Abstract
Some cognitive disturbances accompanying schizophrenia may be due to abnormalities in the thalamus and components of the limbic system. The fornix is an important white-matter relay pathway connecting these structures and is likely to be affected in schizophrenia as well.
Magnetic resonance images of the fornix were analyzed in 15 schizophrenic patients and 15 matched comparison group subjects. Fornix volume was compared between the two groups and was also correlated with the volumes of other neuroanatomical structures, as well as with illness presentation, clinical status, and cognitive/psychological measures.
There was no significant difference in fornix volume between the two groups. Of note, fornix volume correlated significantly with the volumes of the hippocampus, parahippocampus, and the superior temporal gyrus in the schizophrenic subjects, but not in the controls. Moreover, the correlation between fornix and parahippocampal gyrus volumes differed significantly between the two groups. No association was found between fornix volume and illness presentation or between fornix and cognitive/clinical measures.
Results suggest that there are no marked changes in fornix volume in schizophrenia by MRI. The fornix, however, may be part of a network of structures affected in schizophrenia, as indicated by correlated volumetric changes.
Keywords: Fornix, Hippocampus, Image processing, Magnetic resonance imaging, MR, Schizophrenia, Volumetric analysis
1. Introduction
Findings from recent magnetic resonance (MR) studies in schizophrenia suggest that at least some of the characteristic cognitive and affective disturbances observed may be due to abnormalities in the hippocampal formation, a neuroanatomical structure consolidating associative and declarative memory and assisting in assigning meaning to percepts and concepts (see review in Wible et al., 1997; see also Squire, 1992). Abnormalities in the thalamus (e.g. Andreasen et al., 1994; Portas et al., 1998) as well as structures associated with the limbic system and medial temporal region, such as the hippocampus, have also been implicated (e.g. Bogerts et al., 1985; Falkai et al., 1988; Shenton et al., 1997).
Despite frequent focus on memory and affect in schizophrenia research, the fornix — a dense group of axons with cell bodies located in the hippocampus, projecting to the contralateral hippocampus and the thalamus, and terminating in the mamillary bodies (Carpenter, 1991) — has been largely overlooked. In fact, there are no reliable MR studies of the fornix in schizophrenia. This is particularly surprising given that the fornix is a key fiber tract connecting two structures, the hippocampus and thalamus, both of which have been implicated in schizophrenia (for the role of the hippocampus in schizophrenia, see review in Shenton et al., 1997; McCarley et al., 1999; for the role of the thalamus in schizophrenia, see Andreasen et al., 1990, 1994; Corey-Bloom et al., 1995; Flaum et al., 1995; Buchsbaum, 1996; Andreasen, 1997; Shenton et al., 1997, 2000; Portas et al., 1998).
Despite the sparseness of fornix studies in schizophrenia, and some controversy over the precise function of the fornix, there is evidence from a number of sources that it may play a role in functions that are disturbed in schizophrenia. For example, the fornix may be involved in memory retrieval (Calabrese et al., 1995), verbal memory (Calabrese et al., 1995; McMackin et al., 1995), spatial memory (Gaffan, 1994; Parker and Gaffan, 1997), as well as in increased motor activity (Weiner et al., 1998). Furthermore, recent work by Dusek and Eichenbaum (1997) with fornix-lesioned rats suggests that the fornix and hippocampus are also involved in the ability to remember and to flexibly apply relationships among an ordered set of objects (‘transitive inference’). The role of the fornix in these various brain functions thus warrants a closer examination for its possible involvement in schizophrenia.
The present study used contiguous high-resolution thin-slice (1.5 mm) MR imaging, semi-automated and manual segmentation techniques, and three-dimensional volume rendering tools to compare fornix volumes between schizophrenic patients and a comparison group. The in-plane resolution of our MRI images was 0.937 mm, while the average diameter of the fornix in the normal population is approximately 5 mm (Gale et al., 1993). These figures allowed a suitable level of accuracy in image processing, making quantitative analysis of the fornix possible.
2. Methods
2.1. Sample
A complete description of the sample used in this study is described elsewhere (e.g. Shenton et al., 1992; Wible et al., 1995). In summary, the patient population comprised 15 male schizophrenics from the Brockton Veterans Administration Medical Center, 13 of whom were hospitalized. Diagnoses of the patients were made according to criteria in DSM-III-R (American Psychiatric Association, 1987), using information obtained from a review of the patients’ charts and administration of the Schedule for Affective Disorders and Schizophrenia (Spitzer and Endicott, 1978). The clinical research interviews were conducted by the senior author (MES). The patients were between 20 and 54 years of age (mean: 37.6 years; S.D.:±9.3), right-handed, and had never undergone electroconvulsive shock treatment. Furthermore, they had no history of neurological problems, major drug or alcohol abuse within the past 5 years, alcohol dependence, or a DSM-III-R diagnosis of alcohol abuse. They also had not received any medications known to affect the appearance of brain structures on MR. The patients’ mean level of education was 12.5 years, and the parental socio-economic status was lower middle class (Hollingshead, 1965, classification: 3.4±0.8, where a 3.0 represents skilled craftsman/clerical worker/salesperson). The average age of onset of illness was 22.3 (±2.8) years, with a mean duration of 7.1 (±4.6) years. All patients were on neuroleptic medication, receiving an average of 881 mg/day chloropromazine equivalents.
The comparison group consisted of 15 adult males, between 23 and 54 years of age (mean: 37.9; S.D.:±8.8 years), recruited from the surrounding community. These subjects were matched to patients on age (within 2 years), gender (all male), and handedness (all right-handed), and they were group-matched on parental socio-economic status. The comparison subjects had no lifetime history of drug or alcohol addiction, or DSM-III-R abuse within the past 5 years. In addition, they had no diagnosed psychiatric illness in themselves or in their first-degree relatives. They also had no history of steroid use (which can affect the brain), neurological illness, or electroconvulsive shock treatment. The comparison group subjects did not differ significantly from patients in height, weight, head circumference, nor on scores on the WAIS-R information sub-scale (Wechsler, 1981, 1987).
2.2. Clinical measures
Three tests were used to evaluate the type and severity of schizophrenic symptoms: (1) the Scale for the Assessment of Positive Symptoms (SAPS; Andreasen, 1984); (2) the Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1981); and, (3) the Thought Disorder Index (TDI: Johnston and Holzman, 1979). The patients’ mean TDI score was 60±62 (median=44), whereas comparison subjects score less than five (Johnston and Holzman, 1979). Given the lack of psychiatric illness in the comparison group subjects in this study, they were not administered the TDI. Using the SAPS and SANS, and using the categorization scheme described by Andreasen (1981, 1984), 11 of 15 patients were categorized as showing chiefly positive symptoms, the remaining four showed mixed symptoms, and none could be classified as exhibiting mainly negative symptoms. The mean global SAPS score was 11±3.5, and the mean global SANS score for these patients was 9.1±4.1.
Additionally, neuropsychological measures were administered and scored by a neuropsychologist (PG). These measures included the logical memory subset, the visual memory subset, and the verbal paired associates subset of the Wechsler Memory Scale — Revised (Wechsler, 1987).
2.3. Image acquisition and processing
MR images were acquired on a 1.5 Tesla General Electric Scanner (GE Medical Systems, Milwaukee, WI), using a 3D Fourier Transform Spoiled Gradient-Recalled Acquisition sequence (3DFT SPGR), resulting in a coronal series of 124 1.5 mm contiguous slices.
To measure total intracranial contents (ICC), a double-echo spin-echo sequence was used, which resulted in an axial series of 108 interleaved, 3 mm contiguous slices. An automated segmentation algorithm was used to segment the tissue into gray matter, white matter, and cerebrospinal fluid; the sum of the volumes of these three tissue types was used as the ICC value (Shenton et al., 1992; Wible et al., 1995). The ICC was used to correct for differences in head size by computing relative volume for the regions of interest (ROI) (i.e. relative volume=ROI/ICC×100). The left and right portions of certain neuroanatomical structures (e.g. the thalamus) have sometimes been studied separately to determine the presence of lateralized changes. This would not have been a meaningful approach in the case of the fornix, however, because the left and right crura of the fornix merge to form a single body that cannot be divided into left and right portions on MRI (see Fig. 1). Thus, only the total relative fornix volume was used in all calculations, except for the comparison of means where both absolute and relative volumes were compared.
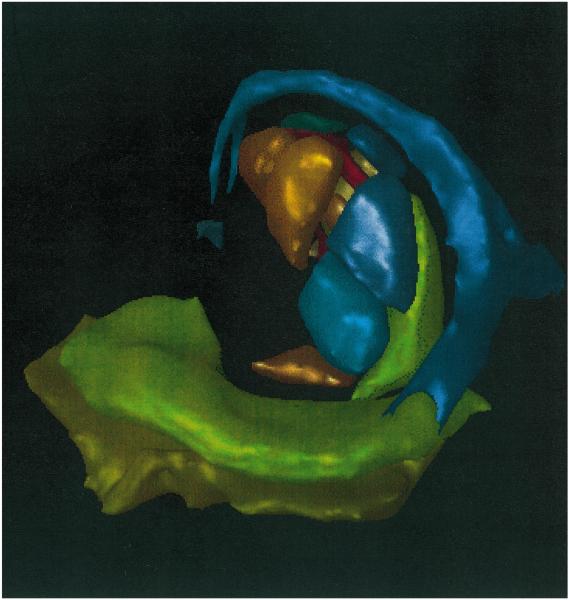
Fig. 1.
Three-dimensional reconstruction of MR of a human brain. This image demonstrates the anatomic location of key structures involved in memory processing. The pathway shown begins with the parahippocampus (olive green), to the hippocampus (pea green), to the fornix (blue), to the thalamus (all thalamic nuclei shown clustered centrally), and ending in the mamillary body (purple). Only left-sided structures are shown for simplicity.
The fornix was segmented in coronal and reformatted axial views. Automated and manual segmentation methods, three-dimensional (3D) slice editing techniques, and 3D volume rendering techniques were applied to the fornix data for visualization and analysis (for details, see Cline et al., 1990; Kikinis et al., 1990). These specialized image-processing software packages were co-developed by General Electric Corporation and members of the Surgical Planning Laboratory.
2.3.1. Fornix landmarks and boundaries
(Fig. 2 illustrates some of these boundaries).
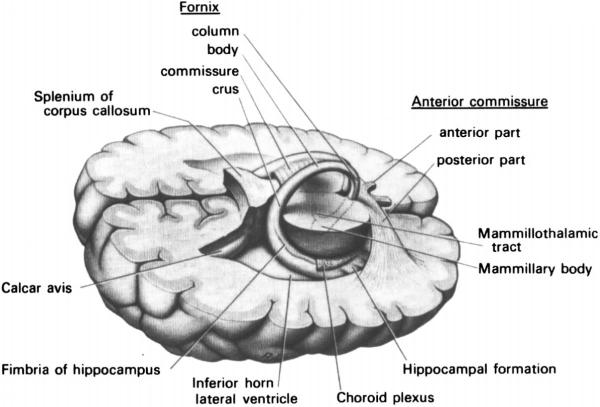
Fig. 2.
Three-dimensional drawing of human brain illustrating the course of the fornix. Not shown are the thalamic, septal, and commissural projections noted in the text. [Used with permission from Carpenter (1991): Core Text of Neuroanatomy. Baltimore: Williams and Wilkins].
2.3.1.1. Anterior boundary
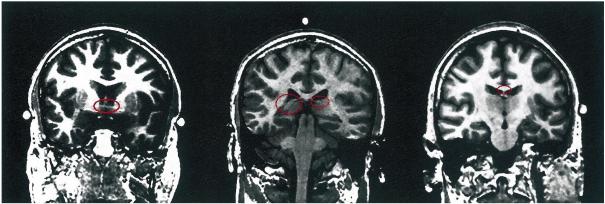
The anterior commissure was used as the landmark for the anterior boundary of the fornix. It lies immediately anterior to the descending columns of the fornix and is visible as a bright horizontal structure on MR images, making it a distinct marker (Fig. 3, left).
Fig. 3.
Coronal images of a brain of a schizophrenic subject. This figure demonstrates landmarks used in fornix ROI delineation. Following radiologic convention, the right side of the image is the left side of the brain. The left image highlights in red the anterior commissure, which marks the anterior extent of fornix; the center image shows the crux of fornix in red, which marks the posterior extent; and the right image shows the fornix body, which marks the superior extent. Note that the red ovals have been added for emphasis and were not part of the image during processing, nor are they part of the image processing itself.
2.3.1.2. Posterior boundary
Neuroanatomically, the posterior aspect of the fornix is directly connected to the hippocampus. Due to partial voluming, it was impossible to define a sharp anatomical boundary between these two structures. For consistency, the first sign of partial voluming between the fornix and hippocampus was used as the posterior boundary for the fornix (Fig. 3, center).
2.3.1.3. Superior boundary
The corpus callosum, cingulate gyrus, and the contour of the lateral ventricles were used as landmarks for the superior boundary for the fornix. These structures were visible on coronal MRI slices (Fig. 3, right).
2.3.1.4. Inferior boundary
The inferior boundary of the body of the fornix was easily discernible due to a large contrast between it and the thalamus inferior to it.
2.3.2. Inter- and intrarater reliability
Two additional trained raters segmented three cases, selected from the 30 cases at random, without any knowledge of group membership. For each case, the fornix was present on approximately 25 coronal MRI slices and 30 reformatted axial MRI slices. The interrater reliability, calculated using an intraclass correlation coefficient, was r=0.826.
The intrarater reliability was also completed for five cases, selected from the 30 cases at random, without any knowledge of group membership. Here, the original rater (J.Z.) repeated the fornix measures for the entire fornix three times. The intrarater reliability, calculated using an intraclass correlation coefficient, was r=0.914. As the fornix was measured for all cases by the same rater (J.Z.), we think that this measure of reliability is more relevant and important to this study than interrater reliability, which measures whether or not others can use the same criteria to reliably measure the fornix.
2.3.3. Data analyses
The relative fornix volume (RFV) of control and patient populations was compared using a two-tailed Student’s t-test for two independent samples, as the data were normally distributed (Shapiro–Wilk statistic: 0.958). The relationship between RFV and other relative brain volumes (previously attained) was also analyzed, in both the schizophrenic and the comparison groups, using Pearson bivariate correlations. Thus, relative volumes of the amygdala, superior temporal gyrus (STG), anterior and posterior temporal lobes, hippocampus, parahippocampal gyrus, and thalamus were entered into the analysis. These structures were chosen, based on previous work that showed a link between them and schizophrenia (e.g. Barta et al., 1990; Shenton et al., 1992; Bogerts et al., 1993; Buchanan et al., 1993; Andreasen et al., 1994; Rossi et al., 1994; Schlaepfer et al., 1994; Zipursky et al., 1994; Portas et al., 1998). To investigate the difference in the correlations between the two groups, a Fischer Z transformation was performed. This statistic provides additional information in that the degree to which correlations within the schizophrenic sample differ from correlations within the normal sample can be evaluated to determine whether or not the correlations likely came from the same population or two different populations.
We also investigated the possible association between RFV and the clinical presentation of schizophrenia. Auditory hallucinations, total negative symptom score (from SANS), total positive symptom score (from SAPS), total thought disorder index score, verbal IQ score, level of medication (measured in thorazine equivalents), and total hospitalization time were all entered into a correlation analysis with RFV. Also entered into this correlation analysis were subset scores from the Wechsler Memory Scale. Once again, normality of the data was determined by the Shapiro–Wilk test. Normally distributed values were evaluated using the Pearson correlation coefficient; and non-normally distributed values were evaluated using Spearman’s Rho correlation. We caution, however, that given the possibility of spuriously significant correlations due to the repeated analyses performed on the same subject group, the analyses performed should be considered exploratory in nature.
3. Results
3.1. Differences in fornix volumes between patients and comparison group subjects
The t-test for two samples showed no significant difference in relative fornix volume between the control and patient groups. There was also no significant difference between the two groups in absolute fornix volume (Table 1).
Table 1.
Absolute and relative volumes and standard deviations (S.D.) of the fornix in schizophrenic and comparison groups
| Mean volume±S.D. |
t-value | P-value | |
|---|---|---|---|
| Absolute fornix volumea | −0.551 | 0.586 | |
| Comparison (n=15) | 680±75 | ||
| Schizophrenics (n=15) | 700±83 | ||
| Relative fornix volume | 0.275 | 0.786 | |
| Comparison (n=15) | 0.44±0.06 | ||
| Schizophrenics (n=15) | 0.43±0.06 |
Volumes are given in ml×10−3. t-values and P-values refer to the comparison between schizophrenic and comparison groups (Student’s t-test; df=28).
3.2. Relationship between fornix volume and other brain region volumes
In the control group, the Pearson bivariate correlation showed no significant relationship between the RFV and the relative volume of any other ROI entered into the analysis. In the schizophrenic group only, RFV correlated positively and significantly with relative volumes of hippocampus (r=0.569, P=0.027), parahippocampal gyrus (r=0.567, P=0.027), and superior temporal gyrus (r=0.586, P=0.022), but not amygdala, anterior temporal lobe, posterior temporal lobe, or thalamus. Also, relative volumes of fornix, superior temporal gyrus, hippocampus, and parahippocampal gyrus all correlated significantly with each other in the schizophrenic group only (hippocampal, parahippocampal and superior temporal gyrus correlations previously reported in Shenton et al., 1992). In contrast, in the control group, none of the volumes of these four structures correlated significantly with the volume of any of the other three (see Table 2 for a summary of significant correlations).
Table 2.
Pearson correlation coefficients for volume correlations among the fornix and other regions of interest in the comparison and schizophrenic subject populations
| Correlation with fornix |
Comparison | Schizophrenics |
|---|---|---|
| Hippocampus | 0.436 (P=0.104) | 0.569 (P=0.027)a |
| Superior temporal gyrus |
0.244 (P=0.380) | 0.586 (P=0.022)a |
| Parahippocampal gyrus |
−0.075 (P=0.790) | 0.567 (P=0.027)a |
| Thalamus | 0.361 (P=0.186) | 0.088 (P=0.754) |
| Amygdala | −0.201 (P=0.474) | 0.395 (P=0.145) |
| Anterior temporal lobe |
−0.155 (P=0.581) | 0.328 (P=0.233) |
| Posterior temporal lobe |
0.345 (P=0.209) | 0.154 (P=0.754) |
Indicates P<0.05, not corrected for multiple comparisons.
Using the Fischer Z transformation to compare population differences in correlations between the schizophrenic and comparison groups, we found that the parahippocampal gyrus/fornix correlation was statistically different between normal controls and schizophrenics (P=0.038) (for the hippocampus: P=0.309, for the superior temporal gyrus: P=0.142).
3.3. Relationship between fornix volume and clinical measures
The Spearman and Pearson bivariate correlation coefficients showed no significant relationship in the schizophrenic group between the RFV and any of the visual, verbal, and memory performance scores or measures of illness presentation entered into the analysis.
4. Discussion
Although we did not find a significant difference in relative fornix volume between schizophrenic and control groups, in the schizophrenic group, we did find a statistically significant correlation between fornix volume and parahippocampal gyrus, hippocampus, and superior temporal gyrus. Such a correlation was not observed in controls. Additionally, the correlation between fornix and parahippocampal volume differed significantly between the two groups. No association was found between fornix volume and illness presentation or between fornix and cognitive/clinical measures.
Our finding of no significant total fornix volume between groups is in line with a recent postmortem study by Chance et al. (1999), in which there was no significant difference reported between schizophrenics and controls in cross-sectional area or total fiber number in the descending columns of the fornix. It should be noted, however, that in the study by Chance et al. (1999), postmortem specimens were used; only the small descending columns of the fornix were analyzed; and the donor population was elderly (mean age for men: 64.9 years; for women: 74.6 years).
We also examined possible correlations among relative fornix volume and the relative volumes of other brain structures noted to be abnormal in schizophrenia. More specifically, we analyzed not only potential differences in fornix volume between groups, but also possible correlations between fornix volume and other brain region volumes, which may reflect an important, albeit more subtle, functional relationship. We acknowledge, however, that such correlations are exploratory and, given the small sample size, may or may not generalize to the larger population of all schizophrenic patients.
With these caveats in mind, we found correlations among the relative volumes of the fornix, hippocampus, and superior temporal gyrus in the schizophrenic group, but not in the controls, which is consistent with other studies that have reported morphometric abnormalities in schizophrenia. Of note, MR volumetric brain abnormalities have been consistently reported in medial temporal and temporal lobe structures (see reviews in Shenton et al., 1997, 2000; McCarley et al., 1999). Currently, the most robust MR findings include enlargement of the lateral ventricles, especially in the left temporal horn (e.g. Johnstone et al., 1989; Dauphinais et al., 1990), and medial temporal lobe reduction (e.g. Woods et al., 1996), which, we note are likely “importantly related to disturbances in associative links in memory” (see review in Squire and Zola-Morgan, 1991; Squire, 1992; Nestor et al., 1993; Shenton et al., 1997, p. 340). Other significant findings include: (1) reductions in the volume of the amygdala-hippocampal complex, parahippocampal gyrus, or both (e.g. Bogerts et al., 1990; Suddath et al., 1990; Egan et al., 1994); (2) reduction in the volume of the superior temporal gyrus (STG) (Barta et al., 1990; Shenton et al., 1992; see also reviews noted above); and (3) a decrease in thalamic volume (e.g. Andreasen et al., 1994; Buchsbaum, 1996). Analyses of other areas of the brain, such as the frontal and parietal lobes, have yielded more equivocal results (again, see our reviews in Shenton et al., 1997, 2000; McCarley et al., 1999).
In addition, Portas et al. (1998), from our laboratory, reported a similar pattern of results. She showed no difference in thalamic volume between schizophrenics and controls, but did report statistically significant correlations between thalamic and other structures’ volumes in schizophrenics but not in controls. Taken together, these data suggest possible subtle abnormalities in connectivity or functional relationships among different brain regions in the absence of volume abnormalities detectable by MR.
As this study is, to the best of our knowledge, the first MR volumetric study of the fornix in a schizophrenic population, more work needs to be done to verify our results, ideally on a larger population. It should also be pointed out that the patients in this study were all male, with predominantly positive symptoms and a chronic course of illness, which may preclude our findings from being generalized to populations of patients with schizophrenia who do not share these characteristics. In addition, some MR systems can now produce image resolutions of up to 1024×1024 (whereas our study used 256×256), which would allow for an even more accurate segmentation procedure, which, in turn, may lead to the detection of heretofore undetected fornix volume reduction in patients diagnosed with schizophrenia compared to controls. Also, an analysis of shape differences may provide information regarding neurodevelopmental alterations in the fornix that may not be as readily detected by volume measurements alone.
In summary, although more work on the fornix is required, these findings suggest that it may be worthwhile for investigators to compare not only isolated brain structures between schizophrenics and comparison subjects, but also groups of structures that may be subtly affected as a functional unit in schizophrenia, without exhibiting a morphological or volumetric abnormality large enough to be detected in each individual structure separately. The brain structures comprising such a group may have a common, functionally related role in producing certain symptoms of schizophrenia.
Acknowledgements
This research was supported in part by funds from the Stanley Scholars Program to support student fellowships in mental health research (Janos Zahajszky), by the National Mental Health Institute, including grants K02MH-01110 and R01 MH-50747 (Dr Shenton), and RO1-40799 (Dr McCarley), by the Medical Research Service and Brockton VA Schizophrenia Center of the Department of Veteran Affairs (Dr McCarley), by VA Merit Awards from the Department of Veterans Affairs (Dr McCarley, Dr Shenton), and by a VA Psychiatry/Neuroscience Research Fellowship from the Department of Veterans Affairs (Dr Dickey).
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. third ed. American Psychiatric Press; Washington, DC: 1987. revised. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) University of Iowa College of Medicine Department of Psychiatry; Iowa City, IA: 1981. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) University of Iowa College of Medicine Department of Psychiatry; Iowa City, IA: 1984. [Google Scholar]
- Andreasen NC, Ehrhardt JC, Swayze VW, 2d, Alliger RJ, Yuh WT, Cohen G, et al. Magnetic resonance imaging of the brain in schizophrenia: The pathophysiologic significance of structural abnormalities. Arch. Gen. Psychiatry. 1990;47:35–44. doi: 10.1001/archpsyc.1990.01810130037006. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Arndt S, Swayze V, Cizadlo T, Flaum M, O’Leary D, et al. Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science. 1994;266:294–298. doi: 10.1126/science.7939669. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. Linking mind and brain in the study of mental illnesses: a project for a scientific psychopathology. Science. 1997;275:1586–1593. doi: 10.1126/science.275.5306.1586. [DOI] [PubMed] [Google Scholar]
- Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE. Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am. J. Psychiatry. 1990;147:1457–1462. doi: 10.1176/ajp.147.11.1457. [DOI] [PubMed] [Google Scholar]
- Bogerts B, Meetz E, Schonfeld-Bausch R. Basal ganglia and limbic system pathology in schizophrenia: A morphometric study of brain volume and shrinkage. Arch. Gen. Psychiatry. 1985;42:784–791. doi: 10.1001/archpsyc.1985.01790310046006. [DOI] [PubMed] [Google Scholar]
- Bogerts B, Ashtari M, Degreef G, Alvir JMJ, Bilder RM, Lieberman JA. Reduced temporal limbic structure volumes on magnetic resonance images in first episode schizophrenia. Psychiatry Res. Neuroimaging. 1990;35:1–13. doi: 10.1016/0925-4927(90)90004-p. [DOI] [PubMed] [Google Scholar]
- Bogerts B, Lieberman JA, Ashtari M, Bilder RM, Degreef G, Lerner G, et al. Hippocampus–amygdala volumes and psychopathology in chronic schizophrenia. Biol. Psychiatry. 1993;33:236–246. doi: 10.1016/0006-3223(93)90289-p. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Breier A, Kirkpatrick B, Elkashef A, Munson RC, Gellad F. Structural abnormalities in deficit and nondeficit schizophrenia. Am. J. Psychiatry. 1993;150:59–65. doi: 10.1176/ajp.150.1.59. [DOI] [PubMed] [Google Scholar]
- Buchsbaum M. PET and MRI of the thalamus in never-medicated patients with schizophrenia. Am. J. Psychiatry. 1996;152:191–199. doi: 10.1176/ajp.153.2.191. [DOI] [PubMed] [Google Scholar]
- Calabrese P, Markowitsch HJ, Harders AG, Scholz M, Gehlen W. Fornix damage and memory — A case report. Cortex. 1995;31:555–564. doi: 10.1016/s0010-9452(13)80066-4. [DOI] [PubMed] [Google Scholar]
- Carpenter MB. Core Text of Neuroanatomy. Williams and Wilkins; Baltimore, MD: 1991. [Google Scholar]
- Chance SA, Highley JR, Esiri MM, Crow TJ. Fiber content of the fornix in schizophrenia: lack of evidence for a primary limbic encephalopathy. Am. J. Psychiatry. 1999;156:1720–1724. doi: 10.1176/ajp.156.11.1720. [DOI] [PubMed] [Google Scholar]
- Cline HE, Lorensen WR, Kikinis R, Jolesz FA. Three-dimensional segmentation of MR images of the head using probability and connectivity. J. Comput. Assist. Tomogr. 1990;14:1037–1045. doi: 10.1097/00004728-199011000-00041. [DOI] [PubMed] [Google Scholar]
- Corey-Bloom J, Jernigan T, Archibald S, Harris MJ, Jeste DV. Quantitative magnetic resonance imaging of the brain in late-life schizophrenia. Am. J. Psychiatry. 1995;152:447–449. doi: 10.1176/ajp.152.3.447. [DOI] [PubMed] [Google Scholar]
- Dauphinais D, DeLisi LE, Crow TJ, Alexandropoulos K, Colter N, Tuma I, et al. Reduction in temporal lobe size in siblings with schizophrenia: A magnetic resonance imaging study. Psychiatry Res. Neuroimaging. 1990;35:137–147. doi: 10.1016/0165-1781(90)90156-y. [DOI] [PubMed] [Google Scholar]
- Dusek JA, Eichenbaum H. The hippocampus and memory for orderly stimulus relations. Proc. Natl. Acad. Sci. USA. 1997;94:7109–7114. doi: 10.1073/pnas.94.13.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Duncan CC, Suddath RL, Kirch DG, Mirsky AF, Wyatt RJ. Event-related potential abnormalities correlate with structural brain alterations and clinical features in patients with chronic schizophrenia. Schizophr. Res. 1994;11:259–271. doi: 10.1016/0920-9964(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Falkai P, Bogerts B, Rozumek M. Limbic pathology in schizophrenia: The entorhinal region — A morphometric study. Biol. Psychiatry. 1988;24:515–521. doi: 10.1016/0006-3223(88)90162-x. [DOI] [PubMed] [Google Scholar]
- Flaum M, Swayze VW, O’Leary DS, Yuh WT, Ehrhardt JC, Arndt SV, et al. Effects of diagnosis and gender on brain morphology in schizophrenia. Am. J. Psychiatry. 1995;152:704–714. doi: 10.1176/ajp.152.5.704. [DOI] [PubMed] [Google Scholar]
- Gaffan D. Dissociated effects of perirhinal cortex ablation, fornix transection and amygdalectomy: Evidence for multiple memory systems in the primate temporal lobe. Exp. Brain Res. 1994;99:411–422. doi: 10.1007/BF00228977. [DOI] [PubMed] [Google Scholar]
- Gale SD, Burr RB, Bigler ED, Blatter D. Fornix degeneration and memory in traumatic brain injury. Brain Res. Bull. 1993;32:345–349. doi: 10.1016/0361-9230(93)90198-k. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Two-factor index of social position. CONN. Yale University Press; New Haven: 1965. [Google Scholar]
- Johnston MH, Holzman PS. Assessing Schizophrenic Thinking. Jossey-Bass; San Francisco, CA: 1979. [Google Scholar]
- Johnstone EC, Owens DGC, Crow TJ, Frith C, Alexandropoulos K, Bydder G, et al. Temporal lobe structure as determined by nuclear magnetic resonance in schizophrenia and bipolar affective disorder. J. Neurol. Neurosurg. Psychiatry. 1989;52:736–741. doi: 10.1136/jnnp.52.6.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikinis R, Jolesz FA, Gerig G, Sandor K, Cline HE, Lorensen WE, et al. Morphometric and morphologic information derived from clinical MR images. In: Hohne KH, Fuchs H, Pizer SM, editors. 3D Imaging in Medicine. Springer; Berlin: 1990. pp. 441–454.pp. F60 [Google Scholar]
- McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, et al. MRI anatomy of schizophrenia. Biol. Psychiatry. 1999;45:1099–1119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMackin D, Cockburn J, Anslow P, Gaffan D. Correlation of fornix damage with memory impairment in six cases of colloid cyst removal. Acta Neurochir. (Wien) 1995;135:12–18. doi: 10.1007/BF02307408. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Shenton ME, McCarley RW, Haimson J, Smith RS, O’Donnell B, Kimble M, Kikinis R, Jolesz FA. Neuropsychological correlates of MRI temporal lobe abnormalities in schizophrenia. Am. J. Psychiatry. 1993;150:1849–1855. doi: 10.1176/ajp.150.12.1849. [DOI] [PubMed] [Google Scholar]
- Parker A, Gaffan D. Mamillary body lesions in monkeys impair object-in-place memory: Functional unity of the fornix–mamillary system. J. Cogn. Neurosci. 1997;9:512–521. doi: 10.1162/jocn.1997.9.4.512. [DOI] [PubMed] [Google Scholar]
- Portas CM, Goldstein JM, Shenton ME, Hokama HH, Wible CG, Fischer I, et al. Volumetric evaluation of the thalamus in schizophrenic male patients using magnetic resonance imaging. Biol. Psychiatry. 1998;43:649–659. doi: 10.1016/s0006-3223(97)00339-9. [DOI] [PubMed] [Google Scholar]
- Rossi A, Stratta P, Mancini F, Gallucci M, Mattei P, Core L, et al. Magnetic resonance imaging findings of amygdala–anterior hippocampus shrinkage in male patients with schizophrenia. Psychiatry Res. 1994;52:43–53. doi: 10.1016/0165-1781(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Harris GJ, Tien AY, Peng LW, Lee S, Federman EB, et al. Decreased regional cortical grey matter volume in schizophrenia. Am. J. Psychiatry. 1994;151:842–848. doi: 10.1176/ajp.151.6.842. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, et al. Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. N. Engl. J. Med. 1992;327:604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Wible CG, McCarley RW. A review of magnetic resonance imaging studies of brain abnormalities in schizophrenia. In: Krishnan KRR, Doraiswamy PM, editors. Brain Imaging in Clinical Psychiatry. Marcel Dekker; New York: 1997. pp. 297–380. [Google Scholar]
- Shenton ME, Frumin M, McCarley RW, Maier S, Westin CF, Fischer IA, et al. MR morphometric findings in schizophrenia. In: Dougherty D, Rauch S, Rosenbaum J, editors. Psychiatric Neuroimaging Strategies: Research and Clinical Applications. American Psychiatric Association; Washington, DC: 2000. pp. 297–380. [Google Scholar]
- Spitzer RL, Endicott J. Schedule for Affective Disorders and Schizophrenia. third ed. New York State Psychiatric Institute — Biometrics Research; New York: 1978. [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Suddath RL, Christison GW, Torrey EF, Casanova MF, Weinberger DR. Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. N. Engl. J. Med. 1990;332:789–794. doi: 10.1056/NEJM199003223221201. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-R Manual: Wechsler Adult Intelligence Scale. Revised. Harcourt Brace Jovanovich; New York: 1981. [Google Scholar]
- Wechsler D. Wechsler Memory Scale — Revised Manual. Psychological Corporation; New York: 1987. [Google Scholar]
- Weiner I, Feldon J, Tarrasch R, Hairston I, Joel D. Fimbria–fornix cut affects spontaneous activity, two-way avoidance and delayed non matching to sample, but not latent inhibition. Behav. Brain Res. 1998;96:59–70. doi: 10.1016/s0166-4328(97)00193-9. [DOI] [PubMed] [Google Scholar]
- Wible CG, Shenton ME, Hokama H, Kikinis R, Jolesz FA, Metcalf D, et al. Prefrontal cortex: A quantitative magnetic resonance imaging study. Arch. Gen. Psychiatry. 1995;43:114–124. doi: 10.1001/archpsyc.1995.03950160029007. [DOI] [PubMed] [Google Scholar]
- Wible CG, Shenton ME, McCarley RW. Functional neuroanatomy of the limbic system and the planum temporale. In: Krishnan KRR, Doraiswamy PM, editors. Brain Imaging in Clinical Psychiatry. Marcel Dekker; New York: 1997. pp. 64–102. [Google Scholar]
- Woods BT, Yurgelun-Todd D, Goldstein JM, Seidman LJ, Tsuang MT. MRI brain abnormalities in chronic schizophrenia: One process or more? Biol. Psychiatry. 1996;40:585–596. doi: 10.1016/0006-3223(95)00478-5. [DOI] [PubMed] [Google Scholar]
- Zipursky RB, Marsh L, Lim KO, DeMent S, Shear PK, Sullivan EV, et al. Volumetric MRI assessment of temporal lobe structures in schizophrenia. Biol. Psychiatry. 1994;35:501–516. doi: 10.1016/0006-3223(94)90097-3. [DOI] [PubMed] [Google Scholar]