Abstract
Rationale
Extracellular signal-regulated protein kinase (ERK1/2) is a member of the mitogen-activated protein kinase (MAPK) signaling pathway and a key molecular target for ethanol (EtOH) and other drugs of abuse.
Objective
The aim of the study was to assess the role of two MAPK pathways, ERK1/2 and c-Jun N-terminal kinase (JNK), on the modulation of EtOH and sucrose self-administration.
Materials and methods
C57BL/6J mice were trained to lever press on a fixed-ratio 4 schedule with 9% EtOH/2% sucrose, or 2% sucrose, as the reinforcer. In experiments 1 and 2, mice were injected with the MEK1/2 inhibitor SL 327 (0–100 mg/kg) and the JNK inhibitor AS 6012452 (0–56 mg/kg) prior to self-administration. In experiment 3, SL 327 (0–100 mg/kg) was administered prior to performance on a progressive ratio (PR) schedule of EtOH reinforcement. In experiment 4, SL 327 and AS 601245 were injected 2 h before a locomotor test.
Results
SL 327 (30 mg/kg) significantly increased EtOH self-administration without affecting locomotion. Higher doses of SL 327 and AS 601245 reduced EtOH-reinforced responding and locomotor activity. Reductions of both ligands on sucrose self-administration were due to decreases in motor activity. SL 327 pretreatment had no effect on PR responding.
Conclusions
ERK1/2 activity is more directly involved in modulating the reinforcing properties of EtOH than JNK activity due to its selective potentiation of EtOH-reinforced responding. The specificity of this effect to EtOH self-administration, rather than sucrose self-administration, suggests that the mechanism by which ERK1/2 increases EtOH-reinforced responding does not generalize to all reinforcing solutions and is not due to increased motivation to consume EtOH.
Keywords: Alcohol drinking, ERK/MAPK, SL 327, AS 601245, Self-administration, Progressive ratio, Motor activity, Reinforcement, Protein kinase, Operant conditioning
Introduction
Alcoholism is a physically and psychologically debilitating disease affecting more than 17.6 million people per year in the USA alone (Grant et al. 2004). The gradual transition from alcohol use to abuse involves escalated intake, increased tolerance and withdrawal symptoms, and behavioral impairments. Despite the widespread social and health-related consequences associated with alcoholism, the molecular neuroadaptations that underlie alcohol self-administration are poorly understood. Recently, the mitogen-activated protein kinase (MAPK) intracellular signaling cascades have been implicated in mechanisms thought to be relevant to dependence and addiction (Chandler 2003; Russo et al. 2008; Zhaiet al. 2008).
MAPKs comprise three distinct signaling pathways, namely, the extracellular signal-regulated (ERK1/2), the c-Jun N-terminal (JNK), and the p38 protein kinases (Boulton and Cobb 1991; Krishna and Narang 2008; Robinson and Cobb 1997). When phosphorylated, or “activated”, MAPK can translocate to the nucleus and facilitate gene transcription (Chen et al. 1992). Of the three subfamilies, the ERK1/2/MAPK pathway has been the most implicated in addictive processes by a mounting body of research demonstrating sensitivity of this pathway in neural plasticity, learning and memory, and drug reinforcement (Adams and Sweatt 2002; Ferguson et al. 2006; Grewal et al. 1999; Mazzucchelli et al. 2002; Thomas and Huganir 2004). In addition, ERK1/2 is abundantly expressed in brain regions that modulate addiction such as the prefrontal cortex, nucleus accumbens, bed nucleus of the stria terminalis and amygdala, and is generally increased by drugs of abuse, including cocaine, d-amphetamine, nicotine, morphine and Δ9-tetrahydrocannabinol (Choe et al. 2002; Lu et al. 2006; Ortiz et al. 1995; Valjent et al. 2004).
The effect of ethanol on ERK1/2 activity has been less extensively studied. Consistent with other abused substances, acute ethanol increases ERK1/2 phosphorylation in the prefrontal cortex, shell of the nucleus accumbens, central and basolateral nucleus of the amygdala, paraventricluar nucleus of the hypothalamus and in the Edinger-Westphal nucleus (Bachtell et al. 2002; Sharko and Hodge 2007). Similarly, ERK1/2 phosphorylation is increased in the basolateral amygdala and nucleus accumbens shell by response contingent presentation of cues formerly paired with ethanol reinforcement (Schroeder et al. 2008). Conversely, other studies have shown have shown a decrease in the phosphorylation of ERK1/2 by acute ethanol (Chandler and Sutton 2005; Hendrickson et al. 1998; Kalluri and Ticku 2002; Sanna et al. 2002; Tsuji et al. 2003). Together, these studies indicate that the relationship between acute ethanol and ERK1/2 activity needs to be further elucidated, and it is possible that the ERK1/2/MAPK pathway functionally regulates ethanol self-administration and other behavioral impairments associated with alcohol abuse and alcoholism.
ERK1/2 is phosphorylated within the activation loop of the kinase on both a threonine and a tyrosine residue by the MAPK/ERK1/2 kinase (MEK1/2; Crews and Erikson 1992; Seger and Krebs 1995). This activated form of the protein (pERK1/2) can induce expression of transcription factors including ELK, CREB, c-fos, and others (Adams et al. 2000; Sgambato et al. 1998; Treisman 1996; Vanhoutte et al. 1999). PD098059 (IC50=2–7 μM), U0126 (IC50=0.06–0.07 μM), and SL 327 (IC50=0.18–0.22 μM) are ligands that inhibit the phosphorylation of MEK1/2, which prevents the downstream phosphorylation of ERK1/2 (Atkins et al. 1998; Favata et al. 1998). Systemic injection of SL 327 decreases levels of pERK1/2 immunoreactivity by 62–91%, in the prefrontal cortex, nucleus accumbens, dorsal striatum, BNST, and amygdala, and a growing number of studies have shown that this class of drugs can significantly modulate behavior (Valjent et al. 2006b). For example, SL 327 blocks acute psychostimulant-induced locomotor activity, the induction of behavioral sensitization to cocaine and d-amphetamine, the expression of conditioned place preference to several drugs of abuse, the consolidation of fear memories, memory retention, long-term potentiation, the parental retrieving behavior of C57BL/6J mice, and cytokine-induced immobility in the forced swim test (Atkins et al. 1998; Cestari et al. 2006; Davis et al. 2000; Kuroda et al. 2007; Mazzucchelli and Brambilla 2000; Salzmann et al. 2003; Schafe et al. 2000; Shi and McGinty 2006; Valjent et al. 2006a, b; Wu and Lin 2008). Likewise, site-specific microinjections of U0126 or PD098059 into the amygdala significantly reduce immobility in the forced swim test, cocaine seeking after 30 days of withdrawal, and consolidation of pavlovian fear conditioning memories (Duvarci et al. 2005; Huang and Lin 2006; Lu et al. 2005; Schafe et al. 2000). Furthermore, intra-ventral tegmental area (VTA) microinjection of U1026 has recently been shown to prevent glial cell line-derived neurotrophic factor (GDNF)-induced decreases in ethanol self-administration (Carnicella et al. 2008). This study provides the most convincing evidence to date to support our central hypothesis that activity of the ERK1/2/MAPK pathway might be involved in modulating the reinforcing properties of ethanol.
To address this hypothesis, the present series of experiments were designed to (1) functionally assess the role of the ERK1/2/MAPK pathway on both fixed- and progressive-ratio responding for ethanol in C57BL/6J mice by inhibiting ERK1/2 phosphorylation with the MEK1/2 inhibitor, SL 327. We chose this inhibitor for our studies because it is centrally active after systemic administration (Atkins et al. 1998; Favata et al. 1998); (2) determine if the ERK1/2 pathway selectively modulates ethanol self-administration by comparing the effects of SL 327 with the effects of a selective, centrally active JNK inhibitor, AS 601245 (IC50 for JNK1–3=0.07–0.22 μM and for MEK1 >10 μM; Carboni et al. 2004; Gaillard et al. 2005); (3) evaluate whether these effects are specific to ethanol as a reinforcer by investigating the effects of both of these ligands on the self-administration of a non-drug reinforcer, sucrose; and (4) determine whether the observed drug effects are specific to ethanol reinforcement or due to non-specific locomotor impairment by testing whether these inhibitors alter open field activity.
Materials and methods
Subjects
Subjects were male C57BL6/J mice (n=42) arriving at 8 weeks of age from Jackson Laboratory (Bar Harbor, ME, USA). Upon arrival, mice were group-housed (four per cage) in clear, polycarbonate cages (28×17×14 cm). Each cage was covered by a stainless steel wire lid through which Purina rodent chow and water was available ad libitum. The vivarium was maintained on a reverse light/dark cycle (lights off at 1000) with temperature at 21±1°C and 23% humidity. All procedures were approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill, and all mice were cared for in accordance with the Division of Laboratory Animal Medicine and the Guide for the Care and Use of Laboratory Animals (National Research Council 1996).
Self-administration apparatus
Self-administration sessions were conducted in 16 operant conditioning chambers (chambers and all components were from Med Associates, St. Albans, VT, USA) that were each housed within a sound-attenuating cubicle equipped with a 28-V fan to provide ventilation and to mask external noise. Opposite walls of the chamber each contained a stainless steel ultra-sensitive retractable lever (required force=0.02 N) located directly below a cue light. Responding on only one of the levers (the “active” lever) was reinforced. Responses on the “inactive” lever were recorded but held no contingencies. A drinking trough was located adjacent to the active lever and was connected to a syringe pump. A photobeam spanned the entrance of the drinking trough allowing for quantification of head-poke entries as an index of temporal patterns of self-administration. Reinforcement delivery was accompanied by a brief illumination of the cue light (800 ms) and pump sound, both of which served as secondary cues. Responses during this time were counted but did not contribute towards the response requirement (time-out period). The chamber and pump were connected to an interface and computer that recorded the input and behavioral output of each mouse (MED-PC for Windows v.4.1).
Ethanol self-administration procedure
Initially, mice were trained in three 16-h sessions to lever press for the delivery of a 10% sucrose (w/v) solution (0.014 ml/reinforcement). To facilitate acquisition of lever pressing, mice were water restricted for 8 h prior to these sessions. During the first session, each lever press on the active lever was reinforced by a delivery of 10% sucrose (fixed-ratio 1; FR 1). Within the second session, the response requirement was increased from FR 1 to FR 2 to FR 4 in increments of 25 (i.e., after receiving the 25th reinforcement, the response requirement increased to FR 2). An FR 4 schedule of reinforcement was maintained for the remainder of the experiment.
Beginning on the fourth test day, drinking sessions were shortened to 2 h in duration and a modified sucrose fading procedure was implemented to facilitate self-administration of ethanol (Samson 1986). Ethanol (v/v) was added in increasing increments (2%–5%–10%) and sucrose (w/v) was faded out of the solution (10%–8%–5%–2%–0%) with a minimum of 3 days at each concentration. After 17 days of unsweetened 10% ethanol, we noted that the mice stopped fully consuming the reinforcer (i.e., fluid remained in the drinking trough at the end of the session). Accordingly, the concentrations of ethanol and sucrose in the reinforcing solution were titrated until all subjects reliably and consistently consumed the reinforcer. The final reinforcing solution was a 9% ethanol/2% sucrose solution. The drinking trough was checked at the end of each session to ensure that all reinforcements were being consumed. An analysis of the cumulative number of lever presses over the 2-h session revealed that the majority of the responses were performed within the 1st half of the session. Thus, on experimental day 32, the length of the session was shortened to and remained at 1 h for the remainder of the study. The operant conditioning chambers were not illuminated and all sessions were conducted in the dark, between 1100 and 1300 h, 6 days/week.
Locomotor activity
Open field activity was measured in Plexiglas activity monitor chambers (27.9 cm2; ENV-510, Med Associates). Two sets of 16 pulse-modulated infrared photobeams were located on opposite walls and recorded X–Y ambulatory movements. The mouse’s position in the open field was assessed every 100 ms to quantify the distance traveled (meter) throughout the session. Each activity monitor was connected to a computer that collected all data.
Blood alcohol assay
Blood was collected at the end of a maintenance self-administration session to assess blood alcohol content (BAC). Each mouse was briefly placed into a restraint tube (Braintree Scientific, Braintree, MA, USA), the tip of the tail was cut (approximately 1 mm) and blood was collected using a heparinized microcapillary tube. Immediately after sample collection, 5 μl of plasma was injected into an Analox GL-5 analyzer machine to quantify BAC (Analox Instruments, Lunenburg, MA, USA).
Experiment 1: Effect of MAPK inhibitors on FR responding for ethanol
Twelve out of 16 mice reliably acquired ethanol self-administration (>10 reinforcements/session). Once stable levels of self-administration were obtained (ca. 73 days; <15% variability for seven test days), mice were injected with vehicle 2 h prior to the start of the session to habituate them to the handing and injection protocol. Once stable levels of responding were obtained after vehicle injection, mice were injected with SL 327 (0, 30, 50, 100 mg/kg) in a counterbalanced manner. A minimum of two non-drug self-administration sessions preceded each test day.
After completion of the SL 327 dose–effect curve, maintenance of ethanol self-administration continued for 3 weeks without drug testing to avoid carryover effects. Next, mice were injected with the JNK inhibitor vehicle 2 h prior to the start of the session to habituate them to the novel vehicle. Once stable levels of responding emerged after vehicle injections, mice were injected with the JNK inhibitor (0, 3, 10, 30, 56 mg/kg) in a counterbalanced manner. A minimum of two non-drug self-administration sessions preceded each test day.
Experiment 2: Effect of MAPK inhibitors on FR responding for sucrose
Experimentally naive mice (n=11) were trained to self-administer sucrose using a modified version of the sucrose fading procedure detailed above. Briefly, mice acquired self-administration of 10% sucrose (w/v) during three 16-h sessions. Next, during 1-h sessions, the concentration of sucrose was gradually reduced from 10%–8%–5%–2% (w/v) with a minimum of 3 days at each concentration. The final solution was maintained at 2% sucrose.
Mice self-administered sucrose for 73 days prior to the initiation of the SL 327 dose–effect curve. Dose effect curves for both SL 327 and AS 601245 were conducted as previously described in experiment 1 with the same dose range, injection interval, and session duration.
Experiment 3: Effect of SL 327 on progressive ratio responding for ethanol
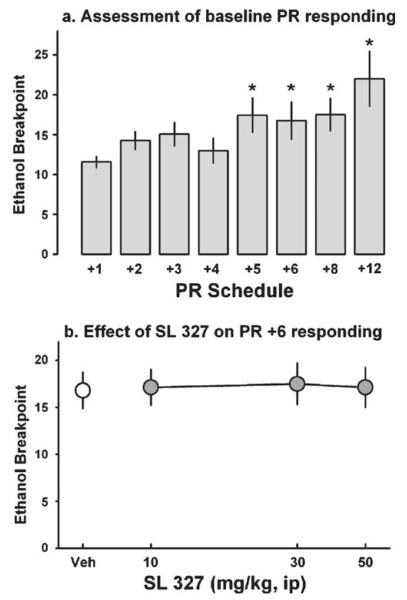
Experimentally naive mice (n=16) were trained to self-administer ethanol as described above. Once stable levels of self-administration were obtained (ca. 62 days), mice were tested for performance on a progressive ratio (PR) schedule of reinforcement, which provides a measure of motivation or reinforcing strength (Markou et al. 1993; Richardson and Roberts 1996; Stafford et al. 1998). The steepness of the PR schedule was first varied over test days with the goal of identifying an optimal PR schedule for testing the effect of SL 327. All of the PR schedules began with an initial response requirement of FR 4 after which the response requirement ascended arithmetically ranging from +1 to +20 (i.e., for a PR +6 schedule the response requirement increased as follows: 4, 10, 16, 22, etc.). Sessions were terminated when 15 min elapsed without obtaining a reinforcer. The breakpoint was defined as the final completed response requirement. A minimum of 4 days separated each PR test to avoid extinction and on intervening days, standard FR sessions were conducted.
Based on the results from the first phase of the experiment, the PR +6 schedule was chosen for testing the effect of SL 327. This PR schedule was chosen because it engendered an intermediate level of responding and breakpoint such that bidirectional drug effects could be observed. Once stable levels of responding were obtained after vehicle injection, mice were injected with SL 327 (0, 10, 30, 50 mg/kg) in a counterbalanced manner. A minimum of four non-drug FR sessions preceded each test.
Experiment 4: Effect of MAPK inhibitors on locomotor activity
Locomotor tests were conducted in ethanol and sucrose self-administering mice (from experiments 1 and 2) to evaluate the behavioral specificity of SL 327 and AS 601245 after completion of both dose–effect curves. Mice were habituated to the locomotor chambers for 2 h, 7–10 days prior to the initiation of the first dose–effect curve. Next, ethanol self-administering mice were injected with SL 327 (0–100 mg/kg; n=12) or AS 601245 (0–56 mg/kg; n=11) 2 h before being placed into the open field. Likewise, sucrose self-administering mice were injected with SL 327 (0–100 mg/kg; n=11) or AS 601245 (0–30 mg/kg, n=10). All tests were initiated at the same time that the self-administration session usually began, and lasted for 1 h. Mice continued to self-administer either ethanol or sucrose on non-test days (5 days/week). A minimum of 1 week separated each test to avoid habituation to the chamber such that a repeated measures design could be utilized. Doses were administered in a counterbalanced manner.
Drugs
All ethanol solutions (v/v) were prepared by diluting 95% ethanol (Pharmco Products Inc., Brookfield, CT, USA) with tap water. The MEK1/2 inhibitor, SL 327 (α-[amino[(4-aminophenyl)thio]methylene]-2-(trifluoromet hyl) benzeneacetonitrile; Tocris Bioscience; Ellisville, MO, USA) was freshly dissolved in 100% dimethlysulfoxide (DMSO; Mallinckrodt Baker, Inc., Phillipsburg, NJ, USA) and was then diluted to 15% DMSO with distilled H2O. Because of solubility concerns at the higher concentrations of SL 327, the injection volume for this dose–effect curve was 2 ml/100 g BW. The c-Jun N-terminal kinase (JNK) inhibitor, AS 601245 (1,3-benzothiazol-2-yl-(2-((2-(3-pyridinyl)ethyl)amino)-4-pyrimidinyl)acetonitrile; EMD Biosciences, San Diego, CA, USA) was freshly suspended in 0.5% carboxymethylcellulose (CMC; Sigma-Aldrich, St. Louis, MO, USA), 0.25% Tween 20 (Sigma-Aldrich), and dH2O. The injection volume for AS 601245 was 1 ml/100 g BW. All drugs were administered via an intra-peritoneal injection (i.p.) with a 27-gauge needle.
Statistical analysis
For each dose–effect curve, a one-way repeated measured analyses of variance (RM ANOVA) was conducted on multiple measures of self-administration including number of reinforcements, number of active and inactive lever responses, number of active headpokes, percent of lever responses on the active lever, and dose consumed (for ethanol self-administering mice). In Figs. 3 and 4, dose consumed and number of reinforcements, respectively, were transformed to percent change from vehicle levels and a RM ANOVA was conducted on the transformed data. A RM ANOVA was also used to analyze the effect of manipulating the PR schedule on subsequent responding. Data from the PR +20 schedules were not included in this analysis because the majority of mice at this schedule received only one reinforcement (i.e., breakpoint = 4). For analysis of open field activity, one-way RM ANOVAs were conducted for each dose–effect curve on total distance traveled. To investigate if there was a dose by time interaction, additional two-way RM ANOVAs (dose × time) were conducted. For post hoc analyses, Dunnett’s test was conducted using the vehicle condition as the common control. A Pearson product moment correlation was conducted for dose consumed (gram per kilogram) and blood alcohol content in a 1-hr session. α was set at 0.05 for all comparisons.
Fig. 3.
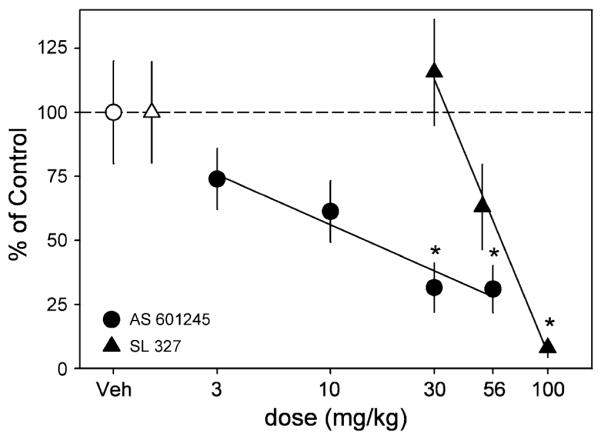
Modulation of 2% sucrose self-administration by MAPK inhibitors. Filled triangles represent the dose–effect curve for SL 327 and filled circles represent the dose–effect curve for AS 601245. Open symbols reflect the vehicle values for SL 327 (triangle) and AS 601245 (circle). Data are presented as a percent change from vehicle levels of means ± SEM (vertical lines) for number of reinforcements. Regression lines are shown that fit the effects of both ligands for this behavior. Asterisks denote significance as compared to vehicle (p<0.05)
Fig. 4.
Effect of SL 327 on the modulation of PR responding for ethanol. a Effect of increasing the PR response requirement on breakpoint (gray bars). b Effect of Veh (white circles) and 10–50 mg/kg SL 327 (gray circles) on PR responding using the PR +6 response requirement schedule. The data reflect the mean (vertical bars) ± SEM. Asterisks denote significance as compared to the +1 PR schedule of reinforcement (a) or to vehicle (b)(p<0.05)
Results
Experiment 1: Effect of MAPK inhibitors on ethanol self-administration
During the week preceding the start of the SL 327 dose–effect curve, the average number of reinforcements was 26.2±2.4, active lever responses was 114.2±10.1, and 77.7±4.3% of responding occurred on the active lever. Blood alcohol content was measured after completion of the AS 601245 dose–effect curve at the end of a 1-h drinking session. An average ethanol intake of 1.31±0.13 g/kg resulted in an average BAC of 92.2±15.5 mg/dl. These data reveal individual variability in dose consumed during a single session and a significant positive correlation between intake and BAC (r2=0.77; Fig. 1).
Fig. 1.
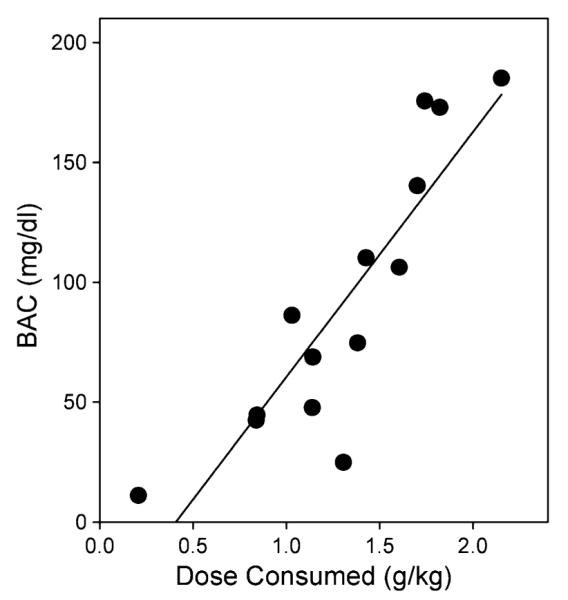
Blood alcohol content after a 1-h self-administration session. Filled circles represent the dose consumed and corresponding blood alcohol content for each individual (n=14). There was a significant positive correlation (r2=0.77) and the average blood alcohol content for the group was 92.2±15.5 mg/dl
SL 327
Administration of the MEK1/2 inhibitor, SL 327 produced a biphasic effect on multiple measures of self-administration including the number of reinforcements obtained (F(11, 33)=13.4, p<0.001), dose-consumed (F(11, 33)=13.9, p<0.001), number of active lever responses (F(11, 33)=11.2, p<0.001), number of headpokes (F(11, 33)=4.4, p=0.01), and number of inactive lever responses (F(11, 33)=4.2, p=0.01). Post hoc analyses revealed that 30 mg/kg significantly increased the number of reinforcements, number of active lever responses, and dose consumed, while 100 mg/kg significantly decreased all of these measures (Fig. 2; Table 1).
Fig. 2.
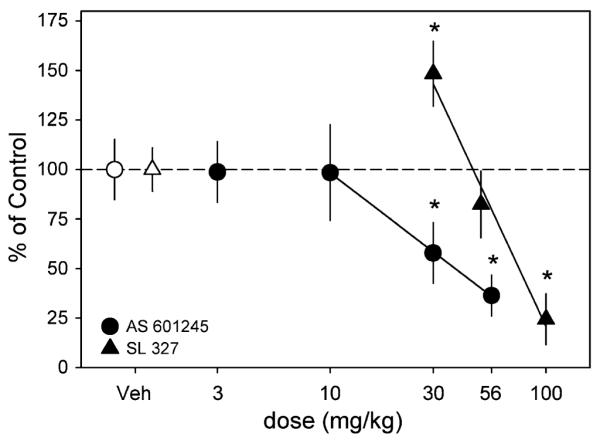
Modulation of ethanol self-administration by MAPK inhibitors. Filled triangles represent the dose–effect curve for SL 327 and filled circles represent the dose–effect curve for AS 601245. Open symbols reflect the vehicle values for SL 327 (triangle) and AS 601245 (circle). Data are presented as a percent change from vehicle levels of means ± SEM (vertical lines) for dose consumed. Regression lines are shown that fit the effects of both ligands for this behavior. Asterisks denote significance as compared to vehicle (p<0.05)
Table 1.
Effect of SL 327 and AS 601245 on ethanol and sucrose self-administration
| SL 327 (mg/kg) |
AS 601245 (mg/kg) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Vehicle | 30 | 50 | 100 | Vehicle | 3 | 10 | 30 | 56 | |
| Ethanol self-administration | |||||||||
| Reinforcements | 19.7±2.1 | 28.4±3.2* | 16.8±3.4 | 11.2±5.9* | 28.8±4.4 | 24.3±2.4 | 20.8±3.5 | 15.3±4.7 | 9.6±3.0* |
| Active lever | 80.3±9.8 | 122.3±14.7* | 71.4±15.0 | 47.2±24.8* | 125.4±20.0 | 104.4±10.0 | 88.3±15.4 | 66.0±20.1* | 41.6±13.1* |
| Inactive lever | 16.6±3.4 | 22.2±8.3 | 14.3±4.3 | 6.0±4.1* | 42.0±13.6 | 41.4±12.6 | 20.9±5.6 | 9.4±2.5* | 8.0±3.6* |
| Percent active responses |
84.3±2.3 | 86.4±3.7 | 83.3±3.9 | 77.5±16.0 | 79.7±3.9 | 76.1±4.7 | 83.4±3.0 | 85.8±2.2 | 82.4±5.0 |
| Headpoke entries |
83.2±12.9 | 93.1±7.6 | 99.8±25.0 | 41.3±18.2* | 94.3±21.7 | 49.8±5.9* | 65.3±14.1 | 54.2±12.6* | 36.6±11.5* |
| Dose (g/kg) | 0.58±0.06 | 0.85±0.10* | 0.50±0.11 | 0.31±0.16* | 0.87±0.13 | 0.70±0.07 | 0.61±0.10 | 0.44±0.13* | 0.28±0.09* |
| Sucrose self-administration | |||||||||
| Reinforcements | 29.6±5.9 | 33.8±8.8 | 18.6±5.0 | 2.3±1.0* | 33.3±6.7 | 25.5±6.0 | 19.5±4.9 | 7.8±2.2* | 7.9±2.2* |
| Active lever | 135.2±29.0 | 145.9±37.6 | 84.1±23.5 | 10.6±4.1* | 150.6 ±32.3 | 116.7±28.6 | 89.3±23.2 | 33.9±9.5* | 35.5±10.4* |
| Inactive lever | 40.2±13.6 | 27.1±9.4 | 44.1±23.6 | 3.2±2.1* | 75.4±25.1 | 54.1±16.8 | 44.6±14.6 | 11.8±4.9* | 10.6±5.2* |
| Percent active responses |
81.3±4.8 | 83.0±5.9 | 78.8±7.5 | 84.4±6.6 | 72.4±6.2 | 74.5±5.1 | 74.9±5.3 | 78.3±7.0 | 82.7±4.5 |
| Headpoke entries |
83.3±12.9 | 93.2±20.1 | 61.3±14.7 | 15.7±4.5* | 79.4±11.5 | 73.1±13.8 | 49.0±9.4 | 33.9±8.5* | 30.5±8.2* |
Frequencies of behaviors and dose consumed (gram per kilogram) during a 1-h session are represented as mean ± SEM
Values in bold emphasis with asterisks indicate statistical significance from vehicle, p<0.05
AS 601245
A reduction in responding for ethanol was seen after systemic administration of the JNK inhibitor, AS 601245. Specifically, the dose-consumed (F(11, 44)=6.1, p<0.001), number of active lever responses (F(11, 44)=5.6, p<0.001), and number of inactive lever responses (F(11, 44)=4.5, p=0.004) were significantly attenuated after administration of the two highest doses, 30 and 56 mg/kg (Fig. 2, Table 1). The number of reinforcements obtained (F(11, 44)=3.07, p=0.026) was similarly reduced by administration of 56 mg/kg AS 601245 and headpoke entries (F(11, 44)=6.1, p<0.001) were significantly reduced by all doses of AS 601245 except for 10 mg/kg.
Experiment 2: Effect of MAPK inhibitors on sucrose self-administration
SL 327
Two mice were excluded from the SL 327 dose–effect curve because they did not demonstrate stable levels of responding for sucrose. Administration of SL 327 dose-dependently decreased several measures of responding including the number of reinforcements obtained (F(8, 24)=8.1, p<0.001), number of active lever responses (F(8, 24)=7.3, p=0.001), number of inactive responses (F(8, 24)=3.3, p=0.036), and number of headpokes (F(8, 24)=5.3, p=0.006). Reduction of these measures was only evident after administration of 100 mg/kg SL 327 (Fig. 3, Table 1).
AS 601245
A reduction in responding for 2% sucrose was observed after administration of AS 601245. Specifically, the number of reinforcements obtained (F(10, 40)=5.1, p=0.002), number of active lever responses (F(10, 40)=4.9, p=0.002), number of inactive lever responses (F(10, 40)=3.3, p=0.019), and number of headpokes (F(10, 40)=4.3, p=0.005) were all significantly lower after administration of the two highest doses, 30 and 56 mg/kg (Fig. 3, Table 1).
Experiment 3: Effect of SL 327 on PR responding for ethanol
As the steepness of the PR schedule increased, the average breakpoint increased and was significantly higher at +5, +6, +8, and +12 PR schedules than for the +1 PR schedule of reinforcement (F(7, 105)=5.74, p<0.001; Fig. 4a). Additionally, the duration of each session varied across PR schedules, ranging from a minimum of 26.3±1.5 min (PR +6) to a maximum of 37.9±1.7 min (PR +3). These values are comparable to those reported in other ethanol studies, which showed pharmacological manipulation of breakpoint, but are lower than breakpoints for other drugs of abuse (Besheer et al. 2008; Brown and Stephens 2002; Czachowski et al. 2003; Economidou et al. 2006; Oster et al. 2006; Richardson and Roberts 1996; Rodd et al. 2003; Stafford et al. 1998; Zghoul et al. 2007).
The PR +6 schedule of reinforcement was used to test the effect of SL 327 on PR responding. Baseline levels of responding under vehicle conditions were as follows: breakpoint (Fig. 4b), number of active lever responses (47.8±8.5), number of reinforcements obtained (3.1±0.3), dose consumed (0.1±0.01 g/kg), number of active headpokes (17.5±1.8), number of inactive lever responses (14.6±8.1), percent responding on the active lever (83.1±4.9), rate of active lever responding (1.58±.26 lever presses/min), and session duration (29.4±2.4 min). Administration of SL 327 (0–50 mg/kg) did not significantly alter any of these measures of PR responding.
Experiment 4: Effect of MAPK inhibitors on locomotor activity
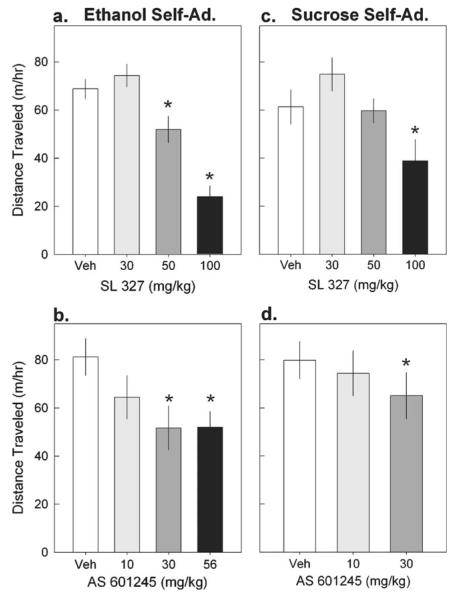
Ethanol self-administering mice
In the mice that had been trained to self-administer ethanol from experiment 1, SL 327 dose-dependently reduced locomotor activity during a 1-h open field test (F(3, 33)=35.2, p<0.001; Fig. 5a). This reduction was evident after injection of 50 and 100 mg/kg SL 327. An additional timecourse analysis did not reveal an interaction between dose and time indicating that the reduction in open field activity by SL 327 (50 and 100 mg/kg) persisted throughout the entire hour. The dose of SL 327 (30 mg/kg) that increased ethanol self-administration did not alter locomotor activity.
Fig. 5.
Effect of MAPK Inhibitors on open field locomotor activity. a Effect of Veh (white), 30 (light gray), 50 (dark grey), and 100 mg/kg SL 327 (black) on the locomotor activity of mice with ethanol self-administration experience. b Effect of Veh (white), 10 (light gray), 30 (dark gray), and 56 mg/kg AS 601245 (black)on the locomotor activity of mice with ethanol self-administration experience. c Effect of Veh (white), 30 (light gray), 50 (dark gray), and 100 mg/kg SL 327 (black) on the locomotor activity of mice with sucrose self-administration experience. d Effect of Veh (white), 10 (light gray), and 30 mg/kg AS 601245 (dark gray) on the locomotor activity of mice with sucrose self-administration experience. The data reflect the mean (vertical bars) ± SEM (vertical lines) of total distance traveled in a 1-h session. Asterisks denote significance as compared to vehicle (p<0.05)
Similarly, administration of AS 601245 significantly reduced open field activity of mice that had been trained to self-administer ethanol (F(3, 30)=4.4, p=0.001; Fig. 5b). This reduction was evident after administration of 30 and 56 mg/kg AS 601245 and persisted throughout the entire hour.
Sucrose self-administering mice
Administration of SL 327 significantly reduced the locomotor activity of mice that had been trained to self-administer 2% sucrose (F(3, 30)=7.3, p<0.001; Fig. 5c). This reduction was observed after injection of 100 mg/kg SL 327 throughout the entire hour.
AS 601245 dose-dependently reduced the locomotor activity of mice that had been trained to self-administer 2% sucrose (F(2, 18)=4.5, p=0.03; Fig. 5d). Post hoc tests revealed that this effect was due to reduced open field activity after administration of 30 mg/kg AS 601245 and was evident throughout the entire hour.
Discussion
The goal of these experiments was to determine whether the ERK/MAPK pathway is functionally involved in modulating the reinforcing properties of ethanol in male C57BL/6J mice. Inhibition of ERK1/2 phosphorylation by the MEK1/2 inhibitor SL 327 led to a biphasic effect on ethanol self-administration; the low dose of SL 327 (30 mg/kg) significantly increased while the higher dose (100 mg/kg) significantly decreased ethanol-reinforced responding. The potentiation of ethanol self-administration by SL 327 appears to be specifically related to a reduction in ERK1/2 activity rather than to MAPK activity in general, because inhibition of the JNK/MAPK pathway by AS 601245 did not increase responding. Rather, AS 601245 dose-dependently reduced operant responding for ethanol. Both inhibitors also reduced sucrose self-administration in a similar, dose-dependent manner although, notably, increased responding was not evident after injection of 30 mg/kg SL 327.
The behavioral implications of modulating MAPK activity are largely unknown. A handful of studies have shown that the MEK1/2 inhibitors SL 327 and U0126 block cue-induced reinstatement, locomotor sensitization to psychostimulants, memory consolidation, LTP, and parental care (Atkins et al. 1998; Cestari et al. 2006; Davis et al. 2000; Kuroda et al. 2007; Lu et al. 2005; Valjent et al. 2006a, b). A recent study by Carnicella et al.( 2008) found that intra-VTA GDNF infusion inhibits ethanol, but not sucrose, self-administration and that this effect is mediated by MAPK signaling since pretreatment with U1026 prevented the GDNF-induced reduction in drinking. Together with our series of experiments, these studies are some of the first to demonstrate that ethanol self-administration is altered due to MEK1/2 inhibition. Furthermore, the effect of SL 327 on ethanol self-administration is intriguing because it suggests that MEK1/2 inhibition can functionally alter behavior by potentiating self-administration behavior. Increases in ethanol self-administration have been found after administration of a wide variety of compounds including allopregnanolone, estradiol, μ opioid agonists, κ opioid antagonists, A2 antagonists and intra-raphe muscimol (Arolfo et al. 2004; Ford et al. 2004, 2005; Hodge et al. 1995; Janak et al. 1998; Mitchell et al. 2005; Quirarte et al. 2007; Reid et al. 2002; Sabino et al. 2007; Sinnott et al. 2002; Tomkins et al. 1994; Ulm et al. 1995; Vacca et al. 2002; Zhang and Kelley 2002). Despite the diverse nature of these ligands, they all have a biphasic effect on self-administration; low doses increase and high doses decrease drinking. This further suggests that there may be a singular common mechanism that underlies escalations in ethanol self-administration, perhaps via an intracellular mechanism such as pERK1/2.
Conceptually, increases in drug-reinforced responding are difficult to interpret because they can result from either increased motivation to self-administer or blockade of the pharmacological effects of ethanol (i.e., requiring more reinforcements to achieve the same subjective effect). In this study, we characterized mouse performance on a variety of PR schedules of alcohol reinforcement as one index of motivation. PR responding was found to be stable across repeated testing and insensitive to a range of SL 327 doses. Importantly, mice were not consuming pharmacologically active amounts of ethanol under this schedule so it is unlikely that the subjective effects of the consumed ethanol or satiety were interacting with their performance. These results on PR responding for ethanol argue against the hypothesis that inhibition of pERK1/2 increased the motivation to consume ethanol. Instead, it is possible that SL 327 (30 mg/kg) might have antagonized the acute pharmacological effects of ethanol to increase ERK1/2 activity, leading to increased ethanol self-administration. The latter interpretation is consistent with a wide body of literature regarding mechanisms of drug self-administration. For instance, a primary pharmacological effect of cocaine is to block the dopamine transporter, which increases synaptic dopamine and activates dopamine receptors. Accordingly, administration of low doses of D1-like or D2-like receptor antagonists has been shown to increase cocaine self-administration (Ettenberg et al. 1982; Koob et al. 1987; Woolverton 1986). Analogous evidence shows that low to moderate doses of the opiate antagonist naloxone increase morphine (Weeks and Collins 1976; Woods et al. 1975) and heroin (Ettenberg et al. 1982; Koob et al. 1984) self-administration. Higher doses of antagonists generally reduce drug self-administration. Thus, one plausible interpretation of the increase in self-administration observed in this study following pretreatment with SL 327 is that ERK1/2 activation is a primary pharmacological effect of ethanol that supports its reinforcing properties.
A secondary behavioral consequence of MEK1/2 inhibition was decreased locomotor behavior after high dose administration of SL 327. Consistent with the literature, open field activity was significantly reduced in ethanol self-administering mice by 50–100 mg/kg SL 327 (Valjent et al. 2006b). However, behaviors during the ethanol self-administration session were unaffected by 50 mg/kg indicating that the motor activation that occurs during a self-administration session is sufficient to overcome the mildly sedative effects of SL 327 at this dose. Interestingly, this context-dependent locomotor-suppressant effect of 50 mg/kg SL 327 was not observed in mice that had been trained to self-administer sucrose (i.e., 50 mg/kg SL 327 did not reduce the frequency of duration of any behavior). Importantly, both ethanol and sucrose mice showed a significantly greater degree of sedation after injection of 100 mg/kg SL 327 in both basal (open field) and stimulated (behavior in the operant conditioning chamber) motor activity. It is known that voluntary home-cage ethanol drinking (Spanos and Hodge 2008) and forced exposure to high doses of ethanol by vapor inhalation (Sanna et al. 2002) leads to a downregulation of pERK1/2 expression. While the effect of daily ethanol intake on pERK1/2 under operant self-administration conditions is not known, the differential response of the ethanol self-administering mice to SL 327 suggests that modest levels of ethanol self-administration might also reduce pERK1/2, which could lead to heightened locomotor sensitivity to ERK1/2 inhibition.
The role of a second MAPK subfamily, JNK, in the regulation of behavior has been less extensively studied but shares many common characteristics with ERK1/2. JNK is widely recognized for its activation in response to neuronal cell death and oxidative stress, and it has recently been shown to be an important downstream effector of glutamate activity (Borsello et al. 2003; Carboni et al. 2008; Herdegen et al. 1998; Mukherjee et al. 1999; Schwarzschild et al. 1997). In striatal tissue, the group 1 metabotropic glutamate receptor (mGluR) agonist DHPG has been shown to cause a threefold increase in JNK and pERK1/2 phosphorylation. This increase is blocked by the group 1 mGluR5 antagonist MPEP, but not the group 1 mGluR1 antagonist CPCCOEt, indicating that this effect is mGluR5 but not mGluR1 dependent (Choe and Wang 2001; Yang et al. 2006). This connection between mGluR5 and JNK activity is relevant because it (1) links JNK phosphorylation to a receptor system that is known to modulate ethanol drinking and (2) suggests that ERK1/2 and JNK can be both activated by the same pharmacological ligands and thus may regulate common processes (Backstrom et al. 2004; Besheer et al. 2006; Hodge et al. 2006; Lominac et al. 2006; McMillen et al. 2005; Schroeder et al. 2005). Because of this relationship, we were interested in investigating whether inhibition of JNK activity by AS 601245 would produce a similar behavioral consequence as ERK1/2 inhibition. At high doses (30 and 56 mg/kg), reductions in both ethanol self-administration and locomotor activity were observed after injection of AS 601245; low doses (3–10 mg/kg) were ineffective. Thus, the potentiation of ethanol self-administration appears to be ERK1/2-specific. This finding is in accordance with an earlier study showing that intra-accumbal amphetamine-conditioned place preference can be blocked by a MEK but not JNK inhibitor (Gerdjikov et al. 2004). Together, these data support the hypothesis that ERK1/2 signaling may be critical for mediating reward-related learning and reinforcement.
A major role of the MAPK pathways in the adult central nervous system is the regulation of neural and behavioral plasticity, including memory processes (Chandler 2003; Impey et al. 1999; Sweatt 2004). For example, SL 327 (100 mg/kg) has been shown to block cued and contextual fear conditioning in a manner that is dissociated from motor effects in rats (Atkins et al. 1998). Similarly, JNK inhibition blocks memory formation and retrieval of an inhibitory avoidance task (Bevilaqua et al. 2003). Thus, it is plausible that the non-specific reductions in ethanol and sucrose self-administration observed after the high doses of the MEK1/2 and JNK inhibitors in this study reflect memory disruption. However, several factors seem to preclude this interpretation. First, it is important to reiterate that doses of the MAPK inhibitors that reduced operant responding also produced significant decreases in spontaneous locomotor activity in both ethanol- and sucrose-exposed mice, which indicates pleiotropic behavioral effects at high doses of these compounds and complicates interpretations related specifically to memory. Second, although SL 327 (30 mg/kg) inhibits spatial memory in mice (Selcher et al. 1999), this dose specifically enhanced ethanol self-administration without effect on sucrose self-administration in the present study, which is inconsistent with memory disruption. Thus, the apparent lack of effect of SL 327 on memory in this study may reflect differential functional regulation or procedural differences between tests of operant conditioned behavior as compared to tests that are commonly used to assess ERK1/2 regulation of memory (e.g., Morris water maze, fear conditioning). Alternatively, the enhancement of ethanol-reinforced responding by SL 327 may reflect neuroplasticity in ERK1/2 function recruited away from memory processes toward regulation of drug reinforcement, which is consistent with other evidence showing that a long history of ethanol self-administration can usurp the function of homeostatic regulatory systems (Kelley et al. 2001).
In sum, the results of this study show that inhibition of ERK1/2 phosphorylation caused a dose-dependent biphasic effect on ethanol-reinforced responding. Systemic administration of a low dose of the MEK1/2 inhibitor SL 327, which crosses the blood–brain barrier and inhibits ERK1/2 phosphorylation, selectively increased ethanol-, but not sucrose-reinforced responding, whereas higher doses were either ineffective or produced non-specific reductions likely due to motor impairments. Importantly, this effect was specific to the ERK1/2/MAPK pathway and to ethanol reinforcement. Since acute ethanol increases ERK1/2 phosphorylation in brain regions that regulate ethanol self-administration (Hodge et al. 1992; Sharko and Hodge 2007), these data are consistent with the hypothesis that ERK1/2 phosphorylation is a primary pharmacological effect of ethanol that supports its reinforcing properties. It will be of interest to examine the functional neuroanatomy of ethanol self-administration to determine if brain regions that show ethanol-induced ERK1/2 activation also regulate its reinforcing properties.
Acknowledgments
This research was supported by NIAAA grants AA011605 (CWH), AA014983 (CWH), and AA007573 (Bowles Center for Alcohol Studies Training Grant). The authors would like to thank Michael C Salling for his outstanding experimental assistance.
References
- Adams JP, Sweatt JD. Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol. 2002;42:135–163. doi: 10.1146/annurev.pharmtox.42.082701.145401. [DOI] [PubMed] [Google Scholar]
- Adams JP, Roberson ED, English JD, Selcher JC, Sweatt JD. MAPK regulation of gene expression in the central nervous system. Acta Neurobiol Exp (Wars) 2000;60:377–394. doi: 10.55782/ane-2000-1357. [DOI] [PubMed] [Google Scholar]
- Arolfo MP, Yao L, Gordon AS, Diamond I, Janak PH. Ethanol operant self-administration in rats is regulated by adenosine A2 receptors. Alcohol Clin Exp Res. 2004;28:1308–1316. doi: 10.1097/01.alc.0000139821.38167.20. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Tsivkovskaia NO, Ryabinin AE. Alcohol-induced c-Fos expression in the Edinger-Westphal nucleus: pharmacological and signal transduction mechanisms. J Pharmacol Exp Ther. 2002;302:516–524. doi: 10.1124/jpet.102.036046. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology. 2004;29:921–928. doi: 10.1038/sj.npp.1300381. [DOI] [PubMed] [Google Scholar]
- Besheer J, Stevenson RA, Hodge CW. mGlu5 receptors are involved in the discriminative stimulus effects of self-administered ethanol in rats. Eur J Pharmacol. 2006;551:71–75. doi: 10.1016/j.ejphar.2006.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Faccidomo S, Grondin JJ, Hodge CW. Regulation of motivation to self-administer ethanol by mGluR5 in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2008;32:209–221. doi: 10.1111/j.1530-0277.2007.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilaqua LR, Kerr DS, Medina JH, Izquierdo I, Cammarota M. Inhibition of hippocampal Jun N-terminal kinase enhances short-term memory but blocks long-term memory formation and retrieval of an inhibitory avoidance task. Eur J Neurosci. 2003;17:897–902. doi: 10.1046/j.1460-9568.2003.02524.x. [DOI] [PubMed] [Google Scholar]
- Borsello T, Croquelois K, Hornung JP, Clarke PG. N-methyl-d-aspartate-triggered neuronal death in organotypic hippocampal cultures is endocytic, autophagic and mediated by the c-Jun N-terminal kinase pathway. Eur J Neurosci. 2003;18:473–485. doi: 10.1046/j.1460-9568.2003.02757.x. [DOI] [PubMed] [Google Scholar]
- Boulton TG, Cobb MH. Identification of multiple extracellular signal-regulated kinases (ERKs) with antipeptide antibodies. Cell Regul. 1991;2:357–371. doi: 10.1091/mbc.2.5.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G, Stephens DN. Effects of cocaine on responding for ethanol or sucrose under a progressive ratio schedule. Behav Pharmacol. 2002;13:157–162. doi: 10.1097/00008877-200203000-00007. [DOI] [PubMed] [Google Scholar]
- Carboni S, Hiver A, Szyndralewiez C, Gaillard P, Gotteland JP, Vitte PA. AS601245 (1,3-benzothiazol-2-yl (2-[[2-(3-pyridinyl) ethyl] amino]-4 pyrimidinyl) acetonitrile): a c-Jun NH2-terminal protein kinase inhibitor with neuroprotective properties. J Pharmacol Exp Ther. 2004;310:25–32. doi: 10.1124/jpet.103.064246. [DOI] [PubMed] [Google Scholar]
- Carboni S, Boschert U, Gaillard P, Gotteland JP, Gillon JY, Vitte PA. AS601245, a c-Jun NH2-terminal kinase (JNK) inhibitor, reduces axon/dendrite damage and cognitive deficits after global cerebral ischaemia in gerbils. Br J Pharmacol. 2008;153:157–163. doi: 10.1038/sj.bjp.0707574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Kharazia V, Jeanblanc J, Janak PH, Ron D. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc Natl Acad Sci U S A. 2008;105:8114–8119. doi: 10.1073/pnas.0711755105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cestari V, Costanzi M, Castellano C, Rossi-Arnaud C. A role for ERK2 in reconsolidation of fear memories in mice. Neurobiol Learn Mem. 2006;86:133–143. doi: 10.1016/j.nlm.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Chandler LJ. Ethanol and brain plasticity: receptors and molecular networks of the postsynaptic density as targets of ethanol. Pharmacol Ther. 2003;99:311–326. doi: 10.1016/s0163-7258(03)00096-2. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Sutton G. Acute ethanol inhibits extracellular signal-regulated kinase, protein kinase B, and adenosine 3′:5′-cyclic monophosphate response element binding protein activity in an age- and brain region-specific manner. Alcohol Clin Exp Res. 2005;29:672–682. doi: 10.1097/01.alc.0000158935.53360.5f. [DOI] [PubMed] [Google Scholar]
- Chen RH, Sarnecki C, Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992;12:915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe ES, Wang JQ. Group I metabotropic glutamate receptors control phosphorylation of CREB, Elk-1 and ERK via a CaMKII-dependent pathway in rat striatum. Neurosci Lett. 2001;313:129–132. doi: 10.1016/s0304-3940(01)02258-3. [DOI] [PubMed] [Google Scholar]
- Choe ES, Chung KT, Mao L, Wang JQ. Amphetamine increases phosphorylation of extracellular signal-regulated kinase and transcription factors in the rat striatum via group I metabotropic glutamate receptors. Neuropsychopharmacology. 2002;27:565–575. doi: 10.1016/S0893-133X(02)00341-X. [DOI] [PubMed] [Google Scholar]
- Crews CM, Erikson RL. Purification of a murine protein-tyrosine/threonine kinase that phosphorylates and activates the Erk-1 gene product: relationship to the fission yeast byr1 gene product. Proc Natl Acad Sci U S A. 1992;89:8205–8209. doi: 10.1073/pnas.89.17.8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czachowski CL, Legg BH, Samson HH. Assessment of sucrose and ethanol reinforcement: the across-session breakpoint procedure. Physiol Behav. 2003;78:51–59. doi: 10.1016/s0031-9384(02)00963-0. [DOI] [PubMed] [Google Scholar]
- Davis S, Vanhoutte P, Pages C, Caboche J, Laroche S. The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J Neurosci. 2000;20:4563–4572. doi: 10.1523/JNEUROSCI.20-12-04563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvarci S, Nader K, LeDoux JE. Activation of extracellular signal-regulated kinase-mitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. Eur J Neurosci. 2005;21:283–289. doi: 10.1111/j.1460-9568.2004.03824.x. [DOI] [PubMed] [Google Scholar]
- Economidou D, Mattioli L, Cifani C, Perfumi M, Massi M, Cuomo V, Trabace L, Ciccocioppo R. Effect of the cannabinoid CB1 receptor antagonist SR-141716A on ethanol self-administration and ethanol-seeking behaviour in rats. Psychopharmacology (Berl) 2006;183:394–403. doi: 10.1007/s00213-005-0199-9. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology (Berl) 1982;78:204–209. doi: 10.1007/BF00428151. [DOI] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Fasano S, Yang P, Brambilla R, Robinson TE. Knockout of ERK1 enhances cocaine-evoked immediate early gene expression and behavioral plasticity. Neuropsychopharmacology. 2006;31:2660–2668. doi: 10.1038/sj.npp.1301014. [DOI] [PubMed] [Google Scholar]
- Ford MM, Eldridge JC, Samson HH. Determination of an estradiol dose–response relationship in the modulation of ethanol intake. Alcohol Clin Exp Res. 2004;28:20–28. doi: 10.1097/01.ALC.0000108647.62718.5A. [DOI] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Phillips TJ, Finn DA. Neurosteroid modulators of GABA(A) receptors differentially modulate ethanol intake patterns in male C57BL/6J mice. Alcohol Clin Exp Res. 2005;29:1630–1640. doi: 10.1097/01.alc.0000179413.82308.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard P, Jeanclaude-Etter I, Ardissone V, Arkinstall S, Cambet Y, Camps M, Chabert C, Church D, Cirillo R, Gretener D, Halazy S, Nichols A, Szyndralewiez C, Vitte PA, Gotteland JP. Design and synthesis of the first generation of novel potent, selective, and in vivo active (benzothiazol-2-yl)acetonitrile inhibitors of the c-Jun N-terminal kinase. J Med Chem. 2005;48:4596–4607. doi: 10.1021/jm0310986. [DOI] [PubMed] [Google Scholar]
- Gerdjikov TV, Ross GM, Beninger RJ. Place preference induced by nucleus accumbens amphetamine is impaired by antagonists of ERK or p38 MAP kinases in rats. Behav Neurosci. 2004;118:740–750. doi: 10.1037/0735-7044.118.4.740. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Grewal SS, York RD, Stork PJ. Extracellular-signal-regulated kinase signalling in neurons. Curr Opin Neurobiol. 1999;9:544–553. doi: 10.1016/S0959-4388(99)00010-0. [DOI] [PubMed] [Google Scholar]
- Hendrickson RJ, Cahill PA, McKillop IH, Sitzmann JV, Redmond EM. Ethanol inhibits mitogen activated protein kinase activity and growth of vascular smooth muscle cells in vitro. Eur J Pharmacol. 1998;362:251–259. doi: 10.1016/s0014-2999(98)00771-7. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Claret FX, Kallunki T, Martin-Villalba A, Winter C, Hunter T, Karin M. Lasting N-terminal phosphorylation of c-Jun and activation of c-Jun N-terminal kinases after neuronal injury. J Neurosci. 1998;18:5124–5135. doi: 10.1523/JNEUROSCI.18-14-05124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Samson HH, Haraguchi M. Microinjections of dopamine agonists in the nucleus accumbens increase ethanol-reinforced responding. Pharmacol Biochem Behav. 1992;43:249–254. doi: 10.1016/0091-3057(92)90665-3. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Chappelle AM, Samson HH. GABAergic transmission in the nucleus accumbens is involved in the termination of ethanol self-administration in rats. Alcohol Clin Exp Res. 1995;19:1486–1493. doi: 10.1111/j.1530-0277.1995.tb01012.x. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Miles MF, Sharko AC, Stevenson RA, Hillmann JR, Lepoutre V, Besheer J, Schroeder JP. The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL/6J mice. Psychopharmacology (Berl) 2006;183:429–438. doi: 10.1007/s00213-005-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TY, Lin CH. Role of amygdala MAPK activation on immobility behavior of forced swim rats. Behav Brain Res. 2006;173:104–111. doi: 10.1016/j.bbr.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Storm DR. Making new connections: role of ERK/MAP kinase signaling in neuronal plasticity. Neuron. 1999;23:11–14. doi: 10.1016/s0896-6273(00)80747-3. [DOI] [PubMed] [Google Scholar]
- Janak PH, Redfern JE, Samson HH. The reinforcing effects of ethanol are altered by the endogenous neurosteroid, allopregnanolone. Alcohol Clin Exp Res. 1998;22:1106–1112. [PubMed] [Google Scholar]
- Kalluri HS, Ticku MK. Ethanol-mediated inhibition of mitogen-activated protein kinase phosphorylation in mouse brain. Eur J Pharmacol. 2002;439:53–58. doi: 10.1016/s0014-2999(01)01599-0. [DOI] [PubMed] [Google Scholar]
- Kelley SP, Nannini MA, Bratt AM, Hodge CW. Neuropeptide-Y in the paraventricular nucleus increases ethanol self-administration. Peptides. 2001;22:515–522. doi: 10.1016/s0196-9781(01)00361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Pettit HO, Ettenberg A, Bloom FE. Effects of opiate antagonists and their quaternary derivatives on heroin self-administration in the rat. J Pharmacol Exp Ther. 1984;229:481–486. [PubMed] [Google Scholar]
- Koob GF, Le HT, Creese I. The D1 dopamine receptor antagonist SCH 23390 increases cocaine self-administration in the rat. Neurosci Lett. 1987;79:315–320. doi: 10.1016/0304-3940(87)90451-4. [DOI] [PubMed] [Google Scholar]
- Krishna M, Narang H. The complexity of mitogen-activated protein kinases (MAPKs) made simple. Cell Mol Life Sci. 2008;65(22):3525–3544. doi: 10.1007/s00018-008-8170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda KO, Meaney MJ, Uetani N, Fortin Y, Ponton A, Kato T. ERK-FosB signaling in dorsal MPOA neurons plays a major role in the initiation of parental behavior in mice. Mol Cell Neurosci. 2007;36:121–131. doi: 10.1016/j.mcn.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Lominac KD, Kapasova Z, Hannun RA, Patterson C, Middaugh LD, Szumlinski KK. Behavioral and neurochemical interactions between Group 1 mGluR antagonists and ethanol: potential insight into their anti-addictive properties. Drug Alcohol Depend. 2006;85:142–156. doi: 10.1016/j.drugalcdep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal models of drug craving. Psychopharmacology (Berl) 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- Mazzucchelli C, Brambilla R. Ras-related and MAPK signalling in neuronal plasticity and memory formation. Cell Mol Life Sci. 2000;57:604–611. doi: 10.1007/PL00000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzucchelli C, Vantaggiato C, Ciamei A, Fasano S, Pakhotin P, Krezel W, Welzl H, Wolfer DP, Pages G, Valverde O, Marowsky A, Porrazzo A, Orban PC, Maldonado R, Ehrengruber MU, Cestari V, Lipp HP, Chapman PF, Pouyssegur J, Brambilla R. Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory. Neuron. 2002;34:807–820. doi: 10.1016/s0896-6273(02)00716-x. [DOI] [PubMed] [Google Scholar]
- McMillen BA, Crawford MS, Kulers CM, Williams HL. Effects of a metabotropic, mglu5, glutamate receptor antagonist on ethanol consumption by genetic drinking rats. Alcohol. 2005;40:494–497. doi: 10.1093/alcalc/agh200. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Liang MT, Fields HL. A single injection of the kappa opioid antagonist norbinaltorphimine increases ethanol consumption in rats. Psychopharmacology (Berl) 2005;182:384–392. doi: 10.1007/s00213-005-0067-7. [DOI] [PubMed] [Google Scholar]
- Mukherjee PK, DeCoster MA, Campbell FZ, Davis RJ, Bazan NG. Glutamate receptor signaling interplay modulates stress-sensitive mitogen-activated protein kinases and neuronal cell death. J Biol Chem. 1999;274:6493–6498. doi: 10.1074/jbc.274.10.6493. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guide for the care and use of laboratory animals. National Academy Press; Washington: 1996. [Google Scholar]
- Ortiz J, Harris HW, Guitart X, Terwilliger RZ, Haycock JW, Nestler EJ. Extracellular signal-regulated protein kinases (ERKs) and ERK kinase (MEK) in brain: regional distribution and regulation by chronic morphine. J Neurosci. 1995;15:1285–1297. doi: 10.1523/JNEUROSCI.15-02-01285.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster SM, Toalston JE, Kuc KA, Pommer TJ, Murphy JM, Lumeng L, Bell RL, McBride WJ, Rodd ZA. Effects of multiple alcohol deprivations on operant ethanol self-administration by high-alcohol-drinking replicate rat lines. Alcohol. 2006;38:155–164. doi: 10.1016/j.alcohol.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Quirarte GL, Reid LD, de la Teja IS, Reid ML, Sanchez MA, Diaz-Trujillo A, Aguilar-Vazquez A, Prado-Alcala RA. Estradiol valerate and alcohol intake: dose–response assessments. BMC Pharmacol. 2007;7:3. doi: 10.1186/1471-2210-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid LD, Marinelli PW, Bennett SM, Fiscale LT, Narciso SP, Oparowski CJ, Reid ML, Merrigan BA, Moricone J, Hubbell CL, Gianoulakis C. One injection of estradiol valerate induces dramatic changes in rats’ intake of alcoholic beverages. Pharmacol Biochem Behav. 2002;72:601–616. doi: 10.1016/s0091-3057(02)00732-3. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Kuc KA, Murphy JM, Lumeng L, Li TK, McBride WJ. Effects of repeated alcohol deprivations on operant ethanol self-administration by alcohol-preferring (P) rats. Neuropsychopharmacology. 2003;28:1614–1621. doi: 10.1038/sj.npp.1300214. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Mazei-Robison MS, Ables JL, Nestler EJ. Neurotrophic factors and structural plasticity in addiction. Neuropharmacology. 2008 doi: 10.1016/j.neuropharm.2008.06.059. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Steardo L, Schmidhammer H, Zorrilla EP. 14-Methoxymetopon, a highly potent mu opioid agonist, biphasically affects ethanol intake in Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 2007;192:537–546. doi: 10.1007/s00213-007-0746-7. [DOI] [PubMed] [Google Scholar]
- Salzmann J, Marie-Claire C, Le Guen S, Roques BP, Noble F. Importance of ERK activation in behavioral and biochemical effects induced by MDMA in mice. Br J Pharmacol. 2003;140:831–838. doi: 10.1038/sj.bjp.0705506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Sanna PP, Simpson C, Lutjens R, Koob G. ERK regulation in chronic ethanol exposure and withdrawal. Brain Res. 2002;948:186–191. doi: 10.1016/s0006-8993(02)03191-8. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J Neurosci. 2000;20:8177–787. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Overstreet DH, Hodge CW. The mGluR5 antagonist MPEP decreases operant ethanol self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats. Psychopharmacology (Berl) 2005;179:262–270. doi: 10.1007/s00213-005-2175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Spanos M, Stevenson JR, Besheer J, Salling M, Hodge CW. Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK1/2 phosphorylation in specific limbic brain regions: blockade by the mGluR5 antagonist MPEP. Neuropharmacology. 2008;55:546–554. doi: 10.1016/j.neuropharm.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzschild MA, Cole RL, Hyman SE. Glutamate, but not dopamine, stimulates stress-activated protein kinase and AP-1-mediated transcription in striatal neurons. J Neurosci. 1997;17:3455–3466. doi: 10.1523/JNEUROSCI.17-10-03455.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- Selcher JC, Atkins CM, Trzaskos JM, Paylor R, Sweatt JD. A necessity for MAP kinase activation in mammalian spatial learning. Learn Mem. 1999;6:478–490. doi: 10.1101/lm.6.5.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgambato V, Vanhoutte P, Pages C, Rogard M, Hipskind R, Besson MJ, Caboche J. In vivo expression and regulation of Elk-1, a target of the extracellular-regulated kinase signaling pathway, in the adult rat brain. J Neurosci. 1998;18:214–226. doi: 10.1523/JNEUROSCI.18-01-00214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharko AC, Hodge CW. Acute ethanol challenge modulates extracellular-signal regulated kinase phosphorylation in vivo. Alcohol Clin Exp Res. 2007;31:193A. [Google Scholar]
- Shi X, McGinty JF. Extracellular signal-regulated mitogen-activated protein kinase inhibitors decrease amphetamine-induced behavior and neuropeptide gene expression in the striatum. Neuroscience. 2006;138:1289–1298. doi: 10.1016/j.neuroscience.2005.12.024. [DOI] [PubMed] [Google Scholar]
- Sinnott RS, Phillips TJ, Finn DA. Alteration of voluntary ethanol and saccharin consumption by the neurosteroid allopregnanolone in mice. Psychopharmacology (Berl) 2002;162:438–447. doi: 10.1007/s00213-002-1123-1. [DOI] [PubMed] [Google Scholar]
- Spanos M, Hodge CW. Neuroadaptive changes in ERK1/2 activity associated with alcohol self-administration in mice. Alcohol Clin Exp Res. 2008;32:162A–162A. [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology (Berl) 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Tomkins DM, Higgins GA, Sellers EM. Low doses of the 5-HT1A agonist 8-hydroxy-2-(di-n-propylamino)-tetralin (8-OH DPAT) increase ethanol intake. Psychopharmacology (Berl) 1994;115:173–179. doi: 10.1007/BF02244769. [DOI] [PubMed] [Google Scholar]
- Treisman R. Regulation of transcription by MAP kinase cascades. Curr Opin Cell Biol. 1996;8:205–215. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- Tsuji R, Guizzetti M, Costa LG. In vivo ethanol decreases phosphorylated MAPK and p70S6 kinase in the developing rat brain. Neuroreport. 2003;14:1395–1399. doi: 10.1097/01.wnr.0000071763.92388.41. [DOI] [PubMed] [Google Scholar]
- Ulm RR, Volpicelli JR, Volpicelli LA. Opiates and alcohol self-administration in animals. J Clin Psychiatry. 1995;56(Suppl 7):5–14. [PubMed] [Google Scholar]
- Vacca G, Serra S, Brunetti G, Carai MA, Gessa GL, Colombo G. Boosting effect of morphine on alcohol drinking is suppressed not only by naloxone but also by the cannabinoid CB1 receptor antagonist, SR 141716. Eur J Pharmacol. 2002;445:55–59. doi: 10.1016/s0014-2999(02)01712-0. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pages C, Herve D, Girault JA, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corbille AG, Bertran-Gonzalez J, Herve D, Girault JA. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci U S A. 2006a;103:2932–2937. doi: 10.1073/pnas.0511030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Trzaskos JM, Girault JA, Herve D. Role of the ERK pathway in psychostimulant-induced locomotor sensitization. BMC Neurosci. 2006b;7:20. doi: 10.1186/1471-2202-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte P, Barnier JV, Guibert B, Pages C, Besson MJ, Hipskind RA, Caboche J. Glutamate induces phosphorylation of Elk-1 and CREB, along with c-fos activation, via an extracellular signal-regulated kinase-dependent pathway in brain slices. Mol Cell Biol. 1999;19:136–146. doi: 10.1128/mcb.19.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks JR, Collins RJ. Changes in morphine self-administration in rats induced by prostaglandin E1 and naloxone. Prostaglandins. 1976;12:11–19. doi: 10.1016/s0090-6980(76)80003-2. [DOI] [PubMed] [Google Scholar]
- Woods JH, Downs DA, Carney J. Behavioral functions of narcotic antagonists: response-drug contingencies. Fed Proc. 1975;34:1777–1784. [PubMed] [Google Scholar]
- Woolverton WL. Effects of a D1 and a D2 dopamine antagonist on the self-administration of cocaine and piribedil by rhesus monkeys. Pharmacol Biochem Behav. 1986;24:531–535. doi: 10.1016/0091-3057(86)90553-8. [DOI] [PubMed] [Google Scholar]
- Wu TH, Lin CH. IL-6 mediated alterations on immobile behavior of rats in the forced swim test via ERK1/2 activation in specific brain regions. Behav Brain Res. 2008;193:183–191. doi: 10.1016/j.bbr.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Yang L, Mao L, Chen H, Catavsan M, Kozinn J, Arora A, Liu X, Wang JQ. A signaling mechanism from G alpha q-protein-coupled metabotropic glutamate receptors to gene expression: role of the c-Jun N-terminal kinase pathway. J Neurosci. 2006;26:971–980. doi: 10.1523/JNEUROSCI.4423-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zghoul T, Abarca C, Sanchis-Segura C, Albrecht U, Schumann G, Spanagel R. Ethanol self-administration and reinstatement of ethanol-seeking behavior in Per1(Brdm1) mutant mice. Psychopharmacology (Berl) 2007;190:13–19. doi: 10.1007/s00213-006-0592-z. [DOI] [PubMed] [Google Scholar]
- Zhai H, Li Y, Wang X, Lu L. Drug-induced alterations in the extracellular signal-regulated kinase (ERK) signalling pathway: implications for reinforcement and reinstatement. Cell Mol Neurobiol. 2008;28:157–172. doi: 10.1007/s10571-007-9240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology (Berl) 2002;159:415–423. doi: 10.1007/s00213-001-0932-y. [DOI] [PubMed] [Google Scholar]