Abstract
Enhanced chemiluminescence (ECL) detection can significantly enhance the sensitivity of immunoassays but often requires expensive and complex detectors. The need for these detectors limits broader use of ECL in immunoassay applications. To make ECL more practical for immunoassays, we utilize a simple cooled charge-coupled device (CCD) detector combined with carbon nanotubes (CNTs) for primary antibody immobilization to develop a simple and portable point-of-care immunosensor. This combination of ECL, CNT, and CCD detector technologies is used to improve the detection of Staphylococcal enterotoxin B (SEB) in food. Anti-SEB primary antibodies were immobilized onto the CNT surface, and the antibody–nanotube mixture was immobilized onto a polycarbonate surface. SEB was then detected by an ELISA assay on the CNT–polycarbonate surface with an ECL assay. SEB in buffer, soy milk, apple juice, and meat baby food was assayed with a LOD of 0.01 ng/mL using our CCD detector, a level similar to the detection limit obtained with a fluorometric detector when using the CNTs. This level is far more sensitive than the conventional ELISA, which has a LOD of ~1 ng/mL. Our simple, versatile, and inexpensive point-of-care immunosensor combined with the CNT-ECL immunoassay method described in this work can also be used to simplify and increase sensitivity for many other types of diagnostics and detection assays.
The enhanced chemiluminescence (ECL) reaction is a widely used detection method for many biological assays. ECL improves the sensitivity of immunoassays and has been used for enzyme-linked immunosorbent assays (ELISA) detection of several microbial toxins (e.g., ricin has been detected in beverages with a sensitivity of 0.04 ng/mL1). ECL-ELISA has also been used for the detection of fumonisin B1 in food samples with a limit of detection (LOD) of 0.09 ng/mL, which is ~10 times more sensitive than that of colorimetric ELISA using the same antibody and horseradish peroxidase (HRP) conjugate.2 Similarly, the ECL immunoassay has been applied to the detection of botulinum type B neurotoxin with a LOD of 0.39–0.78 ng/mL versus a LOD of 1.56 ng/mL for colorimetric ELISA.3 An ECL assay where ruthenylated antibodies were used for the detection of the ricin achieved a LOD of 0.05 ng/mL, which is 10 times more sensitive than colorimetric ELISA utilizing the same pair of antibodies with an alkaline phosphatase conjugate for signal generation.4
In these and other studies, the LOD of ECL for microbial toxins is generally in the range of ~0.09–0.4 ng/mL. However, in order to achieve this range of ECL detection in ELISA assays, relatively complex and expensive fluorometric- or electrochemical-based detectors found mainly in research settings are required. These detectors might be an impediment to broader applications of the ECL technology and the realization of its potential in assay applications especially in clinical, food analysis, and point-of-care settings.
A common approach to enhancing ECL is to increase the signal strength and duration by utilizing the HRP enzyme tethered to a ligand (e.g., antibody, oligonucleotide, aptamer, etc.).5–7 The short-lived nature of peroxidase substrates has been a drawback for ECL detection, so new chemiluminescent substrates for peroxidase that glow stably for long periods of time (e.g., more than 9 h) have enabled sensitive ECL detection.8,9 The longer illuminination times for chemiluminescence have been used for a variety of membrane-based molecular biology methods (e.g., Southern blot, Western blots, etc.). It can be measured by fluorometers, but it is also ideal for use with charge-coupled device (CCD) detectors because they can be exposed to the ECL illumination for long periods of time with low levels of background noise to achieve high signal to background (S/B) ratios.
An alternative ECL chemistry is based on ruthenium(II) trisbipyridal chelate (Ru(bpy)2+3-labeled reporter antibodies. ECL can be evoked from the Ru(bpy)3(2+)-tagged reporter antibodies by application of an electrical potential. Unlike the enzymatic HRP-based ECL detection, Ru detection is not enzymatic. This may simplify the ECL assay; however, the application of an electrical potential needed for a Ru-based ECL detection approach requires integrated electrodes (e.g., screen-printed carbon ink or Au electrodes) on the bottom of the plate wells and a dedicated ECL plate reader to apply the electrical potential. This significantly complicates the detector for Ru-based ECL detection, in contrast to HRP-ECL detection, which utilizes a more conventional fluorometer.
Recently, carbon nanotubes (CNTs)10 have attracted interest for enhancement of biodetection because of their unique mechanical and electronic properties combined with a large specific surface area. CNTs have been primarily used for their electronic properties in electrochemical-based detection11–16 and for the development of various types of transistors.17–23 Although CNTs have very useful properties for biodetection, including a large specific surface area for antibody immobilization and low absorption in the visible range, there has been little use of CNT to enhance ELISA and other optically based assays.
In determining the applicability of these various techniques for biodetection, especially in food safety, a good model toxin system is Staphylococcal enterotoxins (SEs). SEs are a group of 19 (known) heat-labile toxins implicated in several illnesses including food-borne diseases and are also recognized as potential bioweapons.24–27 Many pathogenic Staphylococcus aureus strains generally contain several SE genes.28–31 Foods are often tested for SEs, in particular Staphylococcal enterotoxin B (SEB), as they are a significant cause of food poisoning. Food testing is often complicated by the presence of an inhibitor in the samples. Also, they may contain unrelated cross-reacting materials, which may bind with the antibodies used for detection, leading to false positive results. Such interfering factors vary with food types, and may affect the accuracy of assays. However, SEs have been successfully assayed immunologically using ELISAs,32 which generally use optical detection with LODs in the 0.1–10 ng/mL. Typical levels of detection for commercial colorimetric ELISA systems are from 0.5 to 2 ng/g of food.33–38
Many methods for SE detection have also been developed based on biosensors.39–53 A real-time immunoquantitative PCR method has been developed for the detection of SEB, which consists of immunocapture of the SEB and then real-time PCR amplification of a DNA probe linked to the detection antibody. This method has a LOD of 10 pg mL–1, which is 1000 times more sensitive than the in-house ELISA used.54 Piezocantilevers have also been used to detect SEB with a LOD of 12.5 pg/mL,55 while microcantilever-based methods have demonstrated the best-ever sensitivity reported, down to 2.5 fg/mL.56
To realize the potential of CNT for immunological detection assays, we combined three detection elements: (1) the CNTs for primary antibody immobilization, (2) ECL for light signal generation, and (3) a simple cooled CCD detector to sensitively measure the chemiluminescent signal. In this system, anti-SEB primary antibodies were immobilized onto the CNT surface through electrostatic adsorption, and then the antibody–CNT mixture was immobilized onto a polycarbonate surface. The SEB was then detected by a “sandwich-type” ELISA on the CNT–polycarbonate surface. Initially, the effect of CCD exposure time on detection is investigated. Then, the sensitivity of the new CNT-ECL immunoassay method is evaluated for use in food testing by assaying buffer, soy milk, apple juice, and meat baby food spiked with SEB. Finally, the effects of partial purification using carboxymethylcellulose (CM) on the assay background were investigated.
MATERIALS AND METHODS
Reagents and Chemicals
Reagents
SEB, rabbit anti-SEB affinity purified IgG, and peroxidase (HRP) conjugated anti-SEB IgG were purchased from Toxin Technology (Sarasota, FL). Single-walled CNTs were obtained from Carbon Solutions Inc. (Riverside, CA). Poly(diallyldimethylammonium chloride) polymer (PDDA), o-phenylenediamine dihydrochloride (OPD), and CM were purchased from Sigma-Aldrich (St. Louis, MO). Immun-Star HRP Chemiluminescence Kit was obtained from Bio-Rad (Hercutes, CA). All other reagents were of analytical grade, and deionized water was used throughout. The food used for the analysis (soy milk, apple juice, and meat baby food) were purchased from a local grocery store.
Materials for the fabrication of the 9-Well Sample Chips
Clear 0.25-mm polycarbonate film and ⅛-in. black acrylic were obtained from Piedmont Plastics (Beltsville, MD).
Preparation Equipment
Fisher (FS-14) sonicator was obtained from Fisher Scientific (Pittsburgh, PA), Beckman minicentrifuge was obtained from Beckman (Fullerton, CA).
Preparation of the Immunosensor
Carbon Nanotube Preparation
The CNTs (30 mg) were first shortened and oxidized by mixing with concentrated sulfuric acid and nitric acid mixture (3:1 v/v) and sonicating with a Fisher (FS-14) sonicator for 6 h followed by extensive washing in water (100 mL) until neutralized (pH 7.0). Then the CNTs were dispersed in 100 mL of 1 M NaOH solution for 5 min to achieve net negative charged carboxylic acid groups and washed with water (100 mL).
Antibody Functionalization
A linker molecule to the carbon nanotube was used.57 PDDA is positively charged and SEB antibody is negatively charged, so antibodies are electrostatically adsorbed onto the carbon nanotube. The positively charged polycation was adsorbed by dispersing the CNT in 50 mL of 1 mg/mL PDDA containing 0.5 M NaCl for 30 min followed by centrifugation (10 000 rpm in a Beckman centrifuge for 15 min) and washed with 100 mL of water.
CNT Functionalization
The CNTs were functionalized by dispersing in a rabbit anti-SEB IgG phosphate buffer solution (20 mM, pH 8.0) at a concentration of 0.01 mg/mL for 1 h at room temperature, so that the antibody was adsorbed onto the CNT surface. After centrifugation (15 min) and washing extensively with water (10 mL), the modified CNT was stored at 4 °C in pH 8.0 phosphate buffer at a concentration of ~1 mg/mL for no more than two weeks before use.
Fabrication of the Immunosensor
The 9-well sample chips used for the immunosensor were designed in CorelDraw11 (Corel Corp., Ontario, Canada) and micromachined in ⅛-in. black acrylic using a computer-controlled laser cutter Epilog Legend CO2 65W cutter (Epilog, Golden, CO). Before cutting, both sides of the acrylic sheet were coated with 3 M 9770 adhesive transfer double-sided tape (Piedmont Plastics, Beltsville, MD). The wells were then cleaned using Versa Clean (FisherBrand, Pittsburgh, PA) and washed with MilliQ water before being dried with air. The reaction solution was then added to the top of the sample wells and the top was sealed.
SEB Assays
The immunosensor fluorescence was measured using a standard 96-well plate and a plate reader. One tablet of OPD was dissolved in 20 mL of phosphate buffer (20 mM, pH 7.4) for use as a substrate solution. Before optical detection, hydrogen peroxide (0.01 mL) was added to the substrate solution to a final concentration of 1 mM, and 100 μL of the resulting solution was added to each well of the microtiter plate. Immunosensor assay strips were immersed into the solution. After 5 min, the fluorescence emission at 570 nm was read using excitation at 400 nm.
For the CCD-ECL assay, 15 μL of antibody-modified CNT solution was dropped into the wells of a 9-well sample chip, which were then dried and rinsed with 2 mL of washing buffer (20 mM phosphate buffer, pH 7.4) for 5 min to eliminate any loose or partially immobilized CNTs. The CNT–antibody modified wells were then blocked with 1% BSA for 30 min and incubated with different concentrations of SEB using 15 μL of phosphate buffer solution (20 mM, pH 8.0) buffer for 45 min. After washing again in 2 mL of phosphate buffer, the immunosensor coated with antibody–antigen complex was exposed to HRP-conjugated antirabbit IgG (15 μL, 0.01 mg/mL) in buffer for 1 h. This was followed by 3 more cycles of washing with 2 mL of phosphate buffer.
ECL was achieved by adding 7 μL of ECL buffer (mix the two solutions from the Chemiluminescent Kit in a 1:1 volume ratio) into each well, and the ECL intensity was measured immediately. In the presence of HRP (bound to the secondary antibody), luminol in the ECL reagents will reduce hydrogen peroxide, which emits light as it returns to its basal state and is detectable using a CCD.
Control experiments without CNTs were performed with modified immunosensors. Fifteen microliters of 1 mg/mL PDDA containing 0.5 M NaCl was dropped onto the tip of a polycarbonate strip or wells, incubated for 1 h, and then washed and dried. Fifteen microliters of rabbit anti-SEB IgG solution (pH 8.0) was applied onto the strip in order to adsorb the IgG onto the strip. After 1 h, the immunosensor was blocked with BSA, incubated with SEB and then with HRP conjugated antirabbit IgG as described above.
ECL Detection
Fluorescence measurements were performed with a Spectramax M5 plate reader. For the CCD-based detection, a simple cooled CCD detector was used, as shown schematically in Figure 1. The CCD detector consists of an enclosure and an Atic-16 cooled CCD camera or SXVF-M7 camera (Adirondack Video Astronomy, Hudson Falls, NY). Both cameras employ a Sony ICX-429ALL with 752 × 582 pixel CCD and are equipped with a 5-mm extension tube and a 12-mm Pentax f1.2 lens (Spytown, Utopia, NY). Although the system includes an optical fluorescence detection module to duplicate the capabilities of the plate reader, it was not necessary for ECL detection.
Figure 1.
CCD detector for the point-of-care immunosensor. (A) A schematic configuration of the detector, (B) a photograph of the actual detection platform, and (C) actual nine wells sample chip: plastic enclosure,1 Atic-16 CCD camera.2 A 5-mm extension tube3 is attached to the 12-mm Pentax f1.2 lens.4 A black acrylic shelf box5 was designed to hold the sample chip6 and flurocence illumination module, which was not used in this work.7
The CCD image intensities were quantified and analyzed using ImageJ software, developed and freely distributed by NIH (http://rsb.info.nih.gov/ij/download.html). To measure the signal from each well, a circle 20 pixels in radius (1250 pixel area) was used as a uniform region of interest, which covers roughly 75% of the surface of each well of the chip. The signal for the individual wells was calculated as average of the intensity values of the respective pixels. The value obtained with a concentration of 0 ng/mL SEB was defined as background. The ratio of the signal to background (S/B) ratio was then used to quantify the SEB concentration. The data were then imported into Microsoft Excel (Microsoft, Redmond, WA) for further manipulation.
Food Sample Analysis
Food Sample Spiking
To spike samples of soy milk and apple juice, 1 mL of sample was transferred directly to a 2-mL centrifuge tube and different concentrations of SEB were added. For the meat baby food, 1 g of food sample was transferred to a centrifuge tube, and then buffer and different concentrations of SEB were added. The matrix–toxin was incubated for 30 min.
Cation Exchanger Carboxymethylcellulose for Partial Sample Purification
To detect SEB in food samples, partial sample purification was used to help reduce assay background by reducing the cross-reaction of the antibodies with other components of the food matrix.58,59 In order to establish a widely applicable assay for different foods, the new CNT-ECL immunoassay method was tested in food samples with and without partial toxin purification using the cation exchanger CM.60 To prepare CM for ~100 purification assays, 1 g of CM was equilibrated with 20 mL of loading buffer (loading buffer, 5 mM NaPO3, pH 5.7) until the gel was swollen and then centrifuged at 4000 rpm for 2 min. After centrifugation, the gel was allowed to settle, the buffer was removed, and the CM was washed twice with 20 mL of loading buffer followed by centrifugation as above. After the removal of the buffer in the last centrifugation, loading buffer was added to a final volume of 20 mL. The equilibrated CM was stored at 4 °C before use.
Sample Preparation
The tubes containing the spiked food samples were vortexed briefly, centrifuged at 14 000 rpm for 2 min, and the resulting supernatant solution transferred into a fresh tube. Loading buffer was added into the supernatant solution to reach a sample volume of 1 mL. The sample (400 μL) was mixed with 200 μL of equilibrated CM and vortexed for 30 min, which was following by centrifugation at 14 000 rpm for 2 min, and the soluble material was removed. The material left at the tube was then washed 3 times by repeatedly adding loading buffer and centrifuging. Finally, 100 μL of elution buffer (50 mM NaPO3, pH 6.5; 50 mM NaCl) was added to the matrix, the tubes were centrifuged, and the eluted material was collected for immunoassay.
RESULTS AND DISCUSSION
To simplify ECL detection and to decrease the LOD for SEs, we evaluated the potential of CCD-based detection of ECL using the CNTs functionalized for the detection of SEB. As previously discussed, SEB primary antibodies were immobilized onto a CNT surface through electrostatic adsorption, and then the antibody–nanotube mixture was immobilized onto a polycarbonate surface of a 9-well chip. SEB was then detected by a “sandwich-type” ELISA assay on the CNT–polycarbonate surface using an ECL assay detected by the simple cooled CCD detector shown in Figure 1.
Detection of SEB with the New CNT-ECL Immunoassay Method
To optimize the CCD exposure time for the light-emitting properties of ECL substrate and demonstrate that light emission can occur for long periods of time in our assay, the effect of the CCD exposure on the signal of the CCD-ECL was investigated using the sandwich-type ELISA assay with 1 ng/mL SEB.
In these experiments, the primary antibody was immobilized onto the CNTs prior to the performance of the assay. The analysis time for these assay are as follows: a 45-min incubation time with the SEB sample followed by a brief washing, and then a 1-h incubation time with the secondary antibody labeled with HRP–antibody followed by addition of the ECL reagents and 20 min of exposure. The total amount of time for the assay is ~2 h. The other assays used (fluorescence and chemiluminescence detected by a plate reader) also used the same incubation time.
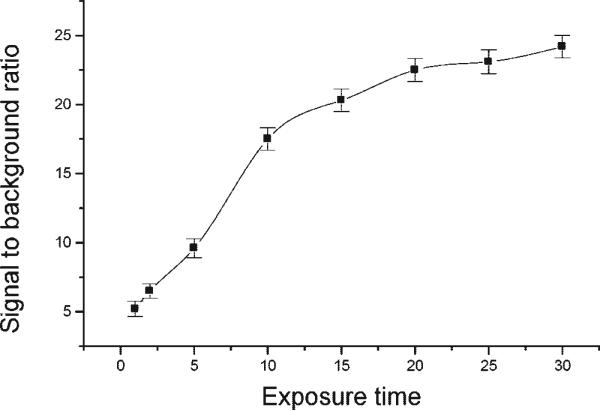
As shown in Figure 2, the light is initially emitted at a very high rate, as evidenced by the increase in the S/B ratio when the exposure time is increased from 1 to 10 min. However, after 20 min, the rate starts to level off relative to the background noise and reaches a steady-state value with relatively little increase in S/B ratio between 20 to 30 min. For ECL detection, we used a 0 ng/mL SEB sample to determine the background and a S/B ratio of 2 to determine the LOD.
Figure 2.
Effect of exposure time on the signal generated by 1 ng/mL SEB during the ECL experiment. Error bar, SD (n = 3).
To determine the assay sensitivity, the new CNT-ECL immunoassay method was tested for detection over a range of SEB concentration using a sandwich-type ELISA assay. In this experiment, various amounts of SEB (0–50 ng/mL) in buffer were added to a 9-well plate with CNT immobilized rabbit anti-SEB IgG. As shown in Figure 3, the intensity of the signal increased with the increase of SEB concentration.
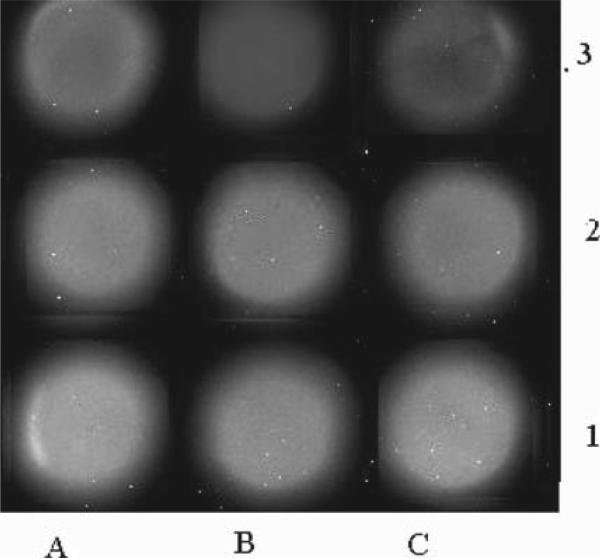
Figure 3.
Immunoassay based on CNT-ECL with different concentrations of SEB detected by the CCD detector (in ng/mL): (1A) 50, (1B) 10, (1C) 5, (2A) 1, (2B) 0.5, (2C) 0.1, (3A) 0.05, (3B) 0.01, and (3C) 0. Exposure time, 20 min.
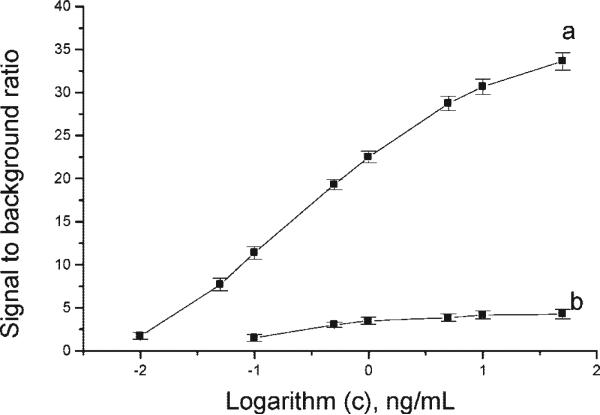
The signal from the three-assay format increases with SEB concentration; as shown in Figure 4, the CNT-ECL immunoassay (a) showed a linear response with SEB concentrations ranging from 0.01 to 10 ng/mL. The control immunosensor without CNT (b) exhibited both lower sensitivity and a narrower linear range 0.1–5 ng/mL. These results suggest that the CNTs enhance SEB immunodetection by as much as 7-fold.
Figure 4.
Calibration curve of the immunoassay to different concentration of SEB with CNT (a) and without CNT (b). Error bar, SD (n = 3).
Assay Reproducibility
To evaluate the reproducibility of the immunosensor, a series of five surfaces with immobilized CNTs were prepared for the detection of 1 ng/mL SEB. The relative standard deviation (RSD) of measurement for the five at any given exposure time was ~6%, suggesting that the assay is reproducible in the tested conditions. The RSDs can be seen in Figure 2. Similar reproducibility experiments were preformed for all of the measurements and yielded similar RSDs, indicating that the reproducibility of the assay is independent of the concentration of SEB as well as exposure time.
Comparison between Various Detection Methods for CNT-Based Assay
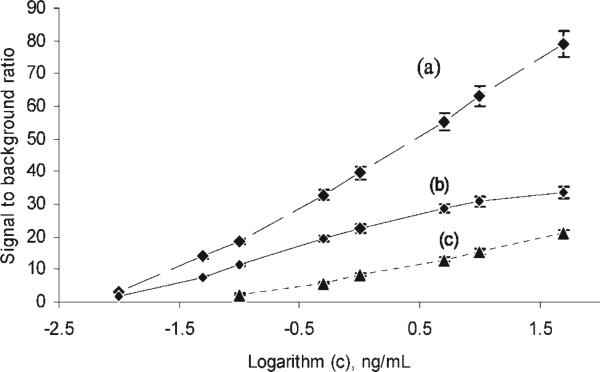
Several methods for HRP detection were compared using the CNT-based assay, including chemiluminescence detected by the point-of-care CCD, chemiluminescence detected by a standard plate reader, and fluorescence detected by the plate reader using OPD as a substrate. Once again, CNT-ECL detection was obtained using a sandwich-type ELISA assay, where SEB was first bound to the anti-SEB primary antibody immobilized on the CNT surfaces followed by binding of the HRP-labeled secondary antibody and HRP assay detection. Figure 5 shows the results from these experiments. Chemiluminescence (a and b) exhibits higher sensitivity than fluorescence detection (c). Chemiluminescence detected by the plate reader (a) and the CCD detector (b) achieved a lower LOD of 0.01 ng/mL, although the S/B ratio values for the plate reader (a) were higher than for the CCD detector (b). The fluorescence detected by the plate reader using OPD as a substrate (c) achieved a LOD of only 0.1 ng/mL, suggesting that chemiluminescence detection is 1 order of magnitude more sensitive than the commonly used OPD-based fluorescence detection. While the plate reader provides a better S/B ratio, it is not more sensitive, and is also more expensive, complex, and not portable.
Figure 5.
Calibration curve of the immunoassay to different concentrations of SEB based on CNTs with different detection methods. (a) Chemiluminescence detected by the plate reader; (b) chemiluminescence detected by the CCD detector; (c) fluorescence detected by plate reader using OPD as substrate; Error bar, SD (n = 3).
Application of CNT-Based Assay for Detection of SEB in Food Samples
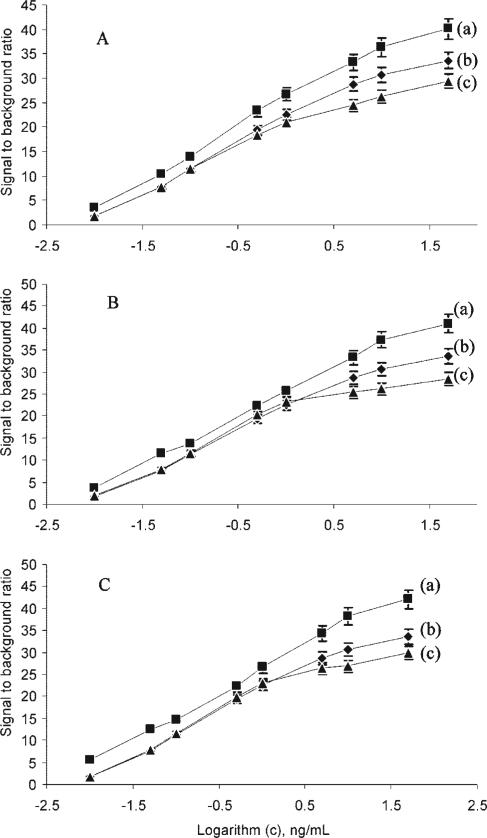
SEB-spiked soy milk samples partially purified with CM and the eluted material were analyzed with the CNTECL immunoassay detected by the point-of-care immunosensor. A serial dilution of SEB in H2O was used as a standard solution control. As seen in Figure 6A, all SEB concentrations of unpurified sample (a) gave higher signals compared to the SEB control solution (b), suggesting some nonspecific adsorption of non-SEB proteins present at the soy milk sample. For lower concentrations of SEB, the control (b), and the CM-purified samples (c) the signals were similar, at higher SEB concentrations of the CM purified material, the signal was lower the than the SEB control solution (b), suggesting that SEB recovery especially at higher concentration of SEB was reduced by ~15%.
Figure 6.
Detection of SEB in soy milk (A) apple juice (B) and baby food (C) with the immunosensor based on CNT. (a) Food without CM purification, (b) standard SEB solution, and (c) with CM purification. Error bar, SD (n = 3).
SEB spiked apple juice (Figure 6B) and meat baby food (Figure 6C) exhibited results similar to that of the soy milk. The new CNT-ECL immunoassay method is able to detect SEB at a variety of concentrations in both apple juice (panel B) and meat baby food (panel C). In both cases, the purified sample (c) exhibits a lower signal than the unpurified material (a), suggesting that the partial purification has removed some cross-reacting materials from the samples. Also, the signal was more consistent with the control (b) at lower concentrations than the unpurified material (a), and at higher concentrations the signal was slightly lower than the control indicating a greater loss of SEB during CM purification.
CONCLUSIONS
A new CNT-ECL immunoassay has been developed with CCD detection that is simpler and can be multiplexed more easily than ECL-ELISA, which utilizes photodiodes and photomultipliers for point detection as well as special assay plates with electrodes in the measuring wells. The new method is more sensitive than the same assay with fluorescence detection (Figure 5) enabling detection of 0.01 ng/mL SEB. This low LOD may enable improved detection of SEB in food and for clinical diagnostics, as well as decreasing the sample size needed for SEB detection assays. We have demonstrated that the new CNT-ECL immunoassay method increases the level of SEB detection (Figure 4), including using either a conventional sandwich-type ELISA assay or a regular optical detector (Figure 5) and detection in food samples (Figure 6).
The ECL substrate used in our assay enables long periods of light emission (Figure 2), and the CCD-based detection can take advantage of this by using longer exposure times. This combination achieves highly sensitive detection levels (Figure 3) with relatively simple and inexpensive equipment (Figure 1). The LOD of our system, 0.01 ng/mL, seems to be far more sensitive than the reported sensitivity for colorimetric ELISA systems and for most reported ECL-ELISA assays. In addition, unlike previous work, which detects ECL using complex and expensive fluorometric or electrochemical-based detectors, our assay method utilizes a relatively simple and inexpensive cooled CCD detector, which has the possibility of broadening the application of ECL for biodetection and the realization of its full potential. The CCD-based detection used here combined with 9-well sample chips reduces the amount of reagents needed: less than 10 μL of sample is needed for ECL detection compared to 50–100 μL needed for ELISA. The simple, relatively inexpensive, and versatile point-of-care immunosensor combined with sensitive CNT-ECL immunoassay makes this system practical for point-of-care diagnostics and for reducing global health inequities.
References
- 1.Garber EA, O'Brien TW. J. AOAC Int. 2008;91:376–382. [PubMed] [Google Scholar]
- 2.Quan Y, Zhang Y, Wang S, Lee N, Kennedy IR. Anal. Chim. Acta. 2006;580:1–8. doi: 10.1016/j.aca.2006.07.063. [DOI] [PubMed] [Google Scholar]
- 3.Guglielmo-Viret V, Attree O, Blanco-Gros V, Thullier P. J. Immunol. Methods. 2005;301:164–172. doi: 10.1016/j.jim.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Guglielmo-Viret V, Thullier P. J. Immunol. Methods. 2007;328:70–78. doi: 10.1016/j.jim.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Marquette CA, Thomas D, Degiuli A, Blum LJ. Anal. Bioanal. Chem. 2003;377:922–928. doi: 10.1007/s00216-003-2112-x. [DOI] [PubMed] [Google Scholar]
- 6.Roda A, Pasini P, Baraldini M, Musiani M, Gentilomi G, Robert C. Anal. Biochem. 1998;257:53–62. doi: 10.1006/abio.1997.2514. [DOI] [PubMed] [Google Scholar]
- 7.Rubtsova M, Kovba GV, Egorov AM. Biosens. Bioelectron. 1998;13:75–85. doi: 10.1016/s0956-5663(97)00072-9. [DOI] [PubMed] [Google Scholar]
- 8.Simmonds AC, Cunningham M, Durrant I, Fowler SJ, Evans MR. Clin. Chem. 1991;37:1527–1528. [PubMed] [Google Scholar]
- 9.Stone T, Durrant I. Genet. Anal. Technol. Appl. 1991;8:230–237. doi: 10.1016/1050-3862(91)90033-n. [DOI] [PubMed] [Google Scholar]
- 10.Iijima S. Nature. 1991;354:56–58. [Google Scholar]
- 11.Panini NV, Messina GA, Salinas E, Fernandez H, Raba J. Biosens. Bioelectron. 2007 doi: 10.1016/j.bios.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Aziz MA, Park S, Jon S, Yang H. Chem. Commun. 2007:2610–2612. doi: 10.1039/b701190c. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez S, Pumera M, Fabregas E. Biosens. Bioelectron. 2007;23:332–340. doi: 10.1016/j.bios.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Okuno J, Maehashi K, Kerman K, Takamura Y, Matsumoto K, Tamiya E. Biosens. Bioelectron. 2007;22:2377–2381. doi: 10.1016/j.bios.2006.09.038. [DOI] [PubMed] [Google Scholar]
- 15.Yu X, Kim SN, Papadimitrakopoulos F, Rusling JF. Mol. Biosyst. 2005;1:70–78. doi: 10.1039/b502124c. [DOI] [PubMed] [Google Scholar]
- 16.Viswanathan S, Wu LC, Huang MR, Ho JA. Anal. Chem. 2006;78:1115–1121. doi: 10.1021/ac051435d. [DOI] [PubMed] [Google Scholar]
- 17.Park DW, Kim YH, Kim BS, So HM, Won K, Lee JO, Kong KJ, Chang H. J. Nanosci. Nanotechnol. 2006;6:3499–3502. [PubMed] [Google Scholar]
- 18.Maehashi K, Katsura T, Kerman K, Takamura Y, Matsumoto K, Tamiya E. Anal. Chem. 2007;79:782–787. doi: 10.1021/ac060830g. [DOI] [PubMed] [Google Scholar]
- 19.Artyukhin AB, Stadermann M, Friddle RW, Stroeve P, Bakajin O, Noy A. Nano Lett. 2006;6:2080–2085. doi: 10.1021/nl061343j. [DOI] [PubMed] [Google Scholar]
- 20.Balasubramanian K, Burghard M. Anal. Bioanal. Chem. 2006;385:452–468. doi: 10.1007/s00216-006-0314-8. [DOI] [PubMed] [Google Scholar]
- 21.Byon HR, Choi HC. J. Am. Chem. Soc. 2006;128:2188–2189. doi: 10.1021/ja056897n. [DOI] [PubMed] [Google Scholar]
- 22.Gruner G. Anal. Bioanal. Chem. 2006;384:322–335. doi: 10.1007/s00216-005-3400-4. [DOI] [PubMed] [Google Scholar]
- 23.Chen RJ, Choi HC, Bangsaruntip S, Yenilmez E, Tang X, Wang Q, Chang YL, Dai H. J. Am. Chem. Soc. 2004;126:1563–1568. doi: 10.1021/ja038702m. [DOI] [PubMed] [Google Scholar]
- 24.Wiener SL. Mil. Med. 1996;161:251–256. [PubMed] [Google Scholar]
- 25.Ler SG, Lee FK, Gopalakrishnakone P. J. Chromatogr., A. 2006;1133:1–12. doi: 10.1016/j.chroma.2006.08.078. [DOI] [PubMed] [Google Scholar]
- 26.Henghold WB., 2nd Dermatol. Clin. 2004;22:257–262. doi: 10.1016/j.det.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Rosenbloom M, Leikin JB, Vogel SN, Chaudry ZA. Am. J. Ther. 2002;9:5–14. doi: 10.1097/00045391-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Sergeev N, Volokhov D, Chizhikov V, Rasooly A. J. Clin. Microbiol. 2004;42:2134–2143. doi: 10.1128/JCM.42.5.2134-2143.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarraud S, Peyrat MA, Lim A, Tristan A, Bes M, Mougel C, Etienne J, Vandenesch F, Bonneville M, Lina G. J. Immunol. 2001;166:669–677. doi: 10.4049/jimmunol.166.1.669. [DOI] [PubMed] [Google Scholar]
- 30.Jarraud S, Mougel C, Thioulouse J, Lina G, Meugnier H, Forey F, Nesme X, Etienne J, Vandenesch F. Infect. Immun. 2002;70:631–641. doi: 10.1128/IAI.70.2.631-641.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Omoe K, Ishikawa M, Shimoda Y, Hu DL, Ueda S, Shinagawa K. J. Clin. Microbiol. 2002;40:857–862. doi: 10.1128/JCM.40.3.857-862.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennett RW. J. Food Prot. 2005;68:1264–1270. doi: 10.4315/0362-028x-68.6.1264. [DOI] [PubMed] [Google Scholar]
- 33.Miyamoto T, Kamikado H, Kobayashi H, Honjoh K, Iio M. J Food Prot. 2003;66:1222–1226. doi: 10.4315/0362-028x-66.7.1222. [DOI] [PubMed] [Google Scholar]
- 34.Pan TM, Yu YL, Chiu SI, Lin SS. Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi. 1996;29:100–107. [PubMed] [Google Scholar]
- 35.Park CE, Akhtar M, Rayman MK. Appl. Environ. Microbiol. 1994;60:677–681. doi: 10.1128/aem.60.2.677-681.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park CE, Warburton D, Laffey PJ. Int. J. Food Microbiol. 1996;29:281–295. doi: 10.1016/0168-1605(95)00046-1. [DOI] [PubMed] [Google Scholar]
- 37.Vernozy-Rozand C, Mazuy-Cruchaudet C, Bavai C, Richard Y. Lett. Appl. Microbiol. 2004;39:490–494. doi: 10.1111/j.1472-765X.2004.01602.x. [DOI] [PubMed] [Google Scholar]
- 38.Wieneke AA. Int. J. Food Microbiol. 1991;14:305–312. doi: 10.1016/0168-1605(91)90122-6. [DOI] [PubMed] [Google Scholar]
- 39.Bergwerff AA, van Knapen F. J. AOAC Int. 2006;89:826–831. [PubMed] [Google Scholar]
- 40.Chatrathi MP, Wang J, Collins GE. Biosens. Bioelectron. 2007;22:2932–2938. doi: 10.1016/j.bios.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen ZG. Bioprocess Biosyst. Eng. 2008;31:345–350. doi: 10.1007/s00449-007-0168-2. [DOI] [PubMed] [Google Scholar]
- 42.Homola J, Dostalek J, Chen S, Rasooly A, Jiang S, Yee SS. Int. J. Food Microbiol. 2002;75:61–69. doi: 10.1016/s0168-1605(02)00010-7. [DOI] [PubMed] [Google Scholar]
- 43.Lin HC, Tsai WC. Biosens. Bioelectron. 2003;18:1479–1483. doi: 10.1016/s0956-5663(03)00128-3. [DOI] [PubMed] [Google Scholar]
- 44.Nedelkov D, Rasooly A, Nelson RW. Int. J. Food Microbiol. 2000;60:1–13. doi: 10.1016/s0168-1605(00)00328-7. [DOI] [PubMed] [Google Scholar]
- 45.Rasooly A. J. Food Prot. 2001;64:37–43. doi: 10.4315/0362-028x-64.1.37. [DOI] [PubMed] [Google Scholar]
- 46.Rasooly A, Herold KE. J. AOAC Int. 2006;89:873–883. [PubMed] [Google Scholar]
- 47.Rasooly L, Rasooly A. Int. J. Food Microbiol. 1999;49:119–127. doi: 10.1016/s0168-1605(99)00053-7. [DOI] [PubMed] [Google Scholar]
- 48.Ruan C, Zeng K, Varghese OK, Grimes CA. Biosens. Bioelectron. 2004;20:585–591. doi: 10.1016/j.bios.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 49.Sapsford KE, Taitt CR, Loo N, Ligler FS. Appl. Environ. Microbiol. 2005;71:5590–5592. doi: 10.1128/AEM.71.9.5590-5592.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shriver-Lake LC, Shubin YS, Ligler FS. J. Food Prot. 2003;66:1851–1856. doi: 10.4315/0362-028x-66.10.1851. [DOI] [PubMed] [Google Scholar]
- 51.Soelberg SD, Chinowsky T, Geiss G, Spinelli CB, Stevens R, Near S, Kauffman P, Yee S, Furlong CE. J. Ind. Microbiol. Biotechnol. 2005;32:669–674. doi: 10.1007/s10295-005-0044-5. [DOI] [PubMed] [Google Scholar]
- 52.Taylor JD, Phillips KS, Cheng Q. Lab Chip. 2007;7:927–930. doi: 10.1039/b618940g. [DOI] [PubMed] [Google Scholar]
- 53.Yacoub-George E, Hell W, Meixner L, Wenninger F, Bock K, Lindner P, Wolf H, Kloth T, Feller KA. Biosens. Bioelectron. 2007;22:1368–1375. doi: 10.1016/j.bios.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 54.Rajkovic A, El-Moualij B, Uyttendaele M, Brolet P, Zorzi W, Heinen E, Foubert E, Debevere J. Appl. Environ. Microbiol. 2006;72:6593–6599. doi: 10.1128/AEM.03068-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Campbell GA, Medina MB, Mutharasan R. Sens. Actuators, B: Chem. 2007;126:354–360. [Google Scholar]
- 56.Maraldo D, Mutharasan R. Anal. Chem. 2007;79:7636–7643. doi: 10.1021/ac070589l. [DOI] [PubMed] [Google Scholar]
- 57.Liu G, Lin Y. Anal. Chem. 2006;78:835–843. doi: 10.1021/ac051559q. [DOI] [PubMed] [Google Scholar]
- 58.Park CE, Akhtar M, Rayman MK. Appl. Environ. Microbiol. 1992;58:2509–2512. doi: 10.1128/aem.58.8.2509-2512.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park CE, Akhtar M, Rayman MK. Appl. Environ. Microbiol. 1993;59:2210–2213. doi: 10.1128/aem.59.7.2210-2213.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Balaban N, Rasooly A. Int. J. Food Microbiol. 2001;64:33–40. doi: 10.1016/s0168-1605(00)00439-6. [DOI] [PubMed] [Google Scholar]