Abstract
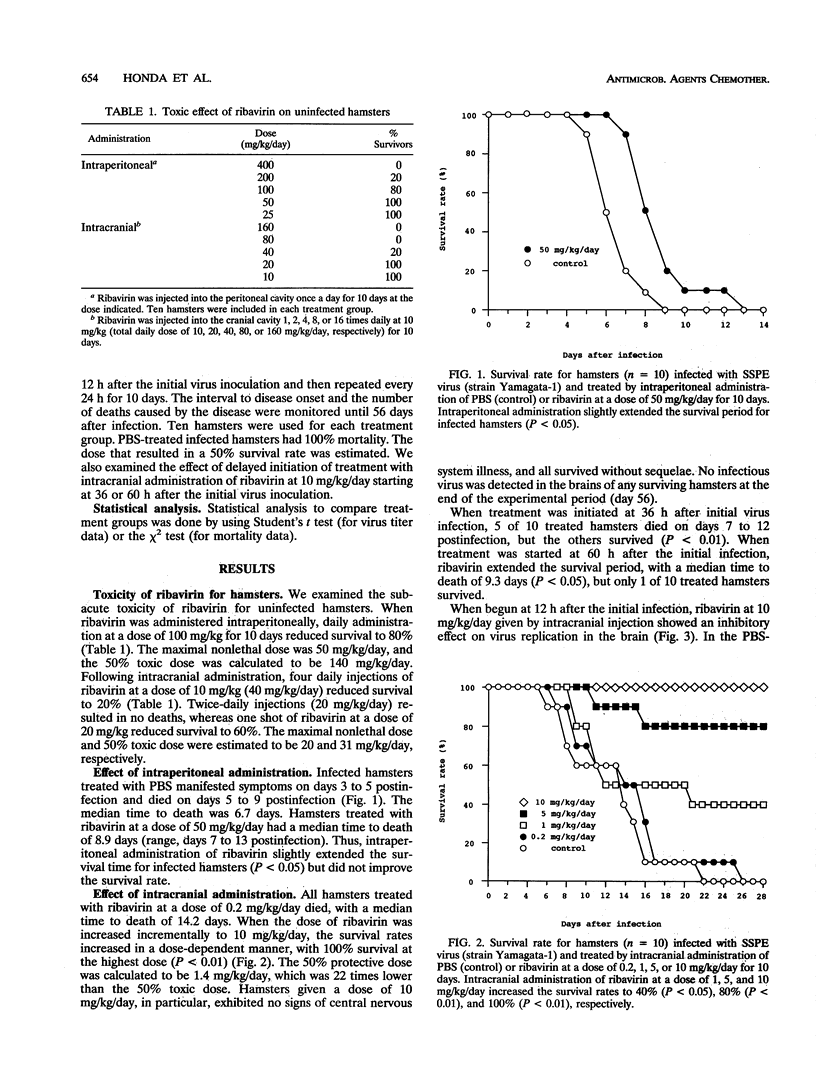
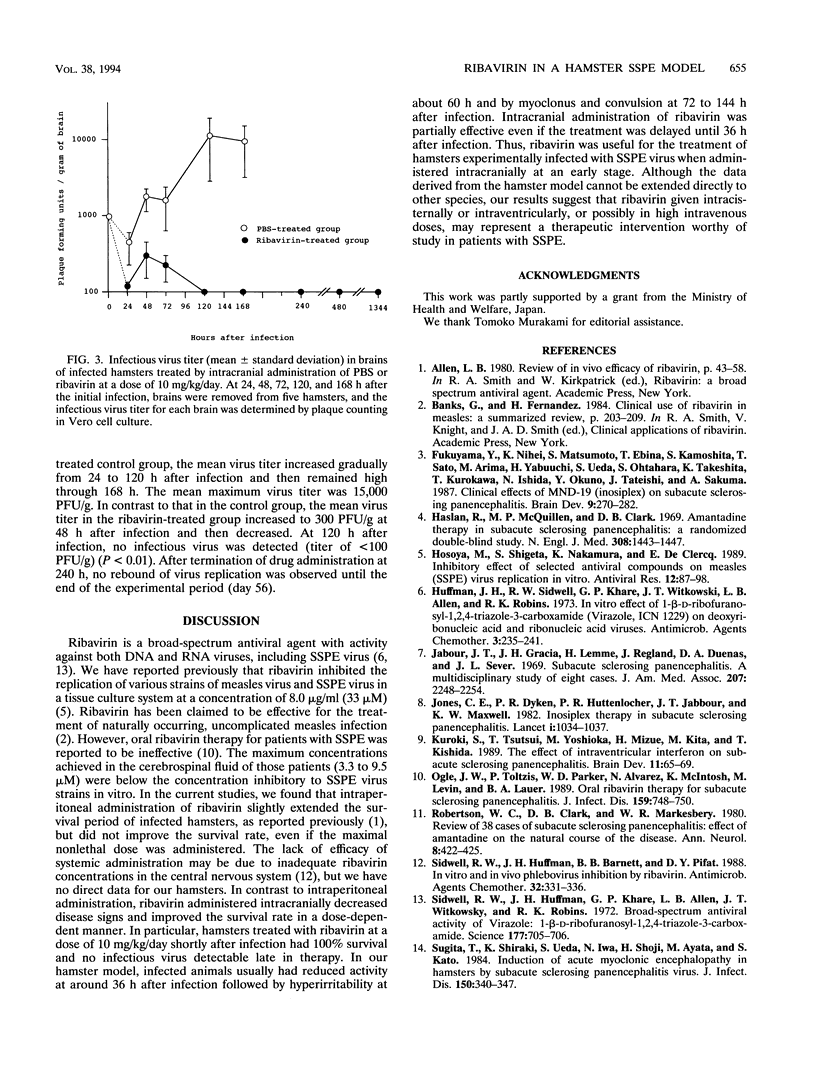
The antiviral activity of ribavirin was studied in hamsters infected with subacute sclerosing panencephalitis (SSPE) virus. Ribavirn did not improve the survival of infected hamsters when administered intraperitoneally at the maximal nonlethal dose of 50 mg/kg/day for 10 days. However, when administered intracranially, ribavirin improved the survival of infected hamsters in a dose-dependent manner. The 50% effective dose was calculated to be 1.4 mg/kg/day, and the selectivity index, based on the ratio of the 50% lethally toxic dose (31 mg/kg/day) to the 50% effective dose, was 22. When begun 12 h, but not 36 h, postinfection, ribavirin at a dose of 10 mg/kg/day completely prevented mortality and inhibited the replication of SSPE virus in brains of infected hamsters. Intrathecal or intraventricular administration of ribavirin should be explored for potential use in the treatment of patients with SSPE.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fukuyama Y., Nihei K., Matsumoto S., Ebina T., Kamoshita S., Sato T., Arima M., Yabuuchi H., Ueda S., Ohtahara S. Clinical effects of MND-19 (Inosiplex) on subacute sclerosing panencephalitis--a multi-institutional collaborative study--The Inosiplex-SSPE Research Committee. Brain Dev. 1987;9(3):270–282. doi: 10.1016/s0387-7604(87)80044-x. [DOI] [PubMed] [Google Scholar]
- Hosoya M., Shigeta S., Nakamura K., De Clercq E. Inhibitory effect of selected antiviral compounds on measles (SSPE) virus replication in vitro. Antiviral Res. 1989 Sep;12(2):87–97. doi: 10.1016/0166-3542(89)90072-7. [DOI] [PubMed] [Google Scholar]
- Huffman J. H., Sidwell R. W., Khare G. P., Witkowski J. T., Allen L. B., Robins R. K. In vitro effect of 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide (virazole, ICN 1229) on deoxyribonucleic acid and ribonucleic acid viruses. Antimicrob Agents Chemother. 1973 Feb;3(2):235–241. doi: 10.1128/aac.3.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbour J. T., Garcia J. H., Lemmi H., Ragland J., Duenas D. A., Sever J. L. Subacute sclerosing panencephalitis. A multidisciplinary study of eight cases. JAMA. 1969 Mar 24;207(12):2248–2254. doi: 10.1001/jama.207.12.2248. [DOI] [PubMed] [Google Scholar]
- Jones C. E., Dyken P. R., Huttenlocher P. R., Jabbour J. T., Maxwell K. W. Inosiplex therapy in subacute sclerosing panencephalitis. A multicentre, non-randomised study in 98 patients. Lancet. 1982 May 8;1(8280):1034–1037. doi: 10.1016/s0140-6736(82)92097-9. [DOI] [PubMed] [Google Scholar]
- Kuroki S., Tsutsui T., Yoshioka M., Mizue H., Kita M., Kishida T. The effect of intraventricular interferon on subacute sclerosing panencephalitis. Brain Dev. 1989;11(1):65–69. doi: 10.1016/s0387-7604(89)80012-9. [DOI] [PubMed] [Google Scholar]
- Ogle J. W., Toltzis P., Parker W. D., Alvarez N., McIntosh K., Levin M. J., Lauer B. A. Oral ribavirin therapy for subacute sclerosing panencephalitis. J Infect Dis. 1989 Apr;159(4):748–750. doi: 10.1093/infdis/159.4.748. [DOI] [PubMed] [Google Scholar]
- Robertson W. C., Jr, Clark D. B., Markesbery W. R. Review of 38 cases of subacute sclerosing panencephalitis: effect of amantadine on the natural course of the disease. Ann Neurol. 1980 Oct;8(4):422–425. doi: 10.1002/ana.410080414. [DOI] [PubMed] [Google Scholar]
- Sidwell R. W., Huffman J. H., Barnett B. B., Pifat D. Y. In vitro and in vivo Phlebovirus inhibition by ribavirin. Antimicrob Agents Chemother. 1988 Mar;32(3):331–336. doi: 10.1128/aac.32.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidwell R. W., Huffman J. H., Khare G. P., Allen L. B., Witkowski J. T., Robins R. K. Broad-spectrum antiviral activity of Virazole: 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science. 1972 Aug 25;177(4050):705–706. doi: 10.1126/science.177.4050.705. [DOI] [PubMed] [Google Scholar]
- Sugita T., Shiraki K., Ueda S., Iwa N., Shoji H., Ayata M., Kato S. Induction of acute myoclonic encephalopathy in hamsters by subacute sclerosing panencephalitis virus. J Infect Dis. 1984 Sep;150(3):340–347. doi: 10.1093/infdis/150.3.340. [DOI] [PubMed] [Google Scholar]



