Abstract
Objective
To examine the effect of an equivalent weight loss, by gastric bypass surgery (GBP) or by diet, on peptide YY3–36 (PYY3–36), ghrelin, and leptin levels and to determine the effect of diabetes status on PYY3–36 levels.
Summary Background Data
The increased PYY3–36 levels after GBP may be involved in the magnitude and the sustainability of weight loss after surgery.
Methods
Of the 30 morbidly obese women who participated in the study, 21 had type 2 diabetes mellitus, and were studied before and after equivalent weight loss of 10 kg by either GBP (n = 11) or by diet (n = 10).
Results
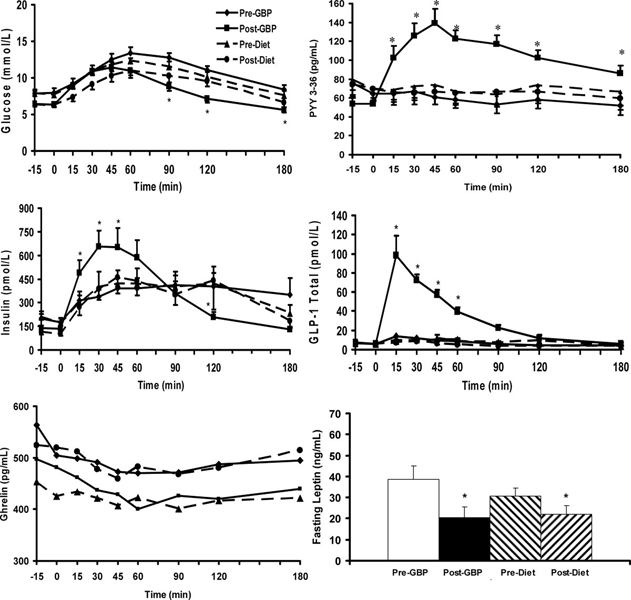
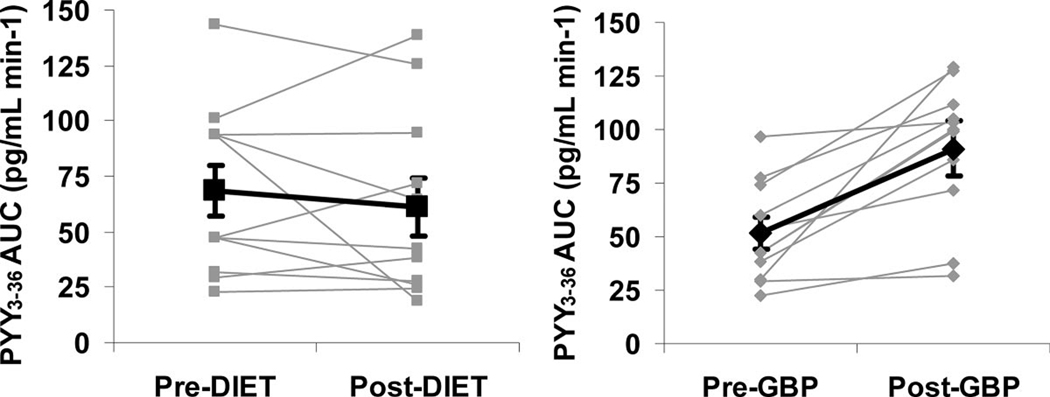
PYY3–36 levels were higher in obese diabetic as compared with nondiabetic individuals (64.1 ± 34.4 pg/mL vs. 39.9 ± 21.1 pg/mL; P < 0.05). PYY3–36 levels increased markedly in response to oral glucose after GBP (peak: 72.3 ± 20.5 pg/mL–132.7 ± 49.7 pg/mL; P < 0.001; AUC0–180: 51.5 ± 23.3 pg/mL.min−1–91.1 ± 32.2 pg/mL.min−1 P < 0.001), but not after diet (peak: 85.5 ± 51.9 pg/mL–84.8 ± 41.13 pg/mL; P = NS; AUC0–180: 68.3 ± 38.5 pg/mL.min−1–61.1 ± 42.2 pg/mL.min−1 P = NS). Fasting ghrelin levels increased after diet (425 ± 91 pg/mL–519 ± 105 pg/mL; P < 0.05), but did not change after GBP (506 ± 121 pg/mL–482 ± 196 pg/mL; P = NS).
Conclusions
Diabetes status seems to be a determinant of PYY3–36 levels. GBP, but not diet-induced weight loss, resulted in markedly increased glucose-stimulated PYY3–36 levels. The increase in stimulated PYY3–36 levels after GBP is likely a result of the surgery rather than a secondary outcome of weight loss. Changes in PYY3–36 levels and ghrelin could contribute to the success of GBP in sustaining weight loss.
Weight loss by diet is often of small magnitude and difficult to sustain over time. On the contrary, bariatric surgery, particularly Roux-en-Y gastric bypass (GBP), typically results in loss of 50% excess body weight within the first year, and the effect is often sustained at 10 years. The rapid and dramatic resolution of type 2 diabetes mellitus (T2DM) after GBP has led to the hypothesis that some of the metabolic effects of the surgery may be independent of weight loss1–3 and may be related to the changes in incretins, gut hormones that stimulate insulin secretion.4,5 The mechanisms by which GBP suppresses appetite are not clear. The marked increase in levels of 2 anorexigenic hormones, peptide YY (PYY),6–10 and glucagon-like peptide 1 (GLP-1),4,7 could explain the increased satiety after GBP. A greater PYY release is associated with greater weight loss after GBP11 and weight regain has been shown with lower PYY levels in a rodent model.12 Previous reports on change in PYY levels after diet intervention are scarce, report only fasting levels, and are inconsistent.13,14
The gastric hormone ghrelin may also be implicated in meal-to-meal regulation.15 Its levels increase in anticipation of a meal and decrease after feeding, suggesting a role in short-term meal-to-meal regulation.16 Ghrelin levels vary as a function of body mass index (BMI) and are low in obese individuals.17 The increase in ghrelin levels may explain weight regain after a diet-induced weight loss.18 Contrary to weight loss by diet, most studies agree that ghrelin levels decrease18,19 or do not increase in proportion to weight loss after GBP,6,20 an effect that could favor a better control of appetite and the decreased food intake observed after this surgery.
In addition to gut hormones, the adipocyte-secreted hormone, leptin, is also an important regulator of energy balance in humans.21 Restriction of food intake or diet-induced weight loss decreases plasma leptin levels. This decrease in leptin signals the hypothalamus a state of negative energy balance and thus triggers food intake.22 After GBP, leptin levels have been found to be lower than expected for a given BMI.6
The first goal of this study was to examine the effect of a short-term equivalent weight loss, by GBP or by diet, on PYY3–36, ghrelin, and leptin levels. Our secondary goal was to determine the effect of BMI and diabetes status on PYY3–36 levels. Partial data on GLP-1 levels after GBP and diet have been published before.2
SUBJECTS AND METHODS
Subjects
A total of 30 women participated in the study: 21 morbidly obese with T2DM and 9 without T2DM. Inclusion criteria for obese patients were BMI >35 kg/m2and age <60 years and normal liver enzymes, thyroid function tests, and blood pressure. Obese individuals without T2DM (n = 9), on no medications that could interfere with glucose homeostasis, served as controls and did not undergo weight loss. Patients with T2DM were diagnosed with T2DM less than 5 years ago, were not on insulin, thiazolinediones, exenatide or dipeptidyl peptidase-IV inhibitors, and had a hemoglobin A1C <8%. All patients with T2DM (n = 21) underwent weight loss and were studied before and after 10 kg weight loss by GBP (surgical group, n = 11) or by diet (diet group, n = 10). Diabetes medications (sulfonylureas and/or metformin) were discontinued 3 days before baseline studies and were adjusted during the diet-induced weight loss to avoid hypoglycemia. Patients after GBP discontinued their diabetes medications the day of the surgery. Patients from the diet and surgical groups were matched for age, body weight, BMI, diabetes duration and control (hemoglobin A1C), and weight loss. The study was approved by the review board of our institution and written informed consent was obtained from all participants before enrollment.
Diet-Induced Weight Loss
As described previously,2 the diet consisted of a meal replacement plan of 1000 kcal/day. A 1-week supply of meal replacement products (Robard Corporation, Mt. Laurel, NJ), including high protein shakes, bars, fruit drinks, and soups, was given to each patient during individual weekly visit at the CRC. Fresh fruits and vegetables were allowed. Body weight was measured weekly and the diet adjusted when necessary. If no weight loss, or if weight gain occurred by the second consecutive weekly visit, the patients were excluded from the study. Although there was no time limit, the expectation was that patients would lose 10 kg in 4 to 8 weeks. During the weight loss, patients were asked to monitor blood glucose levels by finger stick and by keeping daily logs of measurements. Diabetes medications were adjusted by a nurse educator or a diabetologist to avoid hypoglycemia and to fulfill the American Diabetes Association standard of treatment, based on fasting and postprandial glucose levels. Some diabetic patients on sulfonylureas had to decrease their medication and other subjects to stop it to avoid hypoglycemia.
Roux-en-Y Gastric Bypass Protocol
All patients underwent a laparoscopic GBP. In brief, the jejunum was divided 30 cm from the ligament of Treitz and anastomosed to a 30-mL proximal gastric pouch. The jejunum was reanastomosed 150 cm distal to the gastrojejunostomy. All mesenteric defects were closed. The post-GBP diet recommendations included a daily intake of 600 to 800 kcal, 70 g of protein, and 1.8 L of fluid. This was achieved, on an individual basis, with multiple small meals and snacks with various commercial protein supplements. The diet after GBP was monitored by food records, but not directly supervised. The diet in the few days preceding the testing in surgical or diet patients before weight loss was not controlled for.
Three-Hour Oral Glucose Tolerance Test
All patients underwent a 3-hour oral glucose tolerance test (OGTT) with 50 g of glucose (200 mL of noncarbonated glucose drink) in the morning after a 12-hour overnight fast in the CRC. After IV insertion, at 8:00 am, subjects received 50 g of glucose orally. Blood samples were collected at −15, 0, 15, 30,45, 60, 90, 120, and 180 minutes on chilled EDTA tubes with added aprotinin (500 KIU)/mL of blood) and dipeptidyl peptidase-IV inhibitor (Millipore, St. Charles, MO) (10 µL/mL of blood) and centrifuged at 4°C, before storage at −70°C. Baseline values were the average of the blood samples obtained at −15 and 0 minutes before glucose intake.
Hormonal Measurements
PYY3–36, total ghrelin, total GLP-1, leptin, and insulin were measured by radioimmunoassay (Millipore). All intra- and interassay CVs ranged from 3.4% to 7.4% and 4.4% to 7.4%, respectively. Samples for ghrelin assay were only available for 9 patients in the diet group; for the leptin assay, samples were only available for 9 patients in the diet and for 8 in the GBP group. Blood glucose concentrations were measured at the bedside by the glucose oxidase method (Beckman glucose analyzer, Fullerton, CA). All hormonal and metabolite assays were performed at the Hormones and Metabolites Core Laboratory of the New York Obesity Research Center.
Statistical Analysis
Outcome variables were fasting serum glucose, PYY3–36, insulin, GLP-1, leptin, and ghrelin concentrations. The changes in the outcome variables during the OGTT were assessed by peak levels (glucose, PYY3–36, insulin, GLP-1), maximum suppression (ghrelin), and total areas under the curve (AUC0–180) calculated using the trapezoidal method. General linear model with repeated measures was used to detect hormonal changes over time during the OGTT within each condition and before and after GBP. Paired t tests were used to compare data between before and after weight loss within each group. Unpaired t tests were used for comparisons of hormonal levels between obese diabetic and obese nondiabetic patients. Data are expressed as the mean ± SD except in figures, where they appear as mean ± SEM for graphic clarity. Significance was assumed for P < 0.05. Statistical analyses were performed with SPSS, Inc. version 16.0, Chicago, Illinois.
RESULTS
Effect of Weight Loss in Patients With T2DM: Comparison GBP Versus Diet
Clinical Characteristics of Study Groups
Subject characteristics are shown in Table 1. There was no difference in glucose, fasting PYY3–36, GLP-1, ghrelin, and leptin serum concentrations between the GBP and the diet group at baseline. The duration of weight loss was shorter for the GBP group (32.3 ± 13.1 days) compared with the diet group (55.0 ± 9.9 days, P = 0.001) (Table 1).
TABLE 1.
Subject Characteristics Before and After Weight Loss, by Diet (n = 10) and GBP (n = 11)
| Prediet | Postdiet | Pre-GBP | Post-GBP | P | |
|---|---|---|---|---|---|
| Age (yr) | 47.9 ± 7.8 | — | 44.12 ± 10.6 | — | |
| Weight (kg) | 110.6 ± 9.6 | 100.7 ± 9.1* | 117.6 ± 19.3 | 106.4 ± 17.7* | 0.429 |
| BMI (kg/m2) | 42.8 ± 3.8 | 39.0 ± 3.8* | 47.4 ± 10.6 | 41.4 ± 7.6† | 0.233 |
| T2DM duration (mo) | 23.01 ± 18.5 | — | 25.0 ± 19.2 | — | — |
| HbA1C (%) | 6.44 ± 0.61 | — | 6.80 ± 0.57 | — | — |
| Fasting glucose (mmol/L) | 7.84 ± 1.03 | 6.32 ± 0.95* | 7.89 ± 1.56 | 6.45 ± 0.72† | 0.880 |
| 120’ glucose (mmol/L) | 10.29 ± 2.30 | 9.80 ± 2.30 | 10.98 ± 1.60 | 6.96 ± 1.60* | 0.000 |
| Fasting insulin (pmol/L) | 199 ± 105 | 108 ± 48† | 191 ± 95 | 130 ± 49† | 0.344 |
| Peak insulin (pmol/L) | 552 ± 34 | 554 ± 500 | 505 ± 269 | 748 ± 309† | 0.242 |
| Fasting ghrelin (pg/mL)‡ | 425 ± 91 | 519 ± 105† | 506 ± 121 | 482 ± 196 | 0.038 |
| Nadir ghrelin (pg/mL)‡ | 361 ± 91 | 444 ± 96† | 429 ± 122 | 376 ± 131 | 0.004 |
| Max sup.ghrelin (%) | 16.3 ± 12.2 | 15.5 ± 7.6 | 19.0 ± 12.8 | 20.7 ± 7.3 | 0.762 |
| Fasting leptin (ng/mL) | 30.6 ± 11.7 | 22.0 ± 12.3† | 38.8 ± 17.5 | 20.6 ± 14.1* | 0.120 |
| Fasting GLP-1T (pmol/L) | 6.02 ± 2.77 | 6.12 ± 4.19 | 5.95 ± 3.84 | 6.57 ± 3.18 | 0.787 |
| Peak GLP-1T (pmol/L) | 17.8 ± 15.5 | 10.1 ± 5.6 | 16.0 ± 5.9 | 110.3 ± 50.8* | 0.000 |
| Fasting PYY3–36 (pg/mL) | 69.4 ± 44.3 | 69.5 ± 41.3 | 58.7 ± 21.6 | 45.3 ± 20.8† | 0.234 |
| Peak PYY3–36 (pg/mL) | 85.5 ± 51.9 | 84.8 ± 41.3 | 72.3 ± 20.5 | 132.7 ± 49.7* | 0.000 |
| AUC PYY3–36 (pg/mL.min−1) | 68.3 ± 38.5 | 61.1 ± 42.2 | 51.5 ± 23.3 | 91.1 ± 32.2* | 0.001 |
| HOMA-IR | 8.37 ± 4.20 | 3.98 ± 1.80† | 8.96 ± 5.60 | 5.21 ± 2.60† | 0.694 |
Data are expressed as mean ± SD. Fasting, peak, 120 min, AUC (total area under the curve) values were obtained during the OGTT. The reported P value represents the difference between the changes occurring with diet and GBP.
P < 0.001,
P < 0.05, effect of weight loss within each group (diet or GBP).
GBP indicates gastric bypass; BMI, body mass index; T2DM, type 2 diabetes mellitus; AUC, area under the curve; GLP-1T, glucagon like peptide-1 total; PYY3–36, peptide-YY3–36; HbA1C, hemoglobin A1C; HOMA-IR, homeostatic model assessment of insulin resistance.
Side Effects
Five of 11 patients experienced stomach cramping and discomfort, nausea, sweating, flushing, and palpitations 5 to 20 minutes into the 50 g OGTT, after GBP. No severe adverse effects were observed. There were no adverse effects after diet-induced weight loss.
Effect of Weight Loss on Fasting Glucose and Hormone Levels
After a mean weight loss of 10.6 kg (P = NS between groups), fasting glucose and insulin and HOMA-IR decreased significantly and equally in the GBP and the diet group (Table 1). Fasting PYY3–36 levels decreased after GBP (P = 0.016) but not after diet (P = 0.99) (Table 1). Fasting ghrelin levels increased after diet (P = 0.017). On the contrary, after GBP, there was trend of a decrease in ghrelin levels, although this was not significant (P = 0.28) (Table 1).
Leptin decreased by 50% after GBP (38.8 ± 17.5 ng/mL– 20.6 ± 14.1 ng/mL, P < 0.001) and by only 28% after diet (30.6 ± 11.7 ng/mL–22 ± 12.3 ng/mL, P = 0.012) (Table 1). Although fasting leptin levels decreased more in the GBP group as compared with the diet group, the difference in the change between groups was not significant (P = 0.12).
Hormonal Response to Oral Glucose in Patients With T2DM: GBP Versus Diet
PYY3–36 levels increased markedly after GBP during the OGTT. Peak concentrations occurred at 45 minutes and remained significantly elevated compared with baseline for 180 minutes (Table 1, Fig. 1). Stimulated PYY3–36 levels did not change after diet (Table 1, Fig. 1, Fig. 2). Ghrelin levels (fasting and nadir after oral glucose) were higher after diet than after GBP. However, the maximum ghrelin suppression did not change with either weight loss intervention (P = 0.762) (Table 1).
FIGURE 1.
Glucose, insulin, total ghrelin, PYY3–36, total GLP-1 in response to a 50 g glucose load, and fasting leptin levels in morbidly obese subjects with T2DM before and after GBP (n = 11) and before and after a diet-induced weight loss (n = 11). Data are expressed as mean ± SEM, *P < 0.05. Values are indicated for comparison relative to baseline.
FIGURE 2.
Individual changes in PYY3–36 AUC0–180 in response to a 50 g glucose load in morbidly obese patients with T2DM after GBP and diet. Bold line indicates mean ± SEM.
Effect of Diabetes Status on Hormone Levels
Subject characteristics of morbidly obese with and without T2DM are shown in Table 2. Obese with T2DM had higher fasting PYY3–36 levels (P = 0.048) and oral glucose stimulated peak (P = 0.039) and AUC0–180 (P = 0.011) PYY3–36 serum levels than obese without T2DM. PYY3–36 levels did not correlate with any markers of insulin resistance or secretion. Fasting leptin and ghrelin levels were not affected by diabetes status. There was no correlation between leptin and PYY3–36 levels before or after weight loss or between changes in leptin with changes in PYY3–36.
TABLE 2.
Baseline Subject Characteristics of Obese Patients With and Without T2DM
| Obese Without T2DM n = 9 |
Obese With T2DM n = 21 |
|
|---|---|---|
| Age (yr) | 37.4 ± 7.5 | 46.1 ± 9.2* |
| Weight (kg) | 121.1 ± 19.3 | 114.1 ± 15.3 |
| BMI (kg/m2) | 45.5 ± 7.1 | 45.1 ± 8.1 |
| Fasting glucose (mmol/L) | 5.34 ± 0.48 | 7.87 ± 1.29* |
| Fasting insulin (pmol/L) | 167.9 ± 86.4 | 195.2 ± 97.9* |
| HOMA-IR | 4.81 ± 2.57 | 8.67 ± 4.60* |
| Fasting GLP-1T (pmol/L) | 5.23 ± 3.60 | 5.99 ± 3.27 |
| Peak GLP-1T (pmol/L) | 10.1 ± 5.2 | 16.9 ± 11.5 |
| Fasting ghrelin | 409 ± 112 | 516 ± 243 |
| Max sup ghrelin (%) | 24.6 ± 13.5 | 15.9 ± 11.2 |
| Nadir ghrelin (pg/mL) | 315 ± 124 | 428 ± 177 |
| Fasting leptin (ng/mL) | 39.7 ± 11.5 | 34.6 ± 14.0 |
| Fasting PYY3–36 (pg/mL) | 39.9 ± 21.1 | 64.1 ± 34.5* |
| Peak PYY3–36 (pg/mL) | 50.2 ± 24.8 | 78.9 ± 39.1* |
| AUC PYY3–36 (pg/mL/min−1) | 30.0 ± 20.8 | 59.9 ± 32.2* |
Mean ± SD.
Denotes significant difference between groups, P < 0.05.
Max sup ghrelin indicates maximal suppression of ghrelin; AUC, area under the curve.
DISCUSSION
Our goal was to assess changes of gut peptides after short-term weight loss by 2 different methods, diet and GBP surgery. We wished to elucidate possible hormonal mechanisms of energy homeostasis responsible for the greater efficacy of GBP compared with diet.
The main result of this study is that PYY3–36 levels increased markedly in response to oral glucose after GBP surgery, but not after an equivalent weight loss by diet. These results confirm previous data from cross-sectional6,8 and prospective7,9–11 studies showing increased gut peptide levels after GBP or jejuno-ileal bypass.23 Our data demonstrate that weight loss alone does not contribute to the changes in PYY levels observed after GBP, as stimulated PYY3–36 levels did not increase after a diet-induced weight loss. To our knowledge, these are the first data on stimulated PYY3–36 level after diet-induced weight loss. Similarly to our results after diet, PYY levels do not increase after purely restrictive surgeries such as gastric banding,8,24 although they increase after vertical banded gastroplasty.25 The mechanism by which stimulated PYY levels increase after GBP could be related to the more rapid delivery of nutrients, as intestinal transit time is accelerated after GBP7 and as suggested by studies after ileal transposition in rats.26 In our study, fasting PYY3–36 levels decreased after GBP but did not change after diet. These findings are in contrast to those of Roth et al,13 who showed that diet-induced weight loss in obese children increased fasting PYY levels and of Pfluger et al,14 who showed a decrease in fasting PYY levels after diet in adults. Discrepancies between studies could be related to age, sex, diabetes status, and/or energy balance differences.
PYY is rapidly cosecreted with GLP-1 from intestinal L-cells in response to food intake.27,28 We4 and others29 have shown a marked increase in GLP-1 levels in response to oral glucose or a meal after GBP. The administration of PYY3–3630 or of GLP-131–34 reduces food intake in lean and in obese human individuals. GLP-1 and PYY infused simultaneously lower food intake more than either peptide administered alone in lean and obese rodents and in lean humans.35 The increase in the levels of these 2 anorexigenic peptides in concert with the decrease in the orexigenic peptide ghrelin, may act together with pancreatic and adipose hormones to inhibit energy intake and favor weight loss after GBP.12 Examples of these interactions have been demonstrated in rats: ghrelin attenuates, dose-dependently, the anorectic effect of PYY and GLP-1,36 and the coadministration of leptin with PYY3–36 enhances the anoretic effects of PYY3–36.37
The compensatory increase in ghrelin levels after diet-induced weight loss was not observed after GBP. Although conflicting results on the changes in plasma ghrelin after GBP have been reported,6,18,24,38–43 most studies agree that ghrelin levels either decrease or do not increase in proportion to the amount of weight loss after GBP surgery. Ghrelin15,44 and ghrelin analogs45,46 stimulate food intake in lean and obese humans and increase body weight and adiposity in rodents.15 The increase in ghrelin levels after diet may play a role in weight regain and explain, in part, the lower effectiveness of diet for sustained weight loss compared with GBP.
Despite similar weight loss, leptin levels decreased more after GBP than after diet, although this did not reach statistical significance. Others have found lower leptin levels after GBP surgery compared with a BMI-matched group.24 The relatively low levels of leptin after GBP may indicate that factors other than the change in adipose mass may be responsible for the decrease in circulating leptin concentration after GBP. In our study, the level of energy restriction could account for variability in the decrease in leptin levels after GBP and diet.21 Different patterns in gut and pancreatic hormones resulting from GBP could also play a role in the regulation of plasma leptin levels. In vitro, ghrelin stimulates the differentiation of preadipocytes47 and inhibits adipocyte apoptosis.48 The fall of ghrelin levels after GBP could alter its effect on adipose tissue and modulate circulating leptin levels. In addition, leptin is not only produced by adipose tissue but also in small amounts by the stomach,49 which is altered after surgery.
We found higher fasting and postprandial PYY3–36 levels in obese with T2DM compared with obese without T2DM. English et al also reported that fasting PYY levels were significantly higher in T2DM than in controls.50 However, other studies reported low fasting PYY plasma levels in first-degree relatives of subjects with T2DM51 and blunted postprandial PYY levels in early stages of T2DM development in genetically susceptible individuals.52 It is unclear whether PYY levels contribute to the pathogenesis of T2DM and/or whether glucose homeostasis modulates circulating PYY levels. As previously reported,50 we did not find significant correlation between PYY levels and markers of insulin secretion or sensitivity. Future larger studies are needed to clarify the role of PYY, if any, in the physiopathology of T2DM. As shown by others,6,18,24,39–43,53 diabetes status had no effect on leptin or ghrelin levels.
Our study has some limitations. The small amount of glucose administered in our study (50 g glucose, 200 Kcal) may have been insufficient to stimulate PYY3–36 release before weight loss intervention. PYY release is influenced by amount of calories54 and the nutrient composition of the meal, with dietary carbohydrates being weaker stimulants of PYY than protein or fat.27,55–57 However, the same stimulus was able to markedly increase PYY3–36 levels after GBP to levels that could contribute to higher postprandial satiety and weight loss after GBP. Oral glucose is a clinical research tool, used by diabetologists, that reflects poorly on daily food intake. Whether the large increase in PYY levels after oral glucose has any clinical relevance under conditions of normal feeding will remain to be determined. Another limitation of our study is the lack of measurement of hunger or satiety ratings of ad libitum food intake. However, the changes in circulating levels of peptides implicated with meal-to-meal regulation correlates poorly with quantified measures of hunger after GBP6,58 and ad libitum food intake is difficult to study in the early stages after GBP. Another limitation is the absence of perfect matching in calorie restriction between the GBP and the diet group. The diet arm was designed to match the weight loss of the GBP group, not their calorie intake. However, the overall calorie deficit and weight loss were identical.
In summary, we show that the increase in PYY3–36 levels after GBP, but not after diet-induced weight loss, results from the surgical procedure, independently of weight loss. The changes of PYY3–36, ghrelin, and leptin levels, all important regulators of food intake and energy homeostasis, may explain, in part, the greater effectiveness of GBP in sustaining weight loss compared with diet. Understanding the mechanisms involved in the changes of these peptides after the surgery could lead to new treatments for obesity and related metabolic conditions.
ACKNOWLEDGMENTS
The authors thank our volunteer participants, Ping Zhou and Yim Dam for their technical help with hormonal assays, Drs. James McGinty and Ninan Koshy for referring patients to the study, and Antonia Colarusso and Betty Kovacs for their help with recruitment and the diet-induced weight loss.
This work was funded by grants from the American Diabetes Association CR-7-05 CR-18, NIH R01-DK67561, GCRC 1 UL1 RR024156-02, ORC DK-26687, DERC DK-63068-05, and Amylin Investigator Initiated Studies Program. B.L. received grant support through Amylin Investigator Initiated Studies Program in 2007.
Footnotes
J.T., B.B., H.S., T.C., M.B., H.L., and B.O. have nothing to declare.
REFERENCES
- 1.Pories WJ, Caro JF, Flickinger EG, et al. The control of diabetes mellitus (NIDDM) in the morbidly obese with the Greenville Gastric Bypass. Ann Surg. 1987;206:316–323. doi: 10.1097/00000658-198709000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laferrére B, Teixeira J, McGinty J, et al. Effect of weight loss by gastric bypass surgery versus hypocaloric diet on glucose and incretin levels in patients with type 2 diabetes. J Clin Endocrinol Metab. 2008;93:2479–2485. doi: 10.1210/jc.2007-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubino F, Gagner M, Gentileschi P, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–242. doi: 10.1097/01.sla.0000133117.12646.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laferrére B, Heshka S, Wang K, et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709–1716. doi: 10.2337/dc06-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodieux F, Giusti V, D’Alessio DA, et al. Effects of gastric bypass and gastric banding on glucose kinetics and gut hormone release. Obesity (Silver Spring) 2008;16:298–305. doi: 10.1038/oby.2007.83. [DOI] [PubMed] [Google Scholar]
- 6.Korner J, Bessler M, Cirilo LJ, et al. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab. 2005;90:359–365. doi: 10.1210/jc.2004-1076. [DOI] [PubMed] [Google Scholar]
- 7.Morinigo R, Moize V, Musri M, et al. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2006;91:1735–1740. doi: 10.1210/jc.2005-0904. [DOI] [PubMed] [Google Scholar]
- 8.le Roux CW, Aylwin SJ, Batterham RL, et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg. 2006;243:108–114. doi: 10.1097/01.sla.0000183349.16877.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan JL, Mun EC, Stoyneva V, et al. Peptide YY levels are elevated after gastric bypass surgery. Obesity (Silver Spring) 2006;14:194–198. doi: 10.1038/oby.2006.25. [DOI] [PubMed] [Google Scholar]
- 10.Morinigo R, Vidal J, Lacy AM, et al. Circulating peptide YY, weight loss, and glucose homeostasis after gastric bypass surgery in morbidly obese subjects. Ann Surg. 2008;247:270–275. doi: 10.1097/SLA.0b013e31815f6e77. [DOI] [PubMed] [Google Scholar]
- 11.le Roux CW, Welbourn R, Werling M, et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246:780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 12.Meguid MM, Glade MJ, Middleton FA. Weight regain after Roux-en-Y: a significant 20% complication related to PYY. Nutrition. 2008;24:832–842. doi: 10.1016/j.nut.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 13.Roth CL, Enriori PJ, Harz K, et al. Peptide YY is a regulator of energy homeostasis in obese children before and after weight loss. J Clin Endocrinol Metab. 2005;90:6386–6391. doi: 10.1210/jc.2005-1357. [DOI] [PubMed] [Google Scholar]
- 14.Pfluger PT, Kampe J, Castaneda TR, et al. Effect of human body weight changes on circulating levels of peptide YY and peptide YY3-36. J Clin Endocrinol Metab. 2007;92:583–588. doi: 10.1210/jc.2006-1425. [DOI] [PubMed] [Google Scholar]
- 15.Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992–5995. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 16.Cummings DE, Purnell JQ, Frayo RS, et al. A pre-prandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 17.Tschop M, Weyer C, Tataranni PA, et al. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- 18.Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 19.Tritos NA, Mun E, Bertkau A, et al. Serum ghrelin levels in response to glucose load in obese subjects post-gastric bypass surgery. Obes Res. 2003;11:919–924. doi: 10.1038/oby.2003.126. [DOI] [PubMed] [Google Scholar]
- 20.Karamanakos SN, Vagenas K, Kalfarentzos F, et al. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247:401–407. doi: 10.1097/SLA.0b013e318156f012. [DOI] [PubMed] [Google Scholar]
- 21.Havel PJ. Role of adipose tissue in body-weight regulation: mechanisms regulating leptin production and energy balance. Proc Nutr Soc. 2000;59:359–371. doi: 10.1017/s0029665100000410. [DOI] [PubMed] [Google Scholar]
- 22.Flier JS. Clinical review 94: what’s in a name? In search of leptin’s physiologic role. J Clin Endocrinol Metab. 1998;83:1407–1413. doi: 10.1210/jcem.83.5.4779. [DOI] [PubMed] [Google Scholar]
- 23.Naslund E, Gryback P, Hellstrom PM, et al. Gastrointestinal hormones and gastric emptying 20 years after jejunoileal bypass for massive obesity. Int J Obes Relat Metab Disord. 1997;21:387–392. doi: 10.1038/sj.ijo.0800418. [DOI] [PubMed] [Google Scholar]
- 24.Korner J, Inabnet W, Conwell IM, et al. Differential effects of gastric bypass and banding on circulating gut hormone and leptin levels. Obesity (Silver Spring) 2006;14:1553–1561. doi: 10.1038/oby.2006.179. [DOI] [PubMed] [Google Scholar]
- 25.Alvarez BM, Borque M, Martinez-Sarmiento J, et al. Peptide YY secretion in morbidly obese patients before and after vertical banded gastroplasty. Obes Surg. 2002;12:324–327. doi: 10.1381/096089202321088084. [DOI] [PubMed] [Google Scholar]
- 26.Strader AD, Vahl TP, Jandacek RJ, et al. Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am J Physiol Endocrinol Metab. 2005;288:E447–E453. doi: 10.1152/ajpendo.00153.2004. [DOI] [PubMed] [Google Scholar]
- 27.Adrian TE, Ferri GL, Bacarese-Hamilton AJ, et al. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985;89:1070–1077. doi: 10.1016/0016-5085(85)90211-2. [DOI] [PubMed] [Google Scholar]
- 28.Ekblad E, Sundler F. Distribution of pancreatic polypeptide and peptide YY. Peptides. 2002;23:251–261. doi: 10.1016/s0196-9781(01)00601-5. [DOI] [PubMed] [Google Scholar]
- 29.Morinigo R, Lacy AM, Casamitjana R, et al. GLP-1 and changes in glucose tolerance following gastric bypass surgery in morbidly obese subjects. Obes Surg. 2006;16:1594–1601. doi: 10.1381/096089206779319338. [DOI] [PubMed] [Google Scholar]
- 30.Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349:941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 31.Verdich C, Flint A, Gutzwiller JP, et al. A meta-analysis of the effect of glucagon-like peptide-1 (7–36) amide on ad libitum energy intake in humans. J Clin Endocrinol Metab. 2001;86:4382–4389. doi: 10.1210/jcem.86.9.7877. [DOI] [PubMed] [Google Scholar]
- 32.Naslund E, Gutniak M, Skogar S, et al. Glucagon-like peptide 1 increases the period of postprandial satiety and slows gastric emptying in obese men. Am J Clin Nutr. 1998;68:525–530. doi: 10.1093/ajcn/68.3.525. [DOI] [PubMed] [Google Scholar]
- 33.Naslund E, Barkeling B, King N, et al. Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int J Obes Relat Metab Disord. 1999;23:304–311. doi: 10.1038/sj.ijo.0800818. [DOI] [PubMed] [Google Scholar]
- 34.Degen L, Oesch S, Matzinger D, et al. Effects of a preload on reduction of food intake by GLP-1 in healthy subjects. Digestion. 2006;74:78–84. doi: 10.1159/000097585. [DOI] [PubMed] [Google Scholar]
- 35.Neary NM, Small CJ, Druce MR, et al. Peptide YY3-36 and glucagon-like peptide-17–36 inhibit food intake additively. Endocrinology. 2005;146:5120–5127. doi: 10.1210/en.2005-0237. [DOI] [PubMed] [Google Scholar]
- 36.Chelikani PK, Haver AC, Reidelberger RD. Ghrelin attenuates the inhibitory effects of glucagon-like peptide-1 and peptide YY(3–36) on food intake and gastric emptying in rats. Diabetes. 2006;55:3038–3046. doi: 10.2337/db06-0730. [DOI] [PubMed] [Google Scholar]
- 37.Unniappan S, Kieffer TJ. Leptin extends the anorectic effects of chronic PYY(3–36) administration in Ad lib fed rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R51–R58. doi: 10.1152/ajpregu.00234.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geloneze B, Tambascia MA, Pilla VF, et al. Ghrelin: a gut-brain hormone: effect of gastric bypass surgery. Obes Surg. 2003;13:17–22. doi: 10.1381/096089203321136539. [DOI] [PubMed] [Google Scholar]
- 39.Mancini MC, Costa AP, de Melo ME, et al. Effect of gastric bypass on spontaneous growth hormone and ghrelin release profiles. Obesity (Silver Spring) 2006;14:383–387. doi: 10.1038/oby.2006.51. [DOI] [PubMed] [Google Scholar]
- 40.Couce ME, Cottam D, Esplen J, et al. Is ghrelin the culprit for weight loss after gastric bypass surgery? A negative answer. Obes Surg. 2006;16:870–878. doi: 10.1381/096089206777822151. [DOI] [PubMed] [Google Scholar]
- 41.Mingrone G, Granato L, Valera-Mora E, et al. Ultradian ghrelin pulsatility is disrupted in morbidly obese subjects after weight loss induced by malabsorptive bariatric surgery. Am J Clin Nutr. 2006;83:1017–1024. doi: 10.1093/ajcn/83.5.1017. [DOI] [PubMed] [Google Scholar]
- 42.Stratis C, Alexandrides T, Vagenas K, et al. Ghrelin and peptide YY levels after a variant of biliopancreatic diversion with Roux-en-Y gastric bypass versus after colectomy: a prospective comparative study. Obes Surg. 2006;16:752–758. doi: 10.1381/096089206777346772. [DOI] [PubMed] [Google Scholar]
- 43.Lee H, Te C, Koshy S, et al. Does ghrelin really matter after bariatric surgery? Surg Obes Relat Dis. 2006;2:538–548. doi: 10.1016/j.soard.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 44.Druce MR, Wren AM, Park AJ, et al. Ghrelin increases food intake in obese as well as lean subjects. Int J Obes (Lond) 2005;29:1130–1136. doi: 10.1038/sj.ijo.0803001. [DOI] [PubMed] [Google Scholar]
- 45.Laferrére B, Abraham C, Russell CD, et al. Growth hormone releasing peptide-2 (GHRP-2), like ghrelin, increases food intake in healthy men. J Clin Endocrinol Metab. 2005;90:611–614. doi: 10.1210/jc.2004-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laferrére B, Hart A, Bowers C. Obese subjects respond to the stimulatory effect of the ghrelin agonist growth hormone releasing peptide-2 (GHRP-2) on food intake. Obesity. 2006;14:1056–1063. doi: 10.1038/oby.2006.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thompson NM, Gill DA, Davies R, et al. Ghrelin and des-octanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology. 2004;145:234–242. doi: 10.1210/en.2003-0899. [DOI] [PubMed] [Google Scholar]
- 48.Kim MS, Yoon CY, Jang PG, et al. The mitogenic and antiapoptotic actions of ghrelin in 3T3-L1 adipocytes. Mol Endocrinol. 2004;18:2291–2301. doi: 10.1210/me.2003-0459. [DOI] [PubMed] [Google Scholar]
- 49.Sobhani I, Bado A, Vissuzaine C, et al. Leptin secretion and leptin receptor in the human stomach. Gut. 2000;47:178–183. doi: 10.1136/gut.47.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.English PJ, Ashcroft A, Patterson M, et al. Fasting plasma peptide-YY concentrations are elevated but do not rise postprandially in type 2 diabetes. Diabetologia. 2006;49:2219–2221. doi: 10.1007/s00125-006-0344-y. [DOI] [PubMed] [Google Scholar]
- 51.Boey D, Heilbronn L, Sainsbury A, et al. Low serum PYY is linked to insulin resistance in first-degree relatives of subjects with type 2 diabetes. Neuropeptides. 2006;40:317–324. doi: 10.1016/j.npep.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Viardot A, Heilbronn LK, Herzog H, et al. Abnormal postprandial PYY response in insulin sensitive nondiabetic subjects with a strong family history of type 2 diabetes. Int J Obes (Lond) 2008;32:943–948. doi: 10.1038/ijo.2008.24. [DOI] [PubMed] [Google Scholar]
- 53.Geloneze B, Tambascia MA, Pareja JC, et al. Serum leptin levels after bariatric surgery across a range of glucose tolerance from normal to diabetes. Obes Surg. 2001;11:693–698. doi: 10.1381/09608920160558623. [DOI] [PubMed] [Google Scholar]
- 54.Pedersen-Bjergaard U, Host U, Kelbaek H, et al. Influence of meal composition on postprandial peripheral plasma concentrations of vasoactive peptides in man. Scand J Clin Lab Invest. 1996;56:497–503. doi: 10.3109/00365519609088805. [DOI] [PubMed] [Google Scholar]
- 55.Lin HC, Chey WY. Cholecystokinin and peptide YY are released by fat in either proximal or distal small intestine in dogs. Regul Pept. 2003;114:131–135. doi: 10.1016/s0167-0115(03)00115-0. [DOI] [PubMed] [Google Scholar]
- 56.Feinle-Bisset C, Patterson M, Ghatei MA, et al. Fat digestion is required for suppression of ghrelin and stimulation of peptide YY and pancreatic polypeptide secretion by intraduodenal lipid. Am J Physiol Endocrinol Metab. 2005;289:E948–E953. doi: 10.1152/ajpendo.00220.2005. [DOI] [PubMed] [Google Scholar]
- 57.Batterham RL, Heffron H, Kapoor S, et al. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006;4:223–233. doi: 10.1016/j.cmet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 58.Chanoine JP, Mackelvie KJ, Barr SI, et al. GLP-1 and appetite responses to a meal in lean and overweight adolescents following exercise. Obesity (Silver Spring) 2008;16:202–204. doi: 10.1038/oby.2007.39. [DOI] [PubMed] [Google Scholar]