523/JNEUROSCI.22-04-01436.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naudon L, Hotte M, Jay TM. Effects of acute and chronic antidepressant treatments on memory performance: a comparison between paroxetine and imipramine. Psychopharmacology. 2007;191(2):353–364. doi: 10.1007/s00213-006-0660-4. [DOI] [PubMed] [Google Scholar]
- Petracca G, Teson A, Chemerinski E, Leiguarda R, Starkstein SE. A double-blind placebo-controlled study of clomipramine in depressed patients with Alzheimer's disease. The Journal of neuropsychiatry and clinical neurosciences. 1996;8(3):270–275. doi: 10.1176/jnp.8.3.270. [DOI] [PubMed] [Google Scholar]
- Majlessi N, Naghdi N. Impaired spatial learning in the Morris water maze induced by serotonin reuptake inhibitors in rats. Behav Pharmacol. 2002;13(3):237–42. doi: 10.1097/00008877-200205000-00007. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Hesselink M, Hartmann S, Lorenz B, Wollenburg C, Quack G. Modulation of NMDA receptors by glycine--introduction to some basic aspects and recent developments. Amino acids. 1998;14(1-3):207–216. doi: 10.1007/BF01345264. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Quack G. Glutamate in CNS disorders as a target for drug development: an update. Drug news & perspectives. 1998;11(9):523–569. doi: 10.1358/dnp.1998.11.9.863689. [DOI] [PubMed] [Google Scholar]
- Petrie RX, Reid IC, Stewart CA. The N-methyl-D-aspartate receptor, synaptic plasticity, and depressive disorder. A critical review. Pharmacology & therapeutics. 2000;87(1):11–25. doi: 10.1016/S0163-7258(00)00063-2. [DOI] [PubMed] [Google Scholar]
- Stewart CA, Reid IC. Antidepressant mechanisms: functional and molecular correlates of excitatory amino acid neurotransmission. Molecular psychiatry. 2002;7(Suppl 1):S15–22. doi: 10.1038/sj.mp.4001014. [DOI] [PubMed] [Google Scholar]
- Hoey SE, Williams RJ, Perkinton MS. Synaptic NMDA receptor activation stimulates alpha-secretase amyloid precursor protein processing and inhibits amyloid-beta production. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29(14):4442–4460. doi: 10.1523/JNEUROSCI.6017-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesne S, Ali C, Gabriel C, Croci N, MacKenzie ET, Glabe CG, Plotkine M, Marchand-Verrecchia C, Vivien D, Buisson A. NMDA receptor activation inhibits alpha-secretase and promotes neuronal amyloid-beta production. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25(41):9367–9377. doi: 10.1523/JNEUROSCI.0849-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS. Synaptic plasticity and mood disorders. Molecular psychiatry. 2002;7(Suppl 1):S29–34. doi: 10.1038/sj.mp.4001016. [DOI] [PubMed] [Google Scholar]
- Boyer PA, Skolnick P, Fossom LH. Chronic administration of imipramine and citalopram alters the expression of NMDA receptor subunit mRNAs in mouse brain. A quantitative in situ hybridization study. Journal of molecular neuroscience: MN. 1998;10(3):219–233. doi: 10.1007/BF02761776. [DOI] [PubMed] [Google Scholar]
- Nowak G, Li Y, Paul IA. Adaptation of cortical but not hippocampal NMDA receptors after chronic citalopram treatment. European journal of pharmacology. 1996;295(1):75–85. doi: 10.1016/0014-2999(95)00585-4. [DOI] [PubMed] [Google Scholar]
- Nowak G, Trullas R, Layer RT, Skolnick P, Paul IA. Adaptive changes in the N-methyl-D-aspartate receptor complex after chronic treatment with imipramine and 1-aminocyclopropanecarboxylic acid. The Journal of pharmacology and experimental therapeutics. 1993;265(3):1380–1386. [PubMed] [Google Scholar]
- Pallotta M, Segieth J, Whitton PS. Chronic but not acute clomipramine alters the effect of NMDA receptor regulation of dopamine release in rat frontal cortex. Neuroscience letters. 1999;262(3):187–190. doi: 10.1016/S0304-3940(99)00058-0. [DOI] [PubMed] [Google Scholar]
- Paul IA, Layer RT, Skolnick P, Nowak G. Adaptation of the NMDA receptor in rat cortex following chronic electroconvulsive shock or imipramine. European journal of pharmacology. 1993;247(3):305–311. doi: 10.1016/0922-4106(93)90199-J. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, Marek G, Epperson CN, Goddard A, Mason GF. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Molecular psychiatry. 2002;7(Suppl 1):S71–80. doi: 10.1038/sj.mp.4001021. [DOI] [PubMed] [Google Scholar]
- Palucha A, Pilc A. The involvement of glutamate in the pathophysiology of depression. Drug news & perspectives. 2005;18(4):262–268. doi: 10.1358/dnp.2005.18.4.908661. [DOI] [PubMed] [Google Scholar]
- Paul IA, Nowak G, Layer RT, Popik P, Skolnick P. Adaptation of the N-methyl-D-aspartate receptor complex following chronic antidepressant treatments. The Journal of pharmacology and experimental therapeutics. 1994;269(1):95–102. [PubMed] [Google Scholar]
- Cai Z, McCaslin PP. Amitriptyline, desipramine, cyproheptadine and carbamazepine, in concentrations used therapeutically, reduce kainate- and N-methyl-D-aspartate-induced intracellular Ca2+ levels in neuronal culture. European journal of pharmacology. 1992;219(1):53–57. doi: 10.1016/0014-2999(92)90579-S. [DOI] [PubMed] [Google Scholar]
- Sernagor E, Kuhn D, Vyklicky L Jr, Mayer ML. Open channel block of NMDA receptor responses evoked by tricyclic antidepressants. Neuron. 1989;2(3):1221–1227. doi: 10.1016/0896-6273(89)90306-1. [DOI] [PubMed] [Google Scholar]
- Shuto S, Yoshii K, Matsuda A. (1S,2R)-1-Phenyl-2-[(S)-1-aminopropyl]-N,N-diethylcyclopropanecarboxamide (PPDC), a new class of NMDA-receptor antagonist: molecular design by a novel conformational restriction strategy. Japanese journal of pharmacology. 2001;85(3):207–213. doi: 10.1254/jjp.85.207. [DOI] [PubMed] [Google Scholar]
- Szasz BK, Mike A, Karoly R, Gerevich Z, Illes P, Vizi ES, Kiss JP. Direct inhibitory effect of fluoxetine on N-methyl-D-aspartate receptors in the central nervous system. Biological psychiatry. 2007;62(11):1303–1309. doi: 10.1016/j.biopsych.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Layer RT, Popik P, Olds T, Skolnick P. Antidepressant-like actions of the polyamine site NMDA antagonist, eliprodil (SL-82.0715) Pharmacology, biochemistry, and behavior. 1995;52(3):621–627. doi: 10.1016/0091-3057(95)00155-P. [DOI] [PubMed] [Google Scholar]
- Panconi E, Roux J, Altenbaumer M, Hampe S, Porsolt RD. MK-801 and enantiomers: potential antidepressants or false positives in classical screening models? Pharmacology, biochemistry, and behavior. 1993;46(1):15–20. doi: 10.1016/0091-3057(93)90310-P. [DOI] [PubMed] [Google Scholar]
- Skolnick P. Antidepressants for the new millennium. European journal of pharmacology. 1999;375(1-3):31–40. doi: 10.1016/S0014-2999(99)00330-1. [DOI] [PubMed] [Google Scholar]
- Huber TJ, Dietrich DE, Emrich HM. Possible use of amantadine in depression. Pharmacopsychiatry. 1999;32(2):47–55. doi: 10.1055/s-2007-979191. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biological psychiatry. 2000;47(4):351–354. doi: 10.1016/S0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D, Dickson CT. Anxiolytic- and antidepressant-like properties of ketamine in behavioral and neurophysiological animal models. Neuroscience. 2009;161(2):359–369. doi: 10.1016/j.neuroscience.2009.03.038. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends in neurosciences. 2004;27(10):589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Tong L, Thornton PL, Balazs R, Cotman CW. Beta -amyloid-(1-42) impairs activity-dependent cAMP-response element-binding protein signaling in neurons at concentrations in which cell survival Is not compromised. The Journal of biological chemistry. 2001;276(20):17301–17306. doi: 10.1074/jbc.M010450200. [DOI] [PubMed] [Google Scholar]
- Arvanitis DN, Ducatenzeiler A, Ou JN, Grodstein E, Andrews SD, Tendulkar SR, Ribeiro-da-Silva A, Szyf M, Cuello AC. High intracellular concentrations of amyloid-beta block nuclear translocation of phosphorylated CREB. Journal of neurochemistry. 2007;103(1):216–228. doi: 10.1111/j.1471-4159.2007.04704.x. [DOI] [PubMed] [Google Scholar]
- Bezchlibnyk Y, Young LT. The neurobiology of bipolar disorder: focus on signal transduction pathways and the regulation of gene expression. Canadian journal of psychiatry. Revue canadienne de psychiatrie. 2002;47(2):135–148. doi: 10.1177/070674370204700203. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Gould E, Manji H, Buncan M, Duman RS, Greshenfeld HK, Hen R, Koester S, Lederhendler I, Meaney M, Robbins T, Winsky L, Zalcman S. Preclinical models: status of basic research in depression. Biological psychiatry. 2002;52(6):503–528. doi: 10.1016/S0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- Reus VI, Wolkowitz OM. Antiglucocorticoid drugs in the treatment of depression. Expert opinion on investigational drugs. 2001;10(10):1789–1796. doi: 10.1517/13543784.10.10.1789. [DOI] [PubMed] [Google Scholar]
- Pang PT, Lu B. Regulation of late-phase LTP and long-term memory in normal and aging hippocampus: role of secreted proteins tPA and BDNF. Ageing research reviews. 2004;3(4):407–430. doi: 10.1016/j.arr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Egashira N, Iwasaki K, Takashima A, Watanabe T, Kawabe H, Matsuda T, Mishima K, Chidori S, Nishimura R, Fujiwara M. Altered depression-related behavior and neurochemical changes in serotonergic neurons in mutant R406W human tau transgenic mice. Brain research. 2005;1059(1):7–12. doi: 10.1016/j.brainres.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Reed LA, Grabowski TJ, Schmidt ML, Morris JC, Goate A, Solodkin A, Van Hoesen GW, Schelper RL, Talbot CJ, Wragg MA, Trojanowski JQ. Autosomal dominant dementia with widespread neurofibrillary tangles. Annals of Neurology. 1997;42(4):564–572. doi: 10.1002/ana.410420406. [DOI] [PubMed] [Google Scholar]
- Nazarali AJ, Reynolds GP. Monoamine neurotransmitters and their metabolites in brain regions in Alzheimer's disease: a postmortem study. Cellular and molecular neurobiology. 1992;12(6):581–587. doi: 10.1007/BF00711237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altar CA. Neurotrophins and depression. Trends in pharmacological sciences. 1999;20(2):59–61. doi: 10.1016/S0165-6147(99)01309-7. [DOI] [PubMed] [Google Scholar]
- Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry research. 2002;109(2):143–148. doi: 10.1016/S0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- Vaidya VA, Marek GJ, Aghajanian GK, Duman RS. 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17(8):2785–2795. doi: 10.1523/JNEUROSCI.17-08-02785.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandoli C, Sanna A, De Bernardi MA, Follesa P, Brooker G, Mocchetti I. Brain-derived neurotrophic factor and basic fibroblast growth factor downregulate NMDA receptor function in cerebellar granule cells. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1998;18(19):7953–7961. doi: 10.1523/JNEUROSCI.18-19-07953.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SJ, Dawbarn D. Clinical relevance of the neurotrophins and their receptors. Clinical science (London, England: 1979) 2006;110(2):175–191. doi: 10.1042/CS20050161. [DOI] [PubMed] [Google Scholar]
- Arancio O, Chao MV. Neurotrophins, synaptic plasticity and dementia. Current opinion in neurobiology. 2007;17(3):325–330. doi: 10.1016/j.conb.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Matrone C, Di Luzio A, Meli G, D'Aguanno S, Severini C, Ciotti MT, Cattaneo A, Calissano P. Activation of the amyloidogenic route by NGF deprivation induces apoptotic death in PC12 cells. Journal of Alzheimer's disease: JAD. 2008;13(1):81–96. doi: 10.3233/jad-2008-13109. [DOI] [PubMed] [Google Scholar]
- Matrone C, Ciotti MT, Mercanti D, Marolda R, Calissano P. NGF and BDNF signaling control amyloidogenic route and Abeta production in hippocampal neurons. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(35):13139–13144. doi: 10.1073/pnas.0806133105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahara AH, Merrill DA, Coppola G, Tsukada S, Schroeder BE, Shaked GM, Wang L, Blesch A, Kim A, Conner JM, Rockenstein E, Chao MV, Koo EH, Geschwind D, Masliah E, Chiba AA, Tuszynski MH. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer's disease. Nature medicine. 2009;15(3):331–337. doi: 10.1038/nm.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frielingsdorf H, Simpson DR, Thal LJ, Pizzo DP. Nerve growth factor promotes survival of new neurons in the adult hippocampus. Neurobiology of disease. 2007;26(1):47–55. doi: 10.1016/j.nbd.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Thal L, Pay M, Salmon DP, U HS, Bakay R, Patel P, Blesch A, Vahlsing HL, Ho G, Tong G, Potkin SG, Fallon J, Hansen L, Mufson EJ, Kordower JH, Gall C, Conner J. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nature medicine. 2005;11(5):551–555. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- Lazarov O, Robinson J, Tang YP, Hairston IS, Korade-Mirnics Z, Lee VM, Hersh LB, Sapolsky RM, Mirnics K, Sisodia SS. Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell. 2005;120(5):701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Patel NV, Gordon MN, Connor KE, Good RA, Engelman RW, Mason J, Morgan DG, Morgan TE, Finch CE. Caloric restriction attenuates Abeta-deposition in Alzheimer transgenic models. Neurobiology of aging. 2005;26(7):995–1000. doi: 10.1016/j.neurobiolaging.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Nelson RL, Guo Z, Halagappa VM, Pearson M, Gray AJ, Matsuoka Y, Brown M, Martin B, Iyun T, Maudsley S, Clark RF, Mattson MP. Prophylactic treatment with paroxetine ameliorates behavioral deficits and retards the development of amyloid and tau pathologies in 3×TgAD mice. Experimental neurology. 2007;205(1):166–176. doi: 10.1016/j.expneurol.2007.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo-Neustadt A. Brain-derived neurotrophic factor, behavior, and new directions for the treatment of mental disorders. Seminars in clinical neuropsychiatry. 2003;8(2):109–118. doi: 10.1053/scnp.2003.50014. [DOI] [PubMed] [Google Scholar]
- Inbar P, Bautista MR, Takayama SA, Yang J. Assay to screen for molecules that associate with Alzheimer's related beta-amyloid fibrils. Analytical Chemistry. 2008;80(9):3502–3506. doi: 10.1021/ac702592f. [DOI] [PubMed] [Google Scholar]
- Rogers JT, Randall JD, Cahill CM, Eder PS, Huang X, Gunshin H, Leiter L, McPhee J, Sarang SS, Utsuki T, Greig NH, Lahiri DK, Tanzi RE, Bush AI, Giordano T, Gullans SR. An iron-responsive element type II in the 5'-untranslated region of the Alzheimer's amyloid precursor protein transcript. The Journal of biological chemistry. 2002;277(47):45518–45528. doi: 10.1074/jbc.M207435200. [DOI] [PubMed] [Google Scholar]
- Payton S, Cahill CM, Randall JD, Gullans SR, Rogers JT. Drug discovery targeted to the Alzheimer's APP mRNA 5'-untranslated region: the action of paroxetine and dimercaptopropanol. Journal of molecular neuroscience: MN. 2003;20(3):267–275. doi: 10.1385/JMN:20:3:267. [DOI] [PubMed] [Google Scholar]
- Morse LJ, Payton SM, Cuny GD, Rogers JT. FDA-preapproved drugs targeted to the translational regulation and processing of the amyloid precursor protein. Journal of molecular neuroscience: MN. 2004;24(1):129–136. doi: 10.1385/JMN:24:1:129. [DOI] [PubMed] [Google Scholar]
- Tucker S, Ahl M, Bush A, Westaway D, Huang X, Rogers JT. Pilot study of the reducing effect on amyloidosis in vivo by three FDA pre-approved drugs via the Alzheimer's APP 5' untranslated region. Current Alzheimer research. 2005;2(2):249–254. doi: 10.2174/1567205053585855. [DOI] [PubMed] [Google Scholar]
- Tucker S, Ahl M, Cho HH, Bandyopadhyay S, Cuny GD, Bush AI, Goldstein LE, Westaway D, Huang X, Rogers JT. RNA therapeutics directed to the non coding regions of APP mRNA, in vivo anti-amyloid efficacy of paroxetine, erythromycin, and N-acetyl cysteine. Current Alzheimer research. 2006;3(3):221–227. doi: 10.2174/156720506777632835. [DOI] [PubMed] [Google Scholar]
- Pakaski M, Bjelik A, Hugyecz M, Kasa P, Janka Z, Kalman J. Imipramine and citalopram facilitate amyloid precursor protein secretion in vitro. Neurochemistry international. 2005;47(3):190–195. doi: 10.1016/j.neuint.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Arjona AA, Pooler AM, Lee RK, Wurtman RJ. Effect of a 5-HT(2C) serotonin agonist, dexnorfenfluramine, on amyloid precursor protein metabolism in guinea pigs. Brain research. 2002;951(1):135–140. doi: 10.1016/S0006-8993(02)03153-0. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Oishi M, Chen HI, Pinkas-Kramarski R, Jaffe EA, Gandy SE, Greengard P. Cholinergic agonists and interleukin 1 regulate processing and secretion of the Alzheimer beta/A4 amyloid protein precursor. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(21):10075–10078. doi: 10.1073/pnas.89.21.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsch RM, Slack BE, Wurtman RJ, Growdon JH. Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science (New York, N.Y.) 1992;258(5080):304–307. doi: 10.1126/science.1411529. [DOI] [PubMed] [Google Scholar]
- Butterweck V. Mechanism of action of St John's wort in depression: what is known? CNS drugs. 2003;17(8):539–562. doi: 10.2165/00023210-200317080-00001. [DOI] [PubMed] [Google Scholar]
- Mennini T, Gobbi M. The antidepressant mechanism of Hypericum perforatum. Life Sciences. 2004;75(9):1021–1027. doi: 10.1016/j.lfs.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Shelton RC. St John's wort for the treatment of depression. Lancet neurology. 2002;1(5):275. doi: 10.1016/S1474-4422(02)00129-1. [DOI] [PubMed] [Google Scholar]
- Verotta L. Hypericum perforatum, a source of neuroactive lead structures. Current topics in medicinal chemistry. 2003;3(2):187–201. doi: 10.2174/1568026033392589. [DOI] [PubMed] [Google Scholar]
- Singer A, Wonnemann M, Muller WE. Hyperforin, a major antidepressant constituent of St. John's Wort, inhibits serotonin uptake by elevating free intracellular Na+1. The Journal of pharmacology and experimental therapeutics. 1999;290(3):1363–1368. [PubMed] [Google Scholar]
- Butterweck V, Jurgenliemk G, Nahrstedt A, Winterhoff H. Flavonoids from Hypericum perforatum show antidepressant activity in the forced swimming test. Planta Medica. 2000;66(1):3–6. doi: 10.1055/s-2000-11119. [DOI] [PubMed] [Google Scholar]
- Butterweck V, Petereit F, Winterhoff H, Nahrstedt A. Solubilized hypericin and pseudohypericin from Hypericum perforatum exert antidepressant activity in the forced swimming test. Planta Medica. 1998;64(4):291–294. doi: 10.1055/s-2006-957437. [DOI] [PubMed] [Google Scholar]
- Silva BA, Dias AC, Ferreres F, Malva JO, Oliveira CR. Neuroprotective effect of H. perforatum extracts on beta-amyloid-induced neurotoxicity. Neurotoxicity research. 2004;6(2):119–130. doi: 10.1007/BF03033214. [DOI] [PubMed] [Google Scholar]
- Kraus B, Wolff H, Heilmann J, Elstner EF. Influence of Hypericum perforatum extract and its single compounds on amyloid-beta mediated toxicity in microglial cells. Life Sciences. 2007;81(11):884–894. doi: 10.1016/j.lfs.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Klusa V, Germane S, Noldner M, Chatterjee SS. Hypericum extract and hyperforin: memory-enhancing properties in rodents. Pharmacopsychiatry. 2001;34(Suppl 1):S61–9. doi: 10.1055/s-2001-15451. [DOI] [PubMed] [Google Scholar]
- Dinamarca MC, Cerpa W, Garrido J, Hancke JL, Inestrosa NC. Hyperforin prevents beta-amyloid neurotoxicity and spatial memory impairments by disaggregation of Alzheimer's amyloid-beta-deposits. Molecular psychiatry. 2006;11(11):1032–1048. doi: 10.1038/sj.mp.4001866. [DOI] [PubMed] [Google Scholar]
- Sakakibara H, Ishida K, Grundmann O, Nakajima J, Seo S, Butterweck V, Minami Y, Saito S, Kawai Y, Nakaya Y, Terao J. Antidepressant effect of extracts from Ginkgo biloba leaves in behavioral models. Biological & pharmaceutical bulletin. 2006;29(8):1767–1770. doi: 10.1248/bpb.29.1767. [DOI] [PubMed] [Google Scholar]
- Drieu K. Preparation and definition of Ginkgo biloba extract. Presse medicale (Paris, France: 1983) 1986;15(31):1455–1457. [PubMed] [Google Scholar]
- Kondratskaya EL, Pankratov YV, Lalo UV, Chatterjee SS, Krishtal OA. Inhibition of hippocampal LTP by ginkgolide B is mediated by its blocking action on PAF rather than glycine receptors. Neurochemistry international. 2004;44(3):171–177. doi: 10.1016/S0197-0186(03)00126-8. [DOI] [PubMed] [Google Scholar]
- Kalkunte SS, Singh AP, Chaves FC, Gianfagna TJ, Pundir VS, Jaiswal AK, Vorsa N, Sharma S. Antidepressant and antistress activity of GC-MS characterized lipophilic extracts of Ginkgo biloba leaves. Phytotherapy Research: PTR. 2007;21(11):1061–1065. doi: 10.1002/ptr.2212. [DOI] [PubMed] [Google Scholar]
- DeFeudis FV, Drieu K. Ginkgo biloba extract (EGb 761) and CNS functions: basic studies and clinical applications. Current Drug Targets. 2000;1(1):25–58. doi: 10.2174/1389450003349380. [DOI] [PubMed] [Google Scholar]
- Kennedy DO, Scholey AB, Wesnes KA. The dose-dependent cognitive effects of acute administration of Ginkgo biloba to healthy young volunteers. Psychopharmacology. 2000;151(4):416–423. doi: 10.1007/s002130000501. [DOI] [PubMed] [Google Scholar]
- Le Bars PL, Katz MM, Berman N, Itil TM, Freedman AM, Schatzberg AF. A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. North American EGb Study Group. JAMA: the journal of the American Medical Association. 1997;278(16):1327–1332. doi: 10.1001/jama.278.16.1327. [DOI] [PubMed] [Google Scholar]
- Polich J, Gloria R. Cognitive effects of a Ginkgo biloba/vinpocetine compound in normal adults: systematic assessment of perception, attention and memory. Human psychopharmacology. 2001;16(5):409–416. doi: 10.1002/hup.308. [DOI] [PubMed] [Google Scholar]
- Tang F, Nag S, Shiu SY, Pang SF. The effects of melatonin and Ginkgo biloba extract on memory loss and choline acetyltransferase activities in the brain of rats infused intracerebroventricularly with beta-amyloid 1-40. Life Sciences. 2002;71(22):2625–2631. doi: 10.1016/S0024-3205(02)02105-7. [DOI] [PubMed] [Google Scholar]
- Trick L, Boyle J, Hindmarch I. The effects of Ginkgo biloba extract (LI 1370) supplementation and discontinuation on activities of daily living and mood in free living older volunteers. Phytotherapy Research: PTR. 2004;18(7):531–537. doi: 10.1002/ptr.1479. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Williamson JD, Fitzpatrick AL, Kronmal RA, Ives DG, Saxton JA, Lopez OL, Burke G, Carlson MC, Fried LP, Kuller LH, Robbins JA, Tracy RP, Woolard NF, Dunn L, Snitz BE, Nahin RL, Furberg CD, Investigators GEoMGS. Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA: the journal of the American Medical Association. 2008;300(19):2253–2262. doi: 10.1001/jama.2008.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao ZX, Han Z, Drieu K, Papadopoulos V. Ginkgo biloba extract (Egb 761) inhibits beta-amyloid production by lowering free cholesterol levels. The Journal of nutritional biochemistry. 2004;15(12):749–756. doi: 10.1016/j.jnutbio.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Luo Y, Smith JV, Paramasivam V, Burdick A, Curry KJ, Buford JP, Khan I, Netzer WJ, Xu H, Butko P. Inhibition of amyloid-beta aggregation and caspase-3 activation by the Ginkgo biloba extract EGb761. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(19):12197–12202. doi: 10.1073/pnas.182425199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastianetto S, Ramassamy C, Dore S, Christen Y, Poirier J, Quirion R. The Ginkgo biloba extract (EGb 761) protects hippocampal neurons against cell death induced by beta-amyloid. The European journal of neuroscience. 2000;12(6):1882–1890. doi: 10.1046/j.1460-9568.2000.00069.x. [DOI] [PubMed] [Google Scholar]
- Xu Y, Cui C, Pang C, Christen Y, Luo Y. Restoration of impaired phosphorylation of cyclic AMP response element-binding protein (CREB) by EGb 761 and its constituents in Abeta-expressing neuroblastoma cells. The European journal of neuroscience. 2007;26(10):2931–2939. doi: 10.1111/j.1460-9568.2007.05905.x. [DOI] [PubMed] [Google Scholar]
- Tchantchou F, Xu Y, Wu Y, Christen Y, Luo Y. EGb 761 enhances adult hippocampal neurogenesis and phosphorylation of CREB in transgenic mouse model of Alzheimer's disease. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2007;21(10):2400–2408. doi: 10.1096/fj.06-7649com. [DOI] [PubMed] [Google Scholar]
- Benavente-Garcia O, Castillo J. Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. Journal of Agricultural and Food Chemistry. 2008;56(15):6185–6205. doi: 10.1021/jf8006568. [DOI] [PubMed] [Google Scholar]
- Dimpfel W. Rat electropharmacograms of the flavonoids rutin and quercetin in comparison to those of moclobemide and clinically used reference drugs suggest antidepressive and/or neuroprotective action. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology. 2009;16(4):287–294. doi: 10.1016/j.phymed.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Messaoudi M, Bisson JF, Nejdi A, Rozan P, Javelot H. Antidepressant-like effects of a cocoa polyphenolic extract in Wistar-Unilever rats. Nutritional Neuroscience. 2008;11(6):269–276. doi: 10.1179/147683008X344165. [DOI] [PubMed] [Google Scholar]
- Sanchez-Mateo CC, Bonkanka CX, Rabanal RM. Hypericum grandifolium Choisy: a species native to Macaronesian Region with antidepressant effect. Journal of ethnopharmacology. 2009;121(2):297–303. doi: 10.1016/j.jep.2008.10.031. [DOI] [PubMed] [Google Scholar]
- Li Q, Zhao HF, Zhang ZF, Liu ZG, Pei XR, Wang JB, Li Y. Long-term green tea catechin administration prevents spatial learning and memory impairment in senescence-accelerated mouse prone-8 mice by decreasing Abeta(1-42) oligomers and upregulating synaptic plasticity-related proteins in the hippocampus. Neuroscience. 2009. [DOI] [PubMed]
- Matsuzaki K, Yamakuni T, Hashimoto M, Haque AM, Shido O, Mimaki Y, Sashida Y, Ohizumi Y. Nobiletin restoring beta-amyloid-impaired CREB phosphorylation rescues memory deterioration in Alzheimer's disease model rats. Neuroscience letters. 2006;400(3):230–234. doi: 10.1016/j.neulet.2006.02.077. [DOI] [PubMed] [Google Scholar]
- Hou Y, Aboukhatwa MA, Lei DL, Manaye K, Khan I, Luo Y. Anti-depressant natural flavonols modulate BDNF and beta amyloid in neurons and hippocampus of double TgAD mice. Neuropharmacology. 2009. [DOI] [PMC free article] [PubMed]
- Nyth AL, Gottfries CG, Lyby K, Smedegaard-Andersen L, Gylding-Sabroe J, Kristensen M, Refsum HE, Ofsti E, Eriksson S, Syversen S. A controlled multicenter clinical study of citalopram and placebo in elderly depressed patients with and without concomitant dementia. Acta Psychiatrica Scandinavica. 1992;86(2):138–145. doi: 10.1111/j.1600-0447.1992.tb03242.x. [DOI] [PubMed] [Google Scholar]
- Magai C, Kennedy G, Cohen CI, Gomberg D. A controlled clinical trial of sertraline in the treatment of depression in nursing home patients with late-stage Alzheimer's disease. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry. 2000;8(1):66–74. doi: 10.1097/00019442-200002000-00009. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Sheppard JM, Steele CD, Kopunek S, Steinberg M, Baker AS, Brandt J, Rabins PV. Randomized, placebo-controlled, double-blind clinical trial of sertraline in the treatment of depression complicating Alzheimer's disease: initial results from the Depression in Alzheimer's Disease study. The American Journal of Psychiatry. 2000;157(10):1686–1689. doi: 10.1176/appi.ajp.157.10.1686. [DOI] [PubMed] [Google Scholar]
- Thompson S, Herrmann N, Rapoport MJ, Lanctot KL. Efficacy and safety of antidepressants for treatment of depression in Alzheimer's disease: a metaanalysis. Canadian journal of psychiatry. Revue canadienne de psychiatrie. 2007;52(4):248–255. doi: 10.1177/070674370705200407. [DOI] [PubMed] [Google Scholar]
- Palmer AM, DeKosky ST. Monoamine neurons in aging and Alzheimer's disease. Journal of neural transmission. General section. 1993;91(2-3):135–159. doi: 10.1007/BF01245229. [DOI] [PubMed] [Google Scholar]
- Mega MS, Masterman DM, O'Connor SM, Barclay TR, Cummings JL. The spectrum of behavioral responses to cholinesterase inhibitor therapy in Alzheimer disease. Arch Neurol. 1999;56(11):1388–93. doi: 10.1001/archneur.56.11.1388. [DOI] [PubMed] [Google Scholar]
- Francis PT, Ramirez MJ, Lai MK. Neurochemical basis for symptomatic treatment of Alzheimer's disease. Neuropharmacology. 2010. [DOI] [PubMed]
- Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer's disease: a review of progress. J Neurol Neurosurg Psychiatry. 1999;66(2):137–47. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker MW, McGaugh JL. The role of interactions between the cholinergic system and other neuromodulatory systems in learning and memory. Synapse (New York, N.Y.) 1991;7(2):151–168. doi: 10.1002/syn.890070209. [DOI] [PubMed] [Google Scholar]
- Rossor M, Iversen LL. Non-cholinergic neurotransmitter abnormalities in Alzheimer's disease. British medical bulletin. 1986;42(1):70–74. doi: 10.1093/oxfordjournals.bmb.a072101. [DOI] [PubMed] [Google Scholar]
- Lai MK, Tsang SW, Francis PT, Keene J, Hope T, Esiri MM, Spence I, Chen CP. Postmortem serotoninergic correlates of cognitive decline in Alzheimer's disease. Neuroreport. 2002;13(9):1175–1178. doi: 10.1097/00001756-200207020-00021. [DOI] [PubMed] [Google Scholar]
- Riekkinen M, Tolonen R, Riekkinen P Jr. Interaction between 5-HT1A and nicotinic cholinergic receptors in the regulation of water maze navigation behavior. Brain research. 1994;649(1-2):174–180. doi: 10.1016/0006-8993(94)91061-8. [DOI] [PubMed] [Google Scholar]
- Schmitt JA, Wingen M, Ramaekers JG, Evers EA, Riedel WJ. Serotonin and human cognitive performance. Current pharmaceutical design. 2006;12(20):2473–2486. doi: 10.2174/138161206777698909. [DOI] [PubMed] [Google Scholar]
- Mowla A, Mosavinasab M, Haghshenas H, Haghighi AB. Does serotonin augmentation have any effect on cognition and activities of daily living in Alzheimer's dementia? A double-blind, placebo-controlled clinical trial. Journal of clinical psychopharmacology. 2007;27(5):484–487. doi: 10.1097/jcp.0b013e31814b98c1. [DOI] [PubMed] [Google Scholar]
- Finkel SI, Mintzer JE, Dysken M, Krishnan KR, Burt T, McRae T. A randomized, placebo-controlled study of the efficacy and safety of sertraline in the treatment of the behavioral manifestations of Alzheimer's disease in outpatients treated with donepezil. International journal of geriatric psychiatry. 2004;19(1):9–18. doi: 10.1002/gps.998. [DOI] [PubMed] [Google Scholar]
- Altman HJ, Stone WS, Ogren SO. Evidence for a possible functional interaction between serotonergic and cholinergic mechanisms in memory retrieval. Behavioral and neural biology. 1987;48(1):49–62. doi: 10.1016/S0163-1047(87)90574-7. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Segal M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. The Journal of comparative neurology. 1978;179(3):641–667. doi: 10.1002/cne.901790311. [DOI] [PubMed] [Google Scholar]
- Little JT, Broocks A, Martin A, Hill JL, Tune LE, Mack C, Cantillon M, Molchan S, Murphy DL, Sunderland T. Serotonergic modulation of anticholinergic effects on cognition and behavior in elderly humans. Psychopharmacology. 1995;120(3):280–288. doi: 10.1007/BF02311175. [DOI] [PubMed] [Google Scholar]
- Nilsson OG, Leanza G, Bjorklund A. Acetylcholine release in the hippocampus: regulation by monoaminergic afferents as assessed by in vivo microdialysis. Brain research. 1992;584(1-2):132–140. doi: 10.1016/0006-8993(92)90886-E. [DOI] [PubMed] [Google Scholar]
- Smith GS, Kramer E, Ma Y, Hermann CR, Dhawan V, Chaly T, Eidelberg D. Cholinergic modulation of the cerebral metabolic response to citalopram in Alzheimer's disease. Brain: a journal of neurology. 2009;132(Pt 2):392–401. doi: 10.1093/brain/awn326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwolf CH. Near-total loss of 'learning' and 'memory' as a result of combined cholinergic and serotonergic blockade in the rat. Behavioural brain research. 1987;23(1):43–57. doi: 10.1016/0166-4328(87)90241-5. [DOI] [PubMed] [Google Scholar]
- Duman RS. Novel therapeutic approaches beyond the serotonin receptor. Biological psychiatry. 1998;44(5):324–335. doi: 10.1016/S0006-3223(98)00031-6. [DOI] [PubMed] [Google Scholar]
- Palmer AM. Pharmacotherapy for Alzheimer's disease: progress and prospects. Trends in pharmacological sciences. 2002;23(9):426–433. doi: 10.1016/S0165-6147(02)02056-4. [DOI] [PubMed] [Google Scholar]