Abstract
Voxel-based morphometry (VBM) may afford a more rapid and extensive survey of gray matter abnormalities in schizophrenia than manually drawn region of interest (ROI) analysis, the current gold standard in structural MRI. Unfortunately, VBM has not been validated by comparison with ROI analyses, nor used in first-episode patients with schizophrenia or affective psychosis, who lack structural changes associated with chronicity. An SPM99-based implementation of VBM was used to compare a group of 16 patients with first-episode schizophrenia and a group of 18 normal controls and, as a further comparison, 16 first-episode patients with affective psychosis. All groups were matched for age and handedness. High spatial resolution structural images were normalized to the SPM99 template and then segmented, smoothed, and subjected to an ANCOVA. Schizophrenia vs control group comparisons: Voxel-by-voxel comparison of gray matter densities showed that only the left STG region was significantly different when corrected for multiple comparisons P < .05), consistent with our previously reported manual ROI results. Analysis of the extent of voxel clusters, replicated with permutation analyses, revealed group differences in bilateral anterior cingulate gyri and insula (not previously examined by us with manually drawn ROI) and unilateral parietal lobe, but not in medial temporal lobe (where our ROI analysis had shown differences). However, use of a smaller smoothing kernel and a small volume correction revealed left-sided hippocampal group differences. Affective psychosis comparisons: When the same statistical thresholding criteria were used, no significant differences between affective psychosis patients and controls were noted. Since a major interest was whether patients with affective psychosis shared some anatomical abnormalities with schizophrenia, we applied a small volume correction and searched within the regions that were significantly less dense in schizophrenia compared to control subjects. With this statistical correction, the insula showed, bilaterally, the same pattern of differences in affective disorder subjects as that in schizophrenic subjects, whereas both left STG and left hippocampus showed statistical differences between affectives and schizophrenics, indicating the abnormalities specific to first-episode schizophrenia. These findings suggest both the promise and utility of VBM in evaluating gray matter abnormalities. They further suggest the importance of comparing VBM findings with more traditional ROI analyses until the reasons for the differences between methods are determined.
INTRODUCTION
Magnetic resonance imaging (MRI) has been useful in revealing subtle structural brain abnormalities in schizophrenic patients. MR structural analyses of patients diagnosed with schizophrenia have revealed a number of brain abnormalities, including ventricular enlargement, total brain volume reduction, and regional reductions in brain volume within frontal, temporal, and parietal regions (reviewed in Lawrie and Abukmeil, 1998, Shenton et al., 2001, McCarley et al., 1999). Most were small and subtle, rather than large, thus necessitating advanced and accurate measurement tools for their detection.
Thus far the method of choice in investigating the distribution of subtle cerebral pathology in schizophrenia has been an examination of anatomically defined regions of interest (ROI) within the brain. However, this method has limitations. Perhaps most importantly, manual tracing of ROI on successive brain slices is time consuming and hence does not easily allow for the comparison of many brain regions in the same patients, nor does it allow for the use of large subject groups. There is also the question of the validity of investigator-determined ROI, given the complex three-dimensional neuroanatomy of the brain and interindividual variability, even though ROI can be defined so that their measurement is highly reliable.
It is not surprising that methods for automated and more objective definition of brain regions have been eagerly sought. Proposed methods have included linear transformation of brain images into a bounding box of constant size (Andreasen et al., 1994; Sowell et al., 2000), elastic warping of the atlas segmentation to the subject’s brain (Iosifescu et al., 1997), and registration of images with a template brain in stereotactic space (Collins et al., 1994; Wright et al., 1995).
Analogous data analytic problems in PET imaging led to the development of a technique for the spatial transformation of smoothed images of cerebral blood flow into a standard stereotaxic space, followed by three-dimensional voxel-based analysis using statistical parametric mapping (SPM; Friston et al., 1995). Several studies suggested that analysis of structural MRI data using such Voxel-based morphometry (VBM) would be advantageous in offering a more rapid and extensive survey of gray matter abnormalities in schizophrenia than ROI analysis (Sowell et al., 2000; Wright et al., 1999; Sigmudsson et al., 2001). VBM was defined by Ashburner and Friston (2000) as “a voxel-wise comparison of the local concentration of gray matter between two groups of subjects.” This technique is based on normalization of all subject images to the same stereotactic space, segmentation of the normalized images into gray matter, white matter, and CSF, smoothing images using a convolution with a Gaussian kernel, and, finally, statistical analysis using the general linear model to identify regions of gray matter concentration that are significantly related to the variable under study (if normality is not present a nonparametric statistical analysis is used).
The aims of the present study were twofold. First, we sought to evaluate VBM as a more efficient and complete method than ROI for characterization of gray matter abnormalities in patients with first-episode schizophrenia, an important and useful population because of the absence of confounds such as chronic illness and chronic medication, and one, to our knowledge, not previously studied using VBM. Second, we wanted to compare and validate VBM with ROI analysis by using patients who had been previously investigated using ROI methodology (Hirayasu et al., 1998) that found smaller left posterior superior temporal gyrus (STG) and medial temporal lobe gray matter volumes. The addition of patients with first episode affective psychosis (also studied by Hirayasu et al., 1998) was to provide further validation of VBM analysis and also to evaluate the presence of pathology specific to first episode schizophrenia.
MATERIALS AND METHODS
We used VBM to analyze MRI scans collected in our first episode study of schizophrenia, with a contrast group of first episode affective psychosis. These scans had been previously evaluated by traditional ROI analysis, and the report Hirayasu et al. (1998) of this analysis also provides a detailed description of the subjects’ demographic and clinical data. We also present some of the demographic data in Table 1. Briefly, all the patients and comparison subjects met the following criteria: age 18 to 55 years; IQ above 75; right-handedness; negative history of seizures, head trauma with loss of consciousness, and neurological disorder; and no lifetime history of alcohol or drug dependence. Diagnoses were based on the Structured Clinical Interview for DSM-III-R (SCID), review of hospital course, and medical records. The patient group consisted of 16 schizophrenia patients (14 male, 2 female) and 16 patients with affective disorder (13 male and 3 female). The diagnoses of the patients with affective disorder were bipolar disorder, manic (N = 12), bipolar disorder, mixed (N = 2), and major depression (N = 2). All of the patients exhibited psychosis, and all of the patients in the schizophrenia group met the diagnostic criteria for schizophrenia. The median duration of treatment with any psychotropic medication before scanning was short: 1.7 months for the schizophrenic patients and 0.0 months for the patients with affective disorder. Neither duration nor dose of medication was significantly correlated with any MRI volume measure. Technical considerations precluded the analysis of the data from one schizophrenic subject used in the ROI evaluation (Hirayasu et al., 1998).
TABLE 1.
Demographic Data
| Characteristic or test |
Schizophrenic subjects(n = 16) |
Affective psychosis subjects (n = 16) |
Comparison subjects (n = 18) |
|---|---|---|---|
| Age (years) | 26 ± 7.5 | 23.7 ± 4 | 24.0 ± 4.5 |
| Handedness | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.8 ± 0.2 |
| SES | 3.1 ± 1.4* | 2.5 ± 1.3 | 1.8 ± 0.5 |
| PSES | 2.0 ± 1.0* | 1.5 ± 0.7 | 1.3 ± 0.8 |
| Mini-Mental State score |
28.4 ± 2.8 | 29.1 ± 1.4 | 29.1 ± 0.9 |
| Age at first medication |
26.6 ± 7.6 | 23.5 ± 4.1 | |
| Dose of medication |
213.6 ± 217.0 | 197.9 ± 159.4 | |
| BPRS total score |
37.7 ± 9.2 | 33.1 ± 7.8 |
Note. Statistical significance was provided by one-factor ANOVA. Post-hoc Tukey’s honestly significant difference (HSD) test showed significant difference (*P < 0.05) compared to comparison subjects.
The results of Hirayasu et al. showed that patients with first-episode schizophrenia had significantly smaller gray matter volume in the left posterior superior temporal gyrus than did the patients with first-episode affective psychosis or the comparison subjects. The left amygdala–hippocampal complex also demonstrated significant differences between controls and FE schizophrenics but there were no statistical differences between affective disorder patients and first episode schizophrenia subjects.
An age-matched group of 18 psychiatrically well comparison subjects (2 women) were assessed with the SCID editions for nonpatients (SCID-NP) and personality disorders (SCID-II). No subject reported any evidence of psychosis in first-degree relatives. After reading a written description of the study, all participants gave written informed consent.
MRI Method
Acquisition and processing
Magnetic resonance images were obtained on a 1.5-T General Electric scanner (GE Medical Systems, Milwaukee). A series of 124 contiguous coronal images were acquired using an SPGR sequence with the following parameters: TR, 35 ms; TE, 5 ms; 45° flip angle; 24-cm field of view; NEX, 1.0 (number of excitations); matrix, 256 × 256 (192 phase-encoding steps). The voxel (volume of pixel) dimensions were 0.9375 × 0.9375 × 1.5 mm. In the present study these images were used for VBM and in our previous study (Hirayasu et al., 1998) had been used for delineating and measuring temporal lobe ROI.
Image analysis
For VBM analysis we used the latest version of the Statistical Parametric Mapping program (SPM99) developed in the Wellcome Institute (London, UK). VBM methods (Ashburner and Friston, 2000) involved spatial normalization of all images to the same high spatial resolution stereotactic space (1-mm isotropic voxels, SPM 99 T1-weighted template image). Each of the subjects’ images was registered to the same template image by minimizing the residual sum of squared differences between them (Ashburner and Friston, 1997). The spatially normalized images were next partitioned into gray matter, white matter, and cerebrospinal fluid, using a modified mixture model cluster analysis technique with a correction for image intensity nonuniformity.
The gray matter images were then smoothed by convolving with a 12-mm isotropic Gaussian kernel. This step made the subsequent voxel-by-voxel analysis more comparable to a region of interest approach, because each voxel in the smoothed images contained the average concentration of the gray matter from within the selected voxel and, to a lesser extent, from neighboring voxels (the smoothed volume can be thought of as a weighted region of interest). In accord with the central limit theorem, smoothing also had the effect of rendering the data more normally distributed, increasing the validity of parametrical statistical tests (Worsley et al., 1996). Moreover, the smoothing step helped compensate for the inexact nature of spatial normalization.
Statistical analyses (utilizing the theory of Gaussian fields (Friston et al., 1995; Worsley et al., 1996)) were carried out using the general linear model (GLM). Regional (voxel-based) analyses of the images were performed in SPM 99 after covarying for global gray matter intensity (global normalization). These analyses can be regarded as ANCOVAs (Friston et al., 1995). The resulting set of voxel values for this contrast constituted a Statistical Parametric Map of the t statistic (SPM{t}).
The SPM{t} maps, comprising the results of statistical tests on each voxel, were then transformed to the unit normal distribution (Z) and thresholded at P < 0.05 corrected for multiple comparisons (t > 5.75) (P < .000001 when not corrected).
We note the multiple voxelwise comparison correction is quite stringent and prone to false negatives (Ashburner and Friston, 2000). An alternative approach takes into account spatial clustering, testing the probability of chance occurrence of the observed spatial extent of contiguous voxels (Friston et al., 1995). We used this method in a subsequent analysis. First, the SPM{t}, after Z transformation, was thresholded at a higher P value (P < .0001; t > 4.2). Local maxima of Z value were reported as separate regions if they were more than 6 mm apart within a cluster (half-width of the smoothing Gausian kernel). Those regions with P < .05 (corrected for spatial extent) were considered significantly different between groups (Friston et al., 1995; Wright et al., 1999). Some investigators have used this methodology (e.g., Wright et al., 1995, 1999). Other workers have criticized the method, noting that the random field theory used in SPM requires a normal data distribution, an invalid assumption because the preprocessing steps of SPM lead to spatially varying smoothness (Ashburner and Friston, 2000). As permutation tests do not depend on any distributional assumptions (Holmes et al., 1996; Arndt et al., 1996; Bullmore et al., 1999), we decided to use a permutation test to verify cluster level results obtained with SPM. For this, the newest version of SnPM99b(Statistical NonParametric Mapping) was employed (Nichols and Holmes, 2002). We ran permutations of the volumetric data, randomly assigning subjects to the schizophrenia and comparison groups, and stopped at 250 permutations, as the results did not differ from 225 permutations. We used the same criteria for cluster size statistics as we did in SPM cluster analysis. We thresholded the permutation statistic map at P < .001 (t > 4.2) and set the statistical cluster size significance at P < .05.
In the case of regions that were not found to be significantly different with any of these VBM methods, but had been indicated as significantly different in our previous ROI analyses (hippocampus), we considered the possibility that the failure to find a difference might be due to the small volume of the region. For evaluation of this possibility, we utilized a smaller smoothing kernel, 6 mm. Moreover, as this last analysis was hypothesis driven, instead of analyzing the whole brain and correcting results for the huge number of comparisons, we analyzed a region of 40 × 40 × 40 mm, covering both hippocampi, using the small volume correction (SVC) function in SPM99. (This region had 64,000 voxels and, based on the smoothing kernel, 126 resolution elements.) A similar analysis had previously been utilized on different subject populations by Maguire et al. (2000).
With respect to the patients with affective psychosis vs control subjects contrast, we used the same statistical criteria as for the schizophrenia patients vs controls. Statistical maps were thresholded on the 0.001 level, and significant clusters were reported if they survived the 0.05 statistical threshold. In addition, to investigate whether the anatomical differences in first-episode subjects with schizophrenia would be shared by subjects with affective disorder, we utilized a small volume correction, searching only within the regions that were characterized by decrease of gray matter density in schizophrenia when compared to the control group (namely, STG, insula, cingulate gyrus, prefrontal cortex, and parietal lobe.)
For visualization of group differences, the SPM coordinatesand significant voxels were superimposed onto a normalized individual brain. The Talairach Daemon program (Talairach Daemon Client, University of Texas Health Science Center, San Antonio, Texas) was also used to indicate location of significant regions.
RESULTS
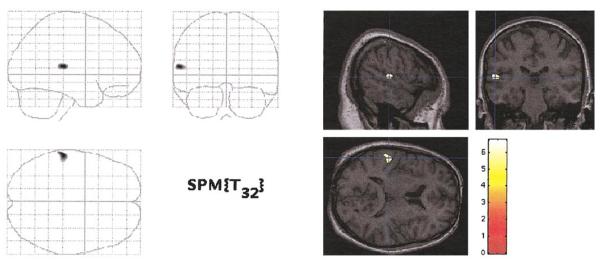
In a voxel-by-voxel comparison, after correcting for multiple comparisons, the only region showing significant decreased gray matter density (Z > 5.27) in schizophrenia patients compared to the comparison subjects was located within the left posterior STG (Talairach coordinates: x = −61, y =−28, z = 11). It lay in and posterior to Heschl’s gyrus (primary auditory cortex) in the STG (Fig. 1).
FIG. 1.
Left posterior superior temporal gyrus gray matter density reduction in first-episode schizophrenia subjects (n = 16) compared with healthy control subjects (n = 18). The SPM maps show statistically significant voxels that survived correction for multiple comparisons (initial threshold, P < 0.000001, t > 5.75; P < 0.05 when corrected for multiple comparisons). The left panel shows voxels mapped onto SPM coordinate space in a “transparent brain.” The right panel shows voxels mapped onto slices of a normalized brain of a control subject. (Neurological convention: the left part of the coronal image is subject left; the upper part of the axial image is subject left). The color scale shows t values for each significant voxel. Dark blue lines indicate the location of a section relative to other section planes.
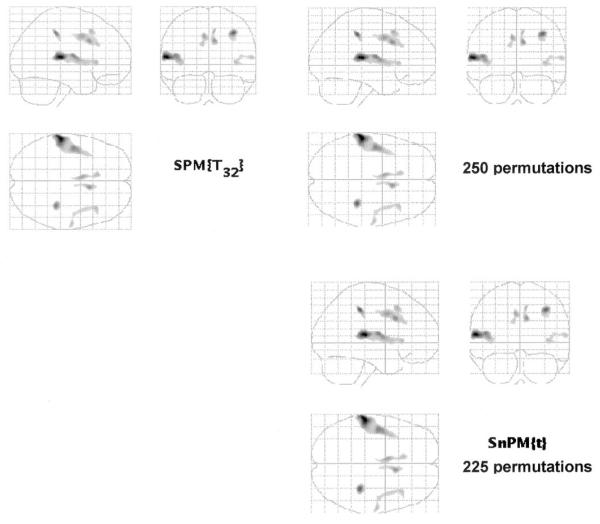
After correction for the spatial extent (see Materials and Methods), five additional regions (see Figs. 2 and 3) of lower gray matter density in the schizophrenia group were localized to the anterior cingulate gyrus and insula on both sides and to right prefrontal and right parietal regions (see Table 2).
FIG. 2.
(Left panel) SPM{t} map of the significant results after thresholding on P < 0.0001 (uncorrected, t > 4.2) and then adjusting probability according to spatial extent of cluster (see text). Note the appearance of an increased number of regions compared with Fig. 1 (see Table 1 for description of region and Z score). Higher probability values are darker. Neurological convention as in Fig. 1. (Right panel) permutation SnPM analysis with the same criteria used for statistical threshold (t statistics map thresholded on P < 0.0001 (t < 4.2) and probability of spatial extend of cluster set at P < 0.05. Upper image, 250 permutations; lower, 225 permutations.
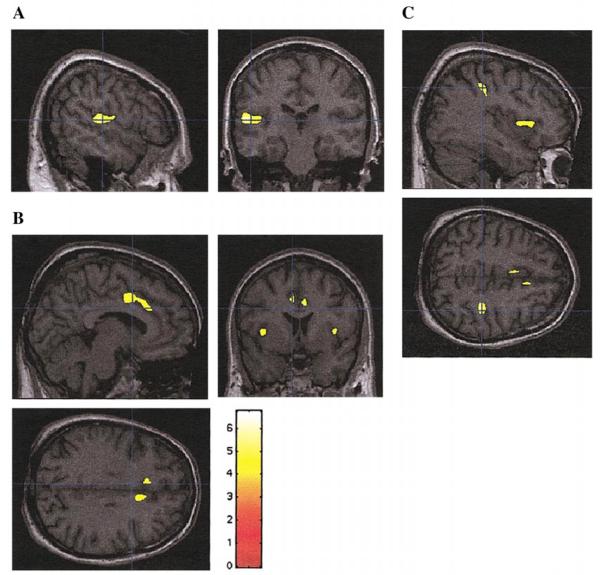
FIG. 3.
SPM{t} statistically significant regions (using spatial extent as in Table 1 and Fig. 2) superimposed on selected sections of a spatially normalized brain from a control subject. (A) Left STG; (B) left and right insula and anterior cingulate gyri; (C) Right parietal lobe, inferior parietal lobule (and insula). Neurological convention as in Fig. 1.
TABLE 2.
Areas of Reduced Regional Gray Matter Density
| NC schizophrenics |
NC affectives |
Affectives schizophrenics |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anatomical location | Talairach coordinates (x, y, z) |
Z score | Talairach coordinates (x, y, z) |
Z score | Talairach coordinates (x, y, z) |
Z score | ||||||
| Right dorsolateral prefrontal cortex | 38 | 22 | 3 | 4.15 | ||||||||
| Left anterior cingulate gyrus | −6 | 2 | 40 | 4.32 | ||||||||
| Right anterior cingulate gyrus | 9 | 14 | 33 | 4.47 | ||||||||
| Left superior temporal gyrus | −60 | −28 | 11 | 5.27 | −58 | −27 | 9 | 4.30 | ||||
| Right superior temporal gyrus | 61 | −12 | 10 | 4.12 | ||||||||
| Left insula | −46 | −8 | 12 | 4.58 | −41 | 0 | −14 | 2.98 | ||||
| Right insula | 39 | 9 | 4 | 3.92 | 39 | 24 | 5 | 3.79 | ||||
| Right inferior parietal lobule | 35 | −34 | 44 | 4.85 | ||||||||
| Left posterior hippocampusa | −18 | −39 | 1 | 4.34 | −34 | −28 | −14 | 3.54 | ||||
Note. The first two columns display areas of reduced density in schizophrenics (n = 16) compared to controls (n = 18), the next two columns display areas of reduced density in affectives (n = 16) compared to controls, and the last two columns display areas of reduced density in schizophrenics reduced in comparison with affectives. The table shows regions that survived correction for spatial extent (see text) with corresponding Z scores for different anatomical locations.
6 mm smoothing kernel.
The permutation test (see Materials and Methods) revealed five clusters of significantly reduced gray matter density in the schizophrenia patients, located in the same regions as the clusters from the previous spatial analysis (see comparison in Fig. 2).
A first episode affective psychosis patient comparison with controls demonstrated no significant differences at both the voxel and cluster levels. We then applied the small volume correction and a limited search to the regions with density reduced in schizophrenia. This revealed, in the bilateral insula but not in the STG, the same pattern of reduction in density as seen in schizophrenia (Fig. 5). In addition schizophrenia patients had significantly less gray matter density in the left STG than affective disorder patients (see Fig. 6).
FIG. 5.
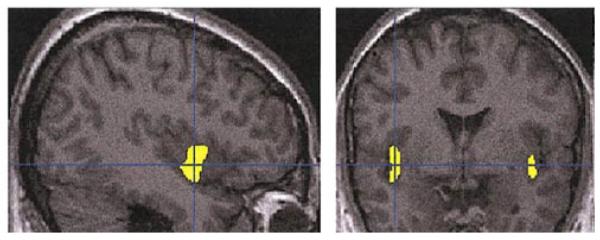
Regions showing decreased gray matter density in affective psychosis patients in comparison to control subjects, when the search was limited to the regions that were also characterized by decreased density in schizophrenia when compared to control subjects. SPM{t} statistically significant regions (using spatial extent as in Table 1 and Fig. 2) are superimposed on selected sections of a spatially normalized brain from a control subject. Neurological convention is as in Fig. 1.
FIG. 6.
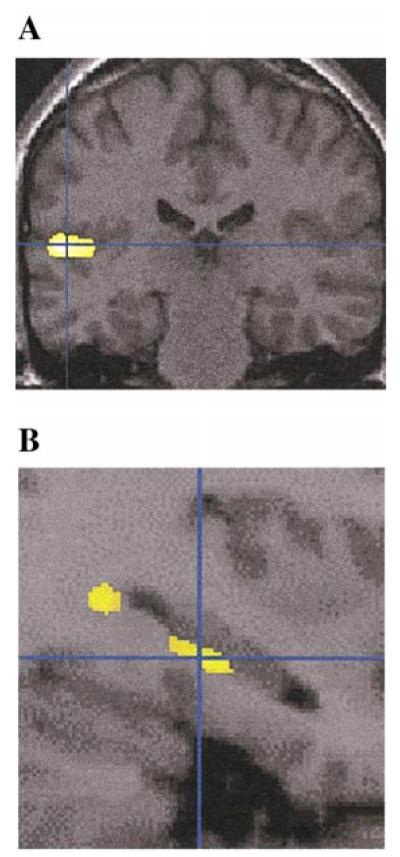
This figure shows SPM{t} regions with gray matter density significantly reduced in schizophrenics, when compared to affectives (using spatial extent as in Table 1 and Fig. 2) superimposed on selected sections of a spatially normalized brain from a control subject. Statistically significant differences are localized within the (A) left temporal lobe and (B) left posterior hippocampus (the latter with use of the 6-mm smoothing kernel and small volume correction, analogous to Fig. 4). Neurological convention is as in Fig. 1.
The last test utilized a smaller smoothing kernel and small volume correction for the hippocampi. It revealed a significant decrease of the gray matter density within the left hippocampus in first episode schizophrenics, compared to comparison subjects (peak difference at (x, y, z): −18, −39, +1 (Z = 4.34), Fig. 4), as well as in schizophrenia patients relative to affective disorder patients (Fig. 6). The anatomical locations of all regions were determined using the Talairach Daemon and further confirmed on MRI brain sections (Figs. 1, 3-6).
FIG. 4.
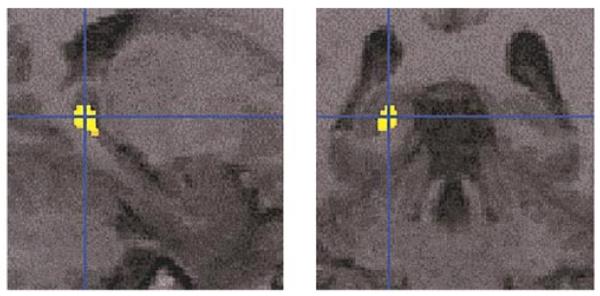
SPM analysis with 6-mm Gaussian smoothing kernel revealed statistically significant (after correcting for small volume) differences between the schizophrenia and control groups within the left posterior hippocampus (x, y, z): −18, −39, +1 (Z = 4.34).
DISCUSSION
Voxel-by-voxel comparison of gray matter density revealed significant reductions in regional gray matter density within left posterior STG in schizophrenia patients but not in affective disorder patients in comparison with control subjects, in agreement with our ROI finding of smaller left posterior STG gray matter volume in schizophrenia compared with both controls and the affective psychosis group Hirayasu et al. (1998). Reduced STG gray matter volume in schizophrenia has been among the most consistent of all structural MRI findings, with all 12 MRI studies concurring (reviewed in Shenton et al., 2001, McCarley et al., 1999, Pearlson, 1997). When P values were adjusted to take into account the spatial extent of voxel clusters, schizophrenia subjects showed decreased gray matter density compared with normal controls in regions previously described to be abnormal in at least some manual ROI analyses, although not always with the same laterality or subdivision. These regions included (see reviews in Shenton et al., 2001, McCarley et al., 1999) (1) right dorsolateral prefrontal cortex (DLPFC), where volume reductions in schizophrenia subjects have been reported in a majority of structural studies (30/50); (2) right inferior parietal lobule (IPL), where ROI studies have shown smaller gray matter volumes (9/15 studies of parietal lobe, especially of IPL); (3) left and right anterior cingulate gyrus (ACG); and (4) left and right insula. The few manual ROI studies of these last two regions reported smaller volumes in schizophrenia for both ACG (Szeszko et al., 2000) and insula (Crespo-Facorro et al., 2000).
With respect to these regions in patients with affective disorder compared with control subjects, the left and right insula were the only regions to show a decrease in gray matter density. This result has not been described before and may suggest that the insula abnormality is present in affective disorder as well as in schizophrenia. On the other hand, the left STG density decrease appears specific to schizophrenia. This left STG result is entirely consistent with the manual ROI findings of (Hirayasu et al., 1998), who did not investigate the insula.
To provide a rigorous statistical test of the validity of the SPM cluster analysis, we conducted a permutation analysis, randomly assigning subjects to different groups (controls and schizophrenics). This analysis, in contrast to cluster analysis by SPM, requires no distibutional assumptions (such as normality) about the data. We used more permutations than other studies using this technique (Sowell et al., 2000; Bullmore et al., 1999), which, together with the consistent results using both 225 and 250 permutations, suggests that our procedure provided a stable and valid estimate of P values.
Motivated by the positive manual ROI findings in medial temporal lobe, we also used a smaller smoothing kernel and SVC and found density reduction in the left medial temporal lobe in the schizophrenia group, consistent with manual ROI analyses, both of the present data set (Hirayasu et al., 1998) and of other sets (Shenton et al., 2001; McCarley et al., 1999). This suggests the importance of smoothing kernel size and small volume correction in evaluation of small regions of interest, a finding underscored by a recent study where a small smoothing kernel and SVC for SPM and manual ROI analysis both showed increased hippocampal densities and volumes in taxi drivers when compared to those of normal controls (Maguire et al., 2000). The present VBM analysis found statistically significant medial temporal gray matter density reduction in schizophrenia compared with affective psychosis, whereas Hirayasu et al. (1998) found smaller, but not statistically significantly different, manual ROI volumes in schizophrenia compared with affective psychosis. This fact further supports the idea that, as stated in this Discussion, factors other than the volume alone (i.e., shape) influence the VBM results and further confirms the importance of comparisons with manual ROI studies as well as careful interpretation of the results.
Our interest in following up on the VBM analysis has led us to implement an ROI analysis on the insula region that showed density reduction in the current study. To check the hypothesis that the insula volumes are reduced bilaterally in schizophrenics, when compared to normal controls, we studied a subsample of 12 subjects and 12 schizophrenics, and the results were consistent with the VBM results. That is, the schizophrenia group was characterized by insula volume de-crease on both left (P(24) = 0.002) and right (P(24) = 0.001, independent sample t test) sides. However, manual ROI analysis of the affective psychosis sample (n = 12) showed close to significant, but not significant differences between controls and affectves on the right (P(23) = 0.071) and no significance on the left (P(23) = 0.222). In addition, insula volumes in FE schizophrenics differed from FE affectives on the left (P(23) = 0.010) and the right (P(23) = 0.037) sides.
The first study comparing manual ROI with VBM in schizophrenia (Wright et al., 1999) evaluated two chronic schizophrenia samples, neither of which showed any gray matter deficits in previous ROI analyses (despite measuring whole brain volume, temporal lobe, and planum temporale volumes (Sharma et al., 1998; Frangou et al., 1997)). The VBM study revealed gray matter deficits located in insula, cingulate gyrus, temporal pole, middle temporal gyrus, and inferior frontal gyrus (note overlap with the present firstepisode study in insula and cingulate gyrus). The reasons for the mismatches between their ROI and VBM methods in temporal lobe ROI were not explained and were not further investigated. Of note, however, was a recent VBM study reporting regional gray matter deficits in superior and medial temporal gyrus, insula, and ACG (Sigmudsson et al., 2001); this study used normalization to the subjects’ brain template with no smoothing and did not provide manual ROI validation.
The differences between these studies’ results, taken together with the present study, further stress the necessity for validation of voxel-based analyses by ROI studies (see discussion of VBM and other automated analyses in McCarley (2001)). Because the validity of VBM has not yet been systematically studied, the sources of differences between VBM and ROI methods remain uncertain. False positive or false negative VBM findings might arise from the changes in the shape or displacement of structures in the course of spatial normalization. Specifically, as the VBM does not tend to match perfectly every possible structure, as some of the other sophisticated methods of registration do (e.g., Fishl et al., 2000; Thompson et al., 2001; Narr et al., 2001), the output of the statistical analysis gives information about the local volume differences. This information is prone to some systematical biases, which could arise from not only the errors caused by the misregistration of anatomical structures, but also movement or gray and white matter intensity patterns that differ between groups (see Bookstein, 2001, and Ashburner and Friston, 2001, discussion in NeuroImage). Recently, methods that attempt to minimize the biases introduced in the analysis by the volume changes during the registration procedure were introduced (Ashburner, 1998; Good et al., 2001). These methods attempt to adjust, or modulate, the voxel values according to the Jacobian determinants derived from the spatial normalization step, thus allowing for analyzing absolute volume (optimized voxel-based morphometry (Good et al., 2001)), relative position of the brain structures (deformation based morphometry), and shape differences (tensor based morphometry) (Ashburner and Friston, 2001). Moreover, some of these potential confounds are not only limited to VBM, but apply also to shape analysis and can be a factor even when perfect warping is performed. As VBM is much faster than these techniques, it makes it possible to obtain reproducible results by studying much bigger populations.
VBM has only recently been thought of as a possible replacement for manual ROI analyses (the current gold standard), but it appears to have several potential advantages. For example, VBM enables regional comparisons throughout the whole brain without restriction to a few selected areas in the typical manual ROI methodology. Indeed, in the present study, VBM pointed to first-episode schizophrenia gray matter differences in regions not examined in our ROI study. Other advantages are the reduction of labor and the ability to use large samples with an attendant increase in statisticalpower. These advantages appear important in disorders such as schizophrenia that have subtle structural changes in several brain regions and many heterogeneous clinical subtypes. The latter consideration would also suggest the importance of studying more homogeneous, larger patient populations, which can be more easily accomplished using VBM analyses.
Despite all these advantages, our study showed that VBM methodology should be chosen with caution, as different hypotheses might require slightly different approaches and parameters. Our data also suggest that negative findings in the VBM analysis do not necessarily preclude manual ROI differences, so the VBM results should be interpreted with caution. A clear example was our need to use a smaller smoothing kernel and SVC to detect hippocampal differences. Had manual ROI results not been available to suggest this analysis, we would not have used it.
Thus, as a general rule, we suggest that each VBM study should be compared with manual ROI analysis until validity is established. We further suggest, since VBM findings point to brain areas not yet systematically studied with ROI analysis, that VBM could be profitably used in an exploratory manner to point to brain regions that might subsequently be evaluated using manual ROI analyses. The example of this approach has been demonstrated on the insula case. Follow up manual ROI analysis was carried out, and similar, although not identical, results to the VBM analysis were obtained—see discussion above.
With respect to the first-episode schizophrenia population, the current VBM data are suggestive of gray matter deficits in a number of regions, although the relatively small sample places limitations on generalizability. These data, derived from patients early in the course of their illness, are compatible with developmental hypotheses of schizophrenic abnormalities. It will be important to follow up these subjects so as to determine if the deficits progress and are thus compatible with a still controversial theory of schizophrenia which posits developmental abnormalities followed by a later neurodegenerative process (see Mednick and McNeil, 1968; McCarley et al., 1996; and reviews in Shenton et al. (2001) and McCarley et al. (1999)).
These VBM data reinforce the concept (Shenton et al., 2001; McCarley et al., 1999) that gray matter abnormalities in schizophrenia are not diffuse, equally distributed in all regions, but rather are concentrated in particular regions. Of these regions, the superior temporal gyrus, and especially on the left (dominant) side, appears to be strongly and frequently affected in schizophrenia.
ACKNOWLEDGMENTS
The authors thank Marie Fairbanks for her administrative assistance. Additionally, we gratefully acknowledge the support of the Department of Veterans Affairs Merit Awards (M.E.S., R.W.M.), the Middleton Award (R.W.M.), the National Institute of Mental Health (K02 MH 01110 and R01 MH 50747 to M.E.S. and R01 MH 40799 and MH 52807 to R.W.M.), and the National Center for Research Resources (11747 to R.K. and P41 1321 to F.A.J.).
REFERENCES
- Andreasen NC, Arndt S, Swayze V, 2nd, Cizadlo T, Flaum M, O’Leary D, Ehrhardt JC, Yuh WT. Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science. 1994;266:294–298. doi: 10.1126/science.7939669. [see comments] [DOI] [PubMed] [Google Scholar]
- Arndt S, Cizadlo T, Andreasen NC, Heckel D, Gold S, O’Leary DS. Tests for comparing images based on randomization and permutation methods. J. Cereb. Blood Flow Metab. 1996;16:1271–1279. doi: 10.1097/00004647-199611000-00023. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston K. Multimodal image coregistration and partitioning—A unified framework. Neuroimage. 1997;6:209–217. doi: 10.1006/nimg.1997.0290. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry— The methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Comments and controversies. Why voxel-based morphometry should be used. Neuroimage. 2001;14:1238–1243. doi: 10.1006/nimg.2001.0961. [DOI] [PubMed] [Google Scholar]
- Bookstein FL. “Voxel based morphometry” should not be used with imperfectly registered images. Neuroimage. 2001;14:1454–1442. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ. Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans. Med. Imaging. 1999;18:32–42. doi: 10.1109/42.750253. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J. Comput. Assist. Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Crespo-Facorro B, Kim J, Andreasen NC, O’Leary DS, Bockholt HJ, Magnotta V. Insular cortex abnormalities in schizophrenia: A structural magnetic resonance imaging study of first-episode patients. Schizophr. Res. 2000;46:35–43. doi: 10.1016/s0920-9964(00)00028-1. [DOI] [PubMed] [Google Scholar]
- Frangou S, Sharma T, Sigmudsson T, Barta P, Pearlson G, Murray RM. The Maudsley Family Study. 4. Normal planum temporale asymmetry in familial schizophrenia. A volumetric MRI study. Br. J. Psychiatry. 1997;170:328–333. doi: 10.1192/bjp.170.4.328. [DOI] [PubMed] [Google Scholar]
- Friston K, Holmes A, KJ W, JP P, CD F, RSJ F. Statistical parametric maps in functional imaging: A general linear approach. Hum. Brain Mapp. 1995;2:189–210. [Google Scholar]
- Hirayasu Y, Shenton M, Salisbury D. First episode schizophrenia differs from first episode affective disorder and a normal comparison group in left temporal lobe MRI volume reduction. Am. J. Psychiatry. 1998;155:1384–1391. doi: 10.1176/ajp.155.10.1384. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Blair RC, Watson JD, Ford I. Nonparametric analysis of statistic images from functional mapping experiments. J. Cereb. Blood Flow Metab. 1996;16:7–22. doi: 10.1097/00004647-199601000-00002. [DOI] [PubMed] [Google Scholar]
- Iosifescu DV, Shenton ME, Warfield SK, Kikinis R, Dengler J, Jolesz FA, McCarley RW. An automated registration algorithm for measuring MRI subcortical brain structures. Neuroimage. 1997;6:13–25. doi: 10.1006/nimg.1997.0274. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Abukmeil SS. Brain abnormality in schizophrenia. A systematic and quantitative review of volumetric magnetic resonance imaging studies. Br. J. Psychiatry. 1998;172:110–120. doi: 10.1192/bjp.172.2.110. [see comments] [DOI] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD. Navigationrelated structural change in the hippocampi of taxi drivers. Proc. Natl. Acad. Sci. USA. 2000;97:4398–403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarley RW. Structural magnetic resonance imaging studies in schizophrenia. In: Davis KL, Charney D, Coyle J, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Lippincott, Williams, & Wilkins; Baltimore: 2001. [Google Scholar]
- McCarley RW, Hsiao JK, Freedman R, Pfefferbaum A, Donchin E. Neuroimaging and the cognitive neuroscience of schizophrenia. Schizophr. Bull. 1996;22:703–725. doi: 10.1093/schbul/22.4.703. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, Shenton ME. MRI anatomy of schizophrenia. Biol. Psychiatry. 1999;45:1099–1119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick SA, McNeil TF. Current methodology in research on the etiology of schizophrenia: Serious difficulties which suggest the use of the high-risk-group method. Psychol. Bull. 1968;70:681–693. doi: 10.1037/h0026836. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum. Brain Mapp. 2002;15(1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlson GD. Superior temporal gyrus and planum temporale in schizophrenia: A selective review. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1997;21:1203–1229. doi: 10.1016/s0278-5846(97)00159-0. [DOI] [PubMed] [Google Scholar]
- Sharma T, Lancaster E, Lee D, Lewis S, Sigmundsson T, Takei N, Gurling H, Barta P, Pearlson G, Murray R. Brain changes in schizophrenia. Volumetric MRI study of families multiply affected with schizophrenia—The Maudsley Family Study 5. Br. J. Psychiatry. 1998;173:132–138. doi: 10.1192/bjp.173.2.132. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey C, Frumin M, McCarley RW. A Review of MRI findings in Schizophrenia. Schizophr. Res. 2001;49(12):1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmudsson T, Suckling J, Maier M, Williams SCR, Bullmore ET, Greenwood KE, Fukuda R, Ron MA, Toone BK. Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am. J. Psychiatry. 2001;158:234–243. doi: 10.1176/appi.ajp.158.2.234. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Levitt J, Thompson PM, Holmes CJ, Blanton RE, Kornsand DS, Caplan R, McCracken J, Asarnow R, Toga AW. Brain abnormalities in early-onset schizophrenia spectrum disorder observed with statistical parametric mapping of structural magnetic resonance images. Am. J. Psychiatry. 2000;157:1475–1484. doi: 10.1176/appi.ajp.157.9.1475. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Bilder RM, Lencz T, Ashtari M, Goldman RS, Reiter G, Wu H, Lieberman JA. Reduced anterior cingulate gyrus volume correlates with executive dysfunction in men with first-episode schizophrenia. Schizophr. Res. 2000;43:97–108. doi: 10.1016/s0920-9964(99)00155-3. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston JJ, Evans AC. A unified statistical approach for determining significant voxels in images of cerebral activation. Hum. Brain. Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Wright IC, Ellison ZR, Sharma T, Friston KJ, Murray RM, McGuire PK. Mapping of grey matter changes in schizophrenia. Schizophr. Res. 1999;3:1–14. doi: 10.1016/s0920-9964(98)00094-2. [DOI] [PubMed] [Google Scholar]
- Wright IC, McGuire PK, Poline JB, Travere JM, Murray RM, Frith CD, Frackowiak RS, Friston KJ. A voxel-based method for the statistical analysis of gray and white matter density applied to schizophrenia. Neuroimage. 1995;2:244–252. doi: 10.1006/nimg.1995.1032. [DOI] [PubMed] [Google Scholar]