Abstract
Plants continuously generate new tissues and organs through the activity of populations of undifferentiated stem cells, called meristems. Here, we discuss the so-called shoot apical meristem (SAM), which generates all the aerial parts of the plant. It has been known for many years that auxin plays a central role in the functioning of this meristem. Auxin is not homogeneously distributed at the SAM and it is thought that this distribution is interpreted in terms of differential gene expression and patterned growth. In this context, auxin transporters of the PIN and AUX families, creating auxin maxima and minima, are crucial regulators. However, auxin transport is not the only factor involved. Auxin biosynthesis genes also show specific, patterned activities, and local auxin synthesis appears to be essential for meristem function as well. In addition, auxin perception and signal transduction defining the competence of cells to react to auxin, add further complexity to the issue. To unravel this intricate signaling network at the SAM, systems biology approaches, involving not only molecular genetics but also live imaging and computational modeling, have become increasingly important.
Auxin dynamically regulates patterning at the shoot apical meristem. Transporters and local biosynthesis are involved in the control of its distribution at the shoot apex, where it is required for formation of new buds.
Plants continuously generate new tissues and organs through the activity of populations of undifferentiated stem cells, called meristems. Because meristems can modulate their activity, they provide the developmental flexibility that allows plants to adapt their development in reaction to the environment (reviews: Lyndon 1998; Traas and Doonan 2001; Aida and Tasaka 2006; Sablowski 2007).
Distinct meristems exist. Apical meristems, positioned at the tip of the shoots and roots, initiate aerial and underground organs, respectively. Along the stems and roots, more diffuse secondary meristems are responsible for secondary thickening of these structures.
The plant hormone auxin plays an instrumental role in meristem biology and we discuss here its role in a particular meristem, the shoot apical meristem (SAM) that generates all the aerial organs including the floral meristems (Fig. 1A–C). In this context, we limit ourselves to the meristems in angiosperm that have been studied in most detail.
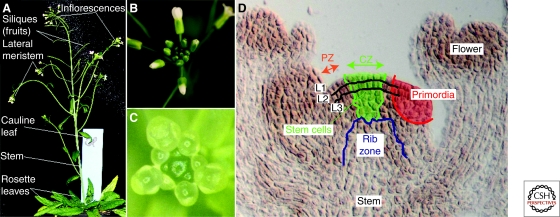
Figure 1.
The shoot apical meristem of Arabidopsis thaliana. (A) Aerial part of a wild-type plant of the Columbia ecotype (Col-0). The SAM is responsible for the production of rosette leaves and, after floral transition, for the production of the stem, cauline leaves, lateral meristems, and flowers of the inflorescence. (B) Details of the tip of the inflorescence, showing the highly organized positioning of flowers around the main axis (a spiral). (C) A dissected inflorescence meristem. Older flowers have been removed to expose the meristem surrounded by young floral buds. (D) Longitudinal section of an inflorescence meristem showing the layered organization (L1, L2, and L3 cell layers). L1 and L2 are also called the tunica and L3 to the corpus. The functional zones are also represented. At the meristem summit the central zone (CZ) contains the stem cells, whereas primordia are initiated in the peripheral zone (PZ). The rib zone (RZ) produces the internal part of the stem.
SHOOT APICAL MERISTEM STRUCTURE AND GENETIC REGULATION
All SAMs have a number of basic characteristics in common (e.g., Lyndon 1998; Traas and Doonan 2001). One of the most prominent features is the presence of a surface layer, called the tunica (Fig. 1D). The tunica—itself often composed of several sublayers—covers the internal cells of the meristem, collectively called corpus. The tunica layers are kept separate as their cells most frequently divide in anticlinal orientations, causing daughter cells to remain in the same layer as their parent. If present, the internal tunica layer—called L2—usually disappears during organ initiation, when L2 cells start to divide in random directions.
Superimposed on this layered organization is a partitioning into zones (Fig. 1D). These zones, characterized by sometimes subtle cytological differences, have well defined functions. At the meristem summit a small group of slowly dividing cells, called the central zone (CZ), ensures a true stem cell function and is responsible for meristem maintenance. The growth rates at the meristem summit usually differ considerably from those at the periphery where cells accelerate their proliferation rates. It is in this peripheral zone (PZ) that new lateral organs are initiated: a restricted number of founder cells increase their proliferation and growth rates resulting in the formation of a rapidly outgrowing primordium, delimited by the organ boundary region, where cell expansion is reduced.
Extensive genetic screens in different species have identified a network of regulators involved in meristem function, organ initiation, and outgrowth, which is described briefly in the following paragraphs (for reviews: Traas and Doonan 2001; Aida and Tasaka 2006; Sablowski 2007; Rast and Simon 2008). Very similar mechanisms are found in different species, and here we discuss what is known on the best-studied model, Arabidopsis thaliana.
A central role in shoot meristem maintenance is played by the transcription factor WUSCHEL (WUS) as in corresponding mutants a SAM is initiated but arrests after having produced a few organs (Laux et al. 1996). Its precise targets and regulators are largely unknown, but it has been well established that it interferes with hormone signaling cascades, in particular cytokinins (Leibfried et al. 2005; Gordon et al. 2009). WUS is involved in a negative feedback loop with the CLAVATA receptor kinase signaling cascade (CLV), whose activity limits the size of the pool of stem cells (see Fig. 2C for expression patterns of the genes).
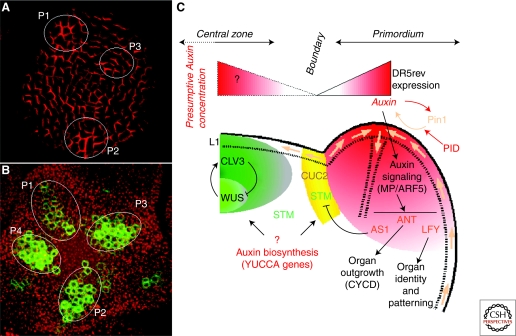
Figure 2.
From dynamic transport to patterning: Auxin and organogenesis at the shoot apical meristem of Arabidopsis thaliana. (A) Immunodetection of PIN1 efflux carrier in the L1 (top view). The image was obtained by confocal microscopy. Note the subcellular polarized localization of the auxin transporter in most cells. The localization suggests that auxin accumulates in these young organ primordia (named P1, P2, and P3 from the oldest to the youngest organ). Adapted from de Reuille et al. (2006). (B) Expression of the synthetic DR5rev::GFP reporter in the inflorescence meristem (top view). Projection of serial optical sections obtained by confocal microscopy. The green corresponds to the GFP and the red to the autofluorescence of meristematic cells. DR5 expression in young emerging primordia indicates activation of auxin induced-genes. (C) Schematic representation of the role of auxin during organogenesis in the inflorescence meristem of Arabidopsis thaliana. See text for details.
At the periphery of the meristem, the initial recruitment of the organ founder cells appears to be the result of two antagonistic processes. First, the homeodomain protein SHOOTMERISTEMLESS (STM), in combination with several members of the so-called CUP SHAPED COTYLEDON (CUC) family of transcription factors, defines meristematic identity, preventing cells from being recruited by the young organs (e.g., Aida et al. 1999). Therefore, as a new organ is initiated, these meristematic identity genes are switched off in Arabidopsis. This involves the transcription factor ASYMMETRIC LEAVES1 (AS1), which maintains the repression of meristem identity factors such as STM in the developing primordia (Byrne et al. 2000). The meristem either generates leaves and lateral meristems or flowers. The identity of the lateral organs produced depends on the activity of LEAFY (LFY), which, by interacting with different transcription factors such as WUS or AGAMOUS (AG), plays a major role in plant and flower architecture (Lenhard et al. 2001; Lohmann et al. 2001).
The patterning of the flower meristem itself largely depends on transcriptional regulation (for reviews: Krizek and Fletcher 2005; Bowman and Floyd 2008). This includes genes that control organ identity, organ number, organ boundaries, and symmetry. In Arabidopsis, for example, APETALA1 (AP1), CAULIFLOWER (CAL), PISTILLATA (PI) SEPALLATA1 (SEP1), and APETALA3 (AP3) direct the development of sepals and petals. Other relevant genes are upstream regulators of these identity genes (e.g., UNUSUAL FLORAL ORGANS and LFY) but also genes that control the number of sepals and petals formed in each flower (PERIANTHIA, WIGGUM), genes required to establish organ boundaries in all shoot meristems (CUC, genes), and genes that participate in organ patterning (KANADIs, YABBYs, and the PHABULOSA/PHAVOLUTA/REVOLUTA group).
Once the founder cell populations of the organs have been identified, they grow out. Our knowledge on the genetic regulation of growth control is more limited and the various signals that integrate and coordinate cell proliferation and expansion remain largely unknown. Some of the connections between the cellular processes and transcriptional regulation have been identified. AINTEGUMENTA (ANT), for instance, is strongly expressed in the outgrowing organs and acts at least partially via regulating genes involved in cell proliferation like CYCLIN D (Mizukami and Fischer 2000). In petals, several genes control growth by affecting cell proliferation and/or cell expansion (Anastasiou and Lenhard 2007). Some of these genes (i.e., JAGGED, ANT, and ARGOS) affect petal growth by positively regulating cell proliferation (Mizukami and Fischer 2000; Hu et al. 2003; Dinneny et al. 2004), whereas other genes (BIGBROTHER, KLUH, and DA1) control final organ size by negatively regulating the duration/period of cell proliferation (Disch et al. 2006; Anastasiou et al. 2007; Li et al. 2008). BIGPETAL, which is regulated downstream of the flower organ identity genes, limits petal growth by controlling cell expansion (Szecsi et al. 2006).
In addition to transcriptional control, posttranscriptional regulation by microRNAs (miRNAs) plays an important role in meristem function. These include, for example, the miR164 and miR156/157 families, which respectively target the CUC and SPL genes (Peaucelle et al. 2007; Wang et al. 2009).
How is this complex regulatory network coordinated? It has been known for many years that auxin plays a central role in meristem function and organ formation. Auxin is not homogeneously distributed at the SAM and it is thought that this distribution is interpreted in terms of differential gene expression and patterned growth. Because the distribution of auxin is largely under the control of auxin transporters, we first discuss auxin transport at the shoot apex.
AUXIN TRANSPORT AND SAM FUNCTION
Auxin Influx and Efflux Carriers Control Auxin Distribution at the SAM
Okada et al. (1991) identified an Arabidopsis mutant called pin-formed 1 (pin1) in reference to its needle-like inflorescence stem, unable to initiate flowers. Identification of the gene showed that it encoded a transmembrane protein (Galweiler et al. 1998) and there is now overwhelming evidence that the PIN1 protein is the founding member of a family of auxin carriers that transport auxin across membranes (for review: Petrasek and Friml 2009). More particularly, PIN1 and closely related family members are involved in auxin efflux out of the cells. PIN1 is strongly expressed in the SAM, where it is mainly found in the tunica and vascular tissues (Fig. 2A). The activity of other PIN-members at the SAM has not been analyzed in detail. Expression studies show that at least PIN 2 and 7 are active in the shoot apex but single mutations in these genes do not significantly perturb meristem function (Muller et al. 1998; Friml et al. 2003). The pin1 phenotype can locally be complemented by local, external applications of high concentrations of auxin, which are able to induce the formation of flower buds (Reinhardt et al. 2000). These applications indicate that high auxin concentrations are required for the initiation of a new organ and, importantly, that PIN transporters are required for the creation of such auxin maxima. Direct evidence demonstrating the existence of auxin maxima at the periphery of the meristem are still lacking because of the absence of a true auxin sensor. However, this scenario is further corroborated by the activation of the synthetic DR5 auxin reporter in the young organs (Fig. 2B). DR5 is composed of a fusion of a synthetic promoter consisting of several auxin response elements (AuxRE) and a minimal 35S promoter with a reporter gene (Sabatini et al. 1999). The AuxREs corresponds to the DNA sequences bound by the AUXIN RESPONSE FACTORS (ARFs; see later for more details) and expression of the reporter gene thus suggests activation of auxin-induced genes during the earlier stages of organ initiation (Benkova et al. 2003; de Reuille et al. 2006; Smith et al. 2006). In addition, the polarity of the PIN transporters has been analyzed to identify the putative direction of auxin fluxes. This has confirmed the hypothesis that auxin fluxes are directed toward the young primordia (Benkova et al. 2003; Reinhardt et al. 2003).
Second sets of transporters associated with auxin distribution at the SAM are the AUX/LAX influx carriers. AUX1, the founding member of the gene family (Bennett et al. 1996), is expressed in the L1 surface layer of the shoot apical meristem (Reinhardt et al. 2003). The protein seems to be evenly localized over all membranes, indicating that the protein is not involved in the creation of hormone fluxes. Instead, it might rather concentrate auxin at the meristem surface. Somewhat surprisingly, its loss of function has only a relatively mild phenotype in the shoot, including perturbations in phyllotactic patterns. The AUX1 sequence shows a high degree of similarity with three other sequences in the Arabidopsis genome, named LAX1, LAX2, and LAX3 (Like AUX1) (Swarup and Bennett 2003), but even the quadruple mutant is still able to produce a viable, fertile plant albeit with important changes in its architecture (Bainbridge et al. 2008). It is therefore conceivable that other proteins, such as certain P-glycoprotein transporters (PGPs), which have also been associated with auxin import, share a redundant function with the members of the LAX family in the meristem (For review: Boutte et al. 2007). Another clue on the precise role of AUX1 comes from a double pin1/aux1 mutant. Auxin applications do not induce single flowers on the apex of such a double mutant, but rather very large, fused organs, suggesting that somehow AUX1 is required for the restriction of organ boundaries (Reinhardt et al. 2003). Altogether, the available data indicate that the formation of local auxin maxima mainly depends on the action of PIN exporters at the meristem surface. AUX and LAX proteins would facilitate organ positioning, probably by guaranteeing a sufficient supply in the L1 layer.
Using Modeling to Explore Polar Auxin Transport Regulation: Canalization or Up-the-Gradient?
The distribution of PIN at the meristem surface is complex and it is not obvious from simple visual inspection what the predicted auxin fluxes would be. Therefore, a set of careful localization studies were performed and the properties of the cellular transport networks analyzed using computer simulations (de Reuille et al. 2006). This confirmed that PIN directs auxin to the sites where young primordia are being formed. The same computer simulations also identified additional properties of the transport network, and in particular a still undefined role for the meristem summit in auxin redistribution was proposed (de Reuille et al. 2006).
What coordinates auxin fluxes in the meristem? The complex and dynamic patterns of PIN distribution could suggest very intricate regulatory mechanisms. Computer models, however, have shown that the actual basis of the observed transport patterns could be very simple. Jönsson et al. (2006) and Smith et al. (2006) proposed models where cells check the auxin concentrations in their direct environment and subsequently pump auxin toward neighbors with a higher concentration, i.e., against the gradient. Because these patterning processes require the interaction of hundreds of cells, it is impossible to estimate on a purely intuitive basis if a particular scenario is plausible or not. Therefore, computational modeling was used as a powerful means to test this hypothesis. Interestingly, these models showed that transport “against the gradient” is sufficient to reproduce realistic PIN1 patterns and to generate different types of phyllotactic patterns (Jonsson et al. 2006; Smith et al. 2006).
It should be noted that, although these models show that the mechanism is plausible, they by no means provide absolute proof. Indeed, other models are able to explain the distribution of auxin transporters. Another hypothesis, for instance, was based on the pioneering work of Sachs who proposed the existence of a positive feedback between flux and transport (Sachs 1969). It was subsequently shown that this mechanism is able to amplify small fluxes and can potentially create canals of auxin between hormone sources and sinks. A range of experiments supports the canalization hypothesis, at least in the inner tissues of the plant where it can account for the formation of venation patterns (Scarpella et al. 2004; Sauer et al. 2006; Scarpella et al. 2006). The existence of canalization would imply the coexistence of two radically different mechanisms for PIN allocation to the membrane, one based on flux sensing (in the inner tissues) and the other on local concentration sensing (at the meristem surface). Therefore, Stoma and colleagues (2008) tested whether canalization could potentially also account for the behavior of auxin transporters at the shoot apical meristem surface. Using a dedicated computer simulation tool, they showed that this was indeed the case, thus providing a unifying concept for the control of auxin distribution in the plant (Stoma et al. 2008). More recently, Bayer and coworkers tested the alternative scenario where both modes of auxin transport (i.e., up-the-gradient and canalization) coexist. One or the other mechanism would prevail, depending on the absolute auxin concentrations (Bayer et al. 2009). This model also reproduced realistic distributions of PIN proteins. Further experiments are now required to distinguish between flux-based polarization and other hypotheses. Although these models remain to be tested, notably through the identification of the molecular mechanism controlling PIN1 polarity, they show that very simple local behaviors of individual cells can generate the complex overall patterns we observe.
Orienting Auxin Transport at the SAM: The Role of PID
Concepts like canalization are still relatively abstract and could represent a combination of processes. Indeed, although the exact mechanisms that control the dynamics of PIN1 polar localization are still unknown, accumulating evidence indicates that it depends on multiple processes, such as membrane traffic and cytoskeleton organization (for review: Kleine-Vehn and Friml 2008). A striking set of data also points at the importance of phosphorylation and the PINOID (PID) serine-threonine protein kinase (Christensen et al. 2000; Benjamins et al. 2001). Arabidopsis mutants perturbed in the PID gene have pin-like inflorescences. Interestingly, this goes along with an apical-to-basal shift in PIN1 polarity (Friml et al. 2004). Accordingly, in the root, ectopic expression of PID induces a basal-to-apical shift in PIN2 and PIN4 polarities. The results so far indicate that the PID kinase controls PIN localization via direct phosphorylation of the transporter (Michniewicz et al. 2007). Interestingly, the kinase does not seem to affect the polarized localization per se, but rather decides on what particular membrane the carrier will be localized. Lee and Cho (2006) also showed that PID may facilitate trafficking of PIN to the plasma membrane, suggesting that it may not only affect the polarity of PIN1 but also the dynamics of polarity changes in meristematic cells (Lee and Cho 2006). Antagonist regulation of PIN1 by PP2A phosphatase has been shown (Michniewicz et al. 2007) and PID activity itself depends on its autophosphorylation status (Zegzouti et al. 2006). Precise modulation of both PIN1 and PID phosphorylation status is thus an essential regulation level of PIN1 localization in the meristem. It was recently shown that PINs are initially delivered to the plasma membrane in a nonpolar manner and that their polarity is established by subsequent endocytic recycling (Dhonukshe et al. 2008). It is thus possible that PID could be involved in directing PIN1 to specific membranes, thus participating in controlling the dynamics of the PIN1 network in the meristem.
Other genes are involved in the PID pathway, like NPY1/ENP/MAB4, a NPH3-like protein first identified as an enhancer of PID during cotyledon development (Treml et al. 2005; Furutani et al. 2007). Mutations in NPY1 induce pin-like inflorescence in the yuc1 yuc4 (yucca) double mutant affected in auxin biosynthesis ([Cheng et al. 2007b]; see part 4 for discussion on the role of the YUCCA genes). NPY1 is expressed in the L1 at the meristem and accumulates in the young organs (Cheng et al. 2007b; Furutani et al. 2007). The inactivation of NPY1 together with two of its closest homologs also completely abolishes organ initiation at the SAM (Cheng et al. 2008). Although the exact role of NPY1 and its homologs still needs to be established, these results suggest that the NPY protein mediates or facilitates PID action on PIN1 localization.
It is interesting to note that PID is expressed at a higher level in the frontiers that separate organs from the meristem (Furutani et al. 2004). It is precisely at these boundaries that PIN undergoes important relocalizations (Heisler et al. 2005). PID could therefore play a decisive role in organ separation.
THE ROLE OF AUXIN METABOLISM AT THE SAM
Although the role of polar auxin transport during developmental processes, notably in the shoot meristem, has been extensively studied, little attention has been paid to the role of auxin metabolism until recent years. However, local concentrations of auxin in the shoot meristem are expected to be under the control of the combined action of polar auxin transport and auxin biosynthesis, but also of auxin catabolism and conjugation. Auxin metabolism is still largely under investigation and its complexity provides one historical reason for focusing primarily on polar auxin transport. Nevertheless, recent studies have identified mutants in key biosynthetic enzymes that highlight the importance of local auxin biosynthesis in the shoot meristem and underline the need to consider this parameter when studying the role of auxin in the shoot meristem and more generally in plant development.
Several excellent reviews have described our current knowledge on auxin metabolism (e.g., Ljung et al. 2002; Woodward and Bartel 2005). We will therefore focus mainly on its importance in shoot apical meristem function.
Local Auxin Biosynthesis is Essential for Shoot Apical Meristem Function
IAA biosynthesis occurs mostly through a tryptophan (Trp)-dependent pathway and involves four parallel and partly interdependent pathways named after the main intermediates: the indole-3-pyruvic acid (IPA), tryptamine (TAM), indole-3-acetaldoxime (IAOx), and indole-3-acetamide (IAM) pathways (Woodward and Bartel 2005). The recent identification of the YUCCA (YUC) family of auxin biosynthetic genes encoding flavin monooxygenases regulating the TAM pathway has been instrumental in demonstrating that auxin biosynthesis is not homogenous in a given tissue and that a fine control of auxin biosynthesis in the shoot apex plays a key role in the regulation of the activity of the shoot and floral meristems.
The YUC family has 11 members and YUC1, its founding member, was isolated based on an increase in hypocotyl length in an activation-tagging screen (Zhao et al. 2001). The yuc1-D gain-of-function mutant displayed typical auxin overproduction phenotype, such as epinastic cotyledons and long hypocotyl, short roots and increased apical dominance. Based on in vitro biochemical assays and on resistance of the mutant to the toxic tryptophan analog, 5-methyl-tryptophan, it was proposed that YUC1 catalyzed the oxidization of tryptamine to N-hydroxyl-tryptamine, a rate-limiting step in the tryptophan-dependent auxin biosynthesis tryptamine pathway (Zhao et al. 2001). The importance of YUC-dependent local auxin biosynthesis in organ initiation was revealed by the analysis of multiple loss-of-function yucca mutants in Arabidopsis (Cheng et al. 2006; Cheng et al. 2007a). Although none of the single mutants showed obvious phenotypes, several double and triple mutant combinations and the quadruple mutant of yuc1, yuc2, yuc4, and yuc6 displayed strong inflorescence and floral developmental defects as well as alterations of leaf vasculature (Cheng et al. 2006). These floral and vascular phenotypes are reminiscent of those observed in the pin1, pid, and ettin/arf3 mutants (Okada et al. 1991; Bennett et al. 1995; Sessions et al. 1997). In the quadruple yuc mutant, pin-like structures were also observed, indicating that these four YUC genes are involved in floral development from the initiation of the flower onwards. The analysis of the expression pattern of the YUC genes further suggested that they regulate flower initiation and development through the spatial and temporal control of auxin biosynthesis. Direct evidence that the developmental defects in the multiple yuc mutant were caused by a local decrease in auxin biosynthesis was shown by complementing the yuc1yuc4 double mutant with a bacterial auxin biosynthesis gene under the control of the YUC1 promoter (Cheng et al. 2006). In addition, Cheng et al. (2007) showed through the analysis of combinations of mutants in yuc1, yuc4, yuc10, and yuc11 that auxin synthesized by the YUC proteins is also necessary for leaf development.
The temporal and spatial regulation of YUC genes thus appears to provide a mechanism for fine-tuning the concentration of auxin during organ initiation and development at the shoot apex. Mutants in YUC homologs of petunia, maize, and rice (Tobena-Santamaria et al. 2002; Fujino et al. 2008; Gallavotti et al. 2008) exhibited similar developmental defects, indicating that the regulation of the TAM pathway for auxin biosynthesis is a conserved mechanism for modulating auxin in the angiosperms. The identification of TRYPTOPHAN AMINOTRANSFERASE of ARABIDOPSIS 1 (TAA1), a long-predicted key enzyme in the indole-3-pyruvic acid (IPA) pathway, and its paralogs (TRYPTOPHAN AMINOTRANSFERASE RELATED or TAR) has recently shown that spatial regulation of auxin biosynthesis through this second Trp-dependent pathway might also play a similar role during development (Stepanova et al. 2008; Tao et al. 2008). Stepanova et al. (2008) notably found that the double taa1/tar2 mutant displays a severe reduction of growth of the aerial tissues and alterations of flower development, suggesting a role in floral organ initiation. As for the YUC genes, TAA1 expression is restricted to specific parts of the flower and the gene might be involved in the local regulation auxin biosynthesis.
At least two nonredundant auxin biosynthesis pathways are thus implicated in organ initiation at the shoot or floral meristem. The phenotype obtained on inactivation of these pathways indicates that they are necessary to control locally auxin homeostasis at the shoot meristem and that auxin transport is probably not the only limiting mechanism for generating the spatial variations in auxin concentration implicated in lateral organ initiation.
Integrating Auxin Metabolism and Transport at the Shoot Meristem: A Challenge for the Future
The results discussed in the previous paragraphs suggest that the integrated action of both auxin transport and metabolism define where and when auxin will accumulate. Ljung et al. (2001) showed the existence of a feedback mechanism between biosynthesis and transport by measuring auxin concentration and synthesis rates on treatment of young Arabidopsis roots with NPA (Ljung et al. 2001). Cheng et al. (2007) suggest that such a feedback mechanism is also active in the shoot meristem because combinations of the pin1 mutation with yuc1 and yuc4 enhances pin1 defects, blocking almost completely leaf and flower initiation. A similar phenotype is obtained by combining aux1 with yuc1, yuc2, yuc4, and yuc6 (Cheng et al. 2007a). Cross-regulations between auxin transport and biosynthesis could be a key feature explaining the robustness of auxin gradients (Weijers et al. 2005) and it would be particularly instructive to analyze the effect of incorporating auxin production and inactivation on the stability of phyllotaxis models. Further work on the spatial control of auxin biosynthesis will probably be essential in this context.
FROM AUXIN SIGNAL TRANSDUCTION TO MORPHOGENESIS AT THE SHOOT APEX
The action of auxin does not only depend on the regulation of its synthesis or transport, but the competence to react to auxin seems also to be controlled in time and space. We now discuss the regulation of auxin signal transduction in the shoot meristem before considering how auxin interacts with the gene networks underlying organ outgrowth.
Spatial Modulation of Auxin Signal Transduction is Involved in Meristem Function
Both biochemical and genetic approaches have identified the members of the Aux/IAA and ARF family of transcriptional regulators as major effectors of auxin signal transduction (for review: Leyser 2006). There are 29 Aux/IAA genes that encode mostly short-lived repressors of auxin-inducible genes. The 23 ARF proteins can be either activators (Q-rich ARFs) or repressors of transcription. Aux/IAA and ARF proteins are able to form homo- and heterodimers both within and between the families. The instability of the Aux/IAAs is intrinsic to the so-called domain II, which interacts directly with SCF-like ubiquitin protein ligases harboring the TIR1 F-box protein or one of the three Auxin-related F-box (AFB) proteins (Dharmasiri et al. 2005a; Dharmasiri et al. 2005b; Kepinski and Leyser 2005; Tan et al. 2007). TIR1 and the AFBs act directly as auxin receptors and their activation leads to auxin-dependent degradation of Aux/IAAs. The current simplified model for auxin transduction is that Aux/IAAs dimerize with the Q-rich ARFs. These complexes bind to auxin-inducible genes, thus preventing transcription. By promoting the degradation of the Aux/IAAs by the SCF, auxin would allow the ARFs to activate transcription of target genes by binding to the AuxREs present in their promoter.
When pin1 meristems are treated with exogenous auxin, all the cells at the periphery of the meristem are competent to respond to auxin to initiate organs (Reinhardt et al. 2000). However, auxin is not able to induce organs at the central zone of the meristem. Mutation in MONOPTEROS/ARF5 induces a phenotype similar to pin1 and blocks the ability of the meristematic cells to respond to exogenous auxin (Hardtke and Berleth 1998; Reinhardt et al. 2003). Because MP/ARF5 is only expressed at the meristem periphery (Hardtke and Berleth 1998), the competence for organ initiation at the periphery of the meristem thus depends, at least in part, on a spatial modulation of auxin signal transduction. Despite an extensive genetic analysis of the ARF and Aux/IAA families (Okushima et al. 2005; Overvoorde et al. 2005), no other ARF and no Aux/IAA has been implicated in shoot meristem function, although indirect evidence suggests that several ARFs could be involved (Hardtke 2004; Pekker 2005; Mallory 2005). The lack of genetic evidence could be because of the demonstrated redundancy between these transcription regulators (Ellis 2005; Pekker 2005; Okushima 2005). It will therefore be important to generate multiple mutant combinations for ARFs as well as dominant negative mutant in all Aux/IAA members. Together with an exploration of the expression patterns of these two gene families, this should give valuable information of the role of signal transduction modulation in the dynamics of auxin responses in the meristem.
Induction of the Morphogenetic Program by Auxin in the Shoot Meristem
The analysis of cell identity in the meristem of the pin1 mutant gave the first clues concerning the role of auxin in the control of cell identity at the meristem (Vernoux et al. 2000). Although meristem structure and maintenance were not severely affected in the mutant, major alterations occurred at the periphery of the pin1 meristem, where organ initiation should occur. A ring-like domain expressing two early markers of organ initiation, LFY and ANT, but also the boundary marker CUC2 was observed around the central zone. This showed that the zone at the meristem periphery has a hybrid identity in pin1. These results also implied that auxin levels control the identities of the cells at the periphery of the meristem, thus impacting organ separation, positioning, and outgrowth. Analysis of the genetic interactions between PIN1 and MP/ARF5 further suggested a differential regulation of the CUC genes by PIN1 and MP during embryogenesis (Aida et al. 2002). Taken together, these data suggest a model where auxin, through PIN1 and MP, could regulate patterning at the meristem partially through the control of CUC gene expression (Fig. 2C).
More recently, live-imaging of Arabidopsis inflorescence meristems allowed detailed analysis of the sequence of gene activation in relation to auxin transport and responses (Heisler et al. 2005). These authors used PIN1-GFP translational fusion in conjunction with the auxin-responsive DR5::VENUS marker, to show that the convergence of PIN1 proteins toward the site of the new organ is likely the first event of organogenesis and is immediately followed by auxin-induced transcription. By combining multiple GFP-protein fusions, their work further suggests that the KNOX gene STM and CUC2 are down-regulated in the early primordia, whereas they are up-regulated in the frontiers in response to specific auxin concentration and/or responses. This elegant analysis not only linked auxin patterns at the meristem to developmental patterning but also supported the proposed role of PIN1 in CUC regulation discussed earlier. In addition to the effects on STM, Hay et al. (2006) demonstrate that BREVIPEDICELLUS (BP), another KNOX gene, is ectopically expressed in the leaves of a pin1 mutant, whereas the bp mutation slightly suppresses both pin1 and pid mutants (Hay et al. 2006). These data support a scenario in which the expression of KNOX genes is first repressed in the incipient primordium by an auxin-dependent mechanism, and is later maintained in a repressed state via the action of regulators such as AS1 (Fig. 2C).
Auxin is not only involved in organ initiation but is also associated with the establishment of symmetry. In vivo imaging suggests that auxin maxima precede and might control the establishment of adaxial/abaxial symmetry by FILAMENTOUS FLOWER and REVOLUTA (Heisler et al. 2005). Such a role was further supported by genetic studies, identifying ETTIN/ARF3 and ARF4 as regulators of organ asymmetry (Pekker et al. 2005). Another link between auxin and organ symmetry involves members of the KANADI (KAN) gene family, which regulate abaxial identity and laminar growth of lateral organs (Bowman and Floyd 2008). Mutations in ARF3/ETTIN were isolated as second site suppressors of ectopic KAN expression (Pekker et al. 2005). Further genetic and expression analysis indicated that ARF3/ETT and ARF4 mediate the KAN pathway but that KAN does not regulate ARF3 and ARF4 activation in lateral organs. ARF3 and ARF4 are expressed during the early stages of organ initiation (Sessions et al. 1997; Pekker et al. 2005), indicating that modulation of auxin activity is likely cooperatively involved with KAN in setting up adaxial/abaxial organ polarity (Pekker et al. 2005).
INTEGRATION OF HORMONE SIGNALING AT THE SHOOT APEX
As in other tissues, SAM function relies on the combinatorial action of several hormones (Shani et al. 2006). We review here briefly the best-characterized hormone interactions involving auxin.
Studies on plant tissue regeneration have shown that a high cytokinin (CK) to auxin ratio can trigger the initiation of shoot meristems from undifferentiated callus but the molecular basis for such interactions in the shoot are just starting to emerge (Gordon et al. 2007; Gordon et al. 2009). In the last few years, it has been shown that high CK signaling is essential for maintenance of the meristem through a direct effect on stem cell activity. However, the identification and characterization of the aberrant pyllotaxy1 (abphyl1) mutant of maize suggest that CKs contributes to organ initiation together with auxin. This mutant shows decussate phyllotaxis, whereas distichous arrangement are usually observed in wild type plants (Jackson and Hake 1999). ABPHYL1 encodes a cytokinin-inducible RESPONSE REGULATOR, a primary cytokinin response gene likely to act in a negative feedback loop to lower CK signaling in the leaf primordia (Giulini et al. 2004). In abphyl1 mutants, auxin contents are lower than in wild type and zmPIN1 expression in the incipient leaf primordia is also greatly reduced (Lee et al. 2009). Unexpectedly, zmPIN1 is rapidly induced in the meristem on exogenous cytokinin applications, suggesting a complex regulation of PIN1 expression by CK. These observations in maize are partly consistent with the effect of CK on PIN expression observed in the Arabidopsis root meristem (Laplaze et al. 2007; Dello Ioio et al. 2008). Together with other recent findings, such as the regulation of CK degradation by auxin in leaf primordia during shade avoidance (Carabelli et al. 2007) or the activation of the CK receptor AHK4 by auxin in hypocotyl explants during the first steps of shoot regeneration (Gordon et al. 2009), these data also further support a possible fundamental role for auxin-CK interactions during organ formation and meristem function.
Auxin is also associated with another key class of hormones in the shoot meristem, the gibberellins (GA). The concentration of active GA is mainly controlled by GA 20-oxydases and GA 3-oxydases, required for GA biosynthesis, and by GA 2-Oxydases, which control GA deactivation. These enzymes are encoded by gene families, some of which show very specific expression patterns in the shoot meristems. In particular, biosynthetic enzymes are excluded from the meristem center and restricted to young organs, whereas deactivation enzymes are expressed at the base of the SAM, below the rib zone (Jasinski et al. 2005; Hu et al. 2008; Rieu et al. 2008). Auxin treatments on seedlings suggest that eight out of 13 GA oxydases (both activating or deactivating) expressed in seedlings are transcriptionaly regulated by auxin (Frigerio et al. 2006). Among those, at least GA20ox1, GA2ox2, and GA2ox4 are expressed in the vegetative SAM and could be targets of auxin signaling in the shoot, although this remains to be shown.
GENERAL CONCLUSION
In the last 10 years, auxin has emerged as a fundamental mobile signal in the shoot meristem as it triggers organ initiation at the periphery of the meristem. The data available so far clearly show that the dynamics of the PIN1 transport network generates localized auxin maxima that are responsible for organ initiation and changes in cell identity. In addition, there is accumulating evidence for an important role of local auxin biosynthesis, although very little is known on how this influences auxin distribution. Likewise, the role of other important regulators of auxin signaling, such as the putative receptor ABP1 (Braun et al. 2008), remains to be determined. The main challenge for the future will be to understand how auxin transport is integrated with auxin biosynthesis and other hormone signaling in order to coordinate cell behavior and differentiation in this complex dynamic structure. The use of systems biology approaches, together with the development of new biological tools to monitor auxin concentration and signaling in live tissues, will likely be the key to reach a new level in our understanding of hormonal signal integration at the shoot meristem.
ACKNOWLEDGMENTS
We would like to thank Olivier Hamant for critical reading of the manuscript. We would also like to apologize to our colleagues whose work was not cited because of space limitations. The research in the author’s laboratory is supported by the European Union, the Human Frontier Science Program Organization, and the Agence Nationale de la Recherche.
Footnotes
Editors: Mark Estelle, Dolf Weijers, Ottoline Leyser, and Karin Ljung
Additional Perspectives on Auxin Signaling available at www.cshperspectives.org
REFERENCES
- Aida M, Tasaka M 2006. Morphogenesis and patterning at the organ boundaries in the higher plant shoot apex u. Plant Mol Biol 60:915–928 [DOI] [PubMed] [Google Scholar]
- Aida M, Ishida T, Tasaka M 1999. Shoot apical meristem and cotyledon formation during Arabidopsis embryogenesis: Interaction among the CUP-SHAPED COTYLEDON and SHOOT MERISTEMLESS genes. Development 126:1563–1570 [DOI] [PubMed] [Google Scholar]
- Aida M, Vernoux T, Furutani M, Traas J, Tasaka M 2002. Roles of PIN-FORMED1 and MONOPTEROS in pattern formation of the apical region of the Arabidopsis embryo. Development 129:3965–3974 [DOI] [PubMed] [Google Scholar]
- Anastasiou E, Lenhard M 2007. Growing up to one’s standard. Current Opinion in Plant Biol 10:63–69 [DOI] [PubMed] [Google Scholar]
- Anastasiou E, Kenz S, Gerstung M, MacLean D, Timmer J, Fleck C, Lenhard M 2007. Control of plant organ size by KLUH/CYP78A5-dependent intercellular signaling. Develop Cell 13:843–856 [DOI] [PubMed] [Google Scholar]
- Bainbridge K, Guyomarc’h S, Bayer E, Swarup R, Bennett M, Mandel T, Kuhlemeier C 2008. Auxin influx carriers stabilize phyllotactic patterning. Genes Dev 22:810–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer EM, Smith RS, Mandel T, Nakayama N, Sauer M, Prusinkiewicz P, Kuhlemeier C 2009. Integration of transport-based models for phyllotaxis and midvein formation. Genes Dev 23:373–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamins R, Quint A, Weijers D, Hooykaas P, Offringa R 2001. The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development 128:4057–4067 [DOI] [PubMed] [Google Scholar]
- Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J 2003. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115:591–602 [DOI] [PubMed] [Google Scholar]
- Bennett SRM, Alvarez J, Bossinger G, Smyth DR 1995. Morphogenesis in pinoid mutants of Arabidopsis-thaliana. Plant Journal 8:505–520 [Google Scholar]
- Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schulz B, Feldmann KA 1996. Arabidopsis AUX1 gene: A permease-like regulator of root gravitropism. Science 273:948–950 [DOI] [PubMed] [Google Scholar]
- Boutte Y, Ikeda Y, Grebe M 2007. Mechanisms of auxin-dependent cell and tissue polarity. Curr Opin Plant Biol 10:616–623 [DOI] [PubMed] [Google Scholar]
- Bowman JL, Floyd SK 2008. Patterning and polarity in seed plant shoots. Annual Rev Plant Biol 59:67–88 [DOI] [PubMed] [Google Scholar]
- Braun N, Wyrzykowska J, Muller P, David K, Couch D, Perrot-Rechenmann C, Fleming AJ 2008. Conditional repression of AUXIN BINDING PROTEIN1 reveals that it coordinates cell division and cell expansion during postembryonic shoot development in Arabidopsis and tobacco. Plant Cell 20:2746–2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne ME, Barley R, Curtis M, Arroyo JM, Dunham M, Hudson A, Martienssen RA 2000. Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature 408:967–971 [DOI] [PubMed] [Google Scholar]
- Carabelli M, Possenti M, Sessa G, Ciolfi A, Sassi M, Morelli G, Ruberti I 2007. Canopy shade causes a rapid and transient arrest in leaf development through auxin-induced cytokinin oxidase activity. Genes Dev 21:1863–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Dai X, Zhao Y 2006. Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev 20:1790–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Dai X, Zhao Y 2007a. Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis. Plant Cell 19:2430–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Qin G, Dai X, Zhao Y 2007b. NPY1, a BTB-NPH3-like protein, plays a critical role in auxin-regulated organogenesis in Arabidopsis. Proc Natl Acad Sci 104:18825–18829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Qin G, Dai X, Zhao Y 2008. NPY genes and AGC kinases define two key steps in auxin-mediated organogenesis in Arabidopsis. Proc Natl Acad Sci 105:21017–21022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen SK, Dagenais N, Chory J, Weigel D 2000. Regulation of auxin response by the protein kinase PINOID. Cell 100:469–478 [DOI] [PubMed] [Google Scholar]
- de Reuille PB, Bohn-Courseau I, Ljung K, Morin H, Carraro N, Godin C, Traas J 2006. Computer simulations reveal properties of the cell-cell signaling network at the shoot apex in Arabidopsis. Proc Natl Acad Sci 103:1627–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dello Ioio R, Nakamura K, Moubayidin L, Perilli S, Taniguchi M, Morita MT, Aoyama T, Costantino P, Sabatini S 2008. A genetic framework for the control of cell division and differentiation in the root meristem. Science 322:1380–1384 [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Estelle M 2005a. The F-box protein TIR1 is an auxin receptor. Nature 435:441–445 [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, Ehrismann JS, Jurgens G, Estelle M 2005b. Plant development is regulated by a family of auxin receptor F box proteins. Dev Cell 9:109–119 [DOI] [PubMed] [Google Scholar]
- Dhonukshe P, Tanaka H, Goh T, Ebine K, Mahonen AP, Prasad K, Blilou I, Geldner N, Xu J, Uemura T, et al. 2008. Generation of cell polarity in plants links endocytosis, auxin distribution and cell fate decisions. Nature 456:962–966 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dinneny JR, Yadegari R, Fischer RL, Yanofsky MF, Weigel D 2004. The role of JAGGED in shaping lateral organs. Development 131:1101–1110 [DOI] [PubMed] [Google Scholar]
- Disch S, Anastasiou E, Sharma VK, Laux T, Fletcher JC, Lenhard M 2006. The E3 ubiquitin ligase BIG BROTHER controls Arabidopsis organ size in a dosage-dependent manner. Current Biol 16:272–279 [DOI] [PubMed] [Google Scholar]
- Ellis CM, Nagpal P, Young JC, Hagen G, Guilfoyle TJ, Reed JW 2005. AUXIN RESPONSE FACTOR1 and AUXIN RESPONSE FACTOR2 regulate senescence and floral organ abscission in Arabidopsis thaliana. Development 132:4563–4574 [DOI] [PubMed] [Google Scholar]
- Frigerio M, Alabadi D, Perez-Gomez J, Garcia-Carcel L, Phillips AL, Hedden P, Blazquez MA 2006. Transcriptional regulation of gibberellin metabolism genes by auxin signaling in Arabidopsis. Plant Physiol 142:553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jurgens G 2003. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426:147–153 [DOI] [PubMed] [Google Scholar]
- Friml J, Yang X, Michniewicz M, Weijers D, Quint A, Tietz O, Benjamins R, Ouwerkerk PB, Ljung K, Sandberg G, et al. 2004. A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science 306:862–865 [DOI] [PubMed] [Google Scholar]
- Fujino K, Matsuda Y, Ozawa K, Nishimura T, Koshiba T, Fraaije MW, Sekiguchi H 2008. NARROW LEAF 7 controls leaf shape mediated by auxin in rice. Mol Genet Genomics 279:499–507 [DOI] [PubMed] [Google Scholar]
- Furutani M, Kajiwara T, Kato T, Treml BS, Stockum C, Torres-Ruiz RA, Tasaka M 2007. The gene MACCHI-BOU 4/ENHANCER OF PINOID encodes a NPH3-like protein and reveals similarities between organogenesis and phototropism at the molecular level. Development 134:3849–3859 [DOI] [PubMed] [Google Scholar]
- Furutani M, Vernoux T, Traas J, Kato T, Tasaka M, Aida M 2004. PIN-FORMED1 and PINOID regulate boundary formation and cotyledon development in Arabidopsis embryogenesis. Development 131:5021–5030 [DOI] [PubMed] [Google Scholar]
- Gallavotti A, Barazesh S, Malcomber S, Hall D, Jackson D, Schmidt RJ, McSteen P 2008. Sparse inflorescence1 encodes a monocot-specific YUCCA-like gene required for vegetative and reproductive development in maize. Proc Natl Acad Sci 105:15196–15201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galweiler L, Guan C, Muller A, Wisman E, Mendgen K, Yephremov A, Palme K 1998. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282:2226–2230 [DOI] [PubMed] [Google Scholar]
- Giulini A, Wang J, Jackson D 2004. Control of phyllotaxy by the cytokinin-inducible response regulator homologue ABPHYL1. Nature 430:1031–1034 [DOI] [PubMed] [Google Scholar]
- Gordon SP, Chickarmane VS, Ohno C, Meyerowitz EM 2009. Multiple feedback loops through cytokinin signaling control stem cell number within the Arabidopsis shoot meristem. Proc Natl Acad Sci 106:16529–16534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SP, Heisler MG, Reddy GV, Ohno C, Das P, Meyerowitz EM 2007. Pattern formation during de novo assembly of the Arabidopsis shoot meristem. Development 134:3539–3548 [DOI] [PubMed] [Google Scholar]
- Hardtke CS, Berleth T 1998. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J 17:1405–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke CS, Ckurshumova W, Vidaurre DP, Singh SA, Stamatiou G, Tiwari SB, Hagen G, Guilfoyle TJ, Berleth T 2004. Overlapping and non-redundant functions of the Arabidopsis auxin response factors MONOPTEROS and NONPHOTOTROPIC HYPOCOTYL 4. Development 131:1089–1100 [DOI] [PubMed] [Google Scholar]
- Hay A, Barkoulas M, Tsiantis M 2006. ASYMMETRIC LEAVES1 and auxin activities converge to repress BREVIPEDICELLUS expression and promote leaf development in Arabidopsis. Development 133:3955–3961 [DOI] [PubMed] [Google Scholar]
- Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, Meyerowitz EM 2005. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr Biol 15:1899–1911 [DOI] [PubMed] [Google Scholar]
- Hu YX, Xie O, Chua NH 2003. The Arabidopsis auxin-inducible gene ARGOS controls lateral organ size. Plant Cell 15:1951–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Mitchum MG, Barnaby N, Ayele BT, Ogawa M, Nam E, Lai WC, Hanada A, Alonso JM, Ecker JR, et al. 2008. Potential sites of bioactive gibberellin production during reproductive growth in Arabidopsis. Plant Cell 20:320–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D, Hake S 1999. Control of phyllotaxy in maize by the abphyl1 gene. Development 126:315–323 [DOI] [PubMed] [Google Scholar]
- Jasinski S, Piazza P, Craft J, Hay A, Woolley L, Rieu I, Phillips A, Hedden P, Tsiantis M 2005. KNOX action in Arabidopsis is mediated by coordinate regulation of cytokinin and gibberellin activities. Curr Biol 15:1560–1565 [DOI] [PubMed] [Google Scholar]
- Jonsson H, Heisler MG, Shapiro BE, Meyerowitz EM, Mjolsness E 2006. An auxin-driven polarized transport model for phyllotaxis. Proc Natl Acad Sci 103:1633–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepinski S, Leyser O 2005. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435:446–451 [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J, Friml J 2008. Polar targeting and endocytic recycling in auxin-dependent plant development. Annu Rev Cell Dev Biol 24:447–473 [DOI] [PubMed] [Google Scholar]
- Krizek BA, Fletcher JC 2005. Molecular mechanisms of flower development: An armchair guide. Nat Rev Gen 6:688–698 [DOI] [PubMed] [Google Scholar]
- Laplaze L, Benkova E, Casimiro I, Maes L, Vanneste S, Swarup R, Weijers D, Calvo V, Parizot B, Herrera-Rodriguez MB, et al. 2007. Cytokinins act directly on lateral root founder cells to inhibit root initiation. Plant Cell 19:3889–3900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux T, Mayer KF, Berger J, Jurgens G 1996. The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122:87–96 [DOI] [PubMed] [Google Scholar]
- Lee SH, Cho HT 2006. PINOID positively regulates auxin efflux in Arabidopsis root hair cells and tobacco cells. Plant Cell 18:1604–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Johnston R, Yang Y, Gallavotti A, Kojima M, Travencolo BA, Costa Lda F, Sakakibara H, Jackson D 2009. Studies of aberrant phyllotaxy1 mutants of maize indicate complex interactions between auxin and cytokinin signaling in the shoot apical meristem. Plant Physiol 150:205–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibfried A, To JP, Busch W, Stehling S, Kehle A, Demar M, Kieber JJ, Lohmann JU 2005. WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature 438:1172–1175 [DOI] [PubMed] [Google Scholar]
- Lenhard M, Bohnert A, Jurgens G, Laux T 2001. Termination of stem cell maintenance in Arabidopsis floral meristems by interactions between WUSCHEL and AGAMOUS. Cell 105:805–814 [DOI] [PubMed] [Google Scholar]
- Leyser O 2006. Dynamic integration of auxin transport and signalling. Current Biology 16:R424–R433 [DOI] [PubMed] [Google Scholar]
- Li Y, Zheng L, Corke F, Smith C, Bevan MW 2008. Control of final seed and organ size by the DA1 gene family in Arabidopsis thaliana. Genes Dev 22:1331–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung K, Bhalerao RP, Sandberg G 2001. Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J 28:465–474 [DOI] [PubMed] [Google Scholar]
- Ljung K, Hull AK, Kowalczyk M, Marchant A, Celenza J, Cohen JD, Sandberg G 2002. Biosynthesis, conjugation, catabolism and homeostasis of indole-3-acetic acid in Arabidopsis thaliana. Plant Mol Biol 49:249–272 [PubMed] [Google Scholar]
- Lohmann JU, Hong RL, Hobe M, Busch MA, Parcy F, Simon R, Weigel D 2001. A molecular link between stem cell regulation and floral patterning in Arabidopsis. Cell 105:793–803 [DOI] [PubMed] [Google Scholar]
- Lyndon RF 1998. The shoot apical meristem: Its growth and development Cambridge University Press, Cambridge [England]; New York [Google Scholar]
- Mallory AC, Bartel DP, Bartel B 2005. MicroRNA-directed regulation of Arabidopsis AUXIN RESPONSE FACTOR17 is essential for proper development and modulates expression of early auxin response genes. Plant Cell 130:1360–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michniewicz M, Zago MK, Abas L, Weijers D, Schweighofer A, Meskiene I, Heisler MG, Ohno C, Zhang J, Huang F, et al. 2007. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 130:1044–1056 [DOI] [PubMed] [Google Scholar]
- Mizukami Y, Fischer RL 2000. Plant organ size control: AINTEGUMENTA regulates growth and cell numbers during organogenesis. Proc Natl Acad Sci 97:942–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller A, Guan C, Galweiler L, Tanzler P, Huijser P, Marchant A, Parry G, Bennett M, Wisman E, Palme K 1998. AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J 17:6903–6911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y 1991. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3:677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okushima Y, Overvoorde PJ, Arima K, Alonso JM, Chan A, Chang C, Ecker JR, Hughes B, Lui A, Nguyen D, et al. 2005. Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: Unique and overlapping functions of ARF7 and ARF19. Plant Cell 17:444–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overvoorde PJ, Okushima Y, Alonso JM, Chan A, Chang C, Ecker JR, Hughes B, Liu A, Onodera C, Quach H, et al. 2005. Functional genomic analysis of the AUXIN/INDOLE-3-ACETIC ACID gene family members in Arabidopsis thaliana. Plant Cell 17:3282–3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peaucelle A, Morin H, Traas J, Laufs P 2007. Plants expressing a miR164-resistant CUC2 gene reveal the importance of post-meristematic maintenance of phyllotaxy in Arabidopsis. Development 134:1045–1050 [DOI] [PubMed] [Google Scholar]
- Pekker I, Alvarez JP, Eshed Y 2005. Auxin response factors mediate Arabidopsis organ asymmetry via modulation of KANADI activity. Plant Cell 17:2899–2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrasek J, Friml J 2009. Auxin transport routes in plant development. Development 136:2675–2688 [DOI] [PubMed] [Google Scholar]
- Rast MI, Simon R 2008. The meristem-to-organ boundary: More than an extremity of anything. Current Opinion in Genetics & Development 18:287–294 [DOI] [PubMed] [Google Scholar]
- Reinhardt D, Mandel T, Kuhlemeier C 2000. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 12:507–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennett M, Traas J, Friml J, Kuhlemeier C 2003. Regulation of phyllotaxis by polar auxin transport. Nature 426:255–260 [DOI] [PubMed] [Google Scholar]
- Rieu I, Ruiz-Rivero O, Fernandez-Garcia N, Griffiths J, Powers SJ, Gong F, Linhartova T, Eriksson S, Nilsson O, Thomas SG, et al. 2008. The gibberellin biosynthetic genes AtGA20ox1 and AtGA20ox2 act, partially redundantly, to promote growth and development throughout the Arabidopsis life cycle. Plant J 53:488–504 [DOI] [PubMed] [Google Scholar]
- Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, et al. 1999. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99:463–472 [DOI] [PubMed] [Google Scholar]
- Sablowski R 2007. Flowering and determinacy in Arabidopsis. Journal of Experimental Botany 58:899–907 [DOI] [PubMed] [Google Scholar]
- Sachs T 1969. Polarity and the induction of organized vascular tissues. Ann Bot 33:263–275 [Google Scholar]
- Sauer M, Balla J, Luschnig C, Wisniewska J, Reinohl V, Friml J, Benkova E 2006. Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity. Genes Dev 20:2902–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpella E, Francis P, Berleth T 2004. Stage-specific markers define early steps of procambium development in Arabidopsis leaves and correlate termination of vein formation with mesophyll differentiation. Development 131:3445–3455 [DOI] [PubMed] [Google Scholar]
- Scarpella E, Marcos D, Friml J, Berleth T 2006. Control of leaf vascular patterning by polar auxin transport. Genes Dev 20:1015–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions A, Nemhauser JL, McColl A, Roe JL, Feldmann KA, Zambryski PC 1997. ETTIN patterns the Arabidopsis floral meristem and reproductive organs. Development 124:4481–4491 [DOI] [PubMed] [Google Scholar]
- Shani E, Yanai O, Ori N 2006. The role of hormones in shoot apical meristem function. Curr Opin Plant Biol 9:484–489 [DOI] [PubMed] [Google Scholar]
- Smith RS, Guyomarc’h S, Mandel T, Reinhardt D, Kuhlemeier C, Prusinkiewicz P 2006. A plausible model of phyllotaxis. Proc Natl Acad Sci 103:1301–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie DY, Dolezal K, Schlereth A, Jurgens G, Alonso JM 2008. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133:177–191 [DOI] [PubMed] [Google Scholar]
- Stoma S, Lucas M, Chopard J, Schaedel M, Traas J, Godin C 2008. Flux-based transport enhancement as a plausible unifying mechanism for auxin transport in meristem development. PLoS Comput Biol 4:e1000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R, Bennett M 2003. Auxin transport: The fountain of life in plants? Dev Cell 5:824–826 [DOI] [PubMed] [Google Scholar]
- Szecsi J, Joly C, Bordji K, Varaud E, Cock JM, Dumas C, Bendahmane M 2006. BIGPETALp, a bHLH transcription factor is involved in the control of Arabidopsis petal size. EMBO J 25:3912–3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Calderon-Villalobos LI, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N 2007. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446:640–645 [DOI] [PubMed] [Google Scholar]
- Tao Y, Ferrer JL, Ljung K, Pojer F, Hong F, Long JA, Li L, Moreno JE, Bowman ME, et al. 2008. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133:164–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobena-Santamaria R, Bliek M, Ljung K, Sandberg G, Mol JN, Souer E, Koes R 2002. FLOOZY of petunia is a flavin mono-oxygenase-like protein required for the specification of leaf and flower architecture. Genes Dev 16:753–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traas J, Doonan H 2001. Cellular basis of shoot apical meristem development. Int Rev Cytol 208:161–206 [DOI] [PubMed] [Google Scholar]
- Treml BS, Winderl S, Radykewicz R, Herz M, Schweizer G, Hutzler P, Glawischnig E, Ruiz RA 2005. The gene ENHANCER OF PINOID controls cotyledon development in the Arabidopsis embryo. Development 132:4063–4074 [DOI] [PubMed] [Google Scholar]
- Vernoux T, Kronenberger J, Grandjean O, Laufs P, Traas J 2000. PIN-FORMED 1 regulates cell fate at the periphery of the shoot apical meristem. Development 127:5157–5165 [DOI] [PubMed] [Google Scholar]
- Wang JW, Czech B, Weigel D 2009. miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 138:738–749 [DOI] [PubMed] [Google Scholar]
- Weijers D, Sauer M, Meurette O, Friml J, Ljung K, Sandberg G, Hooykaas P, Offringa R 2005. Maintenance of embryonic auxin distribution for apical-basal patterning by PIN-FORMED-dependent auxin transport in Arabidopsis. Plant Cell 17:2517–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward AW, Bartel B 2005. Auxin: Regulation, action, and interaction. Ann Bot (Lond) 95:707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegzouti H, Anthony RG, Jahchan N, Bogre L, Christensen SK 2006. Phosphorylation and activation of PINOID by the phospholipid signaling kinase 3-phosphoinositide-dependent protein kinase 1 (PDK1) in Arabidopsis. Proc Natl Acad Sci 103:6404–6409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, Weigel D, Chory J 2001. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291:306–309 [DOI] [PubMed] [Google Scholar]