Abstract
Gene therapy holds considerable promise for the treatment of cardiovascular disease and may provide novel therapeutic solutions for both genetic disorders and acquired pathophysiologies such as arteriosclerosis, heart failure and arrhythmias. Recombinant DNA technology and the sequencing of the human genome have made a plethora of candidate therapeutic genes available for cardiovascular diseases. However, progress in the field of gene therapy for cardiovascular disease has been modest; one of the key reasons for this limited progress is the lack of gene delivery systems for localizing gene therapy to specific sites to optimize transgene expression and efficacy. This review summarizes progress made toward the site-specific delivery of cardiovascular gene therapy and highlights selected promising novel approaches.
Keywords: Angioplasty, local delivery, nanoparticle, stent, transgene, vector
Introduction – The challenges facing gene therapy for cardiovascular disease
Cardiovascular disease remains the leading cause of morbidity and mortality in developed countries. The emergence of human gene therapy in the early 1990s led to numerous attempts, both experimental and clinical, to treat cardiovascular disease with gene therapy strategies. However, despite these considerable efforts, progress in cardiovascular gene therapy has been modest, mainly because of the inability to provide an adequate dose of a therapeutic transgene at the required site of activity. The need for effective gene therapy methods for cardiovascular diseases is further emphasized by the fact that, in March 2009, clinical trials of gene therapy protocols for cardiovascular diseases represented the second largest group of registered human gene therapy protocols worldwide [1].
Because cardiovascular disease is characteristically localized, the site-specific targeting of gene therapy for the cardiovascular system is hypothesized in this review to represent an optimal therapeutic strategy to intervene in this disease.
The suboptimal outcomes of completed human gene therapy trials have led both physicians and scientists to reconsider the basic paradigm of this therapeutic approach. Although a universal consensus regarding gene therapy has not been reached, the approach considered herein is that gene transfer protocols should ideally be based in a hierarchical combination of four distinct elements: a therapeutic transgene; a gene therapy vector; a delivery system; and, in some cases, pharmacological modulation of the transgene product (Figure 1). This review briefly discusses the key aspects of all four elements, with a particular emphasis on the delivery systems employed for site-specific gene therapy in the cardiovascular system.
Figure 1. The principal elements of the gene therapy paradigm.
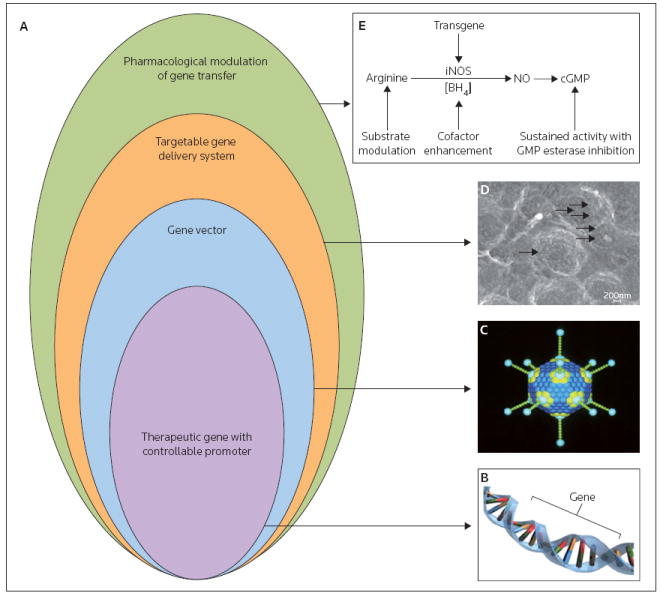
(A) The rational design of a gene therapy strategy requires hierarchical combinations of a regulatable transgene, a targeted gene vector, an appropriate delivery system and, potentially, pharmacological control of the affected metabolic or signaling pathway. Representations of: (B) a transgene and (C) an adenovirus particle, as an example of a gene vector are illustrated. (D) A scanning electron microscopy image of stent-immobilized adenovirus-based vectors (original magnification 75,000-fold) is provided as an example of a delivery system; the arrows point to the individual adenoviruses. (E) The pharmacological regulation of inducible nitric oxide synthase (iNOS) enzymatic function is shown. NO Nitric oxide
Candidate transgenes for cardiovascular gene transfer
A variety of cardiovascular therapeutic gene constructs have been studied in vitro and in vivo. These constructs can be categorized into several groups: antiproliferative (eg, the tumor-suppressor p53 [2]), anti-migratory (eg, metalloprotease inhibitor 3 [TIMP-3] [3]), anti-fibrotic (eg, hepatocyte growth factor [HGF] [4]) antioxidative (eg, superoxide dismutase [SOD] [5]), antithrombotic (eg, tissue factor pathway inhibitor [TFPI] [6]), anti-inflammatory (eg, dominant negative monocyte chemoattractant protein-1 [dnMCP-1] [7]), anti-apoptotic (eg, the NFκB-dependent gene A20 [8]), antiarrhythmic (eg, the cardiac potassium channel missense mutation Q9E-hMiRP1 [9]), procontractile (eg, sarcoplasmic/endoplasmic reticulum calcium ATPase 2 [SERCA-2] [10]), angiogenic (eg, VEGF [11]) and pleiotropic (eg, inducible nitric oxide synthase [iNOS] [12]). Because the mode of action, activity and specificity of transgenes vary significantly, several considerations are important when selecting a therapeutic transgene: whether the protein encoded by the transgene acts intracellularly (eg, p53 or retinoblastoma tumor suppressor [Rb], which interfere directly with intracellular signaling) or extracellularly by engaging autologous or neighboring cell-surface receptors (eg, PDGF, VEGF and other growth factors); whether the sequences providing translocation to the Golgi apparatus and effective secretion are present in the construct; whether the target for the transgene-encoded protein is located on the outer surface of the cells; and whether the restriction of expression to specific cell types is critical. Formerly, most experimental gene therapy studies employed strong heterologous viral promoters (eg, respiratory syncytial virus [RSV] or CMV promoters), which lack tissue specificity and are prone to silencing after a relatively short period of activity [13]. The lack of tissue specificity of these promoters results in the widespread expression of the transgene that primarily reflects the biodistribution of the vector [13]. However, tissue-specific promoters have been increasingly used to achieve the preferential expression of the transgene in myocardium (cardiac troponin T promoter [14]), smooth muscle cells (SMCs; SM22α [15]), endothelial cells (vascular endothelial-cadherin promoter [16]) and macrophages (scavenger receptor A promoter [17]). In addition to the nature and specificity of the promoters used, the inclusion of enhancer sequences such as the Woodchuck hepatitis virus regulatory element can increase significantly the levels of transgene expression driven by the cell-type-specific promoters [18].
Gene vectors
The vectors described in this section of the review are summarized in Table 1.
Table 1.
Selected gene therapy vectors for site-specific delivery of transgenes.
| Vector | Cardiovascular pathology | Gene | Delivery system | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|---|
| Plasmid DNA | In-stent restenosis | VEGF | Stent coating | Non-toxic, ease of production and low immunogenicity | Low level and transient expression | [11] |
| Antisense RNA | In-stent restenosis | cMYC | Catheter | Non-toxic, ease of production and low immunogenicity | Low efficacy of inhibition and non-specific effects | [24] |
| RNAi | Restenosis | HIF1α | Perivascular delivery | Non-toxic with profound gene inhibition | Non-specific effects | [26] |
| Adenovirus (first generation) | In-stent restenosis | iNOS | Polyallylamine bisphosphonate- modified stent | High levels of expression | Limited duration of expression, immune response against the vector and inflammation | [12] |
| Helper-dependent adenovirus | Restenosis | uPA | Intralumenal delivery | Prolonged expression and large capacity of transgene cassette | Low yield of vector and time-consuming production process | [34] |
| Adeno-associated virus | Myocardial infarction | TGFβ | Direct myocardial injection | Prolonged expression and stable genomic integration | Small capacity of transgene cassette | [39] |
| Retrovirus | Restenosis | β-Gal | Intralumenal delivery | Life-long expression | Random genome integration and a risk of oncogenesis | [28] |
| Lentivirus | Heart transplantation | GFP | Direct injection | Transduction of quiescent cells | Low production titers, random genome integration and a risk of oncogenesis | [29] |
Non-viral gene vectors
Plasmid DNA
Because of their low toxicity, non-viral vectors are generally considered to be the safest choice for therapeutic gene transfer. Unfortunately, the inherently low expression levels achieved with plasmid DNA [19] restrict the application of these vectors to limited clinical situations in which low and transient expression of the transgene is required. Ongoing efforts to optimize the plasmid backbone via the inclusion of MAR (matrix attachment region) elements [20] and tissue-specific enhancers [13,18], as well as strategies to prevent premature silencing [21], promise to increase and stabilize the levels of transgene expression obtainable with non-viral vectors.
Antisense and decoy RNA, decoy oligonucleotides and siRNA
Antisense decoy oligonucleotides have been employed extensively to decrease the activation of genes implicated in myocardial [22] and vascular [23] diseases. However, the effectiveness of these agents appears to be insufficient in a clinical setting [24]. More recently, an exponential increase in the use of siRNAs, which provide increased and sustained downregulation of target genes [25], has led to a renewed interest in ‘knockout’ gene therapy approaches for the treatment of cardiovascular diseases [26].
Viral vectors
Retroviruses and lentiviruses
Because the disruption of the host genome caused by the random insertion of the vector genes carries a risk of malignant transformation, the stable integration of retroviral vectors by the host genome is both an advantage (eg, if life-long transgene expression is required) and a serious safety concern [27]. Additionally, only tissues with a high rate of cell proliferation can be effectively transduced with retroviral vectors [28]. Lentivirus vectors, which are derived from a genus of retroviruses that includes HIV-1, efficiently transduce non-dividing cells [29], and generally exhibit a higher tropism to heart and vessels than other Retroviridae species.
Adenoviruses
Adenoviral (Ad) vectors are the most common vector type used in gene therapy clinical trials [1]. A plethora of experimental evidence has demonstrated the successful use of Ad vectors for both reporter and therapeutic transgene expression in the cardiovascular system [30-31]. However, the immunogenic nature of Ad vectors, which cause both cellular and humoral destruction of the vector and vector-transduced cells, precludes substantial transgene expression for more than 2 to 3 weeks after transduction [32]. This major limitation of the first-generation Ad vectors was resolved by the development of the third-generation ‘gutless’ or helper-dependent (HD)/Ad vectors, which are devoid of almost all viral genes [33]. Proof-of-concept in vivo studies have demonstrated significant potential for HD/Ad vectors in the transduction of vasculature [34] and myocardium [35].
Adeno-associated viruses
Vectors from adeno-associated viruses (AAVs) are significantly less immunogenic than other viral vectors, and are naturally capable of prolonged gene expression upon transduction, as a result of the stable integration of the vector genes into the genome of the target cell. The main challenge associated with AAV vectors is the relatively low transduction efficiency of these vectors, and this issue was partially solved by the development of tyrosine mutated [36] and self-complementary [37] AAV vectors. AAV vectors have been investigated as potential vectors for gene transfer in the cardiovascular system and have demonstrated robust long-term expression, particularly with AAV serotypes 6 and 9 [37-40].
Vector targeting
The cells of different tissues and organs each possess a unique repertoire of cell-surface determinants, which would theoretically enable the accurate delivery of vectors to cells of a particular type and location. In practice, a high degree of specific targeting is rarely achieved in vivo, as a result of the overlapping specificities between different cell types from a common lineage. Additionally, the interaction of the vector with blood and tissue en route to the target can effectively mask targeting ligands on the vector surface. Nevertheless, vector-targeting strategies based on either genetic modification of vector surface proteins [41] or on direct chemical modification of the vectors [42] have demonstrated promise in targeting transduction to the cardiovascular tissue.
Gene delivery systems
Generally, gene delivery systems were not thoroughly investigated in previous gene therapy trials. However, an appropriate delivery mode for gene therapeutics is crucial. The main purpose of the gene delivery system is to provide the method of transport to deliver the formulation containing the gene vector to the intended site of action. Minimizing the contact between the gene vector and bodily fluids prior to arrival at the intended location is of particular importance; reducing this contact decreases the dilution of the vector and protects the surface of the vector from non-specific interactions that are typically detrimental to its activity. Furthermore, a successful delivery system must enable physical persistence of the vector in the transfection-competent state at the target site, thereby increasing the probability of delivering the transgene to the cells. Delivery systems can be designed to incorporate elements that facilitate gene transfer to the target cell population by enabling modification of the local extracellular matrix or by rendering target cells more susceptible to transduction with foreign DNA [43]. Finally, the gene delivery system can be coformulated with conventional pharmaceutical agents to optimize the therapeutic activity of the transgene.
Several controlled-release strategies have been successfully investigated for use with gene therapy. For example, biocompatible polymer systems incorporating either plasmid DNA [11] or viral vectors [3] have been included as sustained-release coatings in the design of vascular stents (balloon catheter-deployed metallic devices that are used to relieve vascular obstruction) on the basis that such coatings may provide localized gene therapy to treat the diseased blood vessel. The targeting of gene vectors to specific tissues or cells has also been possible through the incorporation of vectors into polymeric nanoparticles and liposomes with modified surfaces that include targeting ligands, such as antibodies or high-affinity recombinant proteins [44]. Additionally, viral vectors incorporated into nanoparticles are protected from attack by neutralizing antibodies and can exhibit enhanced cell entry, as these encapsulated vectors may bypass receptor-mediated uptake [44], which can be limiting in many cell types. Nevertheless, it should be emphasized that, in general, experience with gene delivery systems is limited, and thus safety and efficacy factors need to be more fully determined.
Myocardial delivery systems
Needle injections
The most straightforward approach to myocardial gene delivery is the direct needle injection of the vector. However, in addition to being technically challenging, this approach has low efficiency; transduced cells are typically observed only along the needle track [45]. Moreover, transgene expression is usually low because of the rapid removal of the vector, which is intensified by the local inflammatory reaction initiated by needle-related tissue damage [46].
Pericardial delivery
The pericardial sac is a natural closed reservoir that can restrain the rapid elimination of the gene vectors. The proximity of the pericardium to the heart muscle was exploited to achieve myocardium transduction [47]. However, the limited permeability of the dense pericardial tissue for Ad required that collagenase and hyaluronidase be coadministered with the vector to achieve detectable levels of myocardial gene transfer [47].
Catheter delivery methods
Coronary delivery
The selective catheterization of coronary vasculature provides an opportunity to target myocardium via the regional circulation. However, the intact endothelium of coronary arteries provides a significant barrier to vector penetration into myocardium; therefore, the transduction results are typically suboptimal unless additional steps are undertaken to increase the permeability of the endothelium with VEGF [48], histamine [48] or a low calcium/serotonin perfusion [49]. Another strategy to augment the effectiveness of coronary gene delivery is to increase delivery pressure, which can be achieved via the brief occlusion of blood outflow from the heart distal to the origin of the coronaries [50]. The coronary venous circulation also was exploited with a retroinfusion technique that demonstrated significant transduction of myocardium with reporter Ad vectors in a porcine model [51]. The highest reported myocardial gene transfer (78% of cardiomyocytes) was achieved with the concurrent delivery of Ad vectors via both the left anterior descending artery and the great cardiac vein [52].
Endocardial delivery
Several types of intracardial catheter devices that enables the injection of 10- to 100-μl volumes of vector formulation have been used for myocardial gene transfer in large animal models. These systems typically have demonstrated improved retention of the vector at the delivery site compared with transepicardial needle injections [53]. The introduction of the electrophysiological mapping system NOGA [54] and the magnetic catheter navigation system Niobe [55] may facilitate further advancements in the localization of myocardial gene delivery.
Vascular delivery systems
Catheters for intralumenal delivery
A double balloon local delivery catheter has two inflatable balloons that are used to isolate a segment of artery [56]. A suspension of the gene vector is instilled via a separate lumen into the hermetically sealed segments of artery. The use of this catheter type requires interruption of the circulation for several minutes, making this strategy unsuitable for coronary delivery. This limitation has been addressed with the Dispatch catheter, which has a spiral coil that allows blood flow through the central lumen to occur simultaneously with the pressure-driven delivery of vector via a side chamber aligned with the arterial wall [57]. Additional catheter technologies include the porous balloon catheter, which employs high-pressure jet streams of the vector suspension [58], and the hydrogel balloon catheter, which is manufactured with a polymer gel coating that is passively loaded with a gene vector immediately prior to use [59]. Upon inflation of the hydrogel balloon catheter, the vector on the interface between the hydrogel and the vessel wall migrates laterally toward the artery. Typically, only the remnant endothelium and the innermost media of the artery are transduced effectively with hydrogel balloons. The infiltrator catheter uses an array of microneedles that can be deployed after balloon inflation to penetrate the surrounding arterial layers [60]. The suspension of gene vector is then injected via the needles into the vessel wall. The infiltrator is the only type of vascular delivery catheter capable of signficant transduction of adventitia and outer media of the artery [61].
Perivascular delivery systems
The vascular adventitia plays an important role in atherosclerosis, restenosis and other vasculoproliferative diseases via several key mechanisms [62]. Perivascular gene delivery presents the simplest strategy to affect pathological processes in the adventitia. Thus, several delivery systems for gene therapeutics have been developed to enable the effective delivery of such therapies to the perivascular region.
Pluronic F-127 gel
Aqueous solutions of the poloxamer polymer Pluronic F-127 undergo reversible gelation at temperatures exceeding 20°C. This property of pluronic gel has been exploited extensively for the creation of a perivascular depot consisting of gene vectors entrapped in the pluronic gel [63]. The release of the vector from the gel is relatively rapid, as a result of the dissolution of the gel in tissue fluid in vivo. However, the gel persists at the delivery site for several hours, which is usually sufficient for effective transduction [64]. An admixture of methylcellulose in the gel further protracted the release rate of the vector [64]. Interestingly, in addition to being capable of sustaining the release of gene vectors, poloxamer polymers of a similar structure increased arterial transduction with Ad vectors [65].
Fibrin glue
A matrix formulated from human fibrinogen, fibronectin and thrombin that is commercially available as Tissucol has been used for the site-specific delivery of non-viral and Ad gene vectors. Only a limited number of studies have examined the potential of this system for perivascular gene transfer [66-67]. The results of these investigations appear promising because, in addition to vector immobilization, the fibrin matrix may affect the transfection competency of the target cells directly via integrin interactions [68].
Collagen wraps
Collagen sheets soaked with Ad vectors and wrapped around balloon-injured pig carotid arteries were used as a perivascular delivery system; a significant increase in reporter gene transduction was observed in arteries treated with this strategy compared with a non-wrapped control [69].
All of the methods for perivascular site-specific gene transfer are highly invasive; thus, their clinical utility with respect to safety and efficacy has not been established. However, these techniques have enabled reliable gene transfer in preclinical models.
Stents
The use of stents for the treatment of cardiovascular disease has resulted in a paradigm shift in the standard of care during the past decade [70]. During the past 5 years, drug-eluting stents (ie, polymer-coated stents with controlled release of potent antiproliferative agents) have also had a significant impact on cardiovascular therapy, and have improved the efficacy of stenting for many patients by reducing the risk of reobstruction of the stented blood vessel, a disease process known as in-stent restenosis (ISR) [71]. However, drug-eluting stents do not address the underlying vascular disease at the stented site and, for many patients, the use of drug-eluting stents poses a risk of delayed stent thrombosis [72], which can result in myocardial infarction or death.
Gene-eluting stents
Stents represent an advantageous platform for local vascular gene therapy for several reasons. For example, an increased local arterial concentration of gene vectors can be achieved with immobilized gene-eluting stents compared with the concentrations obtained with non-immobilized vectors following the administration of a smaller input dose. Vector immobilization on the stent also minimizes the distal spread of gene vectors, limiting the inadvertent inoculation of non-target tissues. Additionally, because cell activation and proliferation in the setting of ISR are almost exclusively observed in the vicinity of stent struts [73], the tethering of gene vectors to those stent wires localizes vectors to the anticipated site of action. Thus, the use of gene delivery stents for atherosclerotic vascular disease may enable the advantageous possibility of site-specific gene delivery with long lasting and controllable transgene expression that could not only prevent ISR, but could also provide therapy for the underlying vascular disease.
Initial investigations into gene delivery stents involved the use of polymer coatings on the surface of metallic stents in a strategy that is comparable to that used for drug-eluting stents [3,11,43]. However, polymer coatings are inflammatory [74]; therefore, unless potent drugs, such as the anticancer agents used commonly in drug-eluting stents, are employed, the inflammatory response to the polymer coating negates the therapeutic effect of gene delivery. Two approaches to minimizing the inflammatory response to stent coatings have been investigated: the use of a hydrophilic coating incorporating phosphorylcholine; and the delivery of gene vectors from the metal surfaces of stents that have been modified with polybisphosphonates (Figure 2). Phosphorylcholine-coated stents were used in several studies of gene delivery stents that demonstrated efficacy in the transduction of therapeutic genes [3,11]. The disadvantage of the phosphorylcholine-based approach relates to the passive-elution mechanism involved in the loading and release of the vector from the coating; an aqueous suspension of the vector is simply absorbed prior to stent deployment [3,11], and presumably is eluted rapidly from the coating during both transit and deployment.
Figure 2. Schematic illustration of adenovirus vector immobilization on the metal surface of stents.
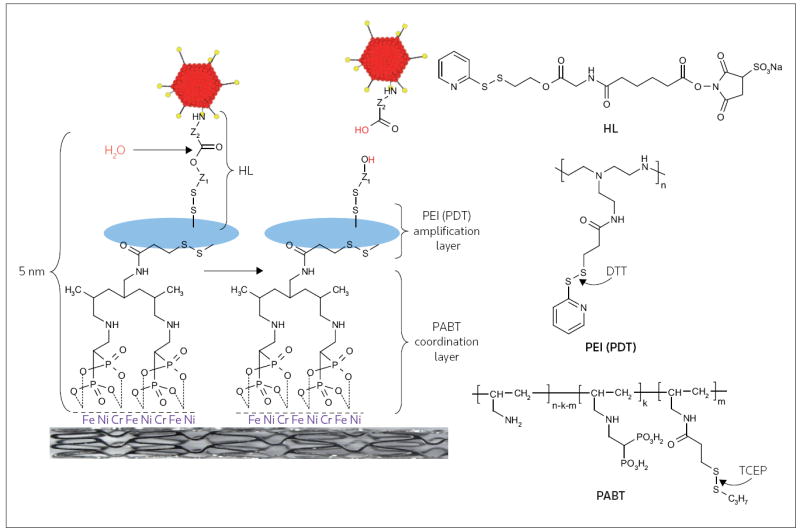
Serotype 5 adenovirus (Ad) vectors were modified by reacting the lysine residues of the viral capsid proteins with a reactive bifunctional amine/thiol hydrolyzable crosslinker (HL) that possessed a hydrolyzable ester bond separating fragments Z1 and Z2. Stainless steel stents were consecutively exposed to a solution of polyallylamine bisphosphonate comprising latent thiol groups (PABT) and the reducing agent tris(2-carboxyethyl)phosphine (TCEP) to activate thiol groups on the PABT-coated surface. To expand the quantity of available thiol functional groups, the stent was also treated with polyethyleneimine modified with pyridyldithio-groups (PEI(PDT)) and dithiothreitol (DTT). Finally, HL-modified Ad vectors were reacted with the thiolated metal surfaces, leading to the covalent tethering of Ad on the stent surface. The release of the covalently immobilized Ad was dependent on the rate of hydrolysis of the ester bond in the HL backbone.
(Adapted from the American Heart Association Inc and Fishbein I, Alferiev I, Bakay M, Stachelek SJ, Sobolewski P, Lai M, Choi H, Chen IW, Levy RJ: Local delivery of gene vectors from bare-metal stents by use of a biodegradable synthetic complex inhibits in-stent restenosis in rat carotid arteries. Circulation (2008) 117(16):2096-2103 © 2008 American Heart Association Inc)
Bare metal stent gene delivery using polybisphosphonates involves the initial formation of the coordination layer on the metal surface of the stent, which is achieved by immersing the stent in an aqueous solution of polybisphosphonate. The molecular monolayer of polybisphosphonate that is permanently appended to the steel can then be modified covalently in order to permit the attachment of vector-binding agents (Figure 2). High-affinity proteins such as antibodies or recombinant receptor fragments can be used to bind either plasmid DNA or viral vectors to the stent surfaces [75]. Synthetic biodegradable linkers that can be used to attach viral vectors to metal stents have been reported (Figure 2) [12]. These novel linkers are particularly promising because they can be synthesized to exhibit a broad variation of hydrolysis durations; therefore, the rate of vector release from the surface of the stent can be programmed to reflect the desired formulation parameters. Additionally, these hydrolyzable linkers also can be used in conjunction with a thiol-amplifying agent to control the magnitude of the dose of vector that is loaded onto the stent (Figure 2). The effective site-specific transduction of arterial substrate with reporter Ad vectors immobilized on the surface of bare metal stents with the poly-bisphosphonate/hydrolyzable linker tethers was demonstrated [12]. Additionally, the immobilization of Ad vectors encoding iNOS resulted in a 40% reduction of neointimal formation in a rat carotid stenting model [12].
Magnetic nanoparticles for targeted delivery to deployed stents
Magnetic nanoparticles (MNPs), which are submicron-sized synthetic biodegradable particles with magnetic responsiveness imparted by nanocrystalline iron oxide and which have the capacity to carry therapeutic content either in the bulk of the particle or attached to the MNP surface [76,77], have been investigated for cardiovascular gene delivery. The magnetizing effect of a strong uniform field, such as that achievable within an MRI scanner environment, on both MNPs and steel stents, enables guided delivery of MNPs to the stented arterial segment and may provide the basis for a novel approach to targeted treatment for in-stent restenosis by addressing the potential need for both an initial delivery and the subsequent readministration of gene vectors that may occur in many clinical circumstances. The feasibility of this targeted gene delivery approach was recently demonstrated using MNPs impregnated with Ad [78]. The superior capacity of MNPs formulated with a reporter Ad to transduce vascular endothelial cells and SMCs in vitro in the presence of a high gradient magnetic field in comparison with non-incorporated Ad was not affected by an Ad-neutralizing antibody, suggesting a Coxsackie-Ad receptor (CAR)-independent mechanism of MNP uptake. This characteristic of the MNP-Ad complexes cell uptake is particularly important in the context of vascular gene transfer because vascular cells are relatively deficient in CAR [44]. Thus, because of the combination of magnetic guidance and a kinetically more favorable cell internalization pathway, this targeted strategy of transgene delivery to stented arteries may both enable a significant reduction in the necessary vector dose and prevent the dissipation of the vector to non-target tissues [79], which could provide the safer delivery and increased efficiency of antirestenotic gene therapy.
The magnetically enhanced transfer of therapeutically relevant genes was demonstrated using MNPs formulated with Ad encoding iNOS [78]. In the magnetic field, the treatment of endothelial cells with Ad-loaded MNPs resulted in a significant, MNP dose-dependent production of nitric oxide (NO). Additional in vitro studies demonstrated that these levels of NO synthesis were therapeutically adequate to inhibit the proliferation of vascular SMCs cocultured with the MNP-treated endothelial cells [80]. The translation of promising in vitro results to the in vivo setting has been accomplished using the previously discussed magnetic targeting approach. This novel targeting scheme was developed as an alternative to the permanent magnet-based guidance strategy, whose utility for therapeutic applications is limited because of the rapid decay and inefficient tissue penetration of the non-uniform fields generated by permanent magnets [81]. Therefore, the use of high gradients induced in a controllable manner on a steel stent by exposure to a strong uniform field (uniform field-induced magnetization effect) has the advantage of providing the direct guidance of MNPs to the stented arterial region under clinically reproducible conditions. The efficiency of in vivo magnetically targeted gene delivery to 304 stainless steel stents was demonstrated in reporter studies in the rat carotid stenting model [78]. Notably, Ad-impregnated MNPs enabled a combination of efficient genetic modification with magnetic cell loading, which is particularly important in the context of cell therapeutic strategies, wherein ex vivo modified cells targeted to magnetizable stents can be used as a platform for site-specific, vascular gene therapy [82].
Pharmacological modulation of gene therapy
The complexity of the signaling pathways impacted by some therapeutic transgenes may result in a requirement for additional therapeutic agents to obtain the desired physiological response. This modulation of gene therapy with conventional pharmaceutical agents is both possible and essential in the case of the induced overexpression of NOS in the cardiovascular system. The pleiotropic nature of the beneficial effects of NO in the vasculature makes the augmentation of NO synthesis via NOS gene transfer a plausible strategy for the prevention of atherosclerosis and ISR [83]. However, the free-radical NO is unstable in physiological conditions; in the presence of reactive oxidative species (ROS), NO is converted rapidly into peroxynitrite. Peroxynitrite exerts direct cytotoxic effects in the vasculature [83,84]. NOS enzymes can be a source of ROS production if the enzyme substrate, arginine, and a principal cofactor, tetrahydrobiopterin (BH4), are deficient [83]. This consideration is particularly relevant to NOS gene therapy, if the augmented production of NOS is not complemented by a similar increase in levels of arginine and BH4. Therefore, the pharmacological supplementation of arginine and BH4 is an essential component of the NOS gene transfer strategy (Figure 1).
Clinical considerations
The choice of the strategy used to implement gene therapy in a clinical setting is dictated by the specific situation. Although gene therapy has been investigated for the treatment of acute medical syndromes, a period of several days between transgene delivery and the maximal therapeutic effect is inevitable with most vector systems. Therefore, gene therapy may be more appropriate for the treatment of conditions with more incipient pathogenesis. Two prominent exceptions to this consideration are the use of gene therapy for the treatment of acute myocardial infarction and for the prevention of ISR. In both of these cases, the pathological processes relating to cellular dynamics and matrix turnover evolve on a weeks-to-months time scale, and thus are amenable to gene therapy. Notably, the acute injuries present in both acute myocardial infarction and ISR render the tissue environment more receptive to lateral vector migration from the delivery site, as a result of tissue edema. Conversely, the low oxygen concentration, low pH and innate immune mediators of the inflammatory environment compromise both the viability and the transduction capacity of the gene vectors. Additionally, the aggressive microenvironment of the acutely injured tissue decreases the survival of transduced cells. For these reasons, therapeutic transgenes with anti-apoptotic effects are of particular interest in the setting of acute myocardial infarction [85]. The acute-phase inflammatory reactions that are detrimental to therapeutic gene transfer are absent in the setting of chronic ischemic and non-ischemic cardiomyopathies. However, with these pathological conditions, the presence of dense connective tissue may limit the local biodistribution of the vector through the myocardium, as was demonstrated for the lateral migration of Ad vectors in tumors [86]. The limited clinical experience with cardiovascular gene therapy in humans does not allow any definite conclusions regarding the advantages and disadvantages of different vectors and delivery systems that have been adapted to specific clinical scenarios.
Future directions
Recent interest in the targeted delivery of stem and progenitor cell populations has encouraged the concept of the synergetic use of gene and cell therapy for the treatment of cardiovascular disease. The central challenge relating to cell delivery in the heart and vasculature is the poor retention and survival of implanted cells in the hostile environment of ischemic tissue [87]. Therefore, the delivery of cells transfected or transduced ex vivo with genes that enhance resistance to apoptosis [88], or provide protection from the stressing microenvironment [89], could improve the function of engrafted cells and consecutive therapeutic outcome. Furthermore, the transduction of the delivered cells with genes capable of modulating the interaction of the cells with the extracellular matrix could potentially increase the retention of delivered cells [88]. Additional progress in the gene therapy field may result, in part, from the merging of gene delivery methods with tissue engineering. The latter technique offers the possibility of using biomaterial scaffolds to guide the ordered repopulation of an implanted material with autologous cells that can be transduced in situ with the scaffold-immobilized gene vectors [90].
Conclusion
Therapeutic gene transfer in the cardiovascular system is of considerable scientific interest both as a strategy to dissect the mechanisms governing the development of common cardiovascular diseases [91] and as a potential treatment modality for patients in whom conventional therapeutic approaches have failed [92]. However, the translation of gene therapies into routine medical practice will require the development and thorough optimization of novel delivery systems capable of the spatial and temporal control of gene vector biodistribution and activity. Delivery systems employing immobilized gene vectors and using induced magnetic fields to target magnetizable implants (such as endovascular steel stents) are examples of delivery approaches that may be capable of reducing the gap between the bench and the bedside.
Acknowledgments
The authors’ research has been supported by grants from the NIH (HL72108), the American Heart Association, the Nanotechnology Institute and the William J Rashkind Endowment of The Children’s Hospital of Philadelphia.
Abbreviations
- Ad
Adenoviral
- iNOS
nitric oxide synthase
- ISR
in-stent restenosis
- HD
helper-dependent
- MNP
magnetic nanoparticle
- NO
nitric oxide
References
-
•
of special interest
-
••
of outstanding interest
- 1.John Wiley & Sons Inc; Hoboken, NJ, USA: 2009. Gene Therapy Clinical Trials Worldwide. www.wiley.co.uk/genmed/clinical/ • A comprehensive searchable online database of gene therapy clinical trials.
- 2.Sanz-González SM, Barquín L, García-Cao I, Roque M, González JM, Fuster JJ, Castells MT, Flores JM, Serrano M, Andrés V. Increased p53 gene dosage reduces neointimal thickening induced by mechanical injury but has no effect on native atherosclerosis. Cardiovasc Res. 2007;75(4):803–812. doi: 10.1016/j.cardiores.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Johnson TW, Wu YX, Herdeg C, Baumbach A, Newby AC, Karsch KR, Oberhoff M. Stent-based delivery of tissue inhibitor of metalloproteinase-3 adenovirus inhibits neointimal formation in porcine coronary arteries. Arterioscler Thromb Vasc Biol. 2005;25(4):754–759. doi: 10.1161/01.ATV.0000157582.33180.a9. [DOI] [PubMed] [Google Scholar]
- 4.Miyagawa S, Sawa Y, Taketani S, Kawaguchi N, Nakamura T, Matsuura N, Matsuda H. Myocardial regeneration therapy for heart failure: Hepatocyte growth factor enhances the effect of cellular cardiomyoplasty. Circulation. 2002;105(21):2556–2561. doi: 10.1161/01.cir.0000016722.37138.f2. [DOI] [PubMed] [Google Scholar]
- 5.Laukkanen MO, Kivelä A, Rissanen T, Rutanen J, Karkkainen MK, Leppanen O, Bräsen JH, Yla-Herttuala S. Adenovirus-mediated extracellular superoxide dismutase gene therapy reduces neointima formation in balloon-denuded rabbit aorta. Circulation. 2002;106(15):1999–2003. doi: 10.1161/01.cir.0000031331.05368.9d. [DOI] [PubMed] [Google Scholar]
- 6.Zoldhelyi P, McNatt J, Shelat HS, Yamamoto Y, Chen ZQ, Willerson JT. Thromboresistance of balloon-injured porcine carotid arteries after local gene transfer of human tissue factor pathway inhibitor. Circulation. 2000;101(3):289–295. doi: 10.1161/01.cir.101.3.289. [DOI] [PubMed] [Google Scholar]
- 7.Egashira K, Nakano K, Ohtani K, Funakoshi K, Zhao G, Ihara Y, Koga J, Kimura S, Tominaga R, Sunagawa K. Local delivery of anti-monocyte chemoattractant protein-1 by gene-eluting stents attenuates in-stent stenosis in rabbits and monkeys. Arterioscler Thromb Vasc Biol. 2007;27(12):2563–2568. doi: 10.1161/ATVBAHA.107.154609. [DOI] [PubMed] [Google Scholar]
- 8.Patel VI, Daniel S, Longo CR, Shrikhande GV, Scali ST, Czismadia E, Groft CM, Shukri T, Motley-Dore C, Ramsey HE, Fisher MD, et al. A20, a modulator of smooth muscle cell proliferation and apoptosis, prevents and induces regression of neointimal hyperplasia. FASEB J. 2006;20(9):1418–1430. doi: 10.1096/fj.05-4981com. [DOI] [PubMed] [Google Scholar]
- 9.Burton DY, Song C, Fishbein I, Hazelwood S, Li Q, DeFelice S, Connolly JM, Perlstein I, Coulter DA, Levy RJ. The incorporation of an ion channel gene mutation associated with the long QT syndrome (Q9E-hMiRP1) in a plasmid vector for site-specific arrhythmia gene therapy: In vitro and in vivo feasibility studies. Hum Gene Ther. 2003;14(9):907–922. doi: 10.1089/104303403765701196. [DOI] [PubMed] [Google Scholar]
- 10.Byrne MJ, Power JM, Preovolos A, Mariani JA, Hajjar RJ, Kaye DM. Recirculating cardiac delivery of AAV2/1SERCA2a improves myocardial function in an experimental model of heart failure in large animals. Gene Ther. 2008;15(23):1550–1557. doi: 10.1038/gt.2008.120. [DOI] [PubMed] [Google Scholar]
- 11.Walter DH, Cejna M, Diaz-Sandoval L, Willis S, Kirkwood L, Stratford PW, Tietz AB, Kirchmair R, Silver M, Curry C, Wecker A, et al. Local gene transfer of phVEGF-2 plasmid by gene-eluting stents. An alternative strategy for inhibition of restenosis. Circulation. 2004;110(1):36–45. doi: 10.1161/01.CIR.0000133324.38115.0A. [DOI] [PubMed] [Google Scholar]
- 12.Fishbein I, Alferiev I, Bakay M, Stachelek SJ, Sobolewski P, Lai M, Choi H, Chen IW, Levy RJ. Local delivery of gene vectors from bare-metal stents by use of a biodegradable synthetic complex inhibits in-stent restenosis in rat carotid arteries. Circulation. 2008;117(16):2096–2103. doi: 10.1161/CIRCULATIONAHA.107.746412. •• Demonstrated the reversible tethering of Ad vectors to polybisphosphonate-modified bare metal stents, as well as both reporter transgene activity and therapeutic (antirestenotic) effects of transgenes delivered via this strategy in a rat carotid stent angioplasty model.
- 13.Papadakis ED, Nicklin SA, Baker AH, White SJ. Promoters and control elements: Designing expression cassettes for gene therapy. Curr Gene Ther. 2004;4(1):89–113. doi: 10.2174/1566523044578077. • A well-written and in-depth review of cell-type-specific and non-specific promoter systems employed in the design of gene vectors.
- 14.Inesi G, Lewis D, Sumbilla C, Nandi A, Strock C, Huff KW, Rogers TB, Johns DC, Kessler PD, Ordahl CP. Cell-specific promoter in adenovirus vector for transgenic expression of SERCA1 ATPase in cardiac myocytes. Am J Physiol. 1998;274(3 Pt 1):C645–C653. doi: 10.1152/ajpcell.1998.274.3.C645. [DOI] [PubMed] [Google Scholar]
- 15.Akyurek LM, Yang ZY, Aoki K, San H, Nabel GJ, Parmacek MS, Nabel EG. SM22α promoter targets gene expression to vascular smooth muscle cells in vitro and in vivo. Mol Med. 2000;6(11):983–991. [PMC free article] [PubMed] [Google Scholar]
- 16.Gory S, Vernet M, Laurent M, Dejana E, Dalmon J, Huber P. The vascular endothelial-cadherin promoter directs endothelial-specific expression in transgenic mice. Blood. 1999;93(1):184–192. [PubMed] [Google Scholar]
- 17.Horvai A, Palinski W, Wu H, Moulton KS, Kalla K, Glass CK. Scavenger receptor A gene regulatory elements target gene expression to macrophages and to foam cells of atherosclerotic lesions. Proc Natl Acad Sci USA. 1995;92(12):5391–5395. doi: 10.1073/pnas.92.12.5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Appleby CE, Kingston PA, David A, Gerdes CA, Umana P, Castro MG, Lowenstein PR, Heagerty AM. A novel combination of promoter and enhancers increases transgene expression in vascular smooth muscle cells in vitro and coronary arteries in vivo after adenovirus-mediated gene transfer. Gene Ther. 2003;10(18):1616–1622. doi: 10.1038/sj.gt.3302044. • Demonstrated that the rational design of promoter and enhancer elements resulted in a 40-fold increase of reporter transgene expression in pig coronary arteries following local intralumenal gene delivery.
- 19.Sarkar N, Blomberg P, Wardell E, Eskandarpour M, Sylven C, Drvota V, Islam KB. Nonsurgical direct delivery of plasmid DNA into rat heart: Time course, dose response, and the influence of different promoters on gene expression. J Cardiovasc Pharmacol. 2002;39(2):215–224. doi: 10.1097/00005344-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Argyros O, Wong SP, Niceta M, Waddington SN, Howe SJ, Coutelle C, Miller AD, Harbottle RP. Persistent episomal transgene expression in liver following delivery of a scaffold/matrix attachment region containing non-viral vector. Gene Ther. 2008;15(24):1593–1605. doi: 10.1038/gt.2008.113. [DOI] [PubMed] [Google Scholar]
- 21.Hyde SC, Pringle IA, Abdullah S, Lawton AE, Davies LA, Varathalingam A, Nunez-Alonso G, Green AM, Bazzani RP, Sumner-Jones SG, Chan M, et al. CpG-free plasmids confer reduced inflammation and sustained pulmonary gene expression. Nat Biotechnol. 2008;26(5):549–551. doi: 10.1038/nbt1399. [DOI] [PubMed] [Google Scholar]
- 22.Kupatt C, Wichels R, Deiss M, Molnar A, Lebherz C, Raake P, von Degenfeld G, Hahnel D, Boekstegers P. Retroinfusion of NFκB decoy oligonucleotide extends cardioprotection achieved by CD18 inhibition in a preclinical study of myocardial ischemia and retroinfusion in pigs. Gene Ther. 2002;9(8):518–526. doi: 10.1038/sj.gt.3301673. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Fukuda N, Kunimoto S, Yokoyama S, Hagikura K, Kawano T, Takayama T, Honye J, Kobayashi N, Mugishima H, Saito S, et al. Stent-based delivery of antisense oligodeoxynucleotides targeted to the PDGF A-chain decreases in-stent restenosis of the coronary artery. J Cardiovasc Pharmacol. 2006;48(4):184–190. doi: 10.1097/01.fjc.0000246940.91191.1f. [DOI] [PubMed] [Google Scholar]
- 24.Kutryk MJ, Foley DP, van den Brand M, Hamburger JN, van der Giessen WJ, deFeyter PJ, Bruining N, Sabate M, Serruys PW. Local intracoronary administration of antisense oligonucleotide against c-myc for the prevention of in-stent restenosis: Results of the randomized Investigation by the Thoraxcenter of Antisense DNA using Local delivery and IVUS after Coronary Stenting (ITALICS) trial. J Am Coll Cardiol. 2002;39(2):281–287. doi: 10.1016/s0735-1097(01)01741-7. [DOI] [PubMed] [Google Scholar]
- 25.de Fougerolles A, Vornlocher HP, Maraganore J, Lieberman J. Interfering with disease: A progress report on siRNA-based therapeutics. Nat Rev Drug Discov. 2007;6(6):443–453. doi: 10.1038/nrd2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karshovska E, Zernecke A, Sevilmis G, Millet A, Hristov M, Cohen CD, Schmid H, Krotz F, Sohn HY, Klauss V, Weber C, et al. Expression of HIF-1α in injured arteries controls SDF-1α mediated neointima formation in apolipoprotein E deficient mice. Arterioscler Thromb Vasc Biol. 2007;27(12):2540–2547. doi: 10.1161/ATVBAHA.107.151050. [DOI] [PubMed] [Google Scholar]
- 27.Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, Clappier E, Caccavelli L, Delabesse E, Beldjord K, Asnafi V, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118(9):3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon EM, Zhu NL, Forney Prescott M, Chen ZH, Anderson WF, Hall FL. Lesion-targeted injectable vectors for vascular restenosis. Hum Gene Ther. 2001;12(10):1277–1287. doi: 10.1089/104303401750270931. • Developed a collagen-targeted retroviral vector, incorporating a tissue-targeting motif containing von Willebrand factor, that demonstrated a 20-fold improvement in the transduction of neointimal cells after both systemic and local delivery compared with the non-targeted vector.
- 29.Zhao J, Pettigrew GJ, Thomas J, Vandenberg JI, Delriviere L, Bolton EM, Carmichael A, Martin JL, Marber MS, Lever AM. Lentiviral vectors for delivery of genes into neonatal and adult ventricular cardiac myocytes in vitro and in vivo. Basic Res Cardiol. 2002;97(5):348–358. doi: 10.1007/s00395-002-0360-0. [DOI] [PubMed] [Google Scholar]
- 30.Wright MJ, Wightman LM, Latchman DS, Marber MS. In vivo myocardial gene transfer: Optimization and evaluation of intracoronary gene delivery in vivo. Gene Ther. 2001;8(24):1833–1839. doi: 10.1038/sj.gt.3301614. [DOI] [PubMed] [Google Scholar]
- 31.Shears LL, 2nd, Kibbe MR, Murdock AD, Billiar TR, Lizonova A, Kovesdi I, Watkins SC, Tzeng E. Efficient inhibition of intimal hyperplasia by adenovirus-mediated inducible nitric oxide synthase gene transfer to rats and pigs in vivo. J Am Coll Surg. 1998;187(3):295–306. doi: 10.1016/s1072-7515(98)00163-x. [DOI] [PubMed] [Google Scholar]
- 32.Bangari DS, Mittal SK. Current strategies and future directions for eluding adenoviral vector immunity. Curr Gene Ther. 2006;6(2):215–226. doi: 10.2174/156652306776359478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmer DJ, Ng P. Helper-dependent adenoviral vectors for gene therapy. Hum Gene Ther. 2005;16(1):1–16. doi: 10.1089/hum.2005.16.1. [DOI] [PubMed] [Google Scholar]
- 34.Wen S, Graf S, Massey PG, Dichek DA. Improved vascular gene transfer with a helper-dependent adenoviral vector. Circulation. 2004;110(11):1484–1491. doi: 10.1161/01.CIR.0000141574.78032.A9. • Compared the utility of first-generation Ad vectors and third-generation (HD) Ad vectors for vascular gene transfer. Following intralumenal dwell delivery in rabbit carotid arteries, the third-generation vectors achieved sustained (≥ 56 days) transgene expression and caused significantly milder inflammation than the corresponding first-generation vectors.
- 35.Fleury S, Driscoll R, Simeoni E, Dudler J, von Segesser LK, Kappenberger L, Vassalli G. Helper-dependent adenovirus vectors devoid of all viral genes cause less myocardial inflammation compared with first-generation adenovirus vectors. Basic Res Cardiol. 2004;99(4):247–256. doi: 10.1007/s00395-004-0471-x. [DOI] [PubMed] [Google Scholar]
- 36.Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M, Herzog RW, Zolotukhin I, Warrington KH, Jr, Weigel-Van Aken KA, Hobbs JA, et al. Next generation of adeno-associated virus 2 vectors: Point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci USA. 2008;105(22):7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bish LT, Sleeper MM, Brainard B, Cole S, Russell N, Withnall E, Arndt J, Reynolds C, Davison E, Sanmiguel J, Wu D, et al. Percutaneous transendocardial delivery of self-complementary adeno-associated virus 6 achieves global cardiac gene transfer in canines. Mol Ther. 2008;16(12):1953–1959. doi: 10.1038/mt.2008.202. [DOI] [PubMed] [Google Scholar]
- 38.Bish LT, Morine K, Sleeper MM, Sanmiguel J, Wu D, Gao G, Wilson JM, Sweeney L. Adeno-associated virus (AAV) serotype 9 provides global cardiac gene transfer superior to AAV1, AAV6, AAV7, and AAV8 in the mouse and rat. Hum Gene Ther. 2008;19(12):1359–1368. doi: 10.1089/hum.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dandapat A, Hu CP, Li D, Liu Y, Chen H, Hermonat PL, Mehta JL. Overexpression of TGFβ1 by adeno-associated virus type-2 vector protects myocardium from ischemia-reperfusion injury. Gene Ther. 2008;15(6):415–423. doi: 10.1038/sj.gt.3303071. [DOI] [PubMed] [Google Scholar]
- 40.Fishbein I, Alferiev IS, Lai M, Levy RJ. AAV2.9 vectors attain robust and sustained gene expression in two models of vascular gene transfer. Circulation. 2007;116:II–64. [Google Scholar]
- 41.Noureddini SC, Curiel DT. Genetic targeting strategies for adenovirus. Mol Pharm. 2005;2(5):341–347. doi: 10.1021/mp050045c. [DOI] [PubMed] [Google Scholar]
- 42.Kreppel F, Kochanek S. Modification of adenovirus gene transfer vectors with synthetic polymers: A scientific review and technical guide. Mol Ther. 2008;16(1):16–29. doi: 10.1038/sj.mt.6300321. [DOI] [PubMed] [Google Scholar]
- 43.Perlstein I, Connolly JM, Cui X, Song C, Li Q, Jones PL, Lu Z, DeFelice S, Klugherz B, Wilensky R, Levy RJ. DNA delivery from an intravascular stent with a denatured collagen-polylactic-polyglycolic acid-controlled release coating: Mechanisms of enhanced transfection. Gene Ther. 2003;10(17):1420–1428. doi: 10.1038/sj.gt.3302043. [DOI] [PubMed] [Google Scholar]
- 44.Chorny M, Fishbein I, Alferiev IS, Nyanguile O, Gaster R, Levy RJ. Adenoviral gene vector tethering to nanoparticle surfaces results in receptor-independent cell entry and increased transgene expression. Mol Ther. 2006;14(3):382–391. doi: 10.1016/j.ymthe.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 45.French BA, Mazur W, Geske RS, Bolli R. Direct in vivo gene transfer into porcine myocardium using replication-deficient adenoviral vectors. Circulation. 1994;90(5):2414–2424. doi: 10.1161/01.cir.90.5.2414. [DOI] [PubMed] [Google Scholar]
- 46.Li JJ, Ueno H, Pan Y, Tomita H, Yamamoto H, Kanegae Y, Saito I, Takeshita A. Percutaneous transluminal gene transfer into canine myocardium in vivo by replication-defective adenovirus. Cardiovasc Res. 1995;30(1):97–105. [PubMed] [Google Scholar]
- 47.Fromes Y, Salmon A, Wang X, Collin H, Rouche A, Hagege A, Schwartz K, Fiszman MY. Gene delivery to the myocardium by intrapericardial injection. Gene Ther. 1999;6(4):683–688. doi: 10.1038/sj.gt.3300853. [DOI] [PubMed] [Google Scholar]
- 48.Logeart D, Hatem SN, Heimburger M, Le Roux A, Michel JB, Mercadier JJ. How to optimize in vivo gene transfer to cardiac myocytes: Mechanical or pharmacological procedures? Hum Gene Ther. 2001;12(13):1601–1610. doi: 10.1089/10430340152528101. [DOI] [PubMed] [Google Scholar]
- 49.Donahue JK, Kikkawa K, Thomas AD, Marban E, Lawrence JH. Acceleration of widespread adenoviral gene transfer to intact rabbit hearts by coronary perfusion with low calcium and serotonin. Gene Ther. 1998;5(5):630–634. doi: 10.1038/sj.gt.3300649. [DOI] [PubMed] [Google Scholar]
- 50.Hajjar RJ, Schmidt U, Matsui T, Guerrero JL, Lee KH, Gwathmey JK, Dec GW, Semigran MJ, Rosenzweig A. Modulation of ventricular function through gene transfer in vivo. Proc Natl Acad Sci USA. 1998;95(9):5251–5256. doi: 10.1073/pnas.95.9.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boekstegers P, von Degenfeld G, Giehrl W, Heinrich D, Hullin R, Kupatt C, Steinbeck G, Baretton G, Middeler G, Katus H, Franz WM. Myocardial gene transfer by selective pressure-regulated retroinfusion of coronary veins. Gene Ther. 2000;7(3):232–240. doi: 10.1038/sj.gt.3301079. [DOI] [PubMed] [Google Scholar]
- 52.Sasano T, Kikuchi K, McDonald AD, Lai S, Donahue JK. Targeted high-efficiency, homogeneous myocardial gene transfer. J Mol Cell Cardiol. 2007;42(5):954–961. doi: 10.1016/j.yjmcc.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grossman PM, Han Z, Palasis M, Barry JJ, Lederman RJ. Incomplete retention after direct myocardial injection. Catheter Cardiovasc Interv. 2002;55(3):392–397. doi: 10.1002/ccd.10136. [DOI] [PubMed] [Google Scholar]
- 54.Vale PR, Losordo DW, Milliken CE, McDonald MC, Gravelin LM, Curry CM, Esakof DD, Maysky M, Symes JF, Isner JM. Randomized, single-blind, placebo-controlled pilot study of catheter-based myocardial gene transfer for therapeutic angiogenesis using left ventricular electromechanical mapping in patients with chronic myocardial ischemia. Circulation. 2001;103(17):2138–2143. doi: 10.1161/01.cir.103.17.2138. [DOI] [PubMed] [Google Scholar]
- 55.Carpi F, Pappone C. Stereotaxis Niobe magnetic navigation system for endocardial catheter ablation and gastrointestinal capsule endoscopy. Expert Rev Med Devices. 2009;6(5):487–498. doi: 10.1586/erd.09.32. [DOI] [PubMed] [Google Scholar]
- 56.Thompson MM, Budd JS, Eady SL, James RF, Bell PR, Steg PG, Feldman LJ, Scoazec JY, Tahlil O, Barry JJ, Boulechfar S, et al. A method to transluminally seed angioplasty sites with endothelial cells using a double balloon catheter. Eur J Vasc Surg. 1993;7(2):113–121. doi: 10.1016/s0950-821x(05)80750-9. [DOI] [PubMed] [Google Scholar]
- 57.Tahlil O, Brami M, Feldman LJ, Branellec D, Steg PG. The Dispatch catheter as a delivery tool for arterial gene transfer. Cardiovasc Res. 1997;33(1):181–187. doi: 10.1016/s0008-6363(96)00188-5. [DOI] [PubMed] [Google Scholar]
- 58.Plante S, Dupuis G, Mongeau CJ, Durand P. Porous balloon catheters for local delivery: Assessment of vascular damage in a rabbit iliac angioplasty model. J Am Coll Cardiol. 1994;24(3):820–824. doi: 10.1016/0735-1097(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 59.Riessen R, Rahimizadeh H, Blessing E, Takeshita S, Barry JJ, Isner JM. Arterial gene transfer using pure DNA applied directly to a hydrogel-coated angioplasty balloon. Hum Gene Ther. 1993;4(6):749–758. doi: 10.1089/hum.1993.4.6-749. [DOI] [PubMed] [Google Scholar]
- 60.Barath P, Popov A, Dillehay GL, Matos G, McKiernan T. Infiltrator angioplasty balloon catheter: A device for combined angioplasty and intramural site-specific treatment. Cathet Cardiovasc Diagn. 1997;41(3):333–341. doi: 10.1002/(sici)1097-0304(199707)41:3<333::aid-ccd15>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 61.Morishige K, Shimokawa H, Yamawaki T, Miyata K, Eto Y, Kandabashi T, Yogo K, Higo T, Egashira K, Ueno H, Takeshita A. Local adenovirus-mediated transfer of C-type natriuretic peptide suppresses vascular remodeling in porcine coronary arteries in vivo. J Am Coll Cardiol. 2000;35(4):1040–1047. doi: 10.1016/s0735-1097(99)00625-7. [DOI] [PubMed] [Google Scholar]
- 62.Maiellaro K, Taylor WR. The role of the adventitia in vascular inflammation. Cardiovasc Res. 2007;75(4):640–648. doi: 10.1016/j.cardiores.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Villa AE, Guzman LA, Poptic EJ, Labhasetwar V, D’Souza S, Farrell CL, Plow EF, Levy RJ, DiCorleto PE, Topol EJ. Effects of antisense c-myb oligonucleotides on vascular smooth muscle cell proliferation and response to vessel wall injury. Circ Res. 1995;76(4):505–513. doi: 10.1161/01.res.76.4.505. [DOI] [PubMed] [Google Scholar]
- 64.Desai SD, Blanchard J. Evaluation of Pluronic F127-based sustained-release ocular delivery systems for pilocarpine using the albino rabbit eye model. J Pharm Sci. 1998;87(10):1190–1195. doi: 10.1021/js980222j. [DOI] [PubMed] [Google Scholar]
- 65.Van Belle E, Maillard L, Rivard A, Fabre JE, Couffinhal T, Kearney M, Branellec D, Feldman LJ, Walsh K, Isner JM. Effects of poloxamer 407 on transfection time and percutaneous adenovirus-mediated gene transfer in native and stented vessels. Hum Gene Ther. 1998;9(7):1013–1024. doi: 10.1089/hum.1998.9.7-1013. [DOI] [PubMed] [Google Scholar]
- 66.Khurana VG, Weiler DA, Witt TA, Smith LA, Kleppe LS, Parisi JE, Simari RD, O’Brien T, Russell SJ, Katusic ZS. A direct mechanical method for accurate and efficient adenoviral vector delivery to tissues. Gene Ther. 2003;10(5):443–452. doi: 10.1038/sj.gt.3301907. [DOI] [PubMed] [Google Scholar]
- 67.Wan L, Li D, Wu Q. Perivenous application of fibrin glue as external support enhanced adventitial adenovirus transfection in rabbit model. J Surg Res. 2006;135(2):312–316. doi: 10.1016/j.jss.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 68.Lei P, Padmashali RM, Andreadis ST. Cell-controlled and spatially arrayed gene delivery from fibrin hydrogels. Biomaterials. 2009;30(22):3790–3799. doi: 10.1016/j.biomaterials.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pakkanen TM, Laitinen M, Hippelainen M, Hiltunen MO, Alhava E, Yla-Herttuala S. Periadventitial lacZ gene transfer to pig carotid arteries using a biodegradable collagen collar or a wrap of collagen sheet with adenoviruses and plasmid-liposome complexes. J Gene Med. 2000;2(1):52–60. doi: 10.1002/(SICI)1521-2254(200001/02)2:1<52::AID-JGM82>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 70.Serruys PW, Kutryk MJ, Ong AT. Coronary-artery stents. N Engl J Med. 2006;354(5):483–495. doi: 10.1056/NEJMra051091. [DOI] [PubMed] [Google Scholar]
- 71.Butt M, Connolly D, Lip GY. Drug-eluting stents: A comprehensive appraisal. Future Cardiol. 2009;5(2):141–157. doi: 10.2217/14796678.5.2.141. [DOI] [PubMed] [Google Scholar]
- 72.Lüscher TF, Steffel J, Eberli FR, Joner M, Nakazawa G, Tanner FC, Virmani R. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation. 2007;115(8):1051–1058. doi: 10.1161/CIRCULATIONAHA.106.675934. [DOI] [PubMed] [Google Scholar]
- 73.Yutani C, Ishibashi-Ueda H, Suzuki T, Kojima A. Histologic evidence of foreign body granulation tissue and de novo lesions in patients with coronary stent restenosis. Cardiology. 1999;92(3):171–177. doi: 10.1159/000006967. [DOI] [PubMed] [Google Scholar]
- 74.van der Giessen WJ, Lincoff AM, Schwartz RS, van Beusekom HM, Serruys PW, Holmes DR, Jr, Ellis SG, Topol EJ. Marked inflammatory sequelae to implantation of biodegradable and nonbiodegradable polymers in porcine coronary arteries. Circulation. 1996;94(7):1690–1697. doi: 10.1161/01.cir.94.7.1690. [DOI] [PubMed] [Google Scholar]
- 75.Fishbein I, Alferiev IS, Nyanguile O, Gaster R, Vohs JM, Wong GS, Felderman H, Chen IW, Choi H, Wilensky RL, Levy RJ. Bisphosphonate-mediated gene vector delivery from the metal surfaces of stents. Proc Natl Acad Sci USA. 2006;103(1):159–164. doi: 10.1073/pnas.0502945102. • Describes a novel polybisphosphonate modification of the stent surface allowing the site-specific delivery of Ad vectors from the stent platform using anti-Ad antibodies or recombinant fragment of CAR as vector tethers.
- 76.Arruebo M, Fernández-Pacheco R, Ibarra MR, Santamaría J. Magnetic nanoparticles for drug delivery. Nano Today. 2007;2(3):22–32. [Google Scholar]
- 77.McBain SC, Yiu HH, Dobson J. Magnetic nanoparticles for gene and drug delivery. Int J Nanomedicine. 2008;3(2):169–180. doi: 10.2147/ijn.s1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chorny M, Fishbein I, Levy RJ. Efficient magnetically driven gene delivery in cultured vascular cells and in the rat carotid stenting model using adenovirus-impregnated nanoparticles. Mol Ther. 2008;16(1S):Abs 325. [Google Scholar]
- 79.Chorny M, Fishbein I, Alferiev I, Levy RJ. Magnetically responsive biodegradable nanoparticles enhance adenoviral gene transfer in cultured smooth muscle and endothelial cells. Mol Pharm. 2009;6(5):1380–1387. doi: 10.1021/mp900017m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chorny M, Fishbein I, Levy RJ. Endothelial cells transduced with magnetically responsive nanoparticles formulated with iNOS encoding adenovirus inhibit proliferation of aortic smooth muscle cells in a direct co-culture model. Mol Ther. 2009;17(1S):Abs 1035. [Google Scholar]
- 81.Yellen BB, Forbes ZG, Halverson DS, Fridman G, Barbee KA, Chorny M, Levy R, Friedman G. Targeted drug delivery to magnetic implants for therapeutic applications. J Magn Magn Mater. 2005;293(1):647–654. [Google Scholar]
- 82.Polyak B, Fishbein I, Chorny M, Alferiev I, Williams D, Yellen B, Friedman G, Levy RJ. High field magnetic gradients can target magnetic nanoparticle-loaded endothelial cells to the surfaces of steel stents. Proc Natl Acad Sci USA. 2008;105(2):698–703. doi: 10.1073/pnas.0708338105. •• This proof-of-principle study demonstrated the capture of regionally delivered Ad-transduced endothelial cells loaded with MNPs onto stainless steel stents in a high-gradient magnetic field induced by paired electromagnets.
- 83.O’Connor DM, O’Brien T. Nitric oxide synthase gene therapy: Progress and prospects. Expert Opin Biol Ther. 2009;9(7):867–878. doi: 10.1517/14712590903002047. [DOI] [PubMed] [Google Scholar]
- 84.Naseem KM. The role of nitric oxide in cardiovascular diseases. Mol Aspects Med. 2005;26(12):33–65. doi: 10.1016/j.mam.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 85.Li W, Ma N, Ong LL, Nesselmann C, Klopsch C, Ladilov Y, Furlani D, Piechaczek C, Moebius JM, Lützow K, Lendlein A, et al. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells. 2007;25(8):2118–2127. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- 86.Cheng J, Sauthoff H, Huang Y, Kutler DI, Bajwa S, Rom WN, Hay JG. Human matrix metalloproteinase-8 gene delivery increases the oncolytic activity of a replicating adenovirus. Mol Ther. 2007;15(11):1982–1990. doi: 10.1038/sj.mt.6300264. [DOI] [PubMed] [Google Scholar]
- 87.Roncalli J, Tongers J, Renault MA, Losordo DW. Biological approaches to ischemic tissue repair: Gene- and cell-based strategies. Expert Rev Cardiovasc Ther. 2008;6(5):653–668. doi: 10.1586/14779072.6.5.653. [DOI] [PubMed] [Google Scholar]
- 88.Penn MS, Mangi AA. Genetic enhancement of stem cell engraftment, survival, and efficacy. Circ Res. 2008;102(12):1471–1482. doi: 10.1161/CIRCRESAHA.108.175174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kong D, Melo LG, Mangi AA, Zhang L, Lopez-Ilasaca M, Perrella MA, Liew CC, Pratt RE, Dzau VJ. Enhanced inhibition of neointimal hyperplasia by genetically engineered endothelial progenitor cells. Circulation. 2004;109(14):1769–1775. doi: 10.1161/01.CIR.0000121732.85572.6F. [DOI] [PubMed] [Google Scholar]
- 90.Spadaccio C, Chello M, Trombetta M, Rainer A, Toyoda Y, Genovese JA. Drug releasing systems in cardiovascular tissue engineering. J Cell Mol Med. 2009;13(3):422–439. doi: 10.1111/j.1582-4934.2008.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Channon KM, Qian H, George SE. Nitric oxide synthase in atherosclerosis and vascular injury: Insights from experimental gene therapy. Arterioscler Thromb Vasc Biol. 2000;20(8):1873–1881. doi: 10.1161/01.atv.20.8.1873. [DOI] [PubMed] [Google Scholar]
- 92.Staudacher DL, Flugelman MY. Cell and gene therapies in cardiovascular disease with special focus on the no option patient. Curr Gene Ther. 2006;6(6):609–623. doi: 10.2174/156652306779010705. [DOI] [PubMed] [Google Scholar]


