Abstract
Background
Many of the neurobehavioral effects of ethanol are mediated by inhibition of excitatory N-methyl-d-aspartate (NMDA) and enhancement of inhibitory γ-amino-butyric-acid (GABA) receptor systems. There is growing interest in drugs that alter these systems as potential medications for problems associated with alcoholism. The drug riluzole, approved for treatment of amyotrophic lateral sclerosis (ALS), inhibits NMDA and enhances GABAA receptor system activity. This study was designed to determine the preclinical efficacy of riluzole to modulate ethanol self-administration and withdrawal.
Methods
Male C57BL/6J mice were trained to lever press on a concurrent fixed-ratio 1 schedule of ethanol (10% v/v) versus water reinforcement during daily 16-hour sessions. Riluzole (1 to 40 mg/kg, IP) was evaluated on ethanol self-administration after acute and chronic (2 week) treatment. To determine if riluzole influences ethanol withdrawal-associated seizures, mice were fed an ethanol-containing or control liquid diet for 18 days. The effects of a single injection of riluzole (30 mg/kg) were examined on handling-induced convulsions after ethanol withdrawal.
Results
Acute riluzole (30 and 40 mg/kg) reduced ethanol self-administration during the first 4 hours of the session, which corresponds to the known pharmacokinetics of this drug. Ethanol self-administration was also reduced by riluzole after chronic treatment. Riluzole (30 mg/kg) significantly decreased the severity of ethanol-induced convulsions 2 hours after ethanol withdrawal.
Conclusions
These results demonstrate that riluzole decreases ethanol self-administration and may reduce ethanol withdrawal severity in mice. Thus, riluzole may have utility in the treatment of problems associated with alcoholism.
Keywords: Alcohol Drinking, Alcoholism, Self-Administration, GABA, NMDA, PKC, Riluzole
Alcoholism is a complex neurobehavioral disorder that is characterized in part by excessive consumption of ethanol and by the development of physical dependence. Many of the neurobiological and behavioral effects of ethanol are mediated by excitatory NMDA and inhibitory GABAA receptor systems (for reviews see Davis and Wu, 2001; Grant and Lovinger, 1995; Koob et al., 1998; Nevo and Hamon, 1995). For example, preclinical studies have shown that GABAA and NMDA receptor systems modulate ethanol drinking (Bienkowski et al., 2001; June et al., 1992), reinforcement (Hodge et al., 1996), reward (Chester and Cunningham, 1999), drug discrimination (Besheer et al., 2003; Grant et al., 1991; Hodge and Cox, 1998), tolerance (Khanna et al., 1991; Mihic et al., 1992), dependence (Morrisett et al., 1990; Morrow et al., 1994), and relapse (Nie and Janak, 2003). Given the involvement of GABAergic and NMDA/glutamatergic systems in a variety of ethanol's neurobiological and behavioral effects, there is growing interest in these systems as potential therapeutic targets in the pharmacological treatment of problems associated with alcoholism (Anton, 2001; Davis and Wu, 2001; Nevo and Hamon, 1995). One efficient strategy that might be used to identify candidate medications for treatment of alcoholism is to use preclinical animal models to evaluate the potential of FDA approved medications with mechanism(s) of action that are known to underlie the neurobehavioral effects of ethanol.
The drug riluzole (2-amino-6-trifluoromethoxy benzothiazole), which is presently an approved medication for the treatment of amyotrophic lateral sclerosis (ALS) (Wagner and Landis, 1997), appears to be one such candidate. That is, riluzole inhibits the presynaptic release of glutamate (Jehle et al., 2000; Martin et al., 1993) and may act postsynaptically to interfere with NMDA receptor function (reviewed by Doble, 1996). Evidence also indicates that riluzole decreases GABA uptake (Mantz et al., 1994) and potentiates function of postsynaptic GABAA receptors (He et al., 2002). In vivo, riluzole has neuroprotective (Debono et al., 1993; Malgouris et al., 1989; Pratt et al., 1992), anticonvulsant (Mizoule et al., 1985; Romettino et al., 1991), anxiolytic-like (Munro et al., 2007), and sedative properties (Mantz et al., 1992) that are consistent with a decrease in CNS excitability via inhibition of NMDA and enhancement of GABAA receptor activity.
Riluzole also appears to have another mechanism of action which may indirectly result in inhibition of CNS excitation. Noh and colleagues (2000) found riluzole to be an effective and direct inhibitor of protein kinase C (PKC). PKC is a family of kinases that regulates a variety of neuronal functions including ion channel activity, neurotransmitter release, receptor desensitization, and differentiation (Tanaka and Nishizuka, 1994). PKC has been shown to positively modulate glutamate channels, as well as sodium and chloride channels (Shearman et al., 1989). Moreover, the finding that riluzole inhibits PKC presents the possibility that riluzole-induced suppression of glutamate release may occur via an indirect mechanism. Previous work from our laboratory has shown that mice lacking the epsilon isoform of PKC (PKCε) show decreased ethanol self-administration (Hodge et al., 1999; Olive et al., 2000), greater sensitivity to ethanol's sedative effects (Hodge et al., 1999), reduced anxiety-like behavior (Hodge et al., 2002), and exhibit diminished ethanol-induced withdrawal seizures (Olive et al., 2001).
Given that riluzole influences glutamate and GABA functioning, and inhibits PKC, the effects of riluzole on ethanol self-administration were tested in inbred C57BL/6J mice, with the prediction that riluzole pretreatment would reduce ethanol self-administration. The pattern of self-administration behavior across the 16-hour session was examined in 4-hour intervals in an effort to relate behavior with the known kinetics of riluzole after IP administration in mice, which shows a dual peak in brain levels at 20 and 80 minutes and is then significantly reduced by 4 hours (Hockly et al., 2006). Further, given riluzole's general suppressant effect on the central nervous system and anticonvulsant properties, suppression of ethanol withdrawal seizures was also predicted. Because riluzole is already approved for human use, reductions in ethanol self-administration and withdrawal seizures may make this drug a viable therapeutic for the treatment of alcohol use disorders.
Method
Animals
Male mice (C57BL/6J; Jackson Laboratories, Bar Harbor, ME) housed in standard Plexiglas cages with at most 4 mice per cage were used in the ethanol self-administration study (n = 12) and the ethanol withdrawal assessments (n = 36). Food and water were continuously available except during self-administration lever press training and the ethanol withdrawal study (see later). The colony room was maintained on a 12-hour light/dark cycle with lights on at 07:00 hours.
Apparatus
Twelve Plexiglas operant chambers (Med Associates, Georgia, VT) measuring 15.9 × 14 × 12.7 cm with stainless steel grid floors were used. Each chamber was housed in a sound-attenuating cubicle equipped with a fan that provided ventilation and masked external noise. The left and right wall of each chamber contained 1 ultra-sensitive stainless steel response lever and a recessed liquid well. One lever press activated a syringe pump that was attached to that liquid well and delivered 0.01 ml of solution. A stimulus light was located above each response lever and was activated each time a reinforcer was delivered. The chambers were illuminated by a house light that was on during the first and last 2 hours of each daily 16-hour session. The chambers were controlled using a computer interface and PC software (Med Associates).
Ethanol Self-Administration
Training Procedures
Operant ethanol self-administration was measured during 16-hour sessions (between 17:00 and 09:00 hours). To correspond with the 12-hour light/dark cycle in the animal colony room, the chambers were illuminated via the house light from 17:00 to 19:00 and 07:00 to 09:00 hours. On the first 2 days of training, mice were weighed and individually placed into a test chamber for overnight 16-hour training sessions to establish reliable lever pressing behavior. Before each of these sessions, water was removed from the home cages 24 hours before the first training session and returned for 2 hours following each training session. During these sessions, both levers were active on a concurrent fixed-ratio 1 (CONC FR1 FR1) schedule with 10% sucrose and water presented as the reinforcer. That is, one lever response resulted in the presentation of 0.01 ml of the solution paired with that lever. On the following days, animals were trained to orally self-administer ethanol using a sucrose substitution procedure (Hodge et al., 1995; Samson, 1986, 1987). Briefly, the FR1 contingency was in place and ethanol was gradually added to the 10% sucrose solution and the sucrose was gradually faded out so that 10% ethanol maintained lever pressing. The mice experienced 4 training sessions a week (Monday through Thursday).
Acute Riluzole Administration
At the beginning of riluzole testing, the mice had approximately 10 months of experience self-administering ethanol and weighed an average of 33.84 ± 0.42 g. The mice had been previously used in another study (2 months earlier) and as such were not drug-naïve. Test sessions were conducted on Tuesday and Thursday of each week. For each 16-hour test session, mice were administered riluzole (0, 1, 10, 30, and 40 mg/kg, IP) and immediately placed in the operant chambers. Riluzole was administered according to a Latin-Square design with the exception that the highest dose (40 mg/kg) was tested last in all animals. Each mouse received a single injection of each dose.
Chronic Riluzole Administration
One week after the final acute riluzole test, mice were divided into 2 groups: control and riluzole, and received daily vehicle (n = 6) or 30 mg/kg riluzole (n = 6) injections, respectively. To ensure that the 2 groups did not differ on baseline ethanol and water responding, the hourly ethanol and water responses for the 2 days before the beginning of the acute riluzole assessments were averaged and compared. No significant main effects or interactions were observed. For the control group, total ethanol and water responses were 237.92 ± 10.32 and 86.17 ± 18.55, respectively. For the Riluzole group, total ethanol and water responses were 208.75 ± 27.19 and 83.08 ± 18.62, respectively. Monday through Thursday self-administration training sessions continued and injections were administered immediately before the sessions. On non-training days (Friday through Sunday), mice were injected and returned to the home cage. This injection protocol continued for 2 weeks.
Ethanol Withdrawal Seizures
Male C57BL/6J mice were maintained on a modified high-protein Lieber-DeCarli liquid control diet (n = 16) or ethanol diet (n = 20; Dyets Inc., Bethlehem, PA) as the sole source of nutrients and fluids for 18 days. Although C57BL/6 mice are not as sensitive to ethanol withdrawal seizures as other mouse strains (Crabbe et al., 1983), this mouse strain was used to maintain consistency with the self-administration studies. Moreover, the liquid diet procedure has been shown to produce 75% mortality in DBA/2J mice, which is an ethanol withdrawal sensitive strain (Goldstein and Arnold, 1976). In the ethanol group, the ethanol concentration in the diet was 2.4% for the first 2 days, and was increased to 4.8% for the remaining 16 days. Each day the mice were weighed and the amount of fluid consumed was recorded. On day 18, the liquid diet was removed 30 minutes after the onset of the light cycle (07:30 hours) and the mice were injected with riluzole (30 mg/kg, IP) or vehicle. Mice were evaluated at 2, 4, 6, and 8 hours following the removal of the diet for withdrawal-induced seizures according to a handling-induced convulsion (HIC) scale (modified from Goldstein, 1972b, as previously reported, Olive et al., 2001; see Table 1). During these seizure assessments, each mouse was lifted by the tail and an observer blind to the treatment conditions of the mice evaluated the severity of the seizures.
Table 1.
Handling-Induced Convulsions Scale Used in the Present Study
| Score | Description |
|---|---|
| 7 | Severe tonic–clonic convulsions prior to tail lift with rapid onset and long duration |
| 6 | Severe tonic–clonic convulsion when lifted by tail with rapid onset and long duration |
| 5 | Tonic–clonic convulsion when lifted by tail; onset delayed by 1–2 s |
| 4 | Tonic convulsion when lifted by tail |
| 3 | Convulsion when lifted by tail and after spin |
| 2 | No convulsion when lifted by tail; tonic convulsion after spin |
| 1 | No convulsion or facial grimace when lifted by the tail; facial grimace after spin |
| 0 | No convulsion or facial grimace |
Drugs
For self-administration, ethanol (95% w/v) was diluted in distilled water to a concentration of 10% (v/v). Riluzole (Sigma, St Louis, MO) was suspended in cyclodextrin (20% w/v) solution which was the vehicle. Riluzole and the cyclodextrin vehicle were injected IP at a volume of 0.01 ml/g of body weight.
Data Analysis
Ethanol intake (g/kg) was estimated from body weight and the number of reinforcers delivered. For the acute riluzole treatment self-administration study, total ethanol intake was analyzed by 1-way repeated measures analysis of variance (RM ANOVA). Total session self-administration and the pattern of responding in 4-hour intervals were analyzed by 2-way RM ANOVA. For the chronic riluzole experiment, self-administration was averaged for each week of the 2 weeks of treatment (i.e., week 1 and week 2). For these studies, total session self-administration and the pattern of responding in 4-hour intervals for week 1 and week 2 were examined by a mixed factors ANOVA, with lever as the within subject factor and treatment (vehicle or riluzole) as the between subject factor. For the withdrawal seizure assessment study, a 3-way mixed factor ANOVA was used. Two-way ANOVAs were used to further explore significant interactions. Post hoc comparisons were conducted using Tukey's procedure.
Results
Ethanol Self-Administration
Acute Riluzole Administration
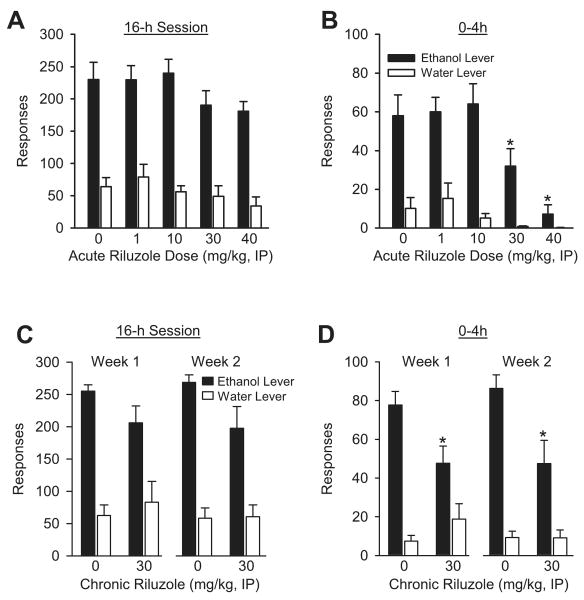
Riluzole significantly reduced total ethanol intake during the 16-hour sessions [F(4,39) = 3.79, p = 0.01; vehicle: 5.34 ± 0.7; 1 mg/kg: 5.03 ± 0.54; 10 mg/kg: 5.58 ± 0.53; 30 mg/kg: 4.10 ± 0.44; 40 mg/kg 3.94 ± 0.36 g/kg]; however, intake at each riluzole dose did not differ significantly from vehicle treatment. Responses on the ethanol and water levers for the 16-hour self-administration session are shown in Fig. 1A. There was a significant main effect of riluzole dose [F(4,36) = 7.66, p < 0.001; vehicle vs. 40 mg/kg], and a significant main effect of lever [F(1,9) = 44.17, p < 0.001]. The interaction was not significant.
Fig. 1.
(A) Mean (±SEM) responses on the ethanol (10% v/v) and water levers during the 16-hour self-administration session after acute riluzole treatment (N = 12). (B) Mean (±SEM) responses on the ethanol and water levers during the first 4-hour interval (0–4 hours) of the 16-hour session after acute riluzole treatment (N = 12). (C) Mean (±SEM) responses on the ethanol (10% v/v) and water levers during the 16-hour self-administration session after the first week (week 1) and the second week (week 2) of chronic riluzole (30 mg/kg) treatment. (N = 6/group). (D) Mean (±SEM) responses on the ethanol and water levers during the first 4-hour interval (0–4 hours) of the 16-hour session during week 1 and week 2 after chronic riluzole (30 mg/kg) treatment (N = 6/group). *Indicates significant difference from vehicle (Tukey, p < 0.05).
In an effort to relate self-administration to the known pharmacokinetics of riluzole, ethanol and water responses were examined in 4-hour intervals (see Table 2). Examination of the first 4 hours of the self-administration session showed a significant main effect of riluzole dose [F(4,36) = 11.53, p < 0.001], a significant main effect of lever [F(1,9) = 27.45, p < 0.001], and a significant interaction [F(4,36) = 6.55, p < 0.001; Fig. 1B]. Ethanol responding was significantly reduced by 30 and 40 mg/kg riluzole, probabilities <0.02. Water responding was not significantly altered by riluzole treatment but visual inspection of the data suggest a trend toward a reduction. For the other time intervals examined (8, 12, and 16 h), significant main effects of lever were observed with greater ethanol responses than water responses [8 h: F(1,9) = 39.52, p < 0.001; 12 h: F(1,9) = 22.57, p < 0.001; 16 h: F(1,9) = 24.12, p < 0.001]. Riluzole treatment did not alter responding at any of these time points (see Table 2).
Table 2.
Self-Administration (Responses) in 4-Hour Intervals After Acute Riluzole Treatment
| Riluzole dose (mg/kg, IP) | |||||
|---|---|---|---|---|---|
| 0 | 1 | 10 | 30 | 40 | |
| 0–4 hours | |||||
| Ethanol lever | 58.00 ± 10.78 | 60.00 ± 7.44 | 64.00 ± 10.49 | 31.91 ± 9.11* | 7.18 ± 4.86* |
| Water lever | 10.10 ± 5.59 | 15.27 ± 7.99 | 5.09 ± 2.44 | 0.82 ± 0.33 | 0.18 ± 0.12 |
| 4–8 hours | |||||
| Ethanol lever | 66.50 ± 11.59 | 68.82 ± 6.98 | 64.00 ± 8.14 | 59.45 ± 10.03 | 60.82 ± 8.69 |
| Water lever | 12.60 ± 4.54 | 20.82 ± 7.88 | 9.45 ± 2.43 | 10.82 ± 5.49 | 7.18 ± 4.73 |
| 8–12 hours | |||||
| Ethanol lever | 35.80 ± 5.98 | 35.27 ± 5.78 | 37.82 ± 5.97 | 39.27 ± 7.50 | 49.45 ± 7.55 |
| Water lever | 4.80 ± 1.39 | 9.18 ± 2.88 | 11.27 ± 4.10 | 11.09 ± 7.66 | 5.55 ± 4.12 |
| 12–16 hours | |||||
| Ethanol lever | 69.91 ± 10.23 | 65.52 ± 8.06 | 74.00 ± 8.62 | 59.73 ± 6.91 | 63.82 ± 6.53 |
| Water lever | 36.80 ± 6.70 | 33.64 ± 8.94 | 30.55 ± 9.21 | 26.36 ± 8.28 | 21.27 ± 11.67 |
Indicates significant difference from 0 (Tukey, p < 0.05).
Chronic Riluzole Administration
Total ethanol intake during the 16-hour sessions for the 2 weeks of chronic riluzole treatment did not differ (vehicle: 5.67 ± 0.24 g/kg; riluzole: 4.41 ± 0.66 g/kg). In order to determine if the chronic riluzole pretreatment regimen altered responding over time, the average of the ethanol and water responses from the first week of the treatment regimen were compared to the average of the ethanol and water responses from the second week of the treatment regimen for each group. Total ethanol and water lever responses during the 16-hour self-administration sessions on week 1 and week 2 of riluzole treatment are shown in Fig. 1C. For week 1 and week 2 significant main effects of lever were observed [week 1: F(1, 10) = 30.83, p < 0.001; week 2: F(1,10) = 43.34, p < 0.001], with greater responding on the ethanol lever. No significant main effects of riluzole dose were observed.
As in the acute riluzole study, self-administration was examined in 4-hour intervals. As shown in Fig. 1D, during the first 4 hour of the self-administration session, ethanol responding was significantly reduced by riluzole during week 1 [F(1,10) = 5.65, p = 0.04) and week 2 (F(1,10) = 10.94, p = 0.008]. A significant main effect of lever was also evident at both weeks [week 1: F(1,10) = 28.98 p < 0.001; week 2: F(1,10) = 45.31, p < 0.001]. A significant lever by treatment interaction was also present at both weeks [week 1: F(1,10) = 5.07, p = 0.048; week 2: F(1,10) = 5.07, p = 0.048]. During both weeks, riluzole treatment significantly reduced ethanol responses (probabilities <0.01), and did not alter water responses. Ethanol and water responses at the other time intervals examined (8, 12, and 16 hours) for weeks 1 and 2 are shown in Table 3. Main effects of lever were observed at each time interval with greater ethanol responses than water responses [week 1 to 8 hours: F(1,10) = 16.50, p = 0.002; 12 hours: F(1,10) = 44.30, p < 0.001; 16 hours: F(1,10) = 10.79, p = 0.008; week 2 to 8 hours: F(1,10) = 28.42, p < 0.001; 12 hours: F(1,10) = 49.59, p < 0.001; 16 hours: F(1,10) = 15.72, p = 0.003]. Riluzole treatment did not alter responding at any of these time points (see Table 3).
Table 3.
Self-Administration (Responses) in 4-Hour Intervals After Chronic Riluzole Treatment
| Week 1 | Week 2 | |||
|---|---|---|---|---|
| Riluzole dose (mg/kg, IP) | Riluzole dose (mg/kg, IP) | |||
| 0 | 30 | 0 | 30 | |
| 0–4 hours | ||||
| Ethanol lever | 77.75 ± 7.04 | 47.68 ± 8.84* | 86.32 ± 7.01 | 47.57 ± 11.84* |
| Water lever | 7.46 ± 3.01 | 18.85 ± 7.97 | 9.29 ± 3.24 | 9.17 ± 4.03 |
| 4–8 hours | ||||
| Ethanol lever | 78.58 ± 2.83 | 65.99 ± 9.98 | 78.75 ± 4.42 | 58.13 ± 10.99 |
| Water lever | 7.75 ± 1.78 | 37.06 ± 15.99 | 9.67 ± 2.07 | 23.75 ± 11.27 |
| 8–12 hours | ||||
| Ethanol lever | 43.08 ± 4.24 | 32.60 ± 6.65 | 41.33 ± 4.80 | 36.29 ± 4.61 |
| Water lever | 6.13 ± 1.44 | 8.99 ± 3.13 | 6.75 ± 1.78 | 11.21 ± 3.87 |
| 12–16 hours | ||||
| Ethanol lever | 55.88 ± 5.90 | 59.94 ± 4.52 | 62.29 ± 5.95 | 55.67 ± 7.64 |
| Water lever | 40.96 ± 16.04 | 17.93 ± 6.70 | 32.33 ± 12.93 | 16.25 ± 5.76 |
Indicates significant difference from 0 (Tukey, p < 0.05).
Ethanol Withdrawal Seizures
Average ethanol intake for the first 2 days of liquid diet (2.4%) was 15.65 ± 0.28 g/kg. Ethanol intake was consistent across the 16 days of exposure to 4.8% ethanol (day 1 of 4.8%: 22.51 ± 0.42 g/kg; day 16 of 4.8% 22.4 ± 0.42 g/kg). The 2 groups (vehicle and riluzole) did not differ on ethanol intake (g/kg per day) across the liquid diet exposure. On the final day of liquid diet exposure ethanol intake for the vehicle group was 22.57 ± 0.7 g/kg and for the riluzole group was 22.26 ± 0.55 g/kg. The time course of withdrawal severity is shown in Fig. 2. The average HIC score during withdrawal was significantly higher for the ethanol-fed mice [F(1,32) = 6.88, p = 0.01] than the control-fed mice. A 3-way mixed design ANOVA showed significant interactions between time and diet type [F(3,96) = 3.02, p = 0.03], and time, diet, and riluzole treatment [F(3,96) = 2.95, p = 0.04]. In the ethanol-fed mice, a subsequent 2-way repeated measures ANOVA showed a significant interaction between time and riluzole treatment [F(3,46) = 3.41, p = 0.03], with a significant riluzole-induced reduction in HIC scores 2 hours following diet removal, p = 0.01. Area under the withdrawal curve after ethanol removal did not differ after riluzole treatment (mean ± SEM: vehicle—11.33 ± 3.12; riluzole—10.20 ± 3.55). In the control-fed mice, a reduction in muscle tone and ataxia was observed after riluzole treatment which likely contributed to a significant main effect of riluzole treatment [two-way RM ANOVA: F(1,17) = 8.05, p = 0.01], with overall lower HIC scores after riluzole treatment, p = 0.01. This effect was likely due to the lack of any withdrawal-like behaviors after riluzole treatment (i.e., scores of 0).
Fig. 2.
Mean (±SEM) Handling-Induced Convulsion (HIC) score in mice maintained on control or ethanol (4.8% v/v)-containing liquid diet. Liquid diet was removed at 07:30 hour and all mice were administered vehicle or 30 mg/kg riluzole. Assessments were made 2, 4, 6, and 8 hours after diet removal. Control/Vehicle (n = 9); Control/Riluzole (n = 10); Ethanol/Vehicle (n = 8); Ethanol/Riluzole (n = 10). *Indicates significant difference from Ethanol/Vehicle (Tukey, p < 0.05).
Discussion
The goal of this study was to conduct a preclinical evaluation of the drug riluzole for potential use as a therapeutic agent to treat problems associated with alcoholism. First, the effects of riluzole on ethanol-reinforced responding by C57BL/6J mice were evaluated after acute and chronic treatment to assess the efficacy of riluzole in reducing the maintenance of ethanol self-administration. Second, the ability of acute riluzole treatment to blunt ethanol-induced withdrawal seizures was also evaluated.
Ethanol Self-Administration
In general, riluzole did not produce significant changes in ethanol self-administration when total responding during the 16-hour session was examined. In healthy human subjects plasma levels of riluzole peak at approximately 1 to 2 hours after oral administration, gradually decline by 4 hours and remain at stable levels until about 12 hours after administration (Le Liboux et al., 1999; Schwenkreis et al., 2000). The pharmacokinetic profile has not been extensively examined in rodents. However, after IP administration of riluzole (30 mg/kg) in the mouse, concentration of the compound in plasma peaks at 20 minutes and in brain peaks at 20 to 80 minutes after injection and both levels are significantly decreased by 4 hours (Hockly et al., 2006). This pattern of drug clearance suggests that riluzole may differentially affect self-administration behavior throughout the 16-hour session. As such, self-administration behavior was examined during the entire 16 hour session and in shorter time intervals (4 hours). During the initial 4 hours of the self-administration session, ethanol responses were significantly reduced by the 2 highest riluzole doses tested (30 and 40 mg/kg). Further, riluzole did not affect ethanol self-administration at any other time interval examined. This pattern of results suggests that the reduction in ethanol self-administration during the early part of the session most likely corresponds to peak riluzole levels.
Chronic treatment with riluzole produced similar reductions in ethanol self-administration as acute treatment. That is, overall ethanol and water responding across the 16-hour session showed no change. However, examination of responding in 4-hour intervals showed a riluzole-induced reduction in ethanol self-administration during the first 4 hours of the session. Further, week 1 and week 2 of riluzole treatment produced similar reductions in ethanol self-administration during the first 4 hours of the session, suggesting no tolerance to riluzole's effects. Further, this suggests that the compound is efficacious at reducing ethanol self-administration after repeated administration, which would be a desirable profile for a potential therapeutic agent.
The behavioral mechanism(s) by which riluzole reduced ethanol self-administration is not clear. Riluzole does not produce stimulus properties similar to ethanol (Hundt et al., 1998), which suggests that the riluzole-induced reductions in ethanol responding are not likely a result of drug substitution. However, riluzole has been shown to have rewarding properties as measured by conditioned place preference procedures (Tzschentke and Schmidt, 1998), suggesting that the drug may produce distinct stimulus properties itself. Indeed, in healthy human subjects, riluzole has been shown to potentiate the subjective properties of amphetamine and produce some amphetamine-like subjective effects (Sofuoglu et al., 2008). Thus, while not producing “ethanol-like” stimulus effects per se riluzole may have produced a distinct subjective or rewarding state that competed/interfered with motivation to self-administer ethanol. Potential abuse liability of riluzole needs to be examined in future studies. Another possible behavioral explanation for the reduction in ethanol self-administration is that riluzole produced a motor impairment. Indeed, riluzole has been shown to decrease spontaneous locomotor activity in naïve rodents (Itzhak and Martin, 2000; Kretschmer et al., 1998). In both the acute study and the chronic treatment study, water responses (total session and 4-hour intervals) were unaffected by riluzole treatment. Responding on an alternate lever (i.e., water lever) typically serves as an index of non-specific motor effects. Thus, the absence of a reduction in water lever responses would make a riluzole-induced motor impairment explanation less likely; however, some issues are raised regarding interpretation of the lack of a riluzole effect on water responses. First, response rate on the water lever was relatively low, which makes it difficult to detect a statistically significant reduction. Second, in the acute study, riluzole produced a greater percent decrease in responding on the water lever than the ethanol lever at 30 and 40 mg/kg, suggesting the low baseline responding did in fact contribute to the lack of statistical significance and a motor reduction may indeed have contributed to the reduction in ethanol responses. Interestingly, however, this was not evident in the chronic riluzole administration study. Previous work has found that mice with ethanol self-administration experience similar to that in the present study are less sensitive to the sedative properties of GABAergic modulation than ethanol-inexperienced mice (Besheer et al., 2004). Thus, given that riluzole has been shown to produce motor impairments in ethanol naïve rodents the effects may not be evident in animals with a history of ethanol self-administration. However, given that the possibility of a motor impairment was not tested directly in these mice this explanation cannot be entirely dismissed.
The riluzole-induced reduction in ethanol self-administration is consistent with studies in which modulators of NMDA/glutamatergic (Bienkowski et al., 1999; Piasecki et al., 1998; Rassnick et al., 1992) and GABAergic systems (Boyle et al., 1993; Ford et al., 2005; Harvey et al., 2002; Hodge et al., 1996; Maccioni et al., 2005; Smith et al., 1992) reduce ethanol self-administration. Further, reductions in ethanol intake using a two-bottle drinking procedure (Besheer et al., 2006) and ethanol self-administration using a similar 16-hour self-administration procedure have been observed in mice lacking PKCε (Olive et al., 2000). Importantly, the general inhibition of PKC by riluzole may have altered sensitivity to ethanol. For example, mice lacking PKCε are more sensitive to the behavioral and biochemical effects of ethanol than wildtype littermates (Hodge et al., 1999).
Ethanol Withdrawal Seizures
Riluzole has anticonvulsant properties and is effective at reducing seizures induced by excitatory amino acids and GABA antagonists (e.g., bicuculline; see Doble, 1996). Riluzole has been shown to reduce the intensity of the withdrawal syndrome (jumping, paw tremors, and body shakes) in morphine-dependent mice (Sepulveda et al., 1999); but had no effect on convulsions induced by an acute cocaine injection (Brackett et al., 2000). This could suggest differential efficacy of riluzole's anticonvulsive effects after chronic and acute drug exposure; however, this suggestion will clearly need further evaluation.
Seizures induced by ethanol withdrawal are generally a result of hyperexcitability in the central nervous system due to changes in GABAergic and glutamatergic systems (Littleton, 1998). Benzodiazepines and other positive modulators of GABAA receptors are commonly used in the suppression of ethanol withdrawal seizures (Goldstein, 1972a; Nutt et al., 1989; Romach and Sellers, 1991); however, NMDA antagonists also show efficacy in attenuating seizures (Grant et al., 1990; Kotlinska and Liljequist, 1996). In the present work, riluzole (30 mg/kg) reduced the severity of HICs during ethanol withdrawal. Interestingly, the blunting of ethanol withdrawal seizures by riluzole was only evident during the first 2 hours of session, which likely parallels peak riluzole levels as previously discussed. Further, it is of interest to note that the control-fed (i.e., ethanol-naïve) mice showed ataxia and reduced muscle tone during these observations; loss of righting reflex was not evaluated in these animals, however, previous work has shown that riluzole can induce a loss of righting reflex (Irifune et al., 2007; Mantz et al., 1992). Overall, the ability of riluzole to inhibit ethanol withdrawal symptoms is consistent with its actions on GABA and NMDA systems.
In this study, the severity of ethanol withdrawal seizures in vehicle treated C57BL/6J mice peaked 2-hour after removal of the ethanol-containing liquid diet. HIC scores at this time point were comparable to what we have previously observed in mice of a hybrid 129SvJae × C57BL/6J genetic background under similar experimental conditions (Olive et al., 2000). However, seizure severity did not increase as a function of time after ethanol removal, which is in contrast to published literature showing the emergence of withdrawal-induced seizures 4 to 8 hours after diet removal (Chan et al., 1981; Goldstein and Arnold, 1976; Olive et al., 2000). One potential explanation for this result was the use of C57BL/6 mice, which are relatively insensitive to ethanol withdrawal seizures (Crabbe et al., 1983). Another explanation is that testing may have been conducted on the descending limb of the HIC curve. This suggests that liquid diet consumption peaked during the early phase of the dark cycle (Hunter et al., 1975; Jelic et al., 1998), which resulted in a progression of the withdrawal syndrome prior to measurement. Further, the area under the withdrawal curve did not differ between the 2 ethanol groups (e.g., withdrawal time course was shifted in the 2 groups) supporting the suggestion that ethanol diet exposed animals were undergoing withdrawal prior to measurement. It will be important to determine the efficacy of riluzole to inhibit ethanol withdrawal seizures in seizure prone mice (i.e., DBA/2J) or under different treatment conditions that allow precise temporal regulation of ethanol exposure and withdrawal.
Another topic that merits discussion is that blood ethanol levels were not evaluated when the ethanol liquid diet was removed from the home cage. Blood samples were not collected at this time due to concerns that procedural stress may interfere with the study. Thus, the treatment groups (vehicle or riluzole) were equated and assigned based on ethanol intake on the final 24-hour and across the liquid diet exposure. While there is no reason to expect a different pattern of liquid diet consumption between the groups (i.e., prior to riluzole treatment), there is the possibility that the riluzole-treated group had lower blood ethanol levels at the time of riluzole administration, which may have contributed to the drug effect.
Conclusions
The results of the present work show that riluzole can selectively reduce ethanol self-administration after acute and chronic administration, and reduce the severity of ethanol withdrawal seizures. Based on this initial study, additional preclinical research is warranted to further assess whether riluzole could be potentially useful as a clinical therapeutic for alcohol use disorders. Although it is unclear if riluzole would be more efficacious than current FDA approved medications for alcohol use disorders, the drug's broad spectrum of effects on glutamate, GABA, and PKC make it an interesting candidate for further study. It will also be important to evaluate potential interactions between riluzole and ethanol, assess effects of riluzole on relapse-like behavior, and determine the side effect profile (i.e., potential motor inhibition) and efficacy of riluzole treatment after a history of prolonged ethanol exposure. Overall, the findings of the present work suggest that riluzole, a drug presently used in the treatment of ALS, may have potential utility in the treatment of problems associated with chronic drinking and ethanol withdrawal symptoms.
Acknowledgments
This work was supported by grants AA014983 and AA011605 from the National Institute on Alcohol Abuse and Alcoholism.
References
- Anton RF. Pharmacologic approaches to the management of alcoholism. J Clin Psychiatry. 2001;62(Suppl 20):11–17. [PubMed] [Google Scholar]
- Besheer J, Cox AA, Hodge CW. Coregulation of ethanol discrimination by the nucleus accumbens and amygdala. Alcohol Clin Exp Res. 2003;27:450–456. doi: 10.1097/01.ALC.0000057036.64169.C1. [DOI] [PubMed] [Google Scholar]
- Besheer J, Lepoutre V, Hodge CW. GABA(B) receptor agonists reduce operant ethanol self-administration and enhance ethanol sedation in C57BL/6J mice. Psychopharmacology (Berl) 2004;174:358–366. doi: 10.1007/s00213-003-1769-3. [DOI] [PubMed] [Google Scholar]
- Besheer J, Lepoutre V, Mole B, Hodge CW. GABAA receptor regulation of voluntary ethanol drinking requires PKCepsilon. Synapse. 2006;60:411–419. doi: 10.1002/syn.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski P, Koros E, Kostowski W, Danysz W. Effects of N-methyl-D-aspartate receptor antagonists on reinforced and nonreinforced responding for ethanol in rats. Alcohol. 1999;18:131–137. doi: 10.1016/s0741-8329(98)00075-5. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Krzascik P, Koros E, Kostowski W, Scinska A, Danysz W. Effects of a novel uncompetitive NMDA receptor antagonist, MRZ 2/579 on ethanol self-administration and ethanol withdrawal seizures in the rat. Eur J Pharmacol. 2001;413:81–89. doi: 10.1016/s0014-2999(01)00743-9. [DOI] [PubMed] [Google Scholar]
- Boyle AE, Segal R, Smith BR, Amit Z. Bidirectional effects of GABAergic agonists and antagonists on maintenance of voluntary ethanol intake in rats. Pharmacol Biochem Behav. 1993;46:179–182. doi: 10.1016/0091-3057(93)90338-t. [DOI] [PubMed] [Google Scholar]
- Brackett RL, Pouw B, Blyden JF, Nour M, Matsumoto RR. Prevention of cocaine-induced convulsions and lethality in mice: effectiveness of targeting different sites on the NMDA receptor complex. Neuropharmacology. 2000;39:407–418. doi: 10.1016/s0028-3908(99)00151-3. [DOI] [PubMed] [Google Scholar]
- Chan AW, Leong FW, Schanley DL, Howe SM. Alcohol withdrawal reactions after chronic intake of chlordiazepoxide and ethanol. Pharmacol Biochem Behav. 1981;15:185–189. doi: 10.1016/0091-3057(81)90175-1. [DOI] [PubMed] [Google Scholar]
- Chester JA, Cunningham CL. GABA(A) receptors modulate ethanol-induced conditioned place preference and taste aversion in mice. Psychopharmacology (Berl) 1999;144:363–372. doi: 10.1007/s002130051019. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Jr, Young ER, Kosobud A. Genetic correlations with ethanol withdrawal severity. Pharmacol Biochem Behav. 1983;18(Suppl 1):541–547. doi: 10.1016/0091-3057(83)90233-2. [DOI] [PubMed] [Google Scholar]
- Davis KM, Wu JY. Role of glutamatergic and GABAergic systems in alcoholism. J Biomed Sci. 2001;8:7–19. doi: 10.1007/BF02255966. [DOI] [PubMed] [Google Scholar]
- Debono MW, Le Guern J, Canton T, Doble A, Pradier L. Inhibition by riluzole of electrophysiological responses mediated by rat kainate and NMDA receptors expressed in Xenopus oocytes. Eur J Pharmacol. 1993;235:283–289. doi: 10.1016/0014-2999(93)90147-a. [DOI] [PubMed] [Google Scholar]
- Doble A. The pharmacology and mechanism of action of riluzole. Neurology. 1996;47(6 Suppl 4):S233–S241. doi: 10.1212/wnl.47.6_suppl_4.233s. [DOI] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Phillips TJ, Finn DA. Neurosteroid modulators of GABA(A) receptors differentially modulate ethanol intake patterns in male C57BL/6J mice. Alcohol Clin Exp Res. 2005;29:1630–1640. doi: 10.1097/01.alc.0000179413.82308.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DB. An animal model for testing effects of drugs on alcohol withdrawal reactions. J Pharmacol Exp Ther. 1972a;183:14–22. [PubMed] [Google Scholar]
- Goldstein DB. Relationship of alcohol dose to intensity of withdrawal signs in mice. J Pharmacol Exp Ther. 1972b;180:203–215. [PubMed] [Google Scholar]
- Goldstein DB, Arnold VW. Drinking patterns as predictors of alcohol withdrawal reactions in DBA/2J mice. J Pharmacol Exp Ther. 1976;199:408–414. [PubMed] [Google Scholar]
- Grant KA, Knisely JS, Tabakoff B, Barrett JE, Balster RL. Ethanol-like discriminative stimulus effects of non-competitive n-methyl-d-aspartate antagonists. Behav Pharmacol. 1991;2:87–95. [PubMed] [Google Scholar]
- Grant KA, Lovinger DM. Cellular and behavioral neurobiology of alcohol: receptor-mediated neuronal processes. Clin Neurosci. 1995;3:155–164. [PubMed] [Google Scholar]
- Grant KA, Valverius P, Hudspith M, Tabakoff B. Ethanol withdrawal seizures and the NMDA receptor complex. Eur J Pharmacol. 1990;176:289–296. doi: 10.1016/0014-2999(90)90022-x. [DOI] [PubMed] [Google Scholar]
- Harvey SC, Foster KL, McKay PF, Carroll MR, Seyoum R, Woods JE, II, Grey C, Jones CM, McCane S, Cummings R, Mason D, Ma C, Cook JM, June HL. The GABA(A) receptor alpha1 subtype in the ventral pallidum regulates alcohol-seeking behaviors. J Neurosci. 2002;22:3765–3775. doi: 10.1523/JNEUROSCI.22-09-03765.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Benz A, Fu T, Wang M, Covey DF, Zorumski CF, Mennerick S. Neuroprotective agent riluzole potentiates postsynaptic GABA(A) receptor function. Neuropharmacology. 2002;42:199–209. doi: 10.1016/s0028-3908(01)00175-7. [DOI] [PubMed] [Google Scholar]
- Hockly E, Tse J, Barker AL, Moolman DL, Beunard JL, Revington AP, Holt K, Sunshine S, Moffitt H, Sathasivam K, Woodman B, Wanker EE, Lowden PA, Bates GP. Evaluation of the benzothiazole aggregation inhibitors riluzole and PGL-135 as therapeutics for Huntington's disease. Neurobiol Dis. 2006;21:228–236. doi: 10.1016/j.nbd.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Chappelle AM, Samson HH. GABAergic transmission in the nucleus accumbens is involved in the termination of ethanol self-administration in rats. Alcohol Clin Exp Res. 1995;19:1486–1493. doi: 10.1111/j.1530-0277.1995.tb01012.x. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Cox AA. The discriminative stimulus effects of ethanol are mediated by NMDA and GABA(A) receptors in specific limbic brain regions. Psychopharmacology (Berl) 1998;139:95–107. doi: 10.1007/s002130050694. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Haraguchi M, Chappelle AM, Samson HH. Effects of ventral tegmental microinjections of the GABAA agonist muscimol on self-administration of ethanol and sucrose. Pharmacol Biochem Behav. 1996;53:971–977. doi: 10.1016/0091-3057(95)02146-9. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, Olive MF, Wang D, Sanchez-Perez AM, Messing RO. Supersensitivity to allosteric GABA(A) receptor modulators and alcohol in mice lacking PKCepsilon. Nat Neurosci. 1999;2:997–1002. doi: 10.1038/14795. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Raber J, McMahon T, Walter H, Sanchez-Perez AM, Olive MF, Mehmert K, Morrow AL, Messing RO. Decreased anxiety-like behavior, reduced stress hormones, and neurosteroid supersensitivity in mice lacking protein kinase Cepsilon. J Clin Invest. 2002;110:1003–1010. doi: 10.1172/JCI15903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundt W, Holter SM, Spanagel R. Discriminative stimulus effects of glutamate release inhibitors in rats trained to discriminate ethanol. Pharmacol Biochem Behav. 1998;59:691–695. doi: 10.1016/s0091-3057(97)00463-2. [DOI] [PubMed] [Google Scholar]
- Hunter BE, Riley JN, Walker DW. Ethanol dependence in the rat: a parametric analysis. Pharmacol Biochem Behav. 1975;3:619–629. doi: 10.1016/0091-3057(75)90183-5. [DOI] [PubMed] [Google Scholar]
- Irifune M, Kikuchi N, Saida T, Takarada T, Shimizu Y, Endo C, Morita K, Dohi T, Sato T, Kawahara M. Riluzole, a glutamate release inhibitor, induces loss of righting reflex, antinociception, and immobility in response to noxious stimulation in mice. Anesth Analg. 2007;104:1415–1421. doi: 10.1213/01.ane.0000263267.04198.36. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL. Effect of riluzole and gabapentin on cocaine- and methamphetamine-induced behavioral sensitization in mice. Psychopharmacology. 2000;151:226–233. doi: 10.1007/s002130000394. [DOI] [PubMed] [Google Scholar]
- Jehle T, Bauer J, Blauth E, Hummel A, Darstein M, Freiman TM, Feuerstein TJ. Effects of riluzole on electrically evoked neurotransmitter release. Br J Pharmacol. 2000;130:1227–1234. doi: 10.1038/sj.bjp.0703424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelic P, Shih MF, Taberner PV. Diurnal variation in plasma ethanol levels of TO and CBA mice on chronic ethanol drinking or ethanol liquid diet schedules. Psychopharmacology (Berl) 1998;138:143–150. doi: 10.1007/s002130050656. [DOI] [PubMed] [Google Scholar]
- June HL, Colker RE, Domangue KR, Perry LE, Hicks LH, June PL, Lewis MJ. Ethanol self-administration in deprived rats: effects of Ro15-4513 alone, and in combination with flumazenil (Ro15-1788) Alcohol Clin Exp Res. 1992;16:11–16. doi: 10.1111/j.1530-0277.1992.tb00628.x. [DOI] [PubMed] [Google Scholar]
- Khanna JM, Wu PH, Weiner J, Kalant H. NMDA antagonist inhibits rapid tolerance to ethanol. Brain Res Bull. 1991;26:643–645. doi: 10.1016/0361-9230(91)90109-w. [DOI] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytièa P, Merlo-Pich E, Weiss F. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22:3–9. [PubMed] [Google Scholar]
- Kotlinska J, Liljequist S. Oral administration of glycine and polyamine receptor antagonists blocks ethanol withdrawal seizures. Psychopharmacology (Berl) 1996;127:238–244. [PubMed] [Google Scholar]
- Kretschmer BD, Kratzer U, Schmidt WJ. Riluzole, a glutamate release inhibitor, and motor behavior. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:181–190. doi: 10.1007/pl00005241. [DOI] [PubMed] [Google Scholar]
- Le Liboux A, Cachia JP, Kirkesseli S, Gautier JY, Guimart C, Montay G, Peeters PA, Groen E, Jonkman JH, Wemer J. A comparison of the pharmacokinetics and tolerability of riluzole after repeat dose administration in healthy elderly and young volunteers. J Clin Pharmacol. 1999;39:480–486. [PubMed] [Google Scholar]
- Littleton J. Neurochemical mechanisms underlying alcohol withdrawal. Alcohol Health Res World. 1998;22:13–24. [PMC free article] [PubMed] [Google Scholar]
- Maccioni P, Serra S, Vacca G, Orru A, Pes D, Agabio R, Addolorato G, Carai MA, Gessa GL, Colombo G. Baclofen-induced reduction of alcohol reinforcement in alcohol-preferring rats. Alcohol. 2005;36:161–168. doi: 10.1016/j.alcohol.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Malgouris C, Bardot F, Daniel M, Pellis F, Rataud J, Uzan A, Blanchard JC, Laduron PM. Riluzole, a novel antiglutamate, prevents memory loss and hippocampal neuronal damage in ischemic gerbils. J Neurosci. 1989;9:3720–3727. doi: 10.1523/JNEUROSCI.09-11-03720.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantz J, Cheramy A, Thierry AM, Glowinski J, Desmonts JM. Anesthetic properties of riluzole (54274 RP), a new inhibitor of glutamate neurotransmission. Anesthesiology. 1992;76:844–848. doi: 10.1097/00000542-199205000-00023. [DOI] [PubMed] [Google Scholar]
- Mantz J, Laudenbach V, Lecharny JB, Henzel D, Desmonts JM. Riluzole, a novel antiglutamate, blocks GABA uptake by striatal synaptosomes. Eur J Pharmacol. 1994;257:R7–R8. doi: 10.1016/0014-2999(94)90716-1. [DOI] [PubMed] [Google Scholar]
- Martin D, Thompson MA, Nadler JV. The neuroprotective agent riluzole inhibits release of glutamate and aspartate from slices of hippocampal area CA1. Eur J Pharmacol. 1993;250:473–476. doi: 10.1016/0014-2999(93)90037-i. [DOI] [PubMed] [Google Scholar]
- Mihic SJ, Kalant H, Liu JF, Wu PH. Role of the gamma-aminobutyric acid receptor/chloride channel complex in tolerance to ethanol and cross-tolerance to diazepam and pentobarbital. J Pharmacol Exp Ther. 1992;261:108–113. [PubMed] [Google Scholar]
- Mizoule J, Meldrum B, Mazadier M, Croucher M, Ollat C, Uzan A, Legrand JJ, Gueremy C, Le Fur G. 2-Amino-6-trifluoromethoxy benzothiazole, a possible antagonist of excitatory amino acid neurotransmission—I. Anticonvulsant properties. Neuropharmacology. 1985;24:767–773. doi: 10.1016/0028-3908(85)90011-5. [DOI] [PubMed] [Google Scholar]
- Morrisett RA, Rezvani AH, Overstreet D, Janowsky DS, Wilson WA, Swartzwelder HS. MK-801 potently inhibits alcohol withdrawal seizures in rats. Eur J Pharmacol. 1990;176:103–105. doi: 10.1016/0014-2999(90)90138-v. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Devaud LL, Bucci D, Smith FD. GABAA and NMDA receptor subunit mRNA expression in ethanol dependent rats. Alcohol Alcohol Suppl. 1994;2:89–95. [PubMed] [Google Scholar]
- Munro G, Erichsen HK, Mirza NR. Pharmacological comparison of anticonvulsant drugs in animal models of persistent pain and anxiety. Neuropharmacology. 2007;53:609–618. doi: 10.1016/j.neuropharm.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Nevo I, Hamon M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. Neurochem Int. 1995;26:305–336. doi: 10.1016/0197-0186(94)00139-l. [DOI] [PubMed] [Google Scholar]
- Nie H, Janak PH. Comparison of reinstatement of ethanol- and sucrose-seeking by conditioned stimuli and priming injections of allopregnanolone after extinction in rats. Psychopharmacology (Berl) 2003;168:222–228. doi: 10.1007/s00213-003-1468-0. [DOI] [PubMed] [Google Scholar]
- Noh KM, Hwang JY, Shin HC, Koh JY. A novel neuroprotective mechanism of riluzole: direct inhibition of protein kinase C. Neurobiol Dis. 2000;7:375–383. doi: 10.1006/nbdi.2000.0297. [DOI] [PubMed] [Google Scholar]
- Nutt D, Adinoff B, Linnoila M. Benzodiazepines in the treatment of alcoholism. Recent Dev Alcohol. 1989;7:283–313. doi: 10.1007/978-1-4899-1678-5_15. [DOI] [PubMed] [Google Scholar]
- Olive MF, Mehmert KK, Messing RO, Hodge CW. Reduced operant ethanol self-administration and in vivo mesolimbic dopamine responses to ethanol in PKCepsilon-deficient mice. Eur J Neurosci. 2000;12:4131–4140. doi: 10.1046/j.1460-9568.2000.00297.x. [DOI] [PubMed] [Google Scholar]
- Olive MF, Mehmert KK, Nannini MA, Camarini R, Messing RO, Hodge CW. Reduced ethanol withdrawal severity and altered withdrawal-induced c-fos expression in various brain regions of mice lacking protein kinase C-epsilon. Neuroscience. 2001;103:171–179. doi: 10.1016/s0306-4522(00)00566-2. [DOI] [PubMed] [Google Scholar]
- Piasecki J, Koros E, Dyr W, Kostowski W, Danysz W, Bienkowski P. Ethanol-reinforced behaviour in the rat: effects of uncompetitive NMDA receptor antagonist, memantine. Eur J Pharmacol. 1998;354:135–143. doi: 10.1016/s0014-2999(98)00442-7. [DOI] [PubMed] [Google Scholar]
- Pratt J, Rataud J, Bardot F, Roux M, Blanchard JC, Laduron PM, Stutzmann JM. Neuroprotective actions of riluzole in rodent models of global and focal cerebral ischaemia. Neurosci Lett. 1992;140:225–230. doi: 10.1016/0304-3940(92)90108-j. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Pulvirenti L, Koob GF. Oral ethanol self-administration in rats is reduced by the administration of dopamine and glutamate receptor antagonists into the nucleus accumbens. Psychopharmacology (Berl) 1992;109:92–98. doi: 10.1007/BF02245485. [DOI] [PubMed] [Google Scholar]
- Romach MK, Sellers EM. Management of the alcohol withdrawal syndrome. Annu Rev Med. 1991;42:323–340. doi: 10.1146/annurev.me.42.020191.001543. [DOI] [PubMed] [Google Scholar]
- Romettino S, Lazdunski M, Gottesmann C. Anticonvulsant and sleep-waking influences of riluzole in a rat model of absence epilepsy. Eur J Pharmacol. 1991;199:371–373. doi: 10.1016/0014-2999(91)90503-i. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol-maintained behavior: a comparison of animal models and their implication to human drinking. In: Thompson T, Dews PB, Barrett JE, editors. Advanced Behavioral Pharmacology, Vol. 6, Neurobehavioral Pharmacology. Lawrence Erlbaum; Hillsdale, NJ: 1987. pp. 221–248. [Google Scholar]
- Schwenkreis P, Liepert J, Witscher K, Fischer W, Weiller C, Malin JP, Tegenthoff M. Riluzole suppresses motor cortex facilitation in correlation to its plasma level. A study using transcranial magnetic stimulation. Exp Brain Res. 2000;135:293–299. doi: 10.1007/s002210000532. [DOI] [PubMed] [Google Scholar]
- Sepulveda J, Astorga JG, Contreras E. Riluzole decreases the abstinence syndrome and physical dependence in morphine-dependent mice. Eur J Pharmacol. 1999;379:59–62. doi: 10.1016/s0014-2999(99)00503-8. [DOI] [PubMed] [Google Scholar]
- Shearman MS, Sekiguchi K, Nishizuka Y. Modulation of ion channel activity: a key function of the protein kinase C enzyme family. Pharmacol Rev. 1989;41:211–237. [PubMed] [Google Scholar]
- Smith BR, Robidoux J, Amit Z. GABAergic involvement in the acquisition of voluntary ethanol intake in laboratory rats. Alcohol Alcohol. 1992;27:227–231. [PubMed] [Google Scholar]
- Sofuoglu M, Waters AJ, Mooney M, Kosten T. Riluzole and D-amphetamine interactions in humans. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:16–22. doi: 10.1016/j.pnpbp.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka C, Nishizuka Y. The protein kinase C family for neuronal signaling. Annu Rev Neurosci. 1994;17:551–567. doi: 10.1146/annurev.ne.17.030194.003003. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Blockade of morphine- and amphetamine-induced conditioned place preference in the rat by riluzole. Neurosci Lett. 1998;242:114–116. doi: 10.1016/s0304-3940(98)00023-8. [DOI] [PubMed] [Google Scholar]
- Wagner ML, Landis BE. Riluzole: a new agent for amyotrophic lateral sclerosis. Ann Pharmacother. 1997;31:738–744. doi: 10.1177/106002809703100614. [DOI] [PubMed] [Google Scholar]