Abstract
Candida albicans is not inhibited by a number of drugs known to affect fungal cells. The basis for this resistance in most cases is unknown but has been attributed to the general impermeability of the fungal cell envelope. A gene (BENr) formerly shown to be responsible for the resistance of C. albicans to benomyl and methotrexate was shown in the present study to confer resistance to four other inhibitory compounds: cycloheximide, benztriazoles, 4-nitroquinoline-N-oxide, and sulfometuron methyl. Analysis of the protein database revealed an apparent similarity of the C. albicans gene to membrane protein genes encoding antibiotic resistance in prokaryotes and eukaryotes and a high degree of identity to a recently cloned gene encoding cycloheximide resistance in Candida maltosa. We propose that BENr encodes a protein that operates in a fashion similar, but not identical, to that described for other multiple-drug resistance proteins.
Full text
PDF

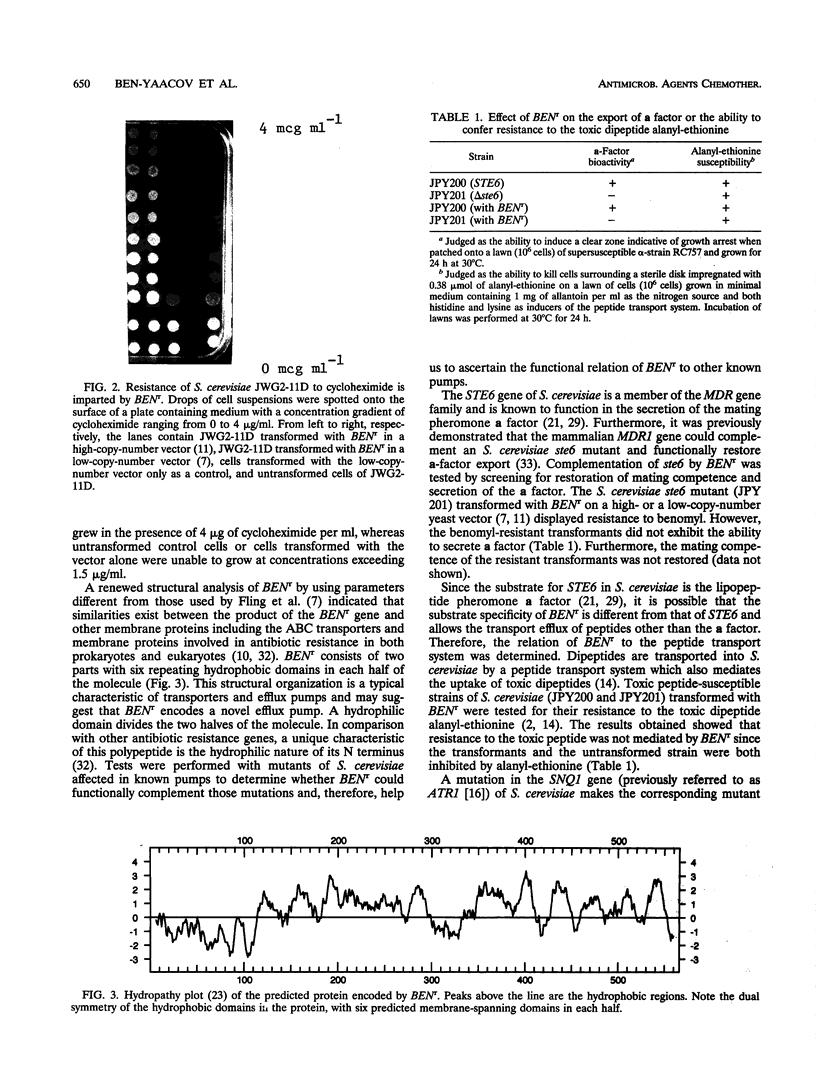


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assaraf Y. G., Schimke R. T. Identification of methotrexate transport deficiency in mammalian cells using fluoresceinated methotrexate and flow cytometry. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7154–7158. doi: 10.1073/pnas.84.20.7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basrai M. A., Zhang H. L., Miller D., Naider F., Becker J. M. Toxicity of oxalysine and oxalysine-containing peptides against Candida albicans: regulation of peptide transport by amino acids. J Gen Microbiol. 1992 Nov;138(11):2353–2362. doi: 10.1099/00221287-138-11-2353. [DOI] [PubMed] [Google Scholar]
- Chan R. K., Otte C. A. Physiological characterization of Saccharomyces cerevisiae mutants supersensitive to G1 arrest by a factor and alpha factor pheromones. Mol Cell Biol. 1982 Jan;2(1):21–29. doi: 10.1128/mcb.2.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K. J., Hill W. B. Studies on the pink, adenine-deficient strains of Candida albicans. I. Cultural and morphological characteristics. Sabouraudia. 1970 May;8(1):48–59. [PubMed] [Google Scholar]
- Dehoux P., Davies J., Cannon M. Natural cycloheximide resistance in yeast. The role of ribosomal protein L41. Eur J Biochem. 1993 Apr 15;213(2):841–848. doi: 10.1111/j.1432-1033.1993.tb17827.x. [DOI] [PubMed] [Google Scholar]
- Fling M. E., Kopf J., Tamarkin A., Gorman J. A., Smith H. A., Koltin Y. Analysis of a Candida albicans gene that encodes a novel mechanism for resistance to benomyl and methotrexate. Mol Gen Genet. 1991 Jun;227(2):318–329. doi: 10.1007/BF00259685. [DOI] [PubMed] [Google Scholar]
- Gömpel-Klein P., Mack M., Brendel M. Molecular characterization of the two genes SNQ and SFA that confer hyperresistance to 4-nitroquinoline-N-oxide and formaldehyde in Saccharomyces cerevisiae. Curr Genet. 1989 Aug;16(2):65–74. doi: 10.1007/BF00393397. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Higgins C. F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Hill J. E., Myers A. M., Koerner T. J., Tzagoloff A. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast. 1986 Sep;2(3):163–167. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]
- Huffaker T. C., Thomas J. H., Botstein D. Diverse effects of beta-tubulin mutations on microtubule formation and function. J Cell Biol. 1988 Jun;106(6):1997–2010. doi: 10.1083/jcb.106.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Island M. D., Naider F., Becker J. M. Regulation of dipeptide transport in Saccharomyces cerevisiae by micromolar amino acid concentrations. J Bacteriol. 1987 May;169(5):2132–2136. doi: 10.1128/jb.169.5.2132-2136.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa S., Driscoll M., Struhl K. ATR1, a Saccharomyces cerevisiae gene encoding a transmembrane protein required for aminotriazole resistance. Mol Cell Biol. 1988 Feb;8(2):664–673. doi: 10.1128/mcb.8.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane S. E., Pastan I., Gottesman M. M. Genetic basis of multidrug resistance of tumor cells. J Bioenerg Biomembr. 1990 Aug;22(4):593–618. doi: 10.1007/BF00762963. [DOI] [PubMed] [Google Scholar]
- Kerridge D., Fasoli M., Wayman F. J. Drug resistance in Candida albicans and Candida glabrata. Ann N Y Acad Sci. 1988;544:245–259. doi: 10.1111/j.1749-6632.1988.tb40410.x. [DOI] [PubMed] [Google Scholar]
- Kerridge D. Mode of action of clinically important antifungal drugs. Adv Microb Physiol. 1986;27:1–72. doi: 10.1016/s0065-2911(08)60303-3. [DOI] [PubMed] [Google Scholar]
- Kuchler K., Sterne R. E., Thorner J. Saccharomyces cerevisiae STE6 gene product: a novel pathway for protein export in eukaryotic cells. EMBO J. 1989 Dec 20;8(13):3973–3984. doi: 10.1002/j.1460-2075.1989.tb08580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Käufer N. F., Fried H. M., Schwindinger W. F., Jasin M., Warner J. R. Cycloheximide resistance in yeast: the gene and its protein. Nucleic Acids Res. 1983 May 25;11(10):3123–3135. doi: 10.1093/nar/11.10.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert G., McDevitt R., Falco S. C., Van Dyk T. K., Ficke M. B., Golin J. Cloning by gene amplification of two loci conferring multiple drug resistance in Saccharomyces. Genetics. 1990 May;125(1):13–20. doi: 10.1093/genetics/125.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack M., Gömpel-Klein P., Haase E., Hietkamp J., Ruhland A., Brendel M. Genetic characterization of hyperresistance to formaldehyde and 4-nitroquinoline-N-oxide in the yeast Saccharomyces cerevisiae. Mol Gen Genet. 1988 Feb;211(2):260–265. doi: 10.1007/BF00330602. [DOI] [PubMed] [Google Scholar]
- Marck C. 'DNA Strider': a 'C' program for the fast analysis of DNA and protein sequences on the Apple Macintosh family of computers. Nucleic Acids Res. 1988 Mar 11;16(5):1829–1836. doi: 10.1093/nar/16.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus S., Caldwell G. A., Miller D., Xue C. B., Naider F., Becker J. M. Significance of C-terminal cysteine modifications to the biological activity of the Saccharomyces cerevisiae a-factor mating pheromone. Mol Cell Biol. 1991 Jul;11(7):3603–3612. doi: 10.1128/mcb.11.7.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. P., Varshavsky A. The yeast STE6 gene encodes a homologue of the mammalian multidrug resistance P-glycoprotein. Nature. 1989 Aug 3;340(6232):400–404. doi: 10.1038/340400a0. [DOI] [PubMed] [Google Scholar]
- Odds F. C. Resistance of yeasts to azole-derivative antifungals. J Antimicrob Chemother. 1993 Apr;31(4):463–471. doi: 10.1093/jac/31.4.463. [DOI] [PubMed] [Google Scholar]
- Orbach M. J., Porro E. B., Yanofsky C. Cloning and characterization of the gene for beta-tubulin from a benomyl-resistant mutant of Neurospora crassa and its use as a dominant selectable marker. Mol Cell Biol. 1986 Jul;6(7):2452–2461. doi: 10.1128/mcb.6.7.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen I. T., Skurray R. A. Topology, structure and evolution of two families of proteins involved in antibiotic and antiseptic resistance in eukaryotes and prokaryotes--an analysis. Gene. 1993 Feb 14;124(1):1–11. doi: 10.1016/0378-1119(93)90755-r. [DOI] [PubMed] [Google Scholar]
- Raymond M., Gros P., Whiteway M., Thomas D. Y. Functional complementation of yeast ste6 by a mammalian multidrug resistance mdr gene. Science. 1992 Apr 10;256(5054):232–234. doi: 10.1126/science.1348873. [DOI] [PubMed] [Google Scholar]
- Rosenbluh A., Mevarech M., Koltin Y., Gorman J. A. Isolation of genes from Candida albicans by complementation in Saccharomyces cerevisiae. Mol Gen Genet. 1985;200(3):500–502. doi: 10.1007/BF00425739. [DOI] [PubMed] [Google Scholar]
- Ruhland A., Brendel M., Haynes R. H. Hyperresistance to DNA damaging agents in yeast. Curr Genet. 1986;11(3):211–215. doi: 10.1007/BF00420609. [DOI] [PubMed] [Google Scholar]
- Sasnauskas K., Jomantiene R., Lebediene E., Lebedys J., Januska A., Janulaitis A. Cloning and sequence analysis of a Candida maltosa gene which confers resistance to cycloheximide. Gene. 1992 Jul 1;116(1):105–108. doi: 10.1016/0378-1119(92)90636-4. [DOI] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured animal cells. Cell. 1984 Jul;37(3):705–713. doi: 10.1016/0092-8674(84)90406-9. [DOI] [PubMed] [Google Scholar]
- Sheir-Neiss G., Lai M. H., Morris N. R. Identification of a gene for beta-tubulin in Aspergillus nidulans. Cell. 1978 Oct;15(2):639–647. doi: 10.1016/0092-8674(78)90032-6. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. H., Neff N. F., Botstein D. Isolation and characterization of mutations in the beta-tubulin gene of Saccharomyces cerevisiae. Genetics. 1985 Dec;111(4):715–734. doi: 10.1093/genetics/111.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesono K., Toda T., Hayashi S., Yanagida M. Cell division cycle genes nda2 and nda3 of the fission yeast Schizosaccharomyces pombe control microtubular organization and sensitivity to anti-mitotic benzimidazole compounds. J Mol Biol. 1983 Aug 5;168(2):271–284. doi: 10.1016/s0022-2836(83)80018-7. [DOI] [PubMed] [Google Scholar]
- Windle B. E., Wahl G. M. Molecular dissection of mammalian gene amplification: new mechanistic insights revealed by analyses of very early events. Mutat Res. 1992 May;276(3):199–224. doi: 10.1016/0165-1110(92)90009-x. [DOI] [PubMed] [Google Scholar]




